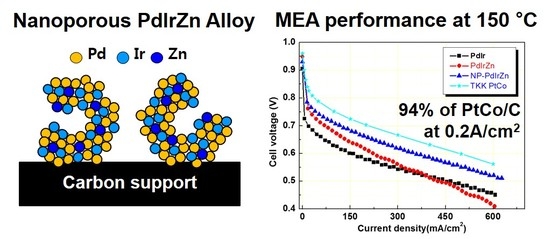
Preparation of Nanoporous PdIrZn Alloy Catalyst by Dissolving Excess ZnO for Cathode of High- Temperature Polymer Electrolyte Membrane Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
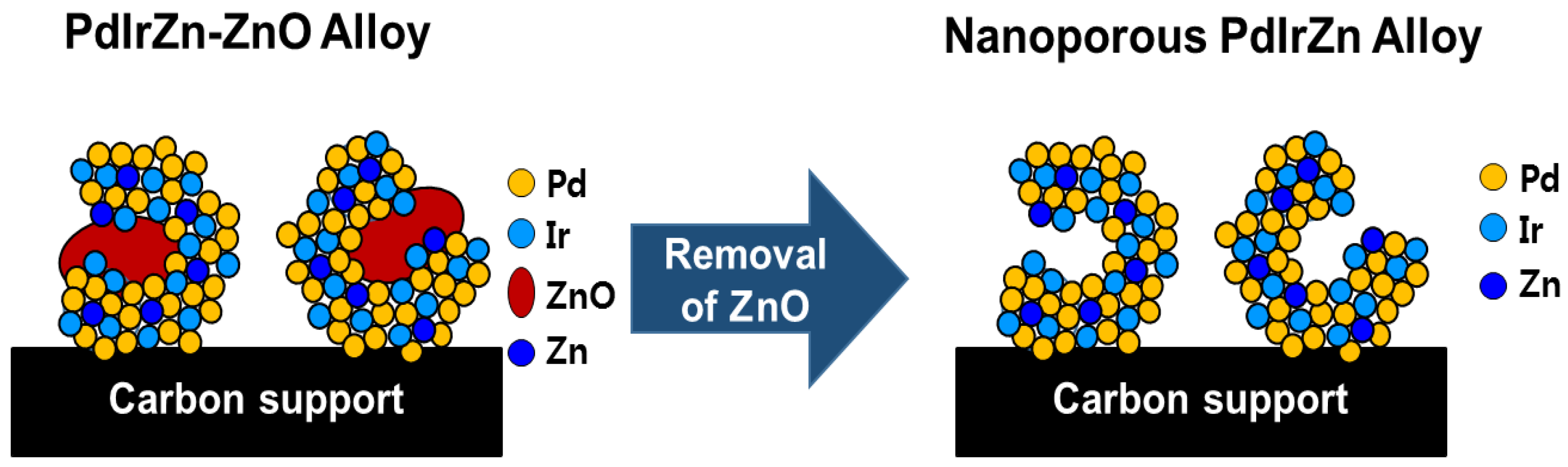
2.1. Preparation of Nanoporous PdIrZn Catalysts
2.2. Physicochemical and Electrochemical Characterization of Catalysts
2.3. Measurement of Membrane Electrode Assembly Performance
3. Results and Discussion
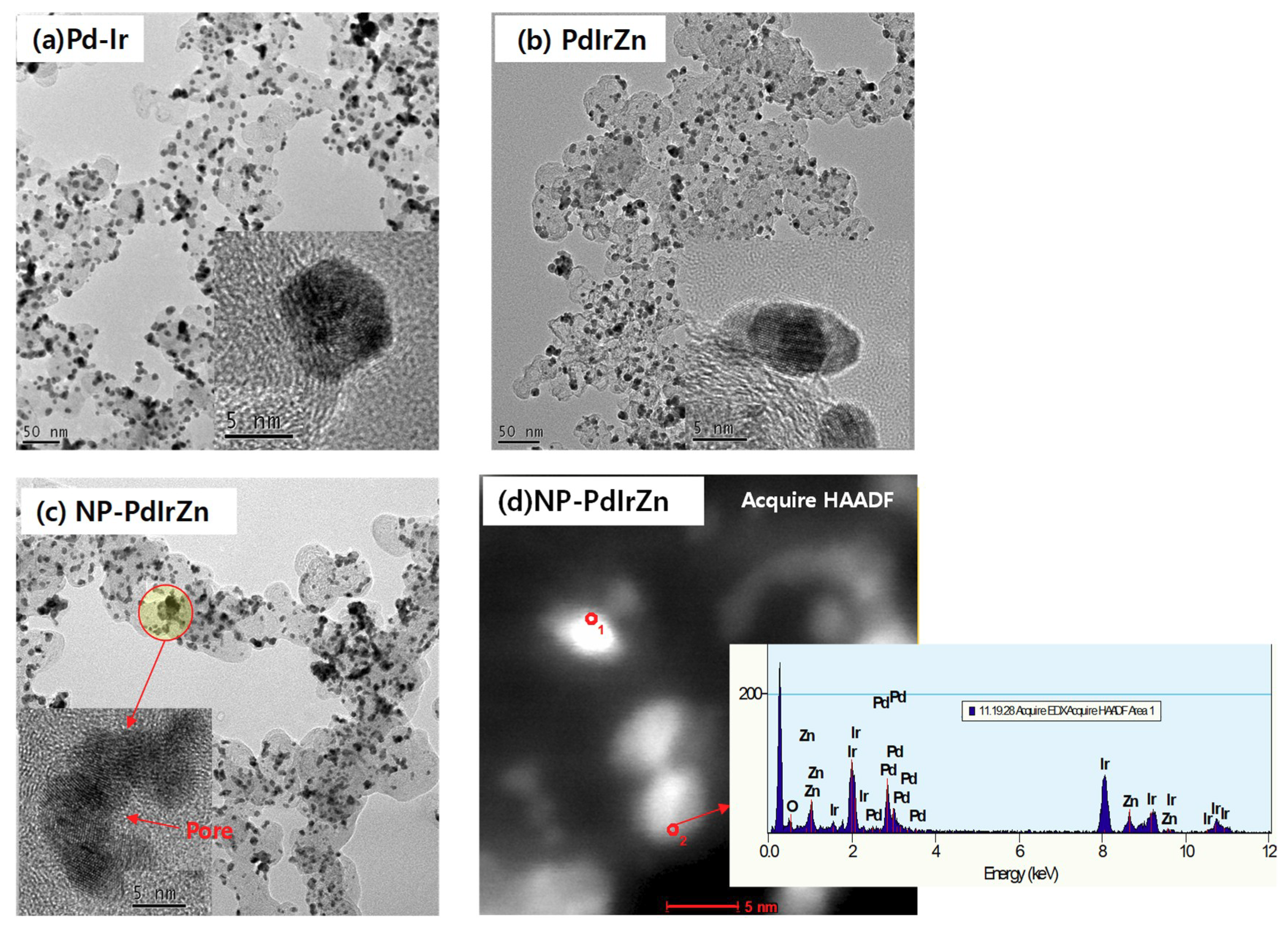
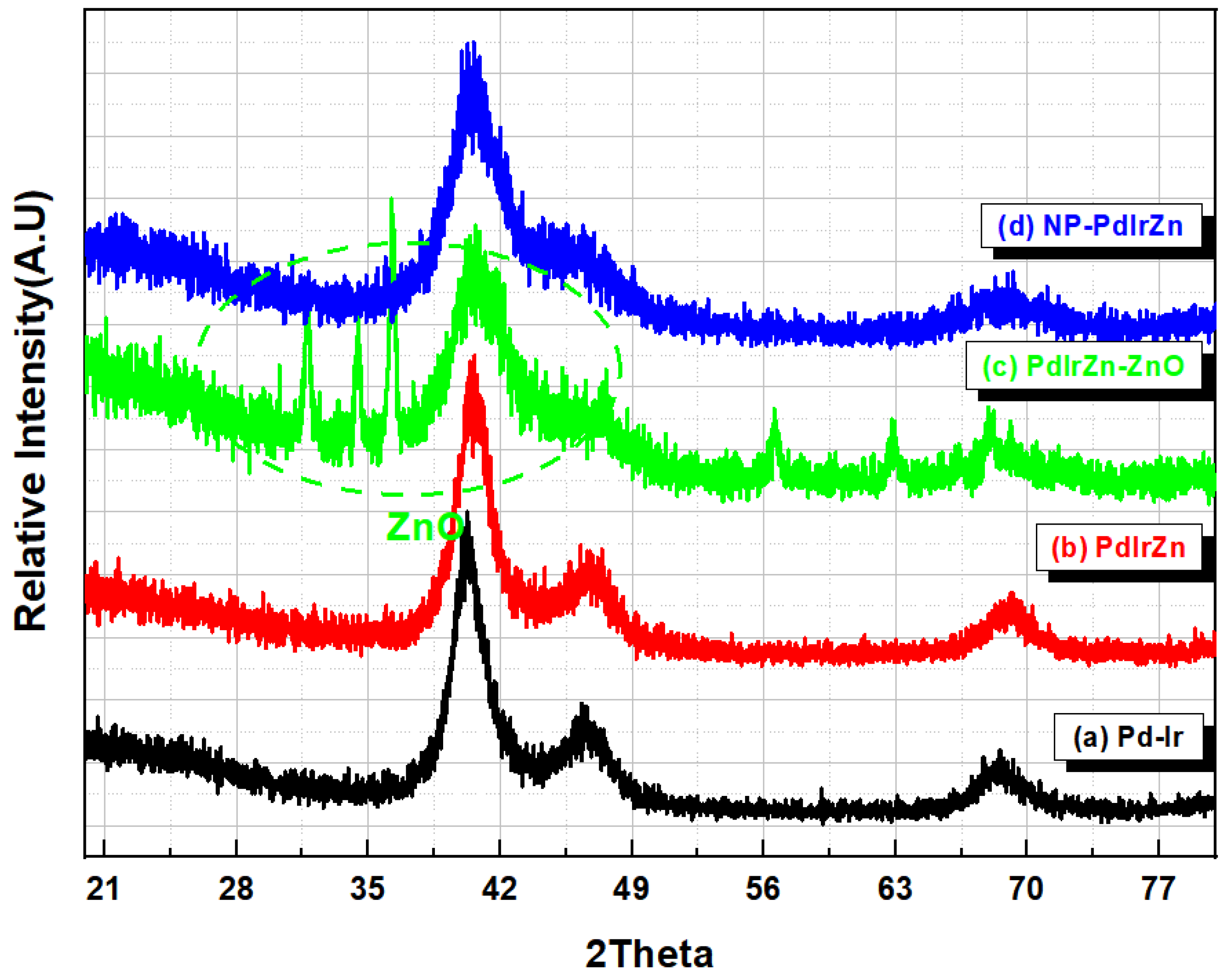
3.1. Physicochemical Characterization of Pd Alloy Catalysts
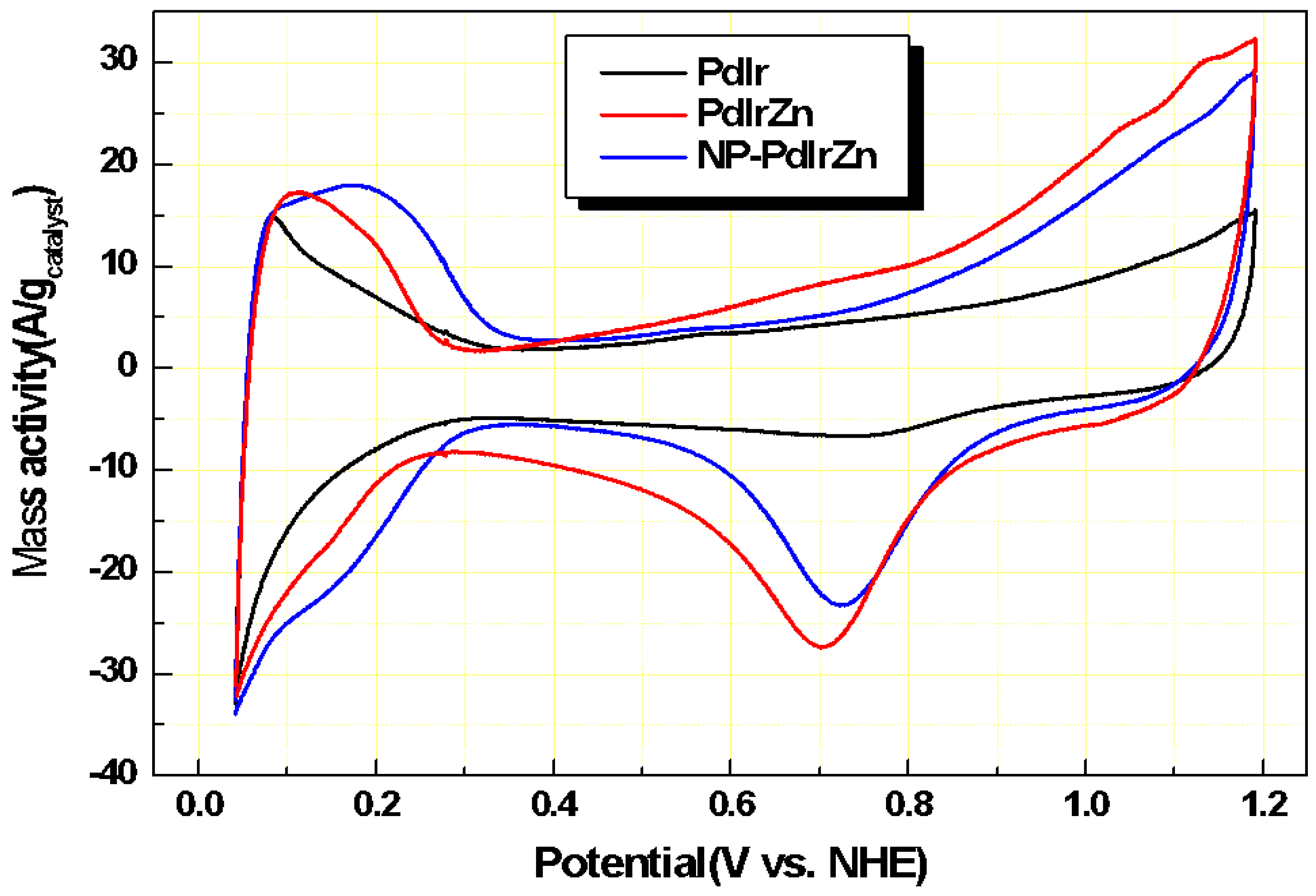
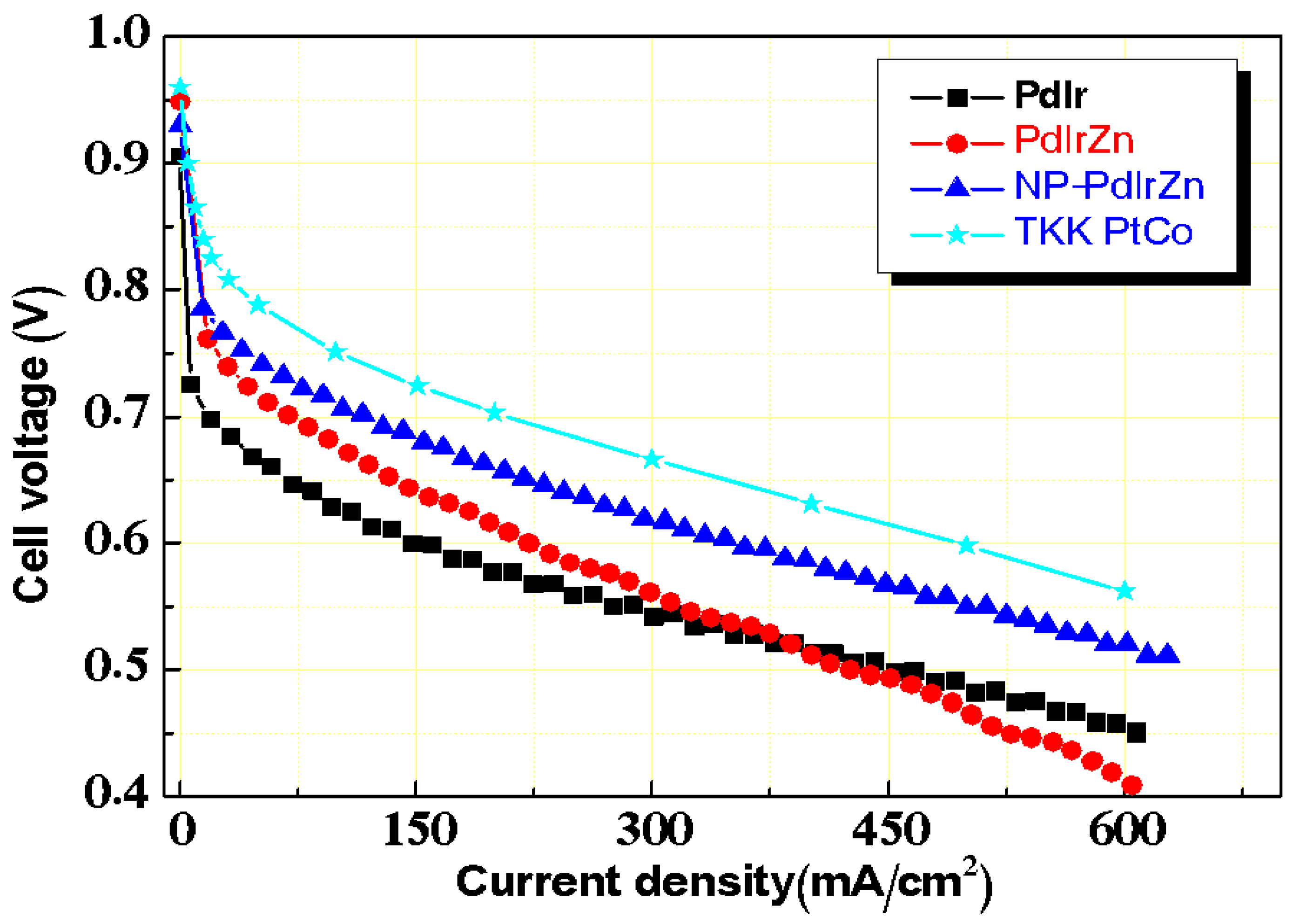
3.2. Electrochemical Properties of Pd Alloy Catalysts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Lehnert, W.; Janßen, H.; Samsun, R.C.; Stolten, D. A review of high-temperature polymer electrolyte membrane fuel-cell (HT-PEMFC)-based auxiliary power units for diesel-powered road vehicles. J. Power Sour. 2016, 311, 91–102. [Google Scholar] [CrossRef]
- Gurz, M.; Baltacioglu, E.; Hames, Y.; Kaya, K. The meeting of hydrogen and automotive: A review. Int. J. Hydrogen Energy 2017, 42, 23334–23346. [Google Scholar] [CrossRef]
- Kang, H.S.; Shin, Y.H. Analytical Study of Tri-Generation System Integrated with Thermal Management Using HT-PEMFC Stack. Energies 2019, 12, 3145. [Google Scholar] [CrossRef]
- Gwak, G.; Kim, M.; Kim, D.; Faizen, M.; Oh, K.; Lee, J.; Choi, J.; Lee, N.; Lim, K.; Ju, H. Performance and Efficiency Analysis of an HT-PEMFC System with an Absorption Chiller for Tri-Generation Applications. Energies 2019, 12, 905. [Google Scholar] [CrossRef]
- Escorihuela, J.; García-Bernabé, A.; Montero, Á.; Sahuquillo, Ó.; Giménez, E.; Compañ, V. Ionic Liquid Composite Polybenzimidazol Membranes for High Temperature PEMFC Applications. Polymers 2019, 11, 732. [Google Scholar] [CrossRef] [PubMed]
- Quartarone, E.; Angioni, S.; Mustarelli, P. Polymer and Composite Membranes for Proton-Conducting, High-Temperature Fuel Cells: A Critical Review. Materials 2017, 10, 687. [Google Scholar] [CrossRef]
- Hwang, K.; Kim, J.-H.; Kim, S.-Y.; Byun, H. Preparation of Polybenzimidazole-Based Membranes and Their Potential Applications in the Fuel Cell System. Energies 2014, 7, 1721–1732. [Google Scholar] [CrossRef]
- Kang, R.-J.; Chen, Y.-S. Experimental Study on the Effect of Hydrogen Sulfide on High-Temperature Proton Exchange Membrane Fuel Cells by Using Electrochemical Impedance Spectroscopy. Catalysts 2018, 8, 441. [Google Scholar] [CrossRef]
- Rosli, R.E.; Sulong, A.B.; Daud, W.R.W.; Zulkifley, M.A.; Husaini, T.; Rosli, M.I.; Majlan, E.H.; Haque, M.A. A review of high-temperature proton exchange membrane fuel cell (HT-PEMFC) system. Int. J. Hydrogen Energy 2017, 42, 9293–9314. [Google Scholar] [CrossRef]
- Chandan, A.; Hattenberger, M.; El-kharouf, A.; Du, S.; Dhir, A.; Self, V.; Pollet, B.G.; Ingram, A.; Bujalski, W. High Temperature (HT) polymer electrolyte membrane fuel cells (PEMFC)–A review. J. Power Sour. 2013, 231, 264–278. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, H.; Zhong, H.; Xu, T.; Jin, H. Carbon-supported Pd–Pt cathode electrocatalysts for proton exchange membrane fuel cells. J. Power Sour. 2011, 196, 3523–3529. [Google Scholar] [CrossRef]
- Zhou, W.P.; Sasaki, K.; Su, D.; Zhu, Y.; Wang, J.X.; Adzic, R.R. Gram-Scale-Synthesized Pd2Co-Supported Pt Monolayer Electrocatalysts for Oxygen Reduction Reaction. J. Phys. Chem. C 2010, 114, 8950–8957. [Google Scholar] [CrossRef]
- Salomé, S.; Ferraria, A.M.; do Rego, A.M.B.; Alcaide, F.; Savadogo, O.; Rego, R. Enhanced activity and durability of novel activated carbon-supported PdSn heat-treated cathode catalyst for polymer electrolyte fuel cells. Electrochim. Acta 2016, 192, 268–282. [Google Scholar] [CrossRef]
- Chandran, P.; Ghosh, A.; Ramaprabhu, S. High-performance Platinum free oxygen reduction reaction and hydrogen oxidation reaction catalyst in polymer electrolyte membrane fuel cell. Sci. Rep. 2018, 8, 3591. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.H.; Choi, S.M.; Lee, D.U.; Kim, W.B.; Chen, Z.W. Correlation between theoretical descriptor and catalytic oxygen reduction activity of graphene supported palladium and palladium alloy electrocatalysts. J. Power Sour. 2015, 300, 1–9. [Google Scholar] [CrossRef]
- Shao, M. Palladium-based electrocatalysts for hydrogen oxidation and oxygen reduction reactions. J. Power Sour. 2011, 196, 2433–2444. [Google Scholar] [CrossRef]
- Kariuki, N.N.; Wang, X.; Mawdsley, J.R.; Ferrandon, M.S.; Niyogi, S.G.; Vaughey, J.T.; Myers, D.J. Colloidal Synthesis and Characterization of Carbon-Supported Pd-Cu Nanoparticle Oxygen Reduction Electrocatalysts. Chem. Mater. 2010, 22, 4144–4152. [Google Scholar] [CrossRef]
- Wang, X.; Kariuki, N.; Vaughey, J.T.; Goodpaster, J.; Kumar, R.; Myers, D.J. Bimetallic Pd–Cu Oxygen Reduction Electrocatalysts. J. Electrochem. Soc. 2008, 155, B602–B609. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.; Wang, L.; Xu, L.; Bian, X.; Ma, H.; Ding, Y. Nanotubular Mesoporous PdCu Bimetallic Electrocatalysts toward Oxygen Reduction Reaction. Chem. Mater. 2009, 21, 3110–3116. [Google Scholar] [CrossRef]
- Zhao, J.; Sarkar, A.; Manthiram, A. Synthesis and characterization of Pd-Ni nanoalloy electrocatalysts for oxygen reduction reaction in fuel cells. Electrochim. Acta 2010, 55, 1756–1765. [Google Scholar] [CrossRef]
- Mandal, K.; Bhattacharjee, D.; Roy, P.S.; Bhattacharya, S.K.; Dasgupta, S. Room temperature synthesis of Pd–Cu nanoalloy catalyst with enhanced electrocatalytic activity for the methanol oxidation reaction. Appl. Catal. A Gen. 2015, 492, 100–106. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Qu, T.; Wang, D.; Kang, Z. Synthesis of Co-Fe-Pd nanoparticles via ultrasonic irradiation and their electro-catalytic activity for oxygen reduction reaction. Appl. Catal. A Gen. 2018, 560, 103–110. [Google Scholar] [CrossRef]
- Ang, S.-Y.; Walsh, D.A. Palladium–vanadium alloy electrocatalysts for oxygen reduction: Effect of heat treatment on electrocatalytic activity and stability. Appl. Catal. B Environ. 2010, 98, 49–56. [Google Scholar] [CrossRef]
- Bampos, G.; Kondarides, D.I.; Bebelis, S. Pd–Zn/C bimetallic electrocatalysts for oxygen reduction reaction. J. Appl. Electrochem. 2018, 48, 675–689. [Google Scholar] [CrossRef]
- Lee, Y.; Jang, J.; Lee, J.G.; Jeon, O.S.; Kim, H.S.; Hwang, H.J.; Shul, Y.G. Optimization of the Pd-Fe-Mo Catalysts for Oxygen Reduction Reaction in Proton-Exchange Membrane Fuel Cells. Electrochim. Acta 2016, 220, 29–35. [Google Scholar] [CrossRef]
- Yuan, W.; Scott, K.; Cheng, H. Fabrication and evaluation of Pt–Fe alloys as methanol tolerant cathode materials for direct methanol fuel cells. J. Power Sour. 2006, 163, 323–329. [Google Scholar] [CrossRef]
- You, D.J.; Jin, S.-A.; Lee, K.H.; Pak, C.; Choi, K.H.; Chang, H. Improvement of activity for oxygen reduction reaction by decoration of Ir on PdCu/C catalyst. Catal. Today 2012, 185, 138–142. [Google Scholar] [CrossRef]
- Yang, T.; Ma, Y.; Huang, Q.; Cao, G. Palladium–iridium nanocrystals for enhancement of electrocatalytic activity toward oxygen reduction reaction. Nano Energy 2016, 19, 257–268. [Google Scholar] [CrossRef]
- Ham, H.C.; Manogaran, D.; Lee, K.H.; Kwon, K.; Jin, S.-A.; You, D.J.; Pak, C.; Hwang, G.S. Communication: Enhanced oxygen reduction reaction and its underlying mechanism in Pd-Ir-Co trimetallic alloys. J. Chem. Phys. 2013, 139, 201104. [Google Scholar] [CrossRef]
- Park, S.H.; Choi, C.H.; Koh, J.K.; Pak, C.; Jin, S.-A.; Woo, S.I. Combinatorial High-Throughput Screening for Highly Active Pd−Ir−Ce Based Ternary Catalysts in Electrochemical Oxygen Reduction Reaction. ACS Comb. Sci. 2013, 15, 572–579. [Google Scholar] [CrossRef]
- Meku, E.; Du, C.; Wang, Y.; Du, L.; Sun, Y.; Kong, F.; Yin, G. Concentration Gradient Pd-Ir-Ni/C Electrocatalyst with Enhanced Activity and Methanol Tolerance for Oxygen Reduction Reaction in Acidic Medium. Electrochim. Acta 2016, 192, 177–187. [Google Scholar] [CrossRef]
- Meku, E.; Du, C.; Sun, Y.; Du, L.; Wang, Y.; Kong, F.; Yin, G. Composition optimization of ternary palladium–iridium–iron alloy catalysts for oxygen reduction reaction in acid medium. RSC Adv. 2016, 6, 22754–22763. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, E.; Pak, C. Comparison of the Characteristics of Pd-Ir-Y Ternary Alloy Catalyst Particles and Oxygen Reduction Activity According to Yttrium Contents. Trans. Korean Hydrogen New Energy Soc. 2018, 29, 260–266. [Google Scholar] [CrossRef]
- Yang, R.; Bian, W.; Strasser, P.; Toney, M.F. Dealloyed PdCu3 thin film electrocatalysts for oxygen reduction reaction. J. Power Sour. 2013, 222, 169–176. [Google Scholar] [CrossRef]
- Zhang, H.; Hao, Q.; Geng, H.; Xu, C. Nanoporous PdCu alloys as highly active and methanol-tolerant oxygen reduction electrocatalysts. Int. J. Hydrogen Energy 2013, 38, 10029–10038. [Google Scholar] [CrossRef]
- Duan, H.; Xu, C. Nanoporous PdZr surface alloy as highly active non-platinum electrocatalyst toward oxygen reduction reaction with unique structure stability and methanol-tolerance. J. Power Sour. 2016, 316, 106–113. [Google Scholar] [CrossRef]
- Duan, H.; Xu, C. Nanoporous PdCr alloys as highly active electrocatalysts for oxygen reduction reaction. Phys. Chem. Chem. Phys. 2016, 18, 4166–4173. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, B.H.; Kim, T.-Y.; Baik, C.; Kim, M.S.; Chai, G.S.; Pak, C. Multifunctional non-Pt ternary catalyst for the hydrogen oxidation and oxygen evolution reactions in reversal-tolerant anode. Catal. Commun. 2019, 103, 105758. [Google Scholar] [CrossRef]
- Park, Y.-B.; You, E.; Pak, C.; Min, M. Preparation and characterization of durable catalyst via diazonium reaction in PEMFC. Electrochim. Acta 2018, 284, 242–252. [Google Scholar] [CrossRef]
- Howard, B.H.; Killmeyer, R.P.; Rothenberger, K.S.; Cugini, A.V.; Morreale, B.D.; Enick, R.M.; Bustamante, F. Hydrogen permeance of palladium–copper alloy membranes over a wide range of temperatures and pressures. J. Membrane Sci. 2004, 241, 207–218. [Google Scholar] [CrossRef]
- You, D.J.; Kim, D.H.; De Lile, J.R.; Li, C.; Lee, S.G.; Kim, J.M.; Pak, C. Pd core-shell alloy catalysts for high-temperature polymer electrolyte membrane fuel cells: Effect of the core composition on the activity towards oxygen reduction reactions. Appl. Catal A Gen. 2018, 562, 250–257. [Google Scholar] [CrossRef]
- Kim, D.-H.; Min, C.-M.; Lee, E.; Lee, J.-S.; Pak, C. Effect of vinylphosphonic acid and polymer binders with phosphate groups on performance of high-temperature polymer electrolyte membrane fuel cell. Catal. Today 2019, in press. [Google Scholar] [CrossRef]
- Polarz, S.; Smarsly, B. Nanoporous Materials. J. Nanosci. Nanotechnol. 2002, 2, 581–612. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, P.; Li, Y. General Preparation for Pt-based alloy nanoporous nanoparticles as potential nanocatalysts. Sci. Rep. 2011, 1, 37. [Google Scholar] [CrossRef] [PubMed]
- Erlebacher, J.; Aziz, M.J.; Karma, A.; Dimitrov, N.; Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 2001, 410, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, X.; Huo, S.; Chen, Z.; Zhao, H.; Xu, J. Facile synthesis of PdIr nanoporous aggregates as highly active electrocatalyst towards methanol and ethylene glycol oxidation. Catal. Toda 2018, 318, 157–166. [Google Scholar] [CrossRef]
- Yi, L.; Wei, W.; Zhao, C.; Yang, C.; Tian, L.; Liu, J.; Wang, X. Electrochemical oxidation of sodium borohydride on carbon supported Pt-Zn nanoparticle bimetallic catalyst and its implications to direct borohydride-hydrogen peroxide fuel cell. Electrochim. Acta 2015, 158, 209–218. [Google Scholar] [CrossRef]
- Behmenyar, G.; Akın, A.N. Investigation of carbon supported Pd-Cu nanoparticles as anode catalysts for direct borohydride fuel cell. J. Power Sour. 2014, 249, 239–246. [Google Scholar] [CrossRef]
- Yogamalar, R.; Srinivasan, R.; Vinu, A.; Ariga, K.; Bose, A.C. X-ray peak broadening analysis in ZnO nanoparticles. Solid State Comm. 2009, 149, 1919–1923. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Bose, A.C.; Rameshbabu, N. X-ray peak broadening studies of nanocrystalline hydroxyapatite by Williamson-Hall analysis. Physica B Conden. Matter 2010, 405, 4256–4261. [Google Scholar] [CrossRef]
- Stevens, D.A.; Dahn, J.R. Electrochemical Characterization of the Active Surface in Carbon-Supported Platinum Electrocatalysts for PEM Fuel Cells. J. Electrochem. Soc. 2003, 150, A770–A775. [Google Scholar] [CrossRef]
- Corradini, P.G.; Pires, F.I.; Paganin, V.A.; Perez, J.; Antolini, E. Effect of the relationship between particle size, inter-particle distance, and metal loading of carbon supported fuel cell catalysts on their catalytic activity. J. Nanopart. Res. 2012, 14, 1080. [Google Scholar] [CrossRef]
- Yang, R.; Leisch, J.; Strasser, P.; Toney, M.F. Structure of Dealloyed PtCu3 Thin Films and Catalytic Activity for Oxygen Reduction. Chem. Mater. 2010, 22, 4712–4720. [Google Scholar] [CrossRef]

| Catalyst | Pd (wt.%) | Ir (wt.%) | Zn (wt.%) |
|---|---|---|---|
| PdIr | 19.8 | 30.1 | 0.0 |
| PdIrZn | 19.1 | 31.0 | 3.0 |
| PdIrZn-ZnO | 18.3 | 31.5 | 10.3 |
| NP-PdIrZn | 19.7 | 34.1 | 3.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, D.J.; Kim, D.-H.; Kim, J.M.; Pak, C. Preparation of Nanoporous PdIrZn Alloy Catalyst by Dissolving Excess ZnO for Cathode of High- Temperature Polymer Electrolyte Membrane Fuel Cells. Energies 2019, 12, 4155. https://doi.org/10.3390/en12214155
You DJ, Kim D-H, Kim JM, Pak C. Preparation of Nanoporous PdIrZn Alloy Catalyst by Dissolving Excess ZnO for Cathode of High- Temperature Polymer Electrolyte Membrane Fuel Cells. Energies. 2019; 12(21):4155. https://doi.org/10.3390/en12214155
Chicago/Turabian StyleYou, Dae Jong, Do-Hyung Kim, Ji Man Kim, and Chanho Pak. 2019. "Preparation of Nanoporous PdIrZn Alloy Catalyst by Dissolving Excess ZnO for Cathode of High- Temperature Polymer Electrolyte Membrane Fuel Cells" Energies 12, no. 21: 4155. https://doi.org/10.3390/en12214155
APA StyleYou, D. J., Kim, D.-H., Kim, J. M., & Pak, C. (2019). Preparation of Nanoporous PdIrZn Alloy Catalyst by Dissolving Excess ZnO for Cathode of High- Temperature Polymer Electrolyte Membrane Fuel Cells. Energies, 12(21), 4155. https://doi.org/10.3390/en12214155