Effects of the Carbon Support Doping with Nitrogen for the Hydrogen Production from Formic Acid over Ni Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Instrumental Methods
2.3. Catalytic Measurements
3. Results and Discussion
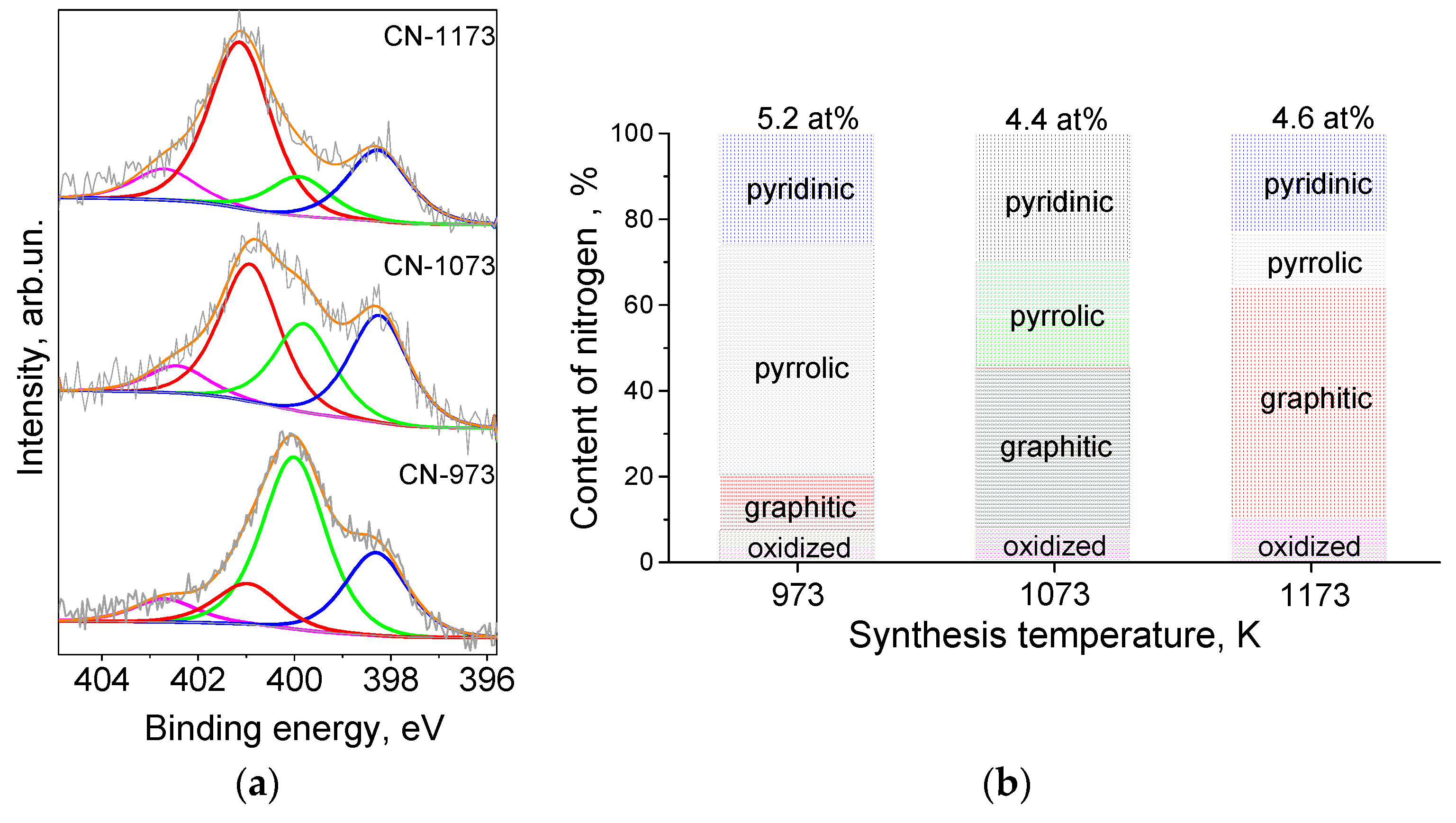
3.1. Characterization of the Supports
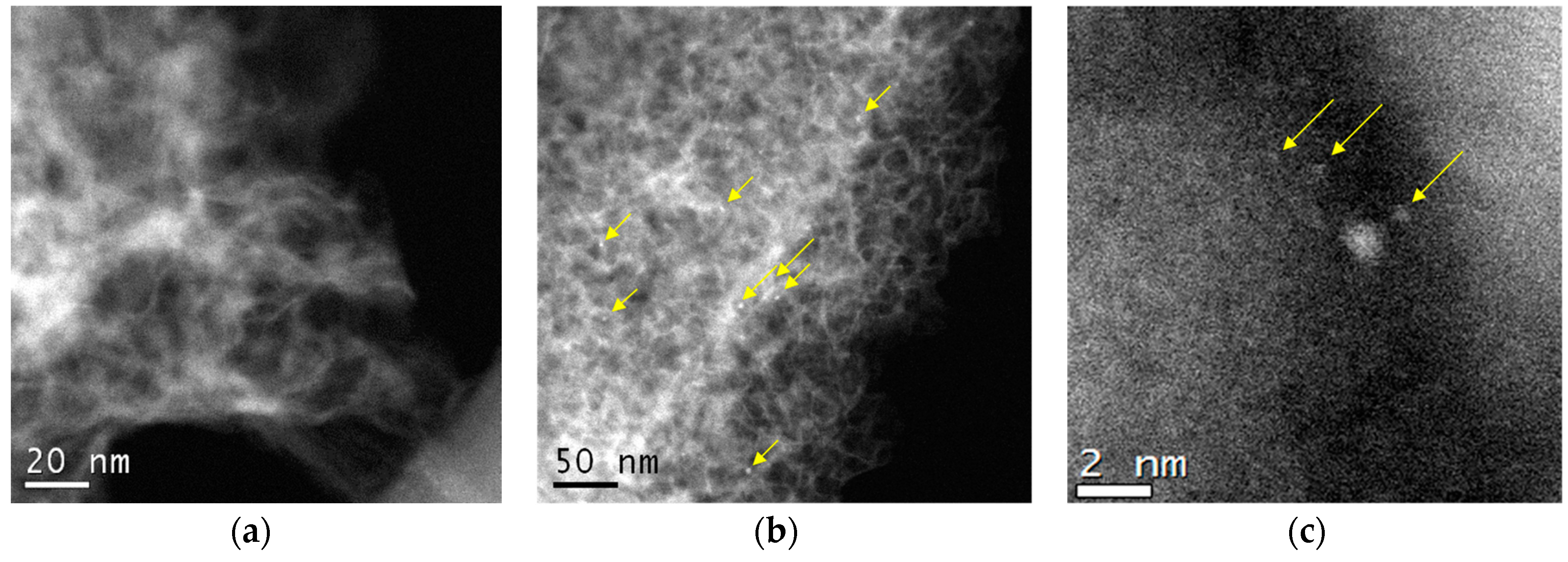
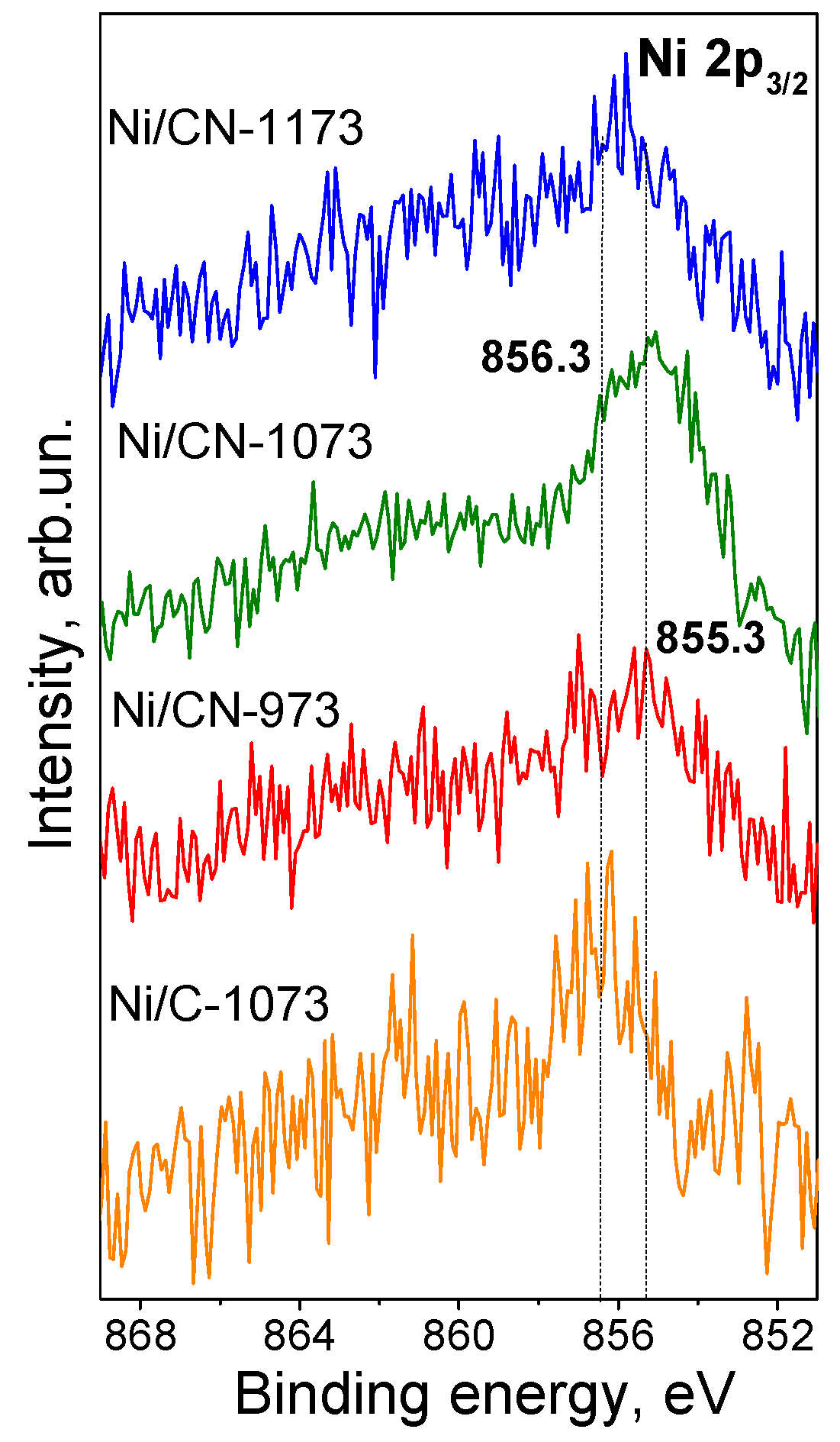
3.2. Characterization of the Catalysts
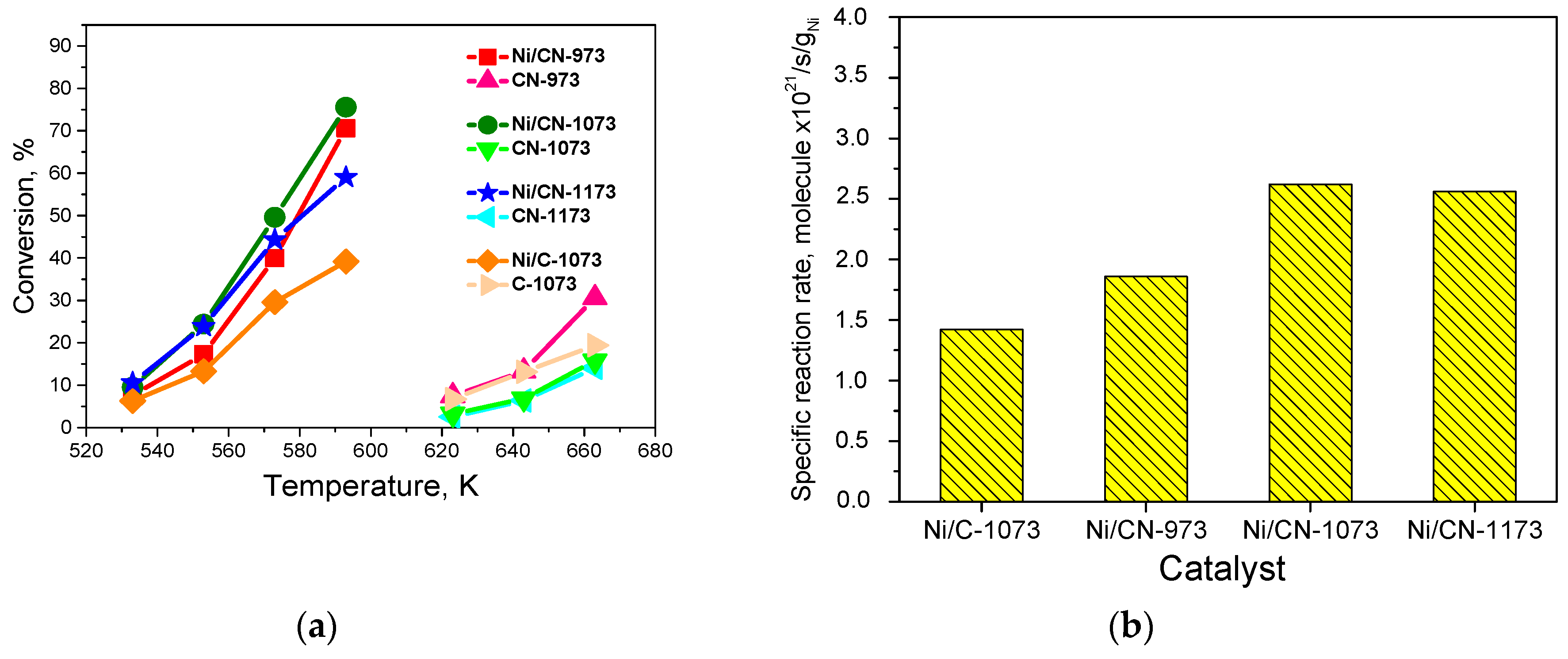
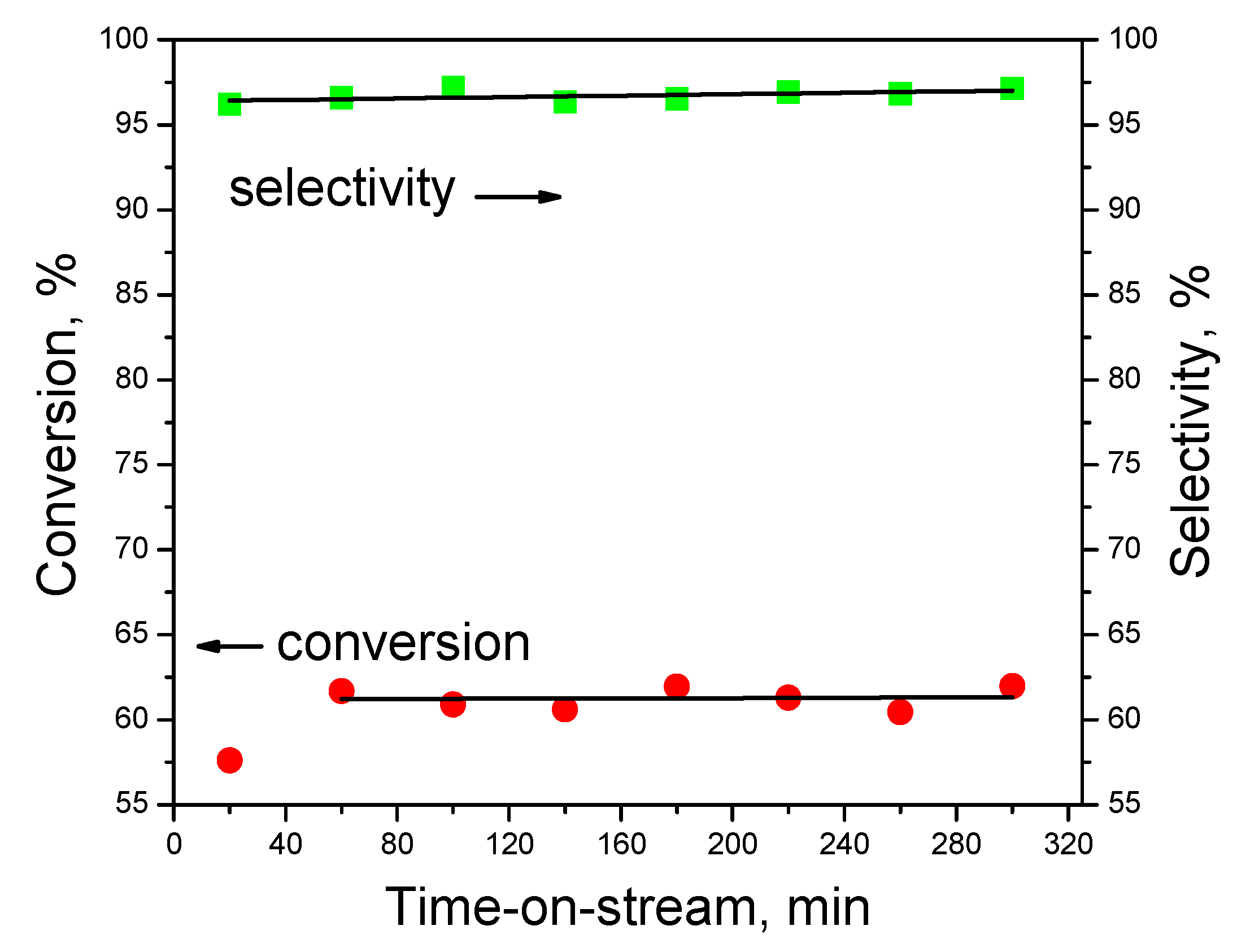
3.3. Catalytic Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| BET | Brunauer–Emmett–Teller |
| DFT | Density Functional Theory |
| EXAFS | Extended X-ray Absorption Fine Structure |
| HAADF/STEM | high angle annular dark field/scanning transmission electron microscopy |
| HRTEM | high resolution transmission electron microscopy |
| XPS | X-ray photoelectron spectroscopy |
References
- Bulushev, D.A.; Ross, J.R.H. Towards Sustainable Production of Formic Acid. ChemSusChem 2018, 11, 821–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulushev, D.A.; Ross, J.R.H. Heterogeneous Catalysts for Hydrogenation of CO2 and Bicarbonates to Formic Acid and Formates. Catal. Rev. 2018, 60, 566–593. [Google Scholar] [CrossRef]
- Koroteev, V.O.; Bulushev, D.A.; Chuvilin, A.L.; Okotrub, A.V.; Bulusheva, L.G. Nanometer-Sized MoS2 Clusters on Graphene Flakes for Catalytic Formic Acid Decomposition. ACS Catal. 2014, 4, 3950–3956. [Google Scholar] [CrossRef]
- Kurnia, I.; Yoshida, A.; Situmorang, Y.A.; Kasai, Y.; Abudula, A.; Guan, G. Utilization of Dealkaline Lignin as a Source of Sodium-Promoted MoS2/Mo2C Hybrid Catalysts for Hydrogen Production from Formic Acid. ACS Sustain. Chem. Eng. 2019, 7, 8670–8677. [Google Scholar] [CrossRef]
- Koós, Á.; Solymosi, F. Production of CO-Free H2 by Formic Acid Decomposition over Mo2C/Carbon Catalysts. Catal. Lett. 2010, 138, 23–27. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Chuvilin, A.L.; Sobolev, V.I.; Stolyarova, S.G.; Shubin, Y.V.; Asanov, I.P.; Ishchenko, A.V.; Magnani, G.; Riccò, M.; Okotrub, A.V.; et al. Copper on Carbon Materials: Stabilization by Nitrogen Doping. J. Mater. Chem. A 2017, 5, 10574–10583. [Google Scholar] [CrossRef]
- Pechenkin, A.; Badmaev, S.; Belyaev, V.; Sobyanin, V. Production of Hydrogen-Rich Gas by Formic Acid Decomposition over Cuo-CeO2/γ-Al2o3 Catalyst. Energies 2019, 12, 3577. [Google Scholar] [CrossRef]
- Bing, Q.M.; Liu, W.; Yi, W.C.; Liu, J.Y. Ni Anchored C2N Monolayers as Low-Cost and Efficient Catalysts for Hydrogen Production from Formic Acid. J. Power Sources 2019, 413, 399–407. [Google Scholar] [CrossRef]
- Fujitsuka, H.; Nakagawa, K.; Hanprerakriengkrai, S.; Nakagawa, H.; Tago, T. Hydrogen Production from Formic Acid Using Pd/C, Pt/C, and Ni/C Catalysts Prepared from Ion-Exchange Resins. J. Chem. Eng. Jpn. 2019, 52, 423–429. [Google Scholar] [CrossRef]
- Iglesia, E.; Boudart, M. Decomposition of Formic Acid on Copper, Nickel, and Copper-Nickel Alloys: Iii. Catalytic Decomposition on Nickel and Copper-Nickel Alloys. J. Catal. 1983, 81, 224–238. [Google Scholar] [CrossRef]
- Yang, G.; Han, H.; Li, T.; Du, C. Synthesis of Nitrogen-Doped Porous Graphitic Carbons Using Nano-CaCO3 as Template, Graphitization Catalyst, and Activating Agent. Carbon 2012, 50, 3753–3765. [Google Scholar] [CrossRef]
- Xia, Y.; Mokaya, R. Synthesis of Ordered Mesoporous Carbon and Nitrogen-Doped Carbon Materials with Graphitic Pore Walls Via a Simple Chemical Vapor Deposition Method. Adv. Mater. 2004, 16, 1553–1558. [Google Scholar] [CrossRef]
- Zacharska, M.; Bulusheva, L.G.; Lisitsyn, A.S.; Beloshapkin, S.; Guo, Y.; Chuvilin, A.L.; Shlyakhova, E.V.; Podyacheva, O.Y.; Leahy, J.J.; Okotrub, A.V.; et al. Factors Influencing the Performance of Pd/C Catalysts in the Green Production of Hydrogen from Formic Acid. ChemSusChem 2017, 10, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Navlani-García, M.; Mori, K.; Salinas-Torres, D.; Kuwahara, Y.; Yamashita, H. New Approaches toward the Hydrogen Production from Formic Acid Dehydrogenation over Pd-Based Heterogeneous Catalysts. Front. Mater. 2019, 6. [Google Scholar] [CrossRef]
- Salinas-Torres, D.; Navlani-García, M.; Mori, K.; Kuwahara, Y.; Yamashita, H. Nitrogen-Doped Carbon Materials as a Promising Platform toward the Efficient Catalysis for Hydrogen Generation. Appl. Catal. A Gen. 2018, 571, 25–41. [Google Scholar] [CrossRef]
- Yamada, Y.; Suzuki, Y.; Yasuda, H.; Uchizawa, S.; Hirose-Takai, K.; Sato, Y.; Suenaga, K.; Sato, S. Functionalized Graphene Sheets Coordinating Metal Cations. Carbon 2014, 75, 81–94. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Y.; Qi, H.; Zhang, L.; Yan, W.; Liu, X.; Yang, X.; Miao, S.; Wang, W.; Liu, C.; et al. A Durable Nickel Single-Atom Catalyst for Hydrogenation Reactions and Cellulose Valorization under Harsh Conditions. Angew. Chem. Int. Ed. 2018, 57, 7071–7075. [Google Scholar] [CrossRef]
- Jiang, K.; Siahrostami, S.; Akey, A.J.; Li, Y.; Lu, Z.; Lattimer, J.; Hu, Y.; Stokes, C.; Gangishetty, M.; Chen, G.; et al. Transition-Metal Single Atoms in a Graphene Shell as Active Centers for Highly Efficient Artificial Photosynthesis. Chem 2017, 3, 950–960. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Zhao, S.; Johannessen, B.; Veder, J.-P.; Saunders, M.; Rowles, M.R.; Cheng, M.; Liu, C.; Chisholm, M.F.; Marco, R.; et al. Atomically Dispersed Transition Metals on Carbon Nanotubes with Ultrahigh Loading for Selective Electrochemical Carbon Dioxide Reduction. Adv. Mater. 2018, 30, 1706287. [Google Scholar] [CrossRef]
- Yang, J.; Qiu, Z.; Zhao, C.; Wei, W.; Chen, W.; Li, Z.; Qu, Y.; Dong, J.; Luo, J.; Li, Z.; et al. In Situ Thermal Atomization to Convert Supported Nickel Nanoparticles into Surface-Bound Nickel Single-Atom Catalysts. Angew. Chem. 2018, 130, 14291–14296. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, S.; Li, H.; He, S.; Veder, J.-P.; Johannessen, B.; Xiao, J.; Lu, S.; Pan, J.; Chisholm, M.F.; et al. Unsaturated Edge-Anchored Ni Single Atoms on Porous Microwave Exfoliated Graphene Oxide for Electrochemical CO2. Appl. Catal. B Environ. 2019, 243, 294–303. [Google Scholar] [CrossRef]
- Zhong, W.; Liu, Y.; Deng, M.; Zhang, Y.; Jia, C.; Prezhdo, O.V.; Yuan, J.; Jiang, J. C2N-Supported Single Metal Ion Catalysts for HCOOH Dehydrogenation. J. Mater. Chem. A 2018, 6, 11105–11112. [Google Scholar] [CrossRef]
- Shlyakhova, E.V.; Bulusheva, L.G.; Kanygin, M.A.; Plyusnin, P.E.; Kovalenko, K.A.; Senkovskiy, B.V.; Okotrub, A.V. Synthesis of Nitrogen-Containing Porous Carbon Using Calcium Oxide Nanoparticles. Phys. Status Solidi B 2014, 251, 2607–2612. [Google Scholar] [CrossRef]
- Golub, F.S.; Beloshapkin, S.; Gusel’nikov, A.V.; Bolotov, V.A.; Parmon, V.N.; Bulushev, D.A. Boosting Hydrogen Production from Formic Acid over Pd Catalysts by Deposition of N-Containing Precursors on the Carbon Support. Energies 2019, 12, 3885. [Google Scholar] [CrossRef]
- Okotrub, A.V.; Fedorovskaya, E.O.; Senkovskiy, B.V.; Bulusheva, L.G. Nitrogen Species in Few-Layer Graphene Produced by Thermal Exfoliation of Fluorinated Graphite Intercalation Compounds. Phys. Status Solidi B 2015, 252, 2444–2450. [Google Scholar] [CrossRef]
- Berríos, C.; Cárdenas-Jirón, G.I.; Marco, J.F.; Gutiérrez, C.; Ureta-Zañartu, M.S. Theoretical and Spectroscopic Study of Nickel(Ii) Porphyrin Derivatives. J. Phys. Chem. A 2007, 111, 2706–2714. [Google Scholar] [CrossRef]
- Iglesia, E.; Boudart, M. Decomposition of Formic-Acid on Copper, Nickel, and Copper Nickel-Alloys. IV. Temperature-Programmed Decomposition of Bulk Nickel Formate and of Formic-Acid Preadsorbed on Nickel Powder. J. Catal. 1984, 88, 325–332. [Google Scholar] [CrossRef]

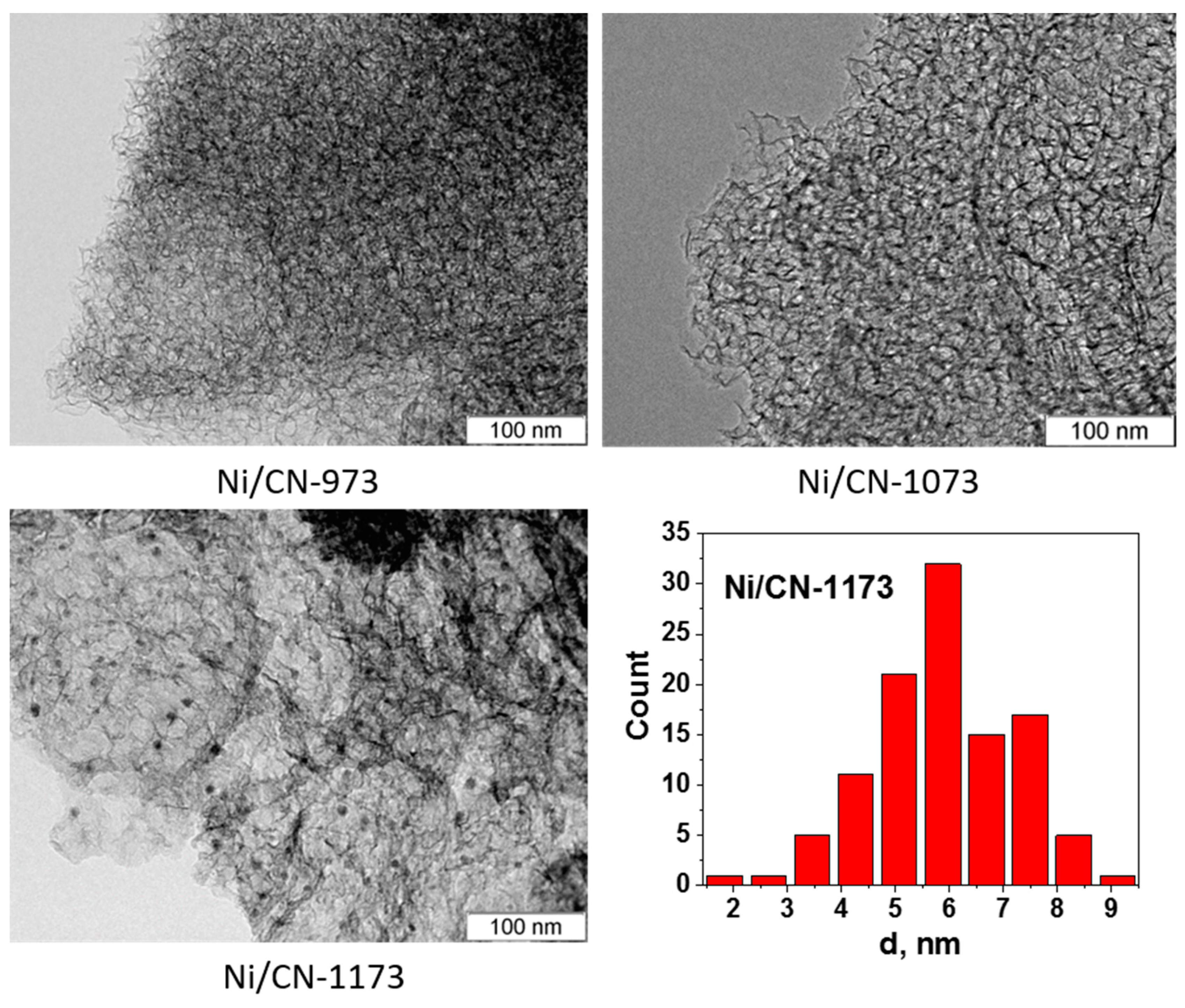
| Support | BET Surface Area, m2 g−1 | Content of N, at% (XPS) | Content of O, at% (XPS) | Catalyst | Mean Ni Particle Size, nm |
|---|---|---|---|---|---|
| C-1073 | 966 | – | 3.0 ± 0.5 | Ni/C-1073 | 3.9 ± 1.2 |
| CN-973 | 866 | 5.2 ± 0.5 | 6.2 ± 0.5 | Ni/CN-973 | <3 nm (rarely seen) |
| CN-1073 | 443 | 4.4 ± 0.5 | 3.2 ± 0.5 | Ni/CN-1073 | <3 nm (rarely seen) |
| CN-1173 | 407 | 4.6 ± 0.5 | 2.2 ± 0.5 | Ni/CN-1173 | 5.5 ± 1.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishchakova, A.D.; Bulushev, D.A.; Stonkus, O.A.; Asanov, I.P.; Ishchenko, A.V.; Okotrub, A.V.; Bulusheva, L.G. Effects of the Carbon Support Doping with Nitrogen for the Hydrogen Production from Formic Acid over Ni Catalysts. Energies 2019, 12, 4111. https://doi.org/10.3390/en12214111
Nishchakova AD, Bulushev DA, Stonkus OA, Asanov IP, Ishchenko AV, Okotrub AV, Bulusheva LG. Effects of the Carbon Support Doping with Nitrogen for the Hydrogen Production from Formic Acid over Ni Catalysts. Energies. 2019; 12(21):4111. https://doi.org/10.3390/en12214111
Chicago/Turabian StyleNishchakova, Alina D., Dmitri A. Bulushev, Olga A. Stonkus, Igor P. Asanov, Arcady V. Ishchenko, Alexander V. Okotrub, and Lyubov G. Bulusheva. 2019. "Effects of the Carbon Support Doping with Nitrogen for the Hydrogen Production from Formic Acid over Ni Catalysts" Energies 12, no. 21: 4111. https://doi.org/10.3390/en12214111
APA StyleNishchakova, A. D., Bulushev, D. A., Stonkus, O. A., Asanov, I. P., Ishchenko, A. V., Okotrub, A. V., & Bulusheva, L. G. (2019). Effects of the Carbon Support Doping with Nitrogen for the Hydrogen Production from Formic Acid over Ni Catalysts. Energies, 12(21), 4111. https://doi.org/10.3390/en12214111







