3.2. Preparation of the Different Electrolytes (NaOH Concentration and Biomass Content)
Table 3 shows the experimental results achieved regarding the first round of experiences in the electrolyser, with and without different liquified biomass contents, for two sodium hydroxide electrolyte concentrations. The parameters measured for each experiment were, the electrolyte temperature in the electrolyser (
T), the input voltage (V), the current intensity (I), the final volumetric flow of gas produced, the initial and final pH (pHi and pHf), as well, the initial and final conductivity (Ki and Kf). All the values pointed in the table are averages values calculated from 4–5 experiences performed for each case, at the end of 120 min.
This table also shows the calculated correspondent relative variations of pH (ΔpH) and conductivity (ΔK). Through these results, it is possible to conclude that, with the exception of the 15% (w/w) of biomass content experiment, the increase in the liquified biomass in the electrolyte decreases the final temperature in the electrolyser and, also, the current intensity, as well, the produced gas volumetric flow, since, both parameters are related. The justification is directly related with the electrolysis conversion and the gas flow observed, which are proportional to the current intensity and, also, to the heat produced in the electrolyser, through Joule’s effect. For the same electrolyte quantity, less current intensity will decrease the heat released and the electrolyser temperature. It’s possible to see, also, that, the presence of organic compounds, such as, the liquified biomass, mixed in the electrolyte, decreases significantly the conductivity values. It’s well known that, organic compounds have very low electrical conductivity values, thus affecting negatively the electrolysis conversion process, decreasing the electrolyte final conductivity, when compared with the sodium hydroxide conductivity values. This is confirmed by these results, which affected also, the final pH of the electrolyte, decreasing it.
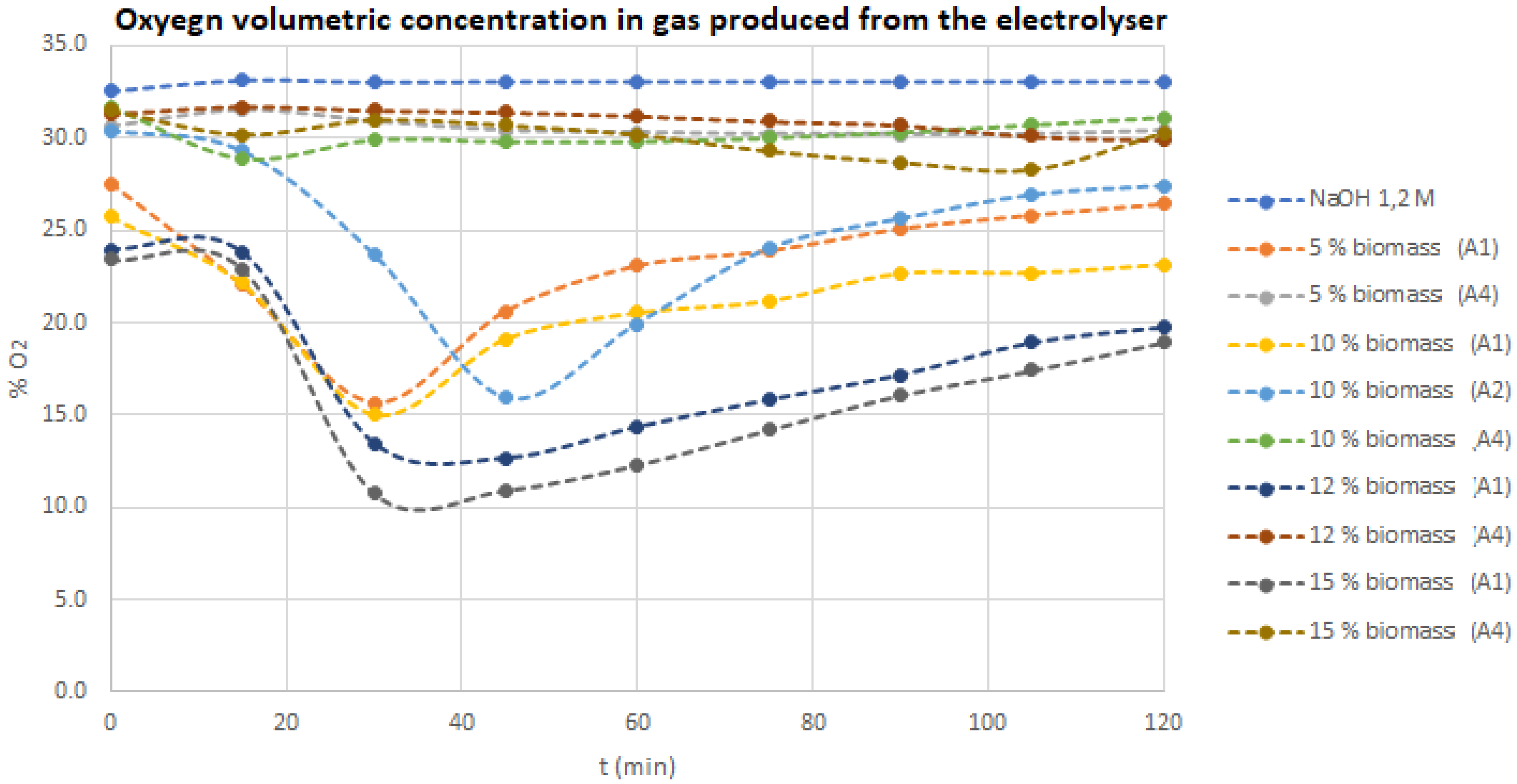
In order to understand the evolution of the oxygen concentration in the electrolyser, over time, for different tested electrolytes (with and without liquified biomass),
Figure 4 shows those evolutions. It is possible to notice that, higher biomass content mixed with the 1.2 M NaOH electrolyte will increase the oxygen consumption, due to its reaction with the carbon from biomass, thus producing CO and CO
2. It is the co-electrolysis processes, like reported elsewhere, by Guerra et al. [
24,
25]. It is, also, interesting to see that, A4 liquified biomass samples shows the same behavior as the 1.2 M NaOH aqueous electrolyte without any biomass content. This is due to the presence in this sample A4, of a large amount of solvent, mainly, water, ascribable to the high solubilization of the sugars compounds. On the contrary, the remaining biomass samples tested (A2) shows a strong oxygen conversion, because this sample only has a little portion of solvent, since it was previously removed in the liquefaction process.
For A2 experiments, it was also observed, a maximum oxygen conversion between 30 and 45 min, although the final oxygen content in the gas produced from the electrolyser, at 120 min, is higher, when compared with the observed at 30–45 min. This difference is due to the non-steady state process, which occurs until it finishes, 10–15 min after the 2 h of each experience performed.
3.3. Experiments in the Syngas/Methane Reactor
Regarding the production of syngas,
Table 4 shows the results obtained with complementary experiences in the methane glass reactor, where this gaseous mixture is produced, with the methane generation, through the reaction between the electrolysis gas and, the liquified biomass. The results achieved and calculated were: the volumetric yields production of solid, liquid and gas phases, respectively, the remaining biomass collected in the reactor (Yield
(liq.biom.)) after the time experience considered, at different temperatures, the condensate (Yield
(cond.)) and, also, the gas mixture produced (Yield
(gas)).
The apparent density of the liquid condensate (ρap. (cond.)) was also calculated. The apparent density of the liquified biomass sample (A2) was previously calculated, giving an average value of 1.14 g·cm−3. From these data, it is possible to conclude that, as expected, the increase of temperature will increase, at shorter times, the volume of produced condensate, thus decreasing the volumetric yield in the remaining liquified biomass. The calculated apparent density of the condensate is decreasing with the increase of the reactor temperature and, those values are similar with the water density, although, as explain more ahead, this condensate has, also, organic compounds, at minor concentrations.
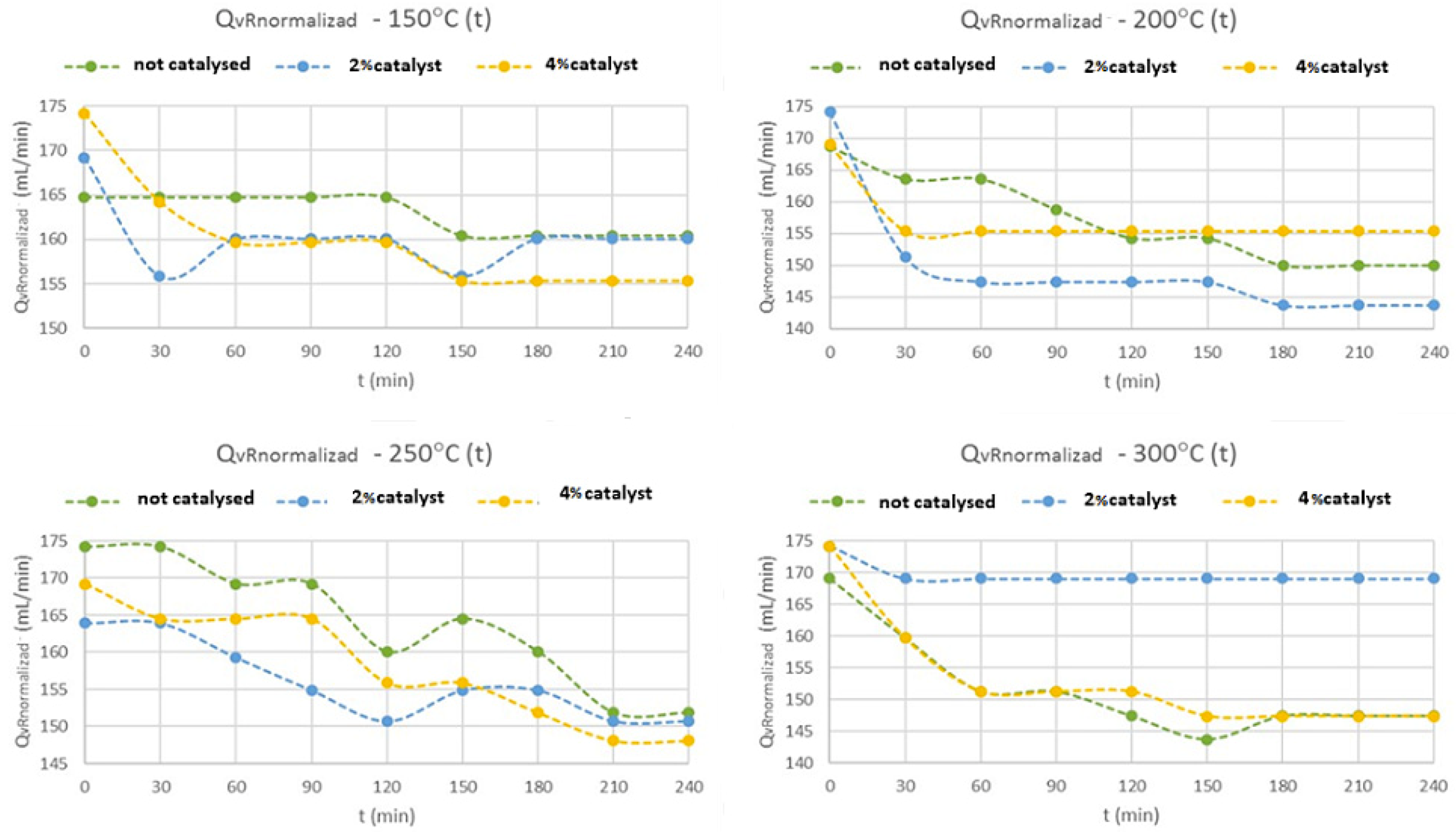
Different operational parameters were studied in the syngas/methane reactor, such as the volumetric flow rate of gas produced over time (Q
vRnormalized), the volumetric percentage of oxygen produced (%O
2) and reacted over time (Q
vRO2consum) and, the volumetric percentage of methane produced (%CH
4). These tests were carried out with different weight contents of zeolite HY catalyst and different temperatures, like described in
Section 2.6. The obtained results are presented in
Figure 5,
Figure 6,
Figure 7 and
Figure 8. All these experimental rounds took place with the following fixed experimental conditions in the electrolyser: 22.4 V of applied voltage, 2.5 A of current intensity, 160 mL/min of electrolyser gas produced (H
2 + O
2), electrolyte of NaOH 0.4 M aqueous solution and, with 4 h in each experience.
Table 5 shows, at the end of 4 hours experience, the correspondent final output values of the gas volumetric flow, as well, the oxygen and methane volumetric contents in the produced gas mixture, for different reaction temperatures and different weight content (z. HY catalyst). To compare with another Y zeolite already prepared, ultra-stabilized with nickel (z. USY), it were also performed, two more experiments with this catalyst, which was supplied from another Portuguese university. The results achieved with USY zeolite doesn’t show any significant improvement, mainly in the %CH
4 content, when compared with the acidified HY zeolite catalyst.
3.3.1. Flow of Produced Gases
Analyzing
Figure 5, the observed flows exhibit the same general behavior. An exception is the flow rate for the test with 2% of HY catalyst at 300 °C, which was constant, due to a leak in the system. This leak did not affect the test at all, but only the flow measurement.
Nevertheless, the final gas flow measurements are basically constant, in all experiments, after 120–150 min of reaction time, when it was reached the steady state, with little differences (<10 mL·min−1) between them, when it was reached the 4 hours of reaction time.
3.3.2. Oxygen Concentration in the Outlet Gas Mixture
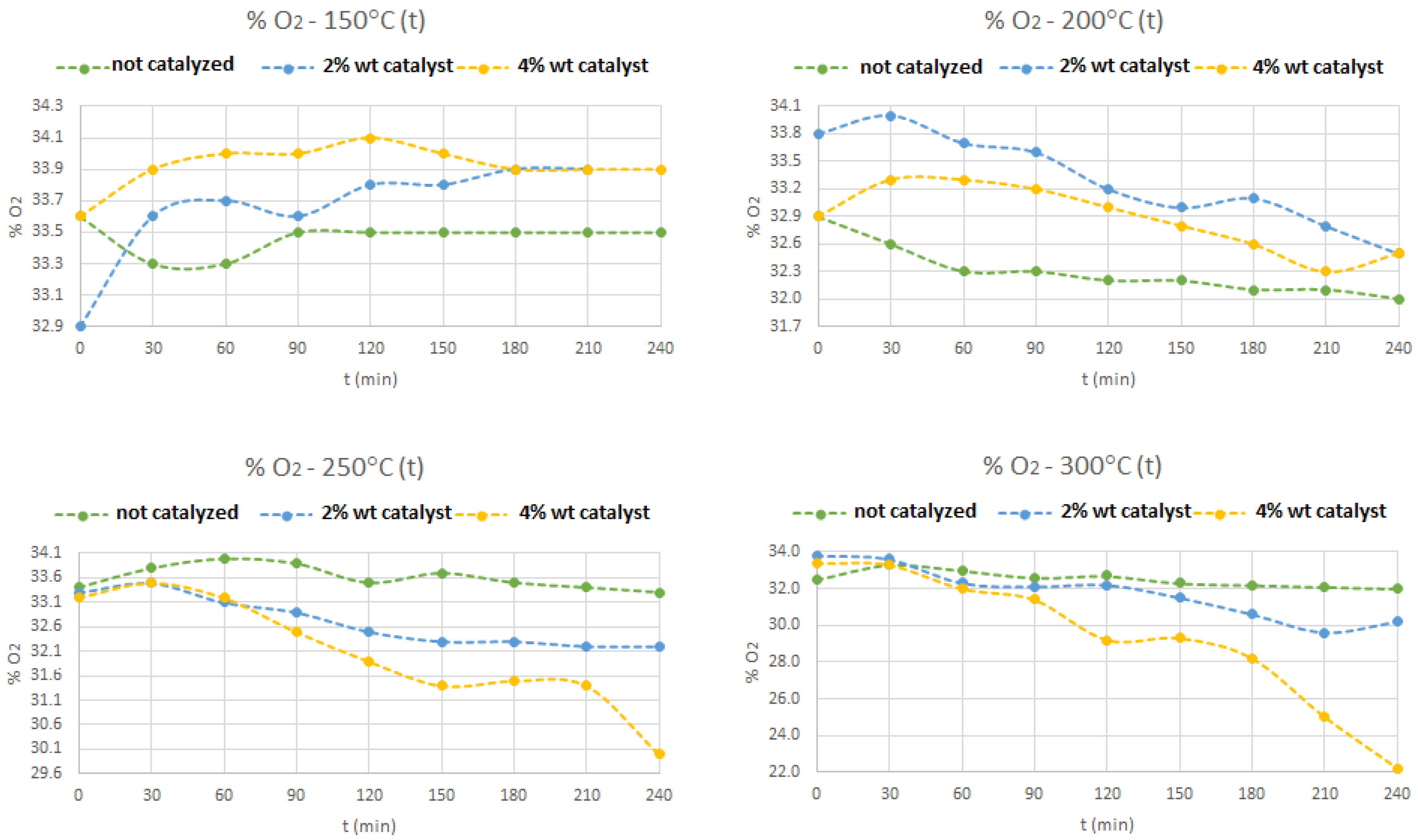
In
Figure 6, it can be seen that, the increase in temperature and the increase in the catalyst weight content affect the percentage of oxygen, i.e., for the temperatures of 250 and 300 °C and with 4% (W
catalyst/W
liq.biom.) of catalyst, it is possible to notice an appreciable decreasing on the oxygen concentration, in the syngas mixture, is more noticeable, after 210 min of reaction time.
At lower temperatures of 150 and 200 °C, the oxygen content only shows slight variations between 32.5 and 33.8% (v/v). It seems that, for temperatures above 250 °C and, with 4% of zeolite HY heterogeneous catalyst, the production of methane gas is enhanced, in the syngas mixture, because the activation energy boundary is being decreased. In all these experiments, slightly concentrations of CO and CO2 were detected, which means that, the gas compound with more and significant concentration is the hydrogen.
3.3.3. Oxygen Flow Rate Consumed
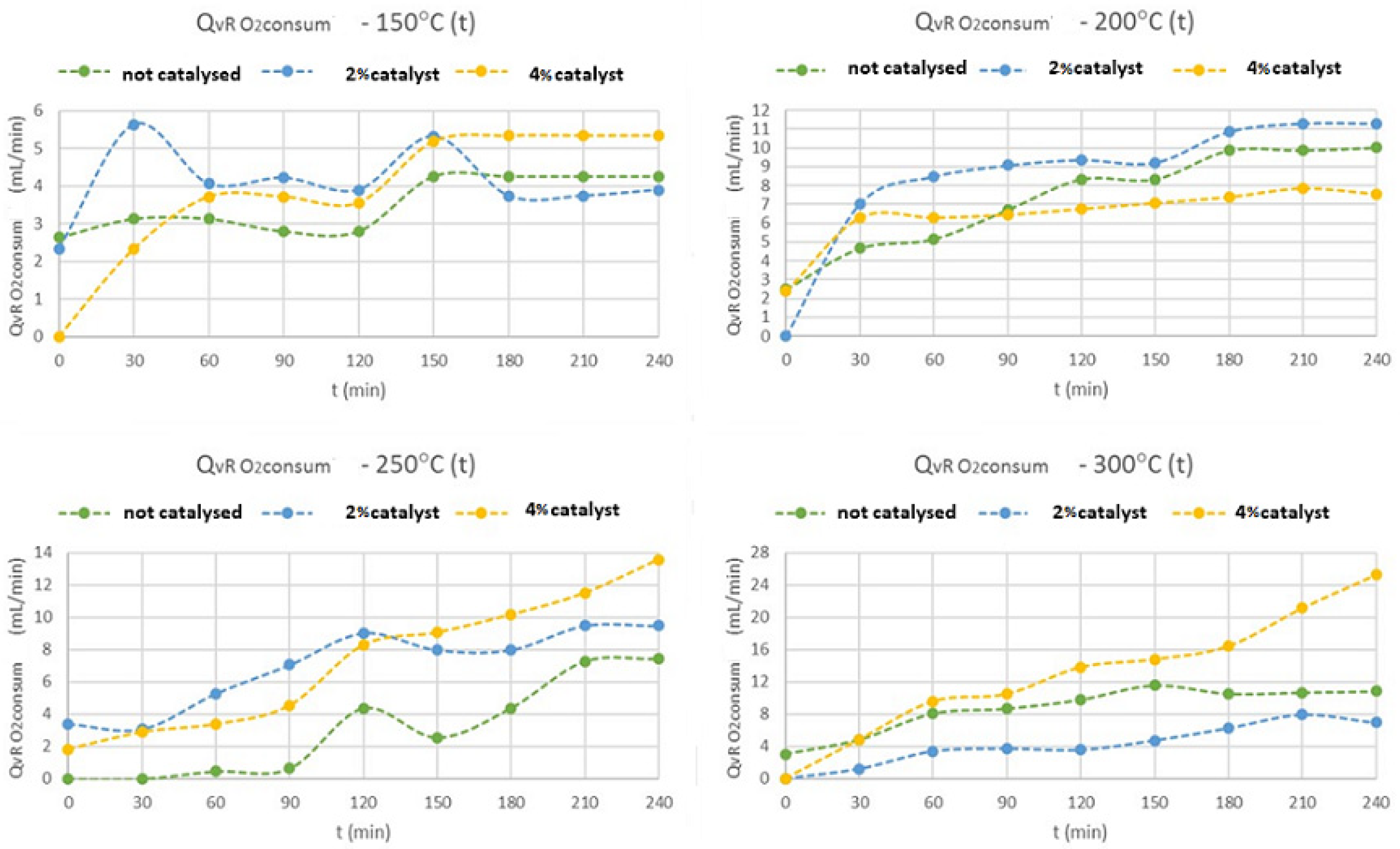
The oxygen consumption evolution is shown in
Figure 7, where it is possible to observe the increase in the reacted oxygen volumetric flow with the increase of temperature, mainly at 250 and 300 °C and, with 4% of z. HY catalyst. At 150 °C, this consumption does not exceed 6 mL·min
−1, and, at 200 °C, this consumption has already reached 12 mL·min
−1. At 250 °C, this consumption increased to 14 mL·min
−1, and, at 300 °C, this consumption is even higher, reaching 28 mL·min
−1, for the test with 4% of z. HY catalyst. The decreasing on the oxygen content in the syngas mixture, will increase the reacted oxygen to produce, mainly, methane gas. These conditions are enhanced with the increase of reaction temperature above 250 °C and, also, with 4% of weighted z. HY catalyst, for the same reasons pointed out above.
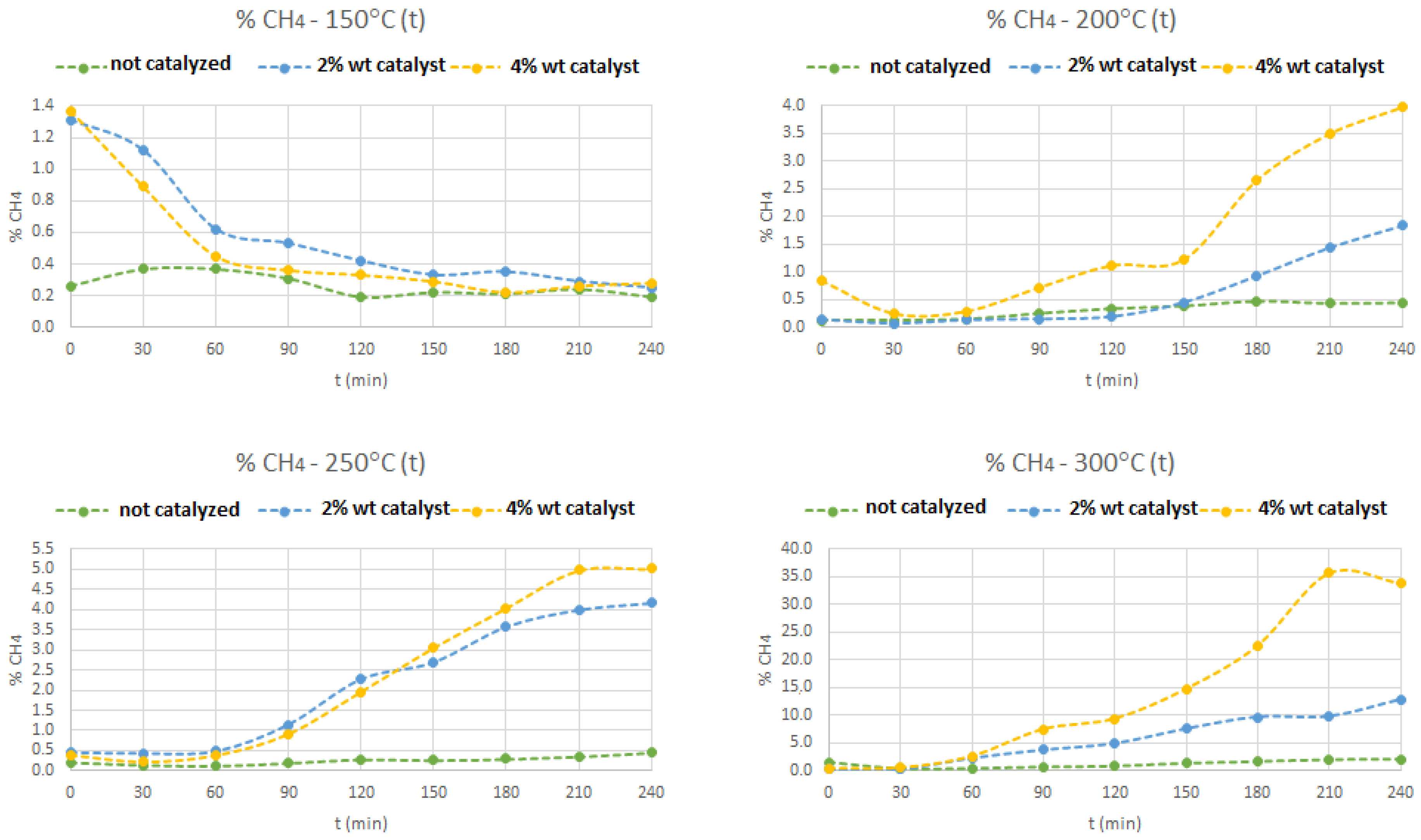
3.3.4. Methane Concentration
Figure 8 shows the production of methane through time, for different temperatures and different contents of zeolite HY catalyst, in the syngas/methanation reactor. At 150 °C, the methane concentration is very low, even with the use of the solid catalyst. Increasing the temperature and the amount of catalyst shows that, the methane production increases, with a maximum of 35%, obtained in the 300 °C test with 4% (
w/
w) of that catalyst. The reasons to explain this behavior of the methane production were explained above in this article, since the production of this fuel gas is directly related with the consumption of oxygen in this reactor, enhanced by the increase of temperature and, with, at least, 4% (
w/
w) of catalyst.
Best conditions which maximized methane concentration (300 °C, 4% (w/w)) were replicated three times and, the same behavior were observed, since, at the end of the 4 hour reaction time, final methane concentration achieved (yield) was 33% and 34% (twice), which gives an overage value of 34%, although, it was achieved also, a maximum concentration of 35% in all replicate experiments. The remaining gas compositions measured were basically the same. After these three replications, z. HY catalyst was calcinated again, to eliminate coal deposition in surface catalyst, to reactivate it, because coal deposition covered the catalyst active sites.
Another experiment with the same best operating conditions was performed, after catalyst recalcination in the same operating conditions and, the achieved results were the same of the previous ones, again with 34% of methane final concentration, at the end of 4 h of reaction time. After recalcination, catalyst acquire the same aspect as used in the first experiments.
In comparison with other similar studies, for instance, with Guerra et al. [
24], it was reached a methane volumetric content of 25.7% of the syngas mixture produced in the 1 kW SYM electrochemical reactor, combined with a follow fixed bed catalytic reactor, under atmospheric pressure and, at 125 °C, using graphite electrodes in the electrolyser and, a Ni/(CaO-Al
2O
3) heterogeneous catalyst. These conditions give a methane gas selectivity of 96.5%, a CO
2 conversion of 44.2% and, residual concentrations of CO. In another study performed also, by the same authors, Guerra et al. [
25] they achieved volumetric CO
2 concentration values of between 2.00–2.50% at 2 bar and 70 °C, but with residual values of methane gas and, with 25% of CO. In this last case, it isn’t occurred any significant production of methane gas, which means, it doesn’t show any relevant processual advantages when compared with the current study. No more similar studies were found in the literature, regarding the electrolytical production of syngas. Besides these two references, only pyrolysis/gasification process shows significant volumetric syngas and methane gas concentrations but, achieved with significant higher temperatures, higher than 400 °C and, in the case of pure syngas, only higher than 700 °C, in gasification process. These higher temperatures will need higher energy inputs for the syngas production, when compared with the electrochemical processes.
3.4. Stoichiometric Analysis
Due to the existence of methane in the final gas produced, it is apparent that the following main reactions occur [
24,
25], between carbon from biomass and the oxygen and hydrogen produced in the alkaline electrolyser. In the electrolyser itself, it occurs, both at the same time, the following reactions:
Anode:
Water alkaline oxidation:
Cathode:
Water alkaline reduction:
In the syngas/methane glass reactor, with the presence of the liquified biomass, attending the production of methane gas, the follow reactions seems to take place, according with [
19,
24,
25]:
Partial carbon oxidation:
After the production of CO and CO
2 gases, in the same methanation reactor, it will be produced methane gas, according with the following reactions, described elsewhere [
19], which is enhanced by temperature and catalyst increases:
Water-gas shift reaction:
The overall reaction from these two (reactions (5) and (6)) leads to the following one:
This means that, in the methanation reactor, the above reactions took placed in the follow order: first, the reactions (3) and (4), simultaneously, then the reactions (5) and (6), which, together, leads to the reaction (7), where, the standard specific enthalpy reaction is equal to − 206 + 41 = −165 kJ.mol−1.
According to the stoichiometry of these reactions, it is possible to obtain some outputs such as: molar flow at the outlet of the electrolyser, molar flow of oxygen and hydrogen at the outlet of the electrolyser, molar flow at the exit of the syngas reactor, molar flow of oxygen, methane, hydrogen, carbon dioxide and carbon monoxide at the exit of these reactor, as well, the molar flow rate of oxygen, methane and hydrogen consumed. From these outputs, in the 300 °C test with 4% of z. HY catalyst, where there is a higher percentage of methane produced, and, since there is a portion of the flow produced that is not justified by the stoichiometry of these reactions, it means that, there are compounds formed in addition to those mentioned before, at the same time, in the reactor. These compounds may be hydrocarbons resulting from cracking processes of biomass itself, enhancing the methane production [
29]. As reported in the literature, the propagation step mechanism of cracking paraffins leads, inevitably, to the co-production of methane gas [
29].
On the other hand, the very low contents of CO and CO2 observed, suggests that, through the temperature reaction and heterogeneous catalyst used conditions, these gases were basically consumed to produce methane, through the Sabatier process. Besides that, the most part of the liquified biomass in the reactor, was converted to liquid condensate.
In the same test with 300 °C and 4% (
w/
w) of z. HY catalyst, it was also observed, after the 240 min of reaction time, the deposition of small black particles, thus covering the catalyst surface, which could be ascribable to the deposition of coke particles, resulting most probably from the follow reaction (Equation (8)), which typically occurs on the methane conversion processes in the presence of steam water and/or oxygen, as well, in the gasification of coal and biomass [
15]:
Although the global process is exothermic, due to the negative values of reaction enthalpies, it’s necessary supply heat in order to achieve the desired temperature. The same procedure occurs in the thermochemical processes of syngas/methane production, like pyrolysis and gasification. Pyrolysis process starts at 400 °C and, syngas production in the gasification process normally occurs from temperatures higher than 700 °C. In order to calculate the theoretical supply heat to the correspondent process (
), it’s necessary to calculate the calorific values of syngas and methane produced in both cases (electrochemical/Sabatier combined process and, both thermochemical processes mentioned above), for the 300 °C achieved in this study and, for 400 °C and 700 °C, which normally occurs in the pyrolysis and in the gasification processes, respectively. To perform this task, it’s necessary to apply the following expression (Equation (9)):
where
is the mean specific calorific capacity between 25 °C and the temperature
(T) used,
is the difference of temperature between 25 °C (room temperature) and the operating one
(T). The product
corresponds to the specific calorific heat which is needed to supply for the process.
To calculate
values, is need, in first place, calculate each
Cp for the correspondent component, between 25 °C and the operating temperature. With Equation (10), it’s possible to calculate each
(Cpi) value, through the thermodynamic values of (a), (b), (c) and (d), which were collected in this study, from the literature (Himmelblau, [
30]). These values are showed in
Table 6. Equation (11) calculate the overall specific calorific capacity (
) for the syngas/methane mixture at the reactor outlet, where (x
i) represents the correspondent volumetric composition of each gas component:
The values of (
) for each case, depending of the operating temperature (
T), applied in Equation (9), gives the values of specific heats.
Table 7 shows those values for the analyzed processes. N
2 was only applied for outlet gases in pyrolysis and gasification processes, while O
2 only in the electrolytic process.
It’s possible to conclude, according with values that, higher temperature process means a significant increase in the input energy and, as consequence, a significant increase with the energetic (operating) costs. Comparing the pyrolysis process (14.15 kJ·mol−1) with the combined electrochemical/Sabatier one (10.38 kJ·mol−1) and considering the same syngas/methane flow and the same gas composition, an decrease of 36% in the input energy was observed. By another hand, the comparison between the same combined process (10.38 kJ·mol−1) with the gasification one (26.92 kJ·mol−1), an decrease of 159% in the input energy was observed, both values applied for each mole of syngas/methane mixture.
3.5. FTIR Analysis
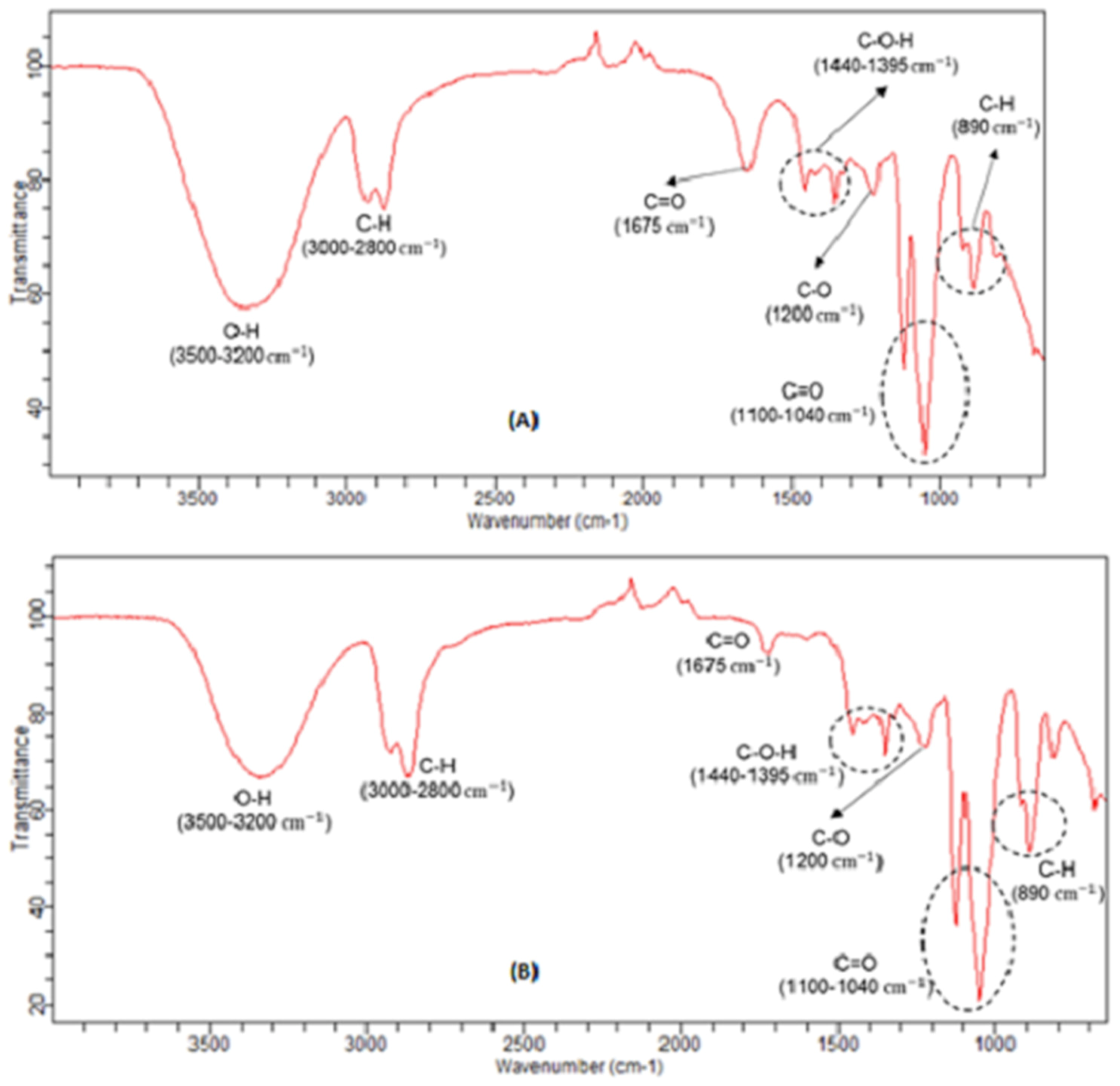
Infrared spectroscopy analyzes were performed on some liquid samples obtained in the previous experiments. These liquid samples refer to the biomass used in the tests, before and after those tests, as well, in the condensate obtained. Analyzing
Figure 9A,B, it can be seen that, the liquified biomass spectra are identical, before and after the trials, respectively, since the absorption peaks detected were almost the same, varying only their intensity.
It can be seen that, the most intensity peak is related with the O-H absorption peaks between 3200 and 3500 cm
−1, mainly ascribable with alcohols, water and, for the case of biomass samples, are related also with O-H bonds of the hydroxyl groups present in the several monomers of the cellulose and hemicellulose structures [
31]. Nevertheless, it’s possible to see a little decreasing in intensity of these absorption peaks of O-H (3200–3500 cm
−1), and also, at 1675 cm
−1 related with C=O bonds (stretching vibrations) of aldehydes and ketones, after the correspondent experiment, due to the evaporation of some of these compounds to the condensate.
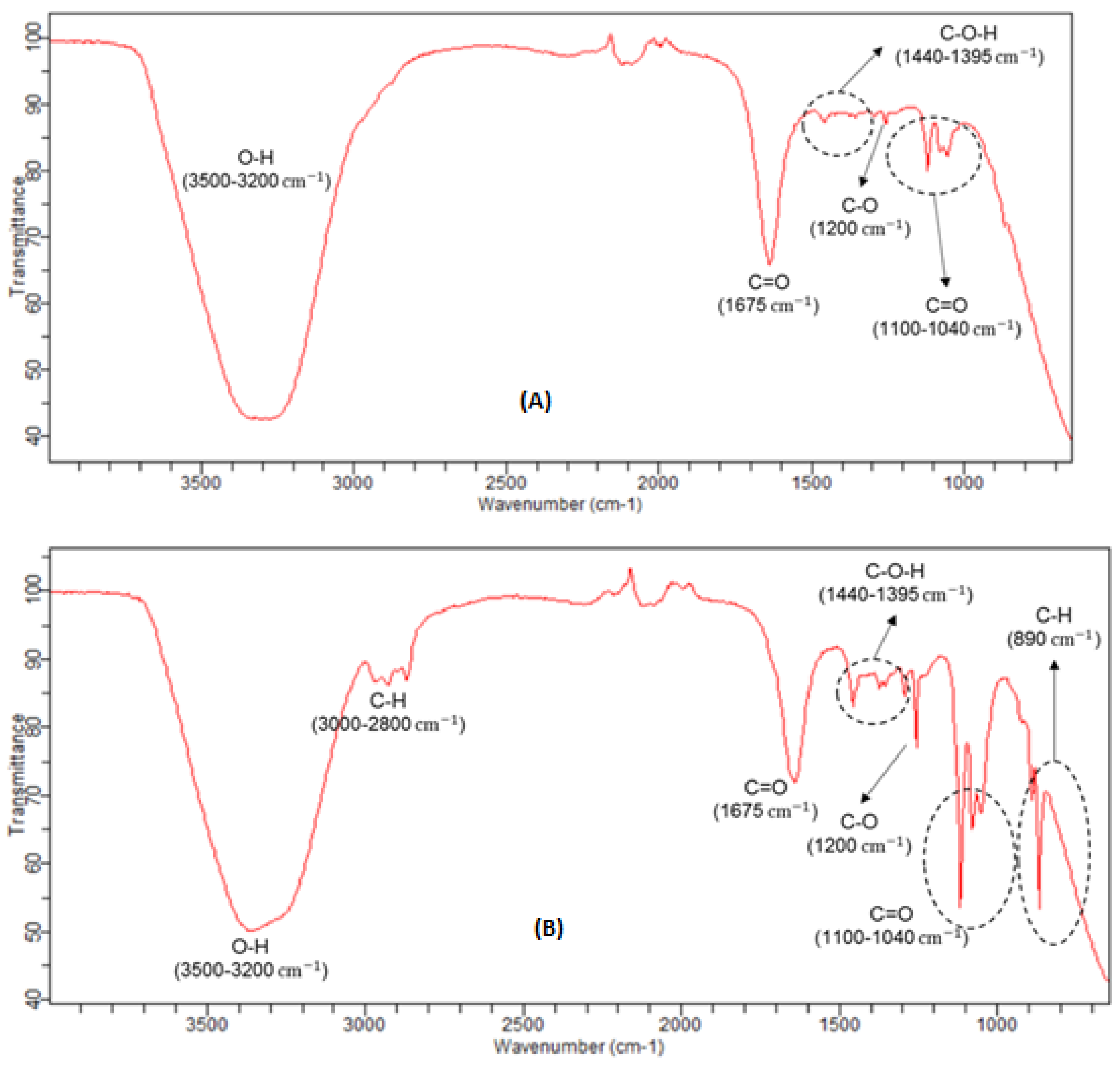
The spectra presented in
Figure 10A,B are related with the liquid condensate samples obtained, respectively, for the trials of 150 °C with 4% of catalyst and, at 300 °C with 2% of catalyst. The remaining trials performed give similar condensate FTIR spectra to these two cases. For low reaction temperatures, it’s possible to detect the O-H stretching vibrations bonds, typical in water, alcohols and similar compounds, but also, the C=O absorption peaks, at 1675 cm
−1, typical of aldehydes and ketones. For higher temperatures (250 and 300 °C), it is also possible to detect, besides these absorption peaks mentioned before, other ones, mainly at, 2800–3000 cm
−1 and at 890 cm
−1, which correspond to the stretching and bending vibrations of the C-H bonds of the aldehydes, as well, at 1040–1100 cm
−1, for stretching vibrations of the C=O bonds of the carboxylic acids, 1200 cm
−1, addressed to stretching vibrations of the C-O bonds of the alcohols and, finally, at 1395–1440 cm
−1, ascribable to stretching vibrations of the C-O-H bonds of the carboxylic acids, also.
When comparing the FTIR spectra of liquified biomass and condensate liquid samples, it’s possible to verify that, the functional groups which are decreasing its intensity in the biomass samples, increase in the condensate samples.
This fact was to be expected, since the most volatile constituents with O-H, C-O and C=O bounds evaporate during the reaction and, therefore, are collected in condensate tank. Since the evaporation of the condensate previous collected was almost complete at 100 °C, suggests that the major quantity of those alcohols, aldehydes and carboxylic acids have boiling temperatures below 100 °C, which could be ascribable to formaldehyde, methanol, ethanol and, formic acid, since these compounds have, all, normal boiling temperatures below than 100 °C.
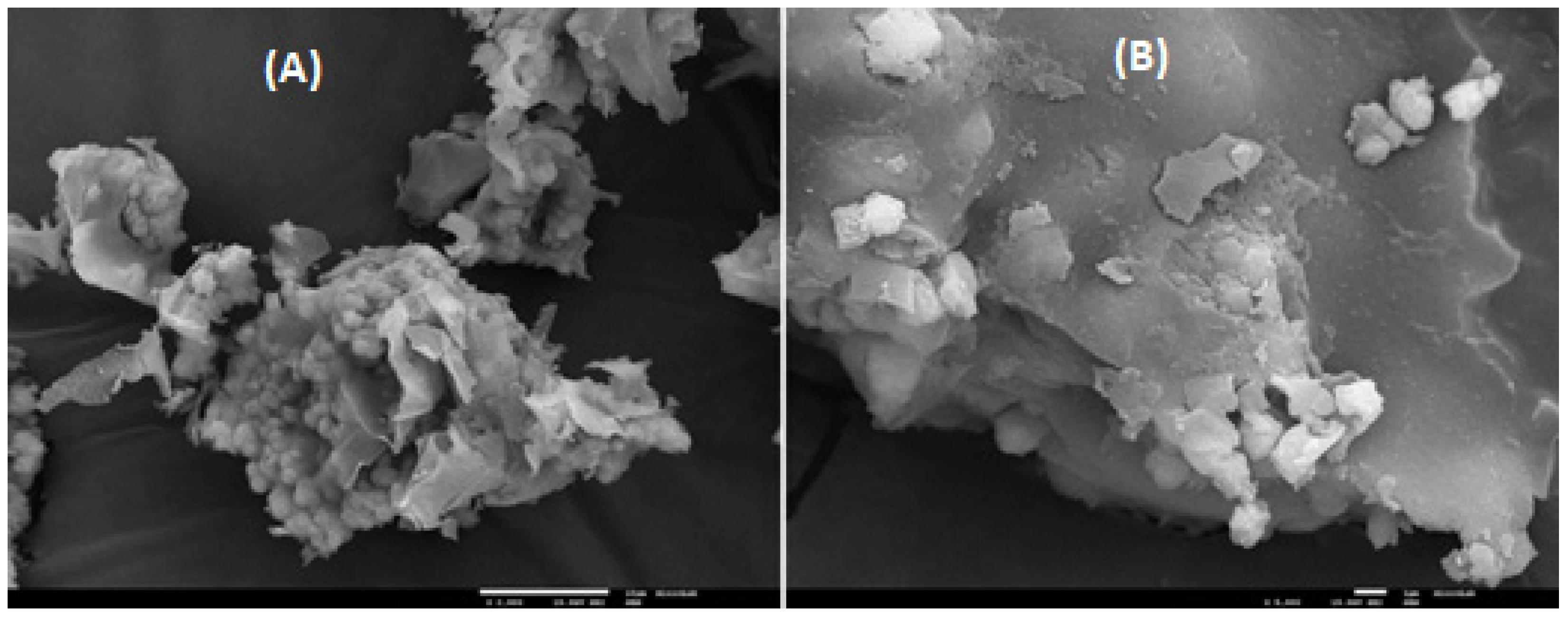
3.6. SEM-EDS Analysis
By the end of the syngas production test at 300 °C, with 4 g of z. HY catalyst, a solid was obtained, with some black particles, ascribable to coke deposition. In order to observe and characterize morphologically, this sample, before and after the acidification and calcination processes, as well, after the reaction at those conditions, SEM-EDS analysis was used, as shown in
Figure 11 and
Figure 12 (SEM images) and
Figure 13 and
Figure 14 (EDS spectra – atomic percentages).
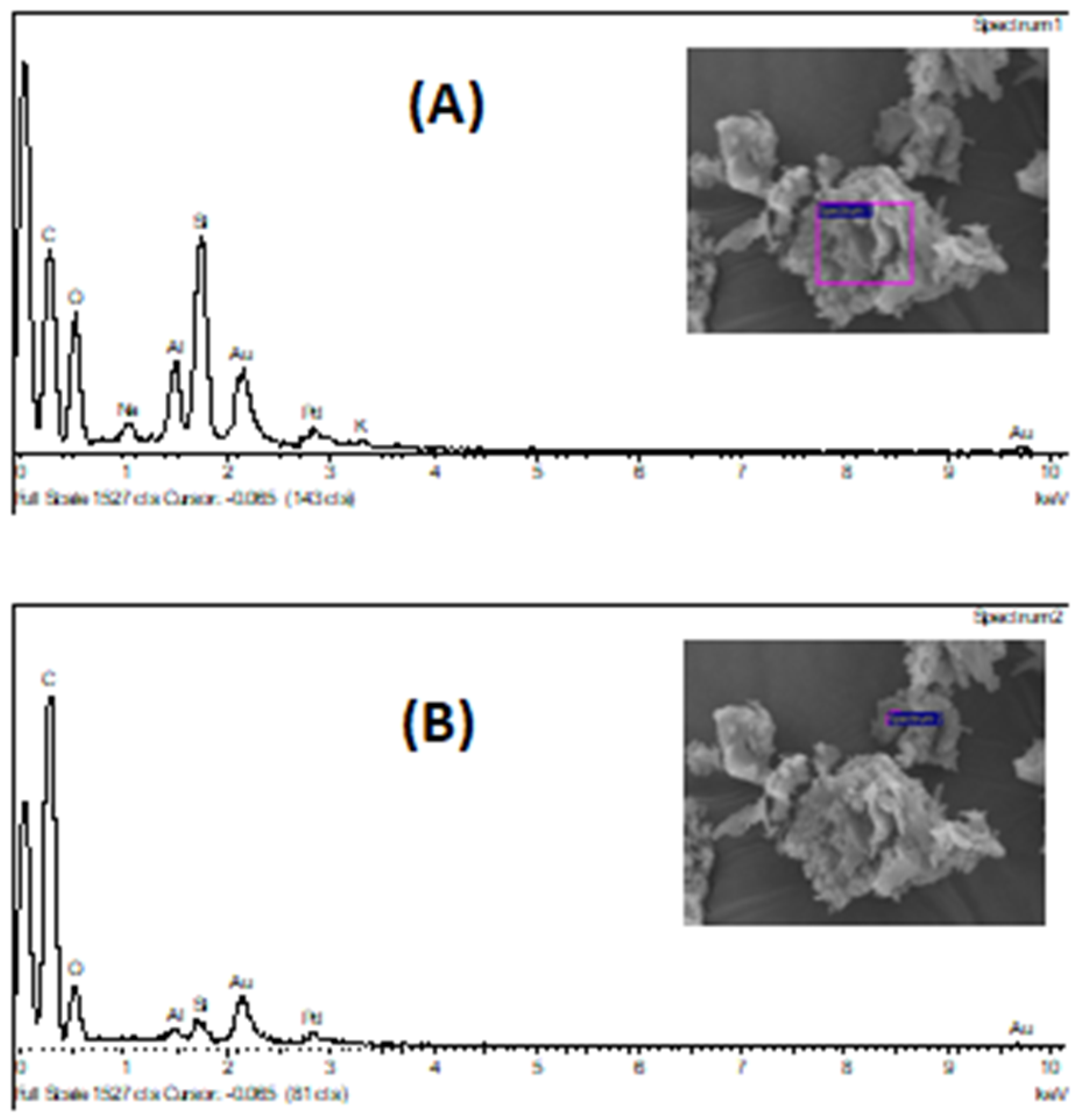
Analyzing the several SEM micrographics of these figures, it is possible to conclude that the solid sample is not homogeneous in its constitution, mainly in the solid sample collected after the reaction process, at 300 °C with 4% of weighted catalyst. The grey areas of post-reaction catalyst, according with SEM image of
Figure 11B can be ascribable to the carbonaceous residue deposited on the surface catalyst, with a significant content, since the carbon atomic content increase significantly, from 3.4% to 68.1% and 76.2%, in two different points of the solid surface analyzed, according with
Table 8.
Several researchers pointed out in their articles that, this coke can be eliminated to CO
2, regenerating the zeolite HY catalyst, to be active again in this reaction. The same phenomena was also observed in this work. It is well known that, this catalyst is the same that is currently used in the fluid catalytic cracking of heavy diesel fuels, in the crude oil refining industry [
32]. The catalyst has the same behavior, in both processes.
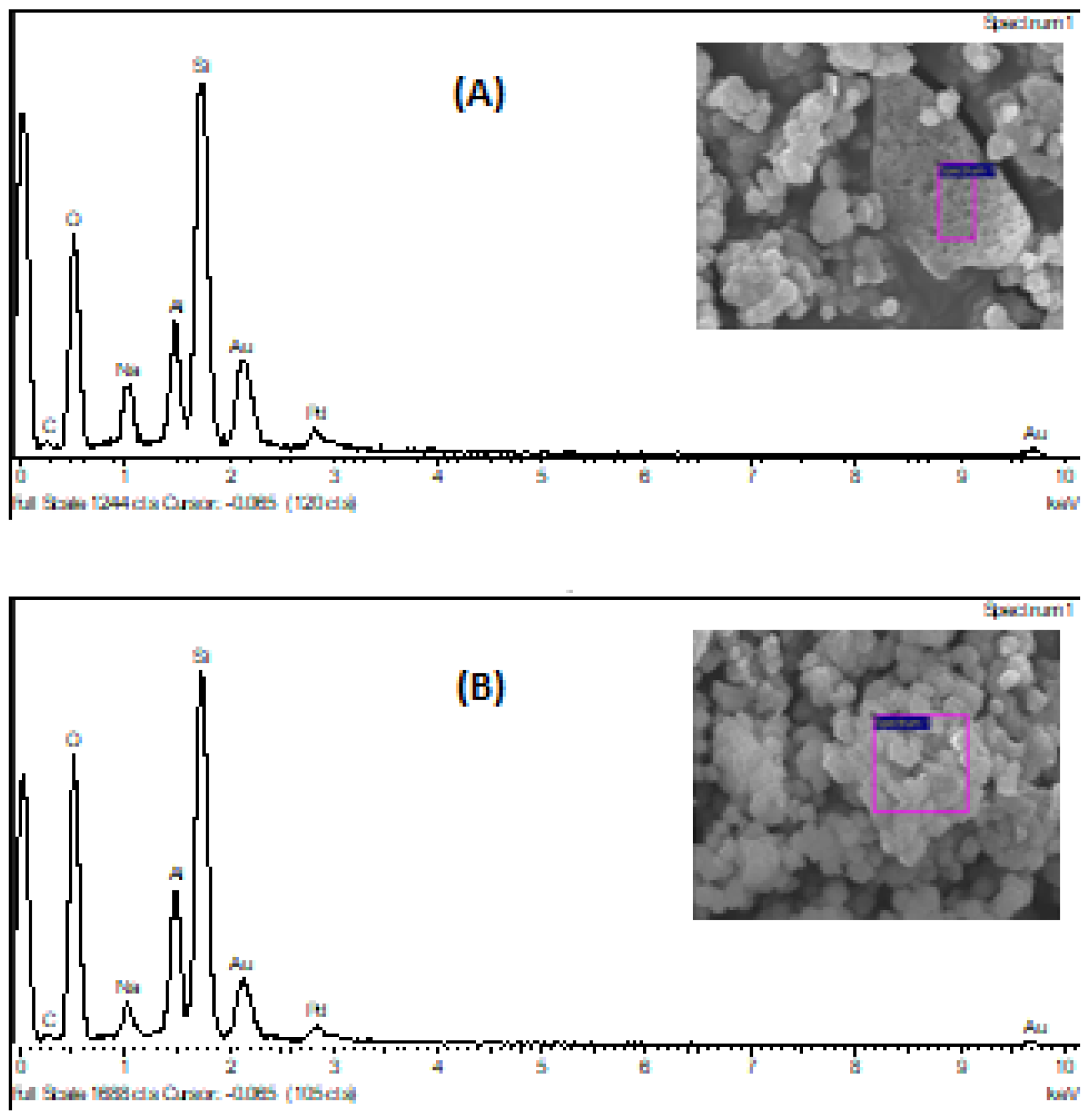
Besides this, when comparing SEM images and EDS analysis of atomic contents, before and after the catalyst activation process (acidification and calcination), there aren’t significant changes in the morphology of the catalyst surface, with a little exception in the decreasing of the sodium content, which was expectable, due to the ionic exchange performed, where the sodium cation was leached.
The carbon atomic content decreased also, probably due to the calcination process, where the adsorbed CO2 was released of the catalyst surface, to the atmosphere and/or, due to the decomposition of some sodium carbonate adsorbed, which was converted to sodium oxide with CO2 released.