Microalgae Cultivation in Pilot Scale for Biomass Production Using Exhaust Gas from Thermal Power Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Conditions of Microalgae Cultivation in Batch Scale
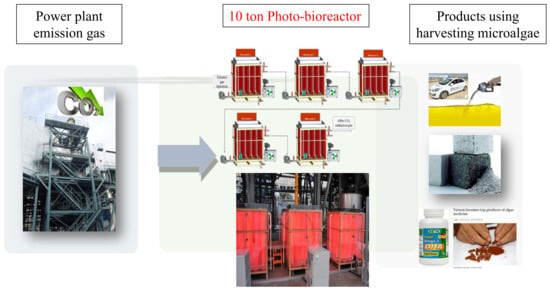
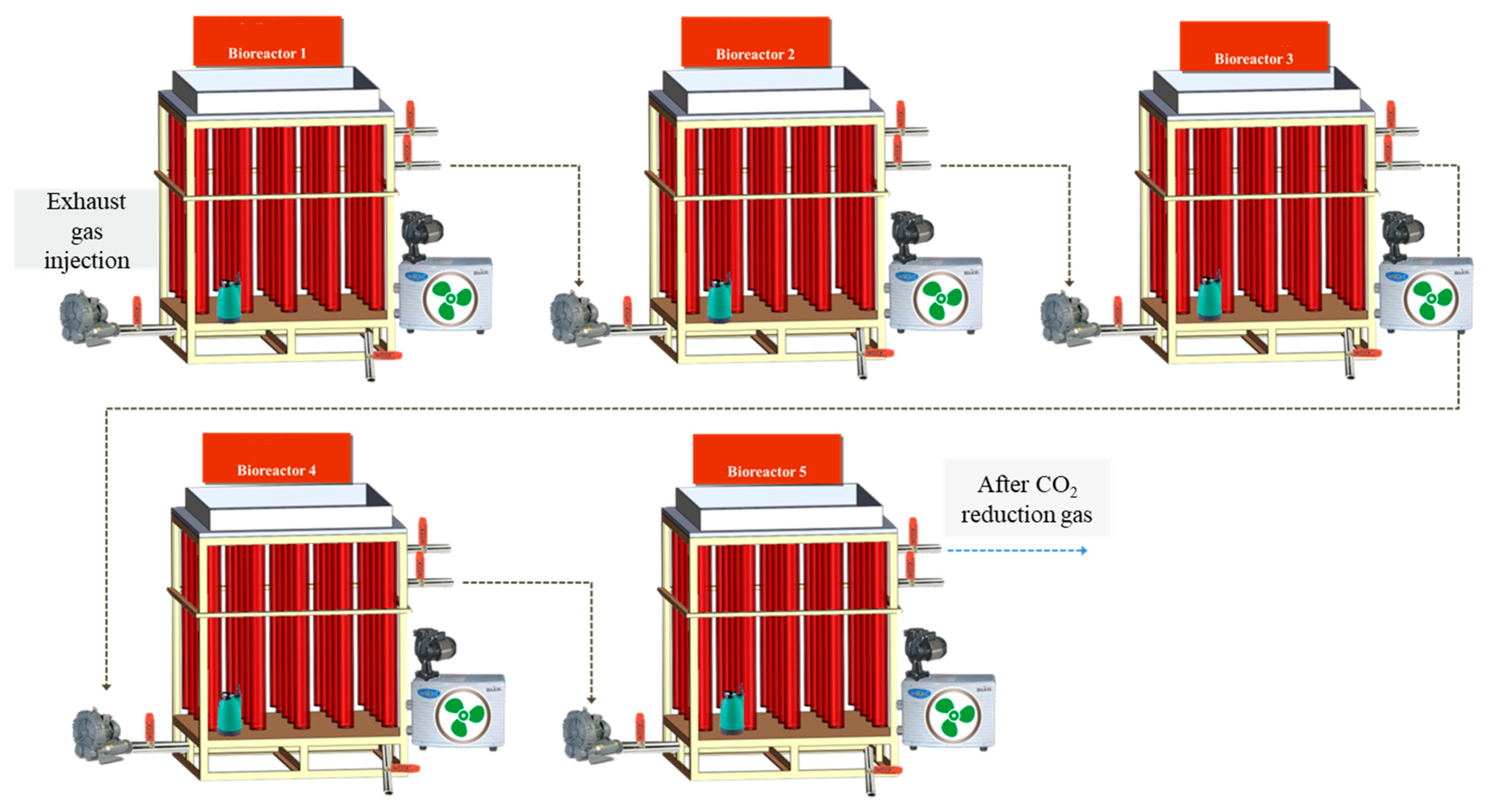
2.2. Cultivation of Microalgae in Photobioreactor (PBR)
2.3. Analysis of Microalgal Growth
2.4. Algal Harvest
2.5. Analysis of Lipid and C16–C18 Fatty Acid Methyl Ester (FAME)
3. Results and Discussion
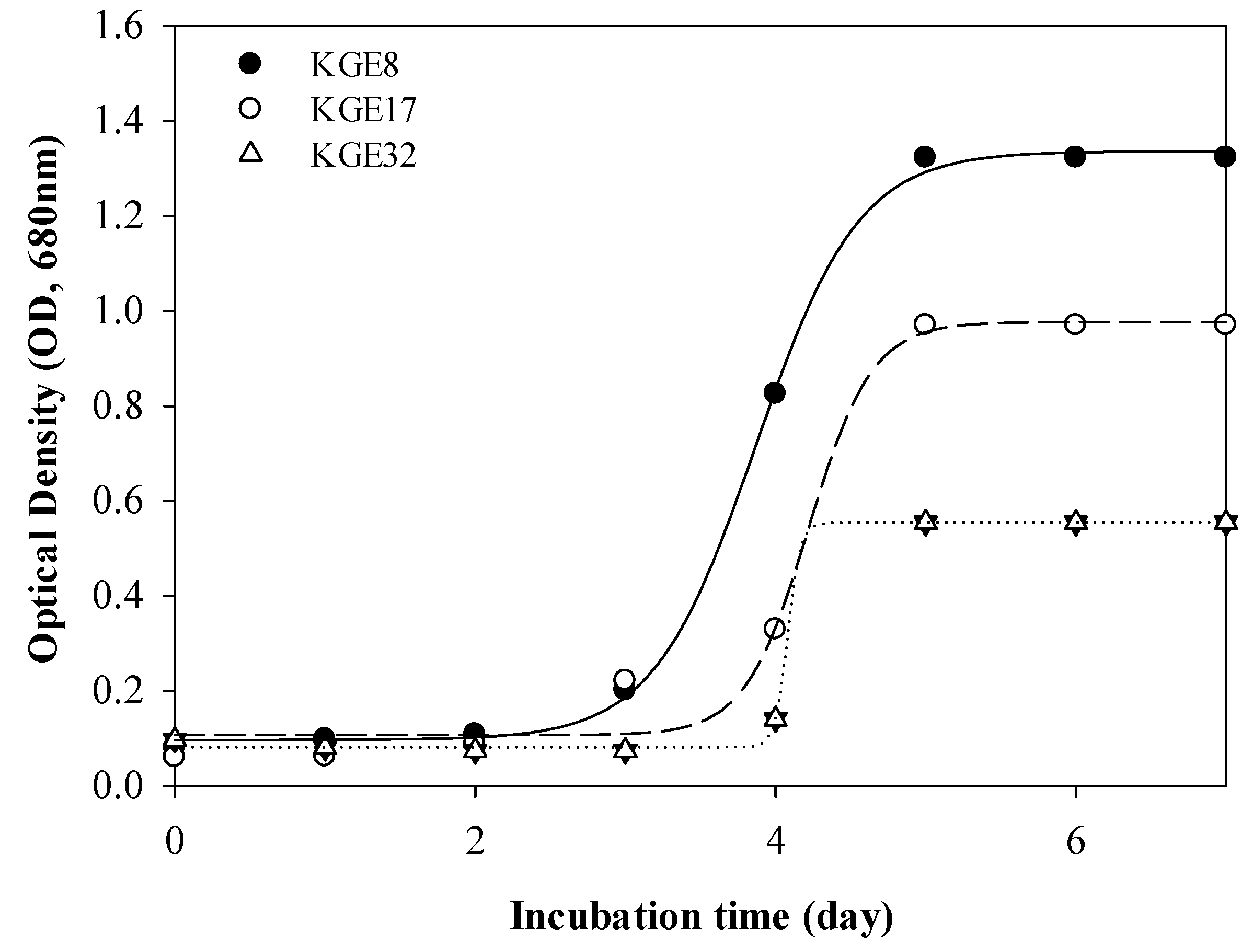
3.1. Growable Microalgae in Exhaust Gas Condition
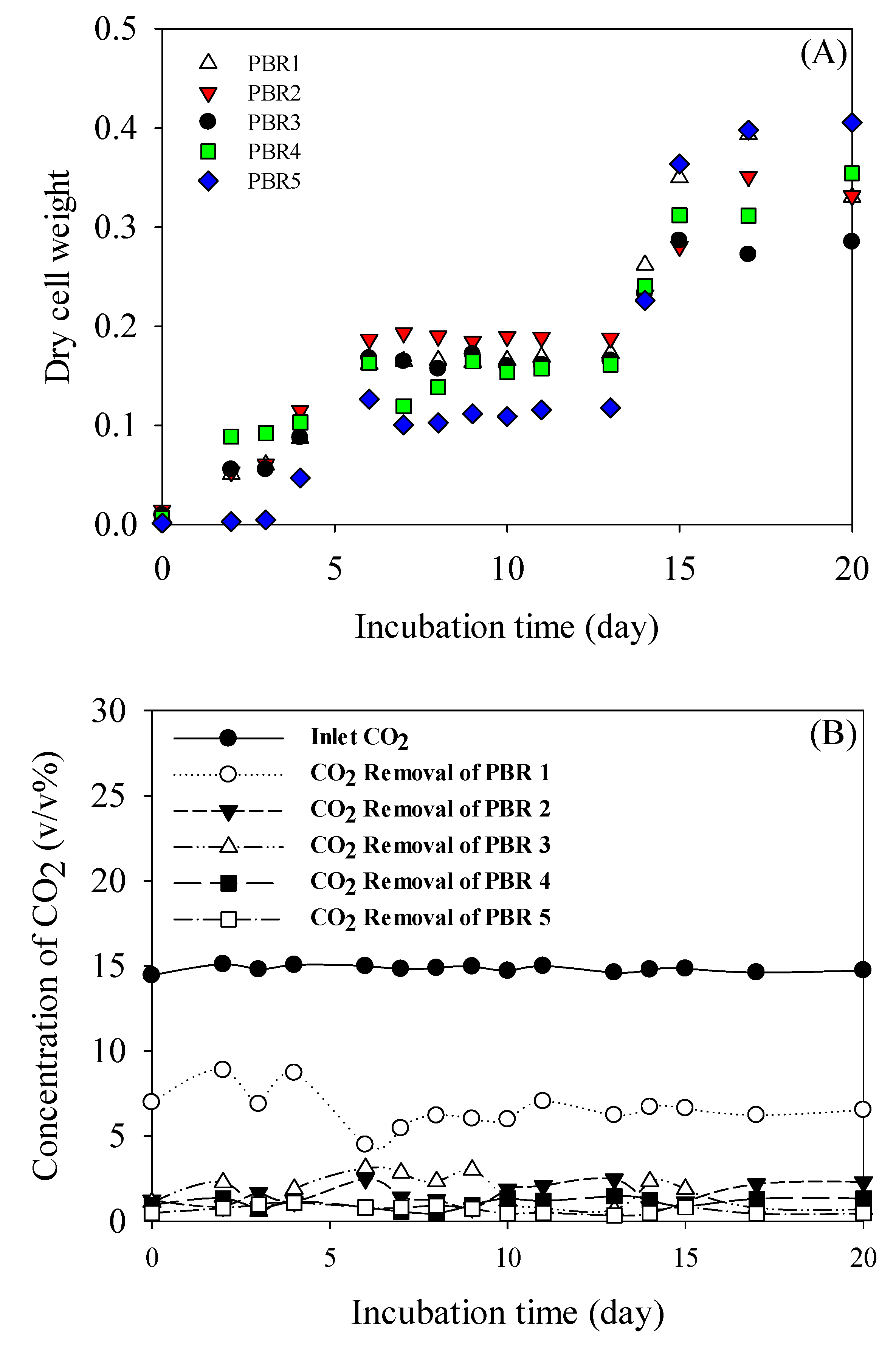
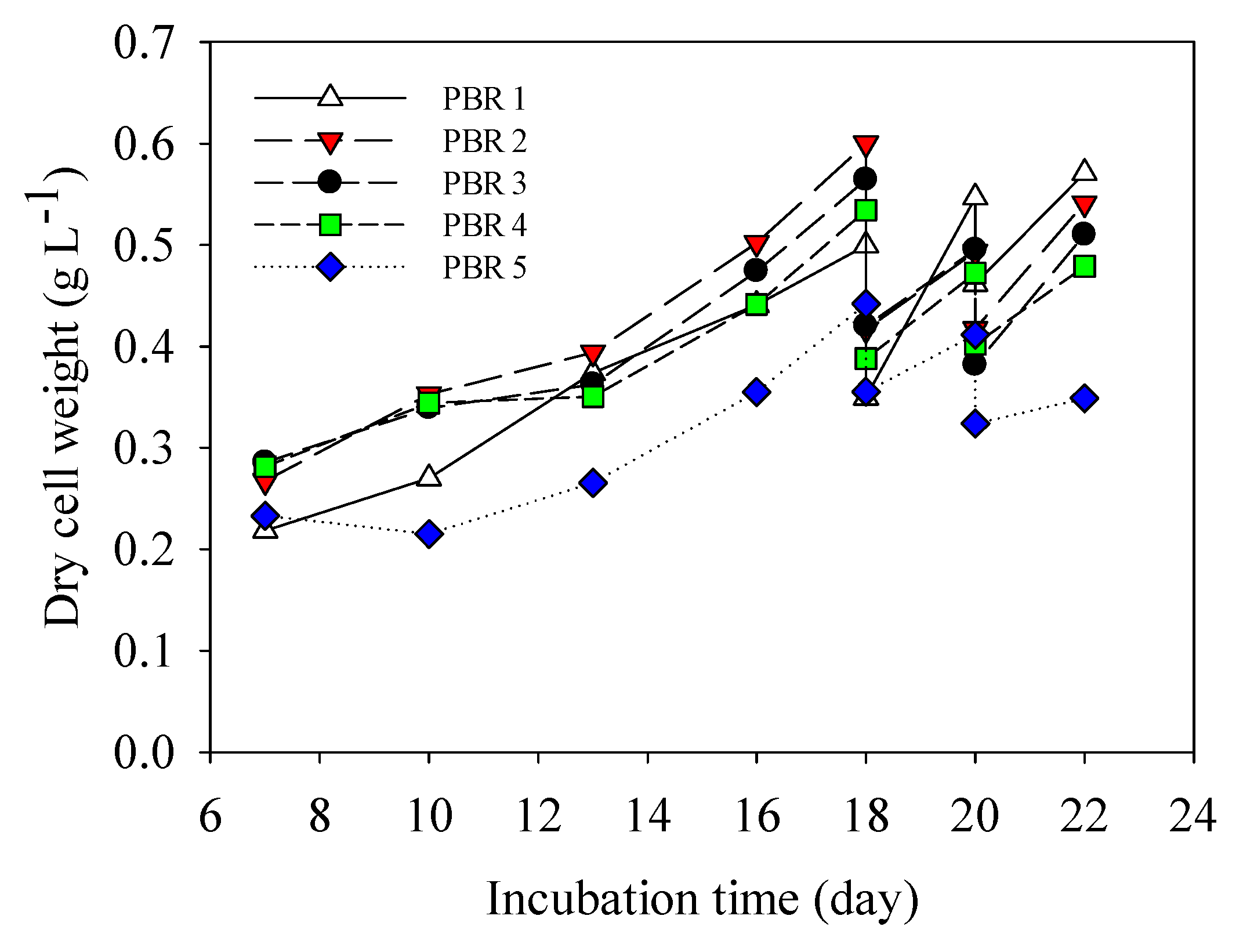
3.2. Pilot Scale Cultivation
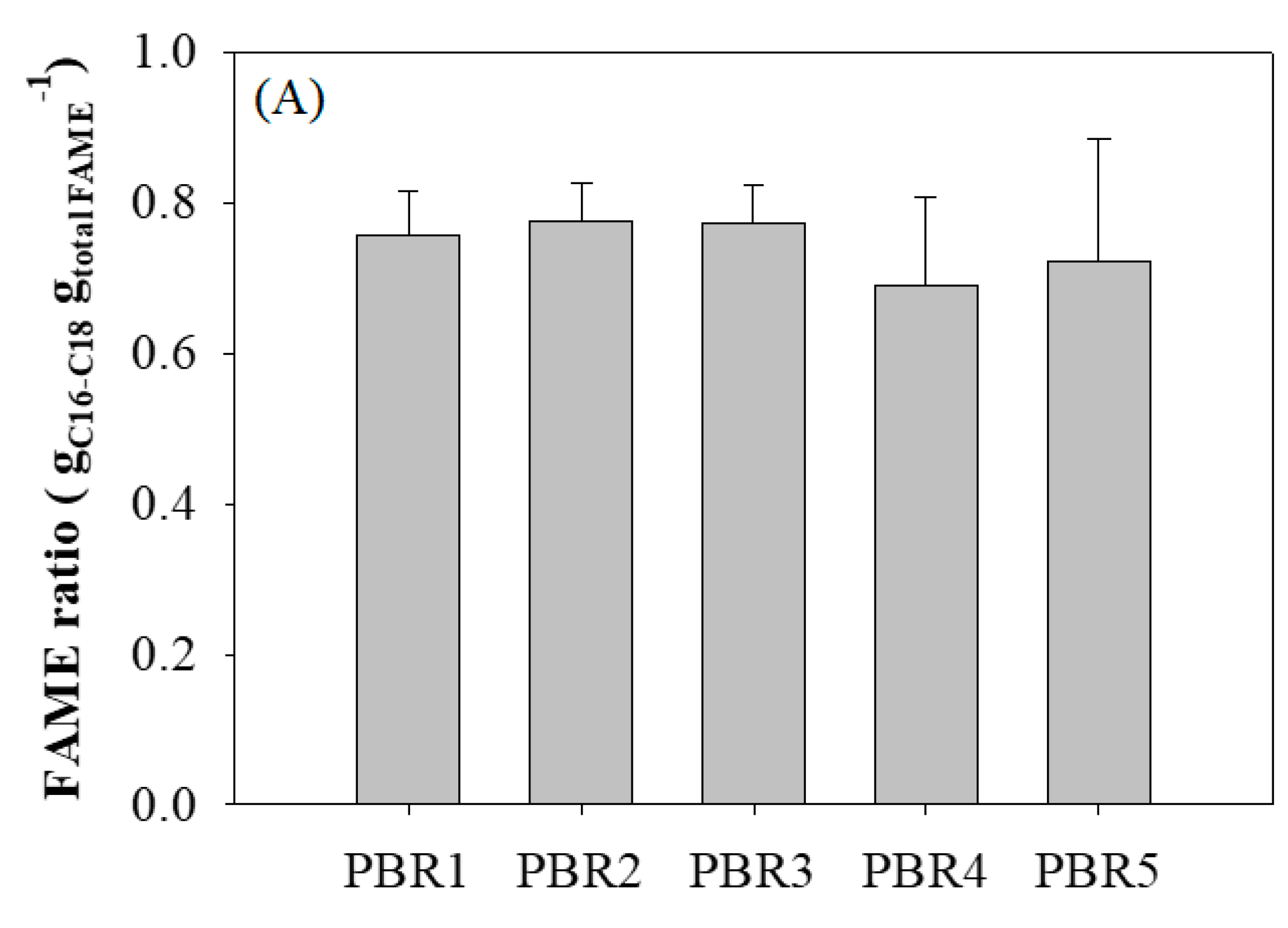
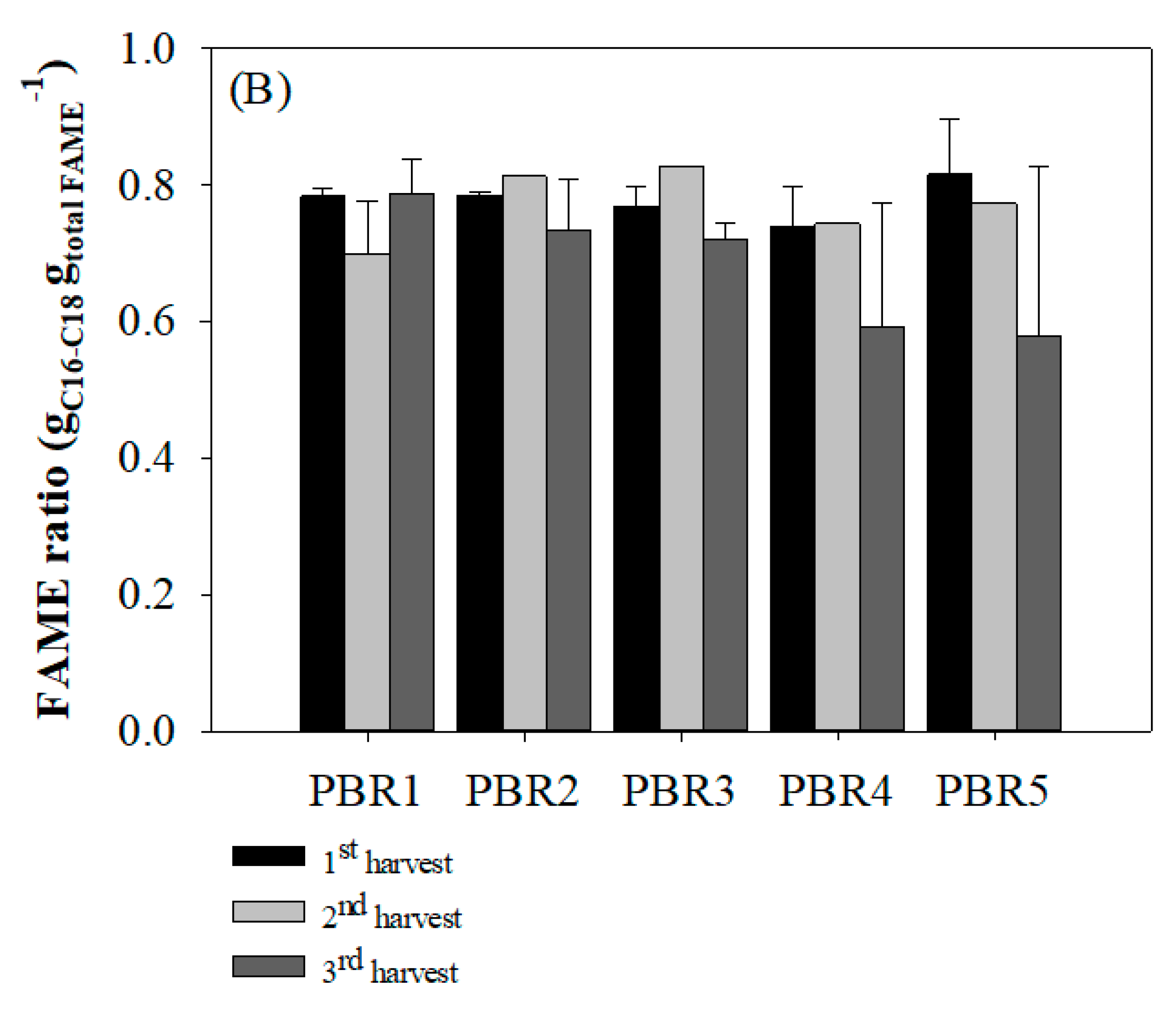
3.3. Lipid and Fatty Acid Productivity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shen, Y. Carbon dioxide bio-fixation and wastewater treatment via algae photochemical synthesis for biofuels production. RSC Adv. 2014, 4, 49672–49722. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, S.; Cao, B.; Hu, Y.; Abomohra, A.E.-F.; Wang, Q.; Qian, L.; Liu, L.; Liu, X.; He, Z.; et al. Optimization of hydrothermal co-liquefaction of seaweeds with lignocellulosic biomass: Merging 2nd and 3rd generation feedstocks for enhanced bio-oil production. Energy 2019, 173, 413–422. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Garcia-Perez, J.S.; Rittmann, B.E.; Parra-Saldívar, R. Photosynthetic bioenergy utilizing CO2: An approach on flue gases utilization for third generation biofuels. J. Clean. Prod. 2015, 98, 53–65. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Yun, H. Carbon Dioxide Sequestration of Exhaust Fumes by Freshwater Microalgae with Massive Biomass Production. Bachelor’s Thesis, Yonsei University, Seoul, Korea, 2015. [Google Scholar]
- Zhao, B.; Su, Y.; Zhang, Y.; Cui, G. Carbon dioxide fixation and biomass production from combustion flue gas using energy microalgae. Energy 2015, 89, 347–357. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Show, P.L.; Yap, Y.J.; Ling, T.C.; Chang, J.-S. Effects of water culture medium, cultivation systems and growth modes for microalgae cultivation: A review. J. Taiwan Inst. Chem. Eng. 2018, 91, 332–344. [Google Scholar] [CrossRef]
- Zhao, B.; Su, Y. Process effect of microalgal-carbon dioxide fixation and biomass production: A review. Renew. Sustain. Energy Rev. 2014, 31, 121–132. [Google Scholar] [CrossRef]
- Aslam, A.; Thomas-Hall, S.R.; Mughal, T.A.; Schenk, P.M. Selection and adaptation of microalgae to growth in 100% unfiltered coal-fired flue gas. Bioresour. Technol. 2017, 233, 271–283. [Google Scholar] [CrossRef]
- Kassim, M.A.; Meng, T.K. Carbon dioxide (CO2) biofixation by microalgae and its potential for biorefinery and biofuel production. Sci. Total Environ. 2017, 584, 1121–1129. [Google Scholar] [CrossRef]
- Bischoff, H.W. Phycological studies. IV. Some Algae from Enchanted Rock and Related Algal Species. Univ. Texas Publ. 1963, 6318, 95. [Google Scholar]
- Blair, M.F.; Kokabian, B.; Gude, V.G. Light and growth medium effect on Chlorella vulgaris biomass production. J. Environ. Chem. Eng. 2014, 2, 665–674. [Google Scholar] [CrossRef]
- Ji, M.-K.; Yun, H.-S.; Hwang, B.S.; Kabra, A.N.; Jeon, B.-H.; Choi, J. Mixotrophic cultivation of Nephroselmis sp. using industrial wastewater for enhanced microalgal biomass production. Ecol. Eng. 2016, 95, 527–533. [Google Scholar] [CrossRef]
- Yun, H.S.; Ji, M.K.; Park, Y.T.; Salama el, S.; Choi, J. Microalga, Acutodesmus obliquus KGE 30 as a potential candidate for CO2 mitigation and biodiesel production. Environ. Sci. Pollut. Res. Int. 2016, 23, 17831–17839. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.-Y.; Kao, C.-Y.; Huang, T.-T.; Lin, C.-J.; Ong, S.-C.; Chen, C.-D.; Chang, J.-S.; Lin, C.-S. Microalgal biomass production and on-site bioremediation of carbon dioxide, nitrogen oxide and sulfur dioxide from flue gas using Chlorella sp. cultures. Bioresour. Technol. 2011, 102, 9135–9142. [Google Scholar] [CrossRef] [PubMed]
- Praveenkumar, R.; Kim, B.; Choi, E.; Lee, K.; Cho, S.; Hyun, J.-S.; Park, J.-Y.; Lee, Y.-C.; Lee, H.U.; Lee, J.-S.; et al. Mixotrophic cultivation of oleaginous Chlorella sp. KR-1 mediated by actual coal-fired flue gas for biodiesel production. Bioprocess Biosyst. Eng. 2014, 37, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Praveenkumar, R.; Kim, B.; Choi, E.; Lee, K.; Park, J.-Y.; Lee, J.-S.; Lee, Y.-C.; Oh, Y.-K. Improved biomass and lipid production in a mixotrophic culture of Chlorella sp. KR-1 with addition of coal-fired flue-gas. Bioresour. Technol. 2014, 171, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.K.; Yun, H.S.; Hwang, J.H.; Salama, E.S.; Jeon, B.H.; Choi, J. Effect of flue gas CO2 on the growth, carbohydrate and fatty acid composition of a green microalga Scenedesmus obliquus for biofuel production. Environ. Technol. 2017, 38, 2085–2092. [Google Scholar] [CrossRef]
- Tang, D.; Han, W.; Li, P.; Miao, X.; Zhong, J.-J. CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour. Technol. 2011, 102, 3071–3076. [Google Scholar] [CrossRef]
- Borowitzka, M.A. The ‘stress’ concept in microalgal biology—Homeostasis, acclimation and adaptation. Environ. Boil. Fishes 2018, 30, 2815–2825. [Google Scholar] [CrossRef]
- Cheng, D.; Li, X.; Yuan, Y.; Yang, C.; Tang, T.; Zhao, Q.; Sun, Y. Adaptive evolution and carbon dioxide fixation of Chlorella sp. in simulated flue gas. Sci. Total Environ. 2019, 650, 2931–2938. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.B.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Wong, C.Y.; Ramli, A.; Kiew, P.L.; Lee, K.T. Semi-continuous cultivation of Chlorella vulgaris using chicken compost as nutrients source: Growth optimization study and fatty acid composition analysis. Energy Convers. Manag. 2018, 164, 363–373. [Google Scholar] [CrossRef]
- Sun, X.-M.; Ren, L.-J.; Zhao, Q.-Y.; Ji, X.-J.; Huang, H. Microalgae for the production of lipid and carotenoids: A review with focus on stress regulation and adaptation. Biotechnol. Biofuels 2018, 11, 272. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.M. Uptake of carbon dioxide from flue gas by microalgae. Energy Convers. Manag. 1996, 37, 1363–1367. [Google Scholar] [CrossRef]
- Park, Y.-T.; Lee, H.; Yun, H.-S.; Song, K.-G.; Yeom, S.-H.; Choi, J. Removal of metal from acid mine drainage using a hybrid system including a pipes inserted microalgae reactor. Bioresour. Technol. 2013, 150, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.K.; Schuhmann, H.; Schenk, P.M. High Lipid Induction in Microalgae for Biodiesel Production. Energies 2012, 5, 1532–1553. [Google Scholar] [CrossRef]
- Nayak, M.; Dhanarajan, G.; Dineshkumar, R.; Sen, R. Artificial intelligence driven process optimization for cleaner production of biomass with co-valorization of wastewater and flue gas in an algal biorefinery. J. Clean. Prod. 2018, 201, 1092–1100. [Google Scholar] [CrossRef]
- Singh, S.; Singh, P. Effect of CO2 concentration on algal growth: A review. Renew. Sustain. Energy Rev. 2014, 38, 172–179. [Google Scholar] [CrossRef]
| Gas Contents | Initial Gas Concentration |
|---|---|
| CO2 (v/v %) | 14.9 (±0.2) |
| NOx (ppmv) | 220.2 (±10.5) |
| SOx (ppmv) | 32 (±5) |
| CO (ppmv) | 1549 (±242) |
| O2 (%) | 5.47 (±0.03) |
| Stage | CO2 (v/v %) | NOx (ppmv) | CO (ppmv) | O2 (v/v %) |
|---|---|---|---|---|
| PBR1 | 14.90 (±0.18) | 220.2 (±10.5) | 1548.5 (±242) | 5.47 (±0.03) |
| PBR2 | 8.08 (±0.52) | 124.4 (±21.8) | 941.1 (±41.9) | 12.42 (±0.93) |
| PBR3 | 6.72 (±0.73) | 99.3 (±16.0) | 703.3 (±51.4)) | 13.89 (±0.99) |
| PBR4 | 4.99 (±0.52) | 72.3 (±12.1) | 537.2 (±21.6) | 15.81 (±0.86) |
| PBR5 | 3.87 (±0.52) | 51.9 (±9.3) | 421.1 (±35.2) | 17.03 (±0.95) |
| Out | 3.11 (±0.60) | 51.8 (±8.18) | 337.0 (±5.1) | 16.86 (±1.41) |
| Species | Carbon Dioxide Concentration (%) | Incubation Condition | Medium | Lipid Contents (%) | Specific Growth Rate (μmax, d−1) |
|---|---|---|---|---|---|
| Scenedesmus obliquus KGE 9 a | 14.1 | Batch | BBM | 22.8 | 1.00 |
| Chlorella pyrenoidosaSJTU-2b | 10.0 | Batch | BG11 | 24.2 | 0.78 |
| Acutodesmus obliquus KGE 30 c | 14.1 | Batch | BBM | 17.5 | 1.09 |
| Acutodesmus obliquus KGE 32 d | 14.1 | Batch | BBM | - | 1.08 |
| Acutodesmus obliquus KGE 17 d | 14.1 | Batch | BBM | - | 1.37 |
| Nephroselmis sp. KGE 8 d | 14.1 | Batch | BBM | 59.4 | 1.41 |
| 0 Nephroselmis sp. KGE 8 d | 14.1 | Pilot scale | BBM | 60.9 | 0.26 |
| Strain | Volumetric Productivity of Biomass at μmax (g L−1 day−1) | C16–C18 Ratio (wt %) | Lipid Content (wt %) |
|---|---|---|---|
| KGE8 cultivated in Laboratory | 0.696 | 95.1 | 59.4 |
| KGE8 Cultivated in Pilot scale | 0.163 | 77.8 | 60.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Ahn, Y.; Pandi, K.; Ji, M.-K.; Yun, H.-S.; Choi, J.-Y. Microalgae Cultivation in Pilot Scale for Biomass Production Using Exhaust Gas from Thermal Power Plants. Energies 2019, 12, 3497. https://doi.org/10.3390/en12183497
Park S, Ahn Y, Pandi K, Ji M-K, Yun H-S, Choi J-Y. Microalgae Cultivation in Pilot Scale for Biomass Production Using Exhaust Gas from Thermal Power Plants. Energies. 2019; 12(18):3497. https://doi.org/10.3390/en12183497
Chicago/Turabian StylePark, Sanghyun, Yongtae Ahn, Kalimuthu Pandi, Min-Kyu Ji, Hyun-Shik Yun, and Jae-Young Choi. 2019. "Microalgae Cultivation in Pilot Scale for Biomass Production Using Exhaust Gas from Thermal Power Plants" Energies 12, no. 18: 3497. https://doi.org/10.3390/en12183497
APA StylePark, S., Ahn, Y., Pandi, K., Ji, M.-K., Yun, H.-S., & Choi, J.-Y. (2019). Microalgae Cultivation in Pilot Scale for Biomass Production Using Exhaust Gas from Thermal Power Plants. Energies, 12(18), 3497. https://doi.org/10.3390/en12183497