Techno-Economic Optimization of CO2-to-Methanol with Solid-Oxide Electrolyzer
Abstract
1. Introduction
2. System Description and Modeling
2.1. Solid-Oxide Electrolysis Process
2.2. Methanol Synthesis and Upgrading
2.3. Heat Exchanger Network and Steam Turbine Network
3. Methodology
3.1. Thermodynamic Performance Indicators
3.2. Economic Performance Indicators
3.2.1. Capital Investment
3.2.2. Operational Cost
3.2.3. Payback Time and Levelized Methanol Production Cost
3.3. Optimization Methodology and Problem Definition
4. Results and Discussion
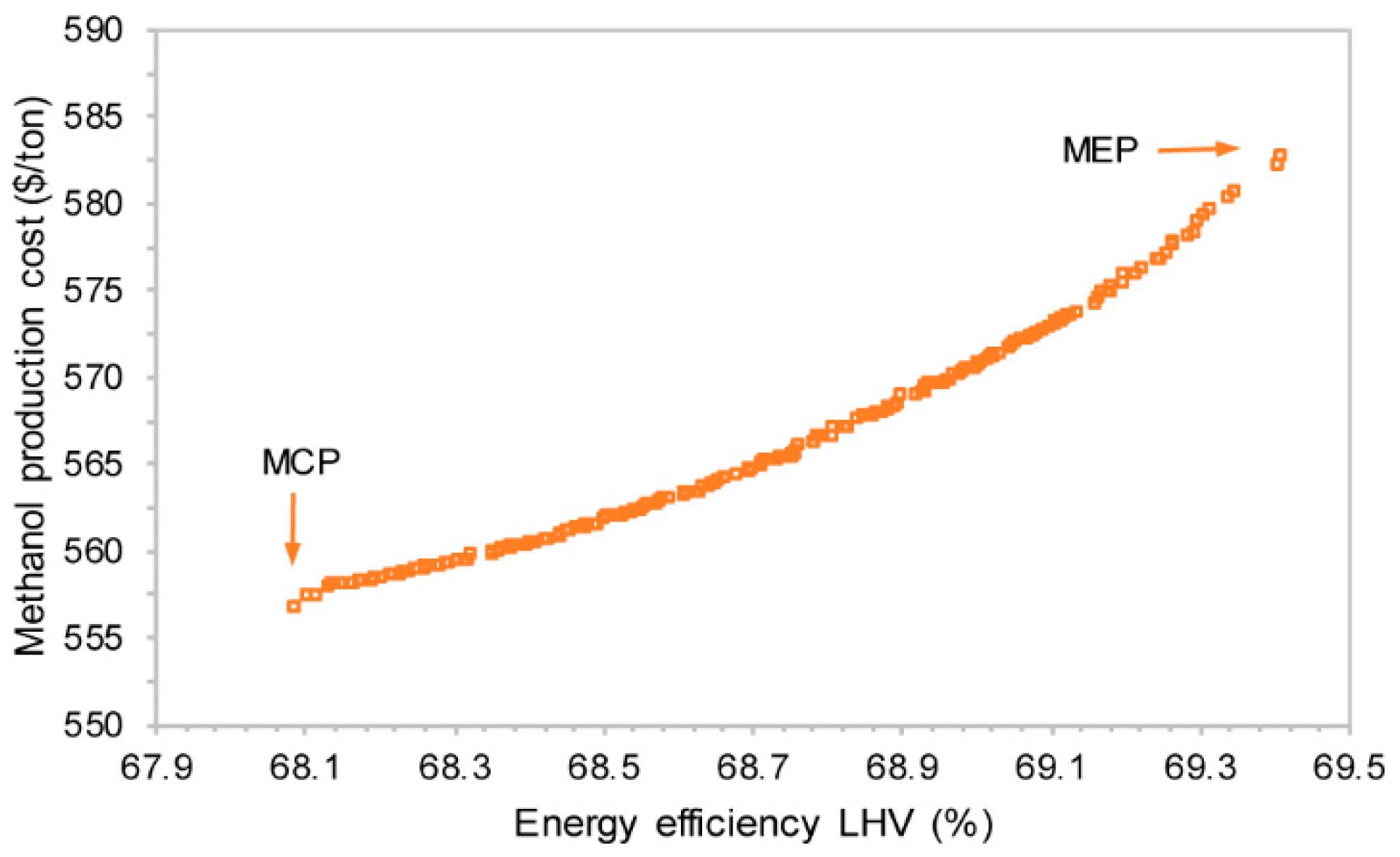
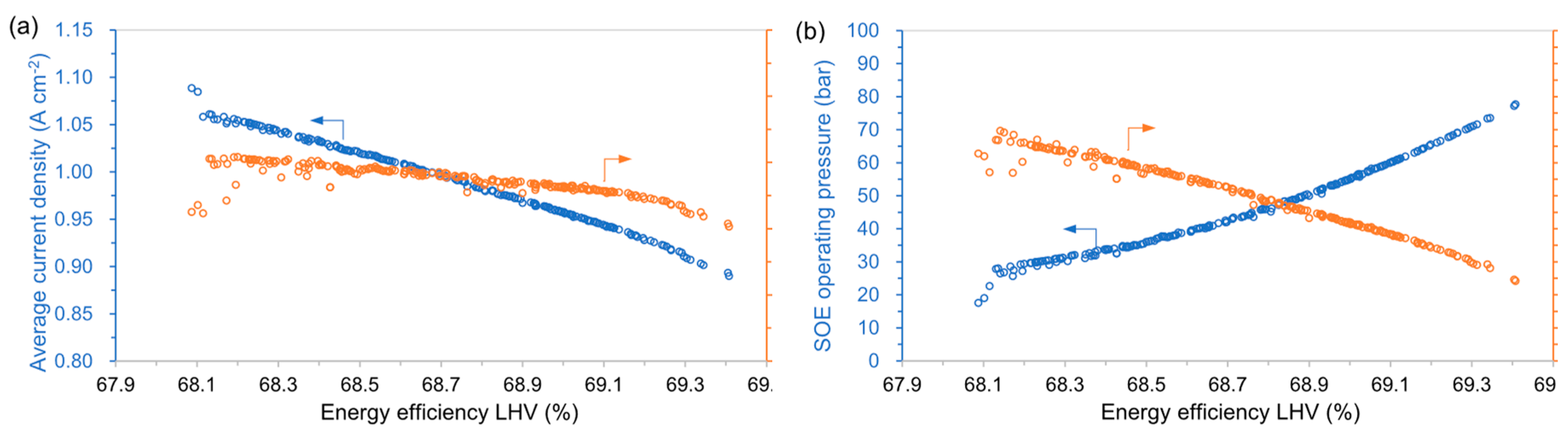
4.1. Trade-Off between Efficiency and Cost
4.2. Heat Integration
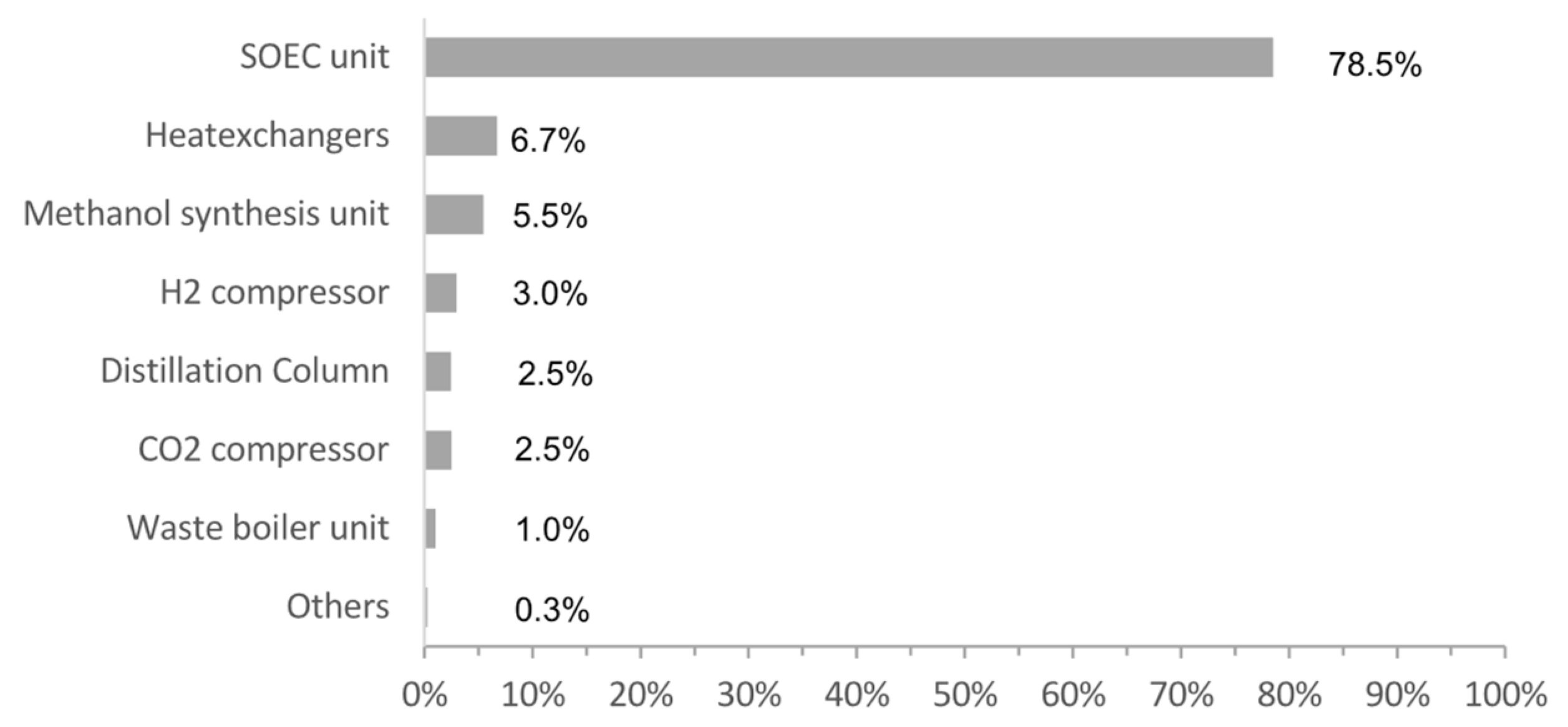
4.3. Cost Distribution
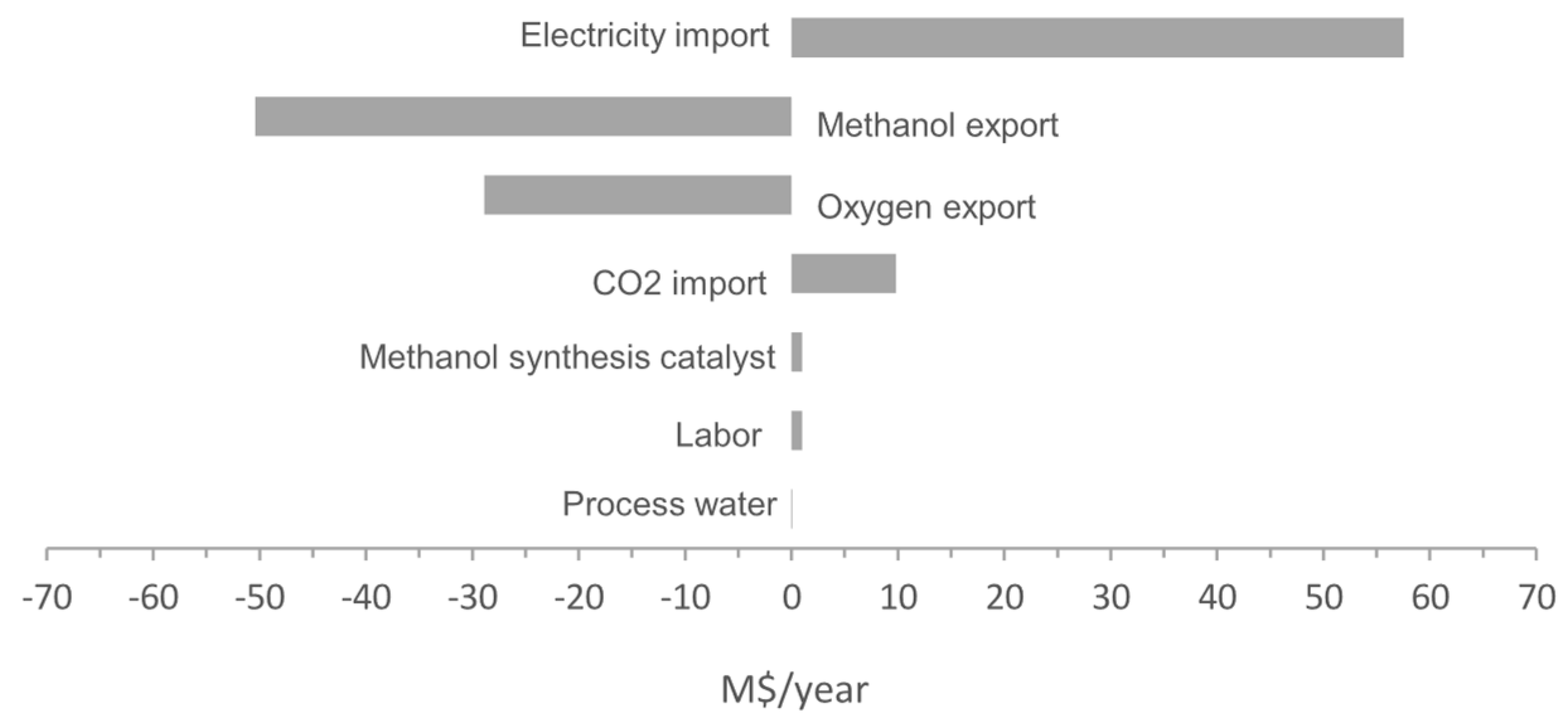
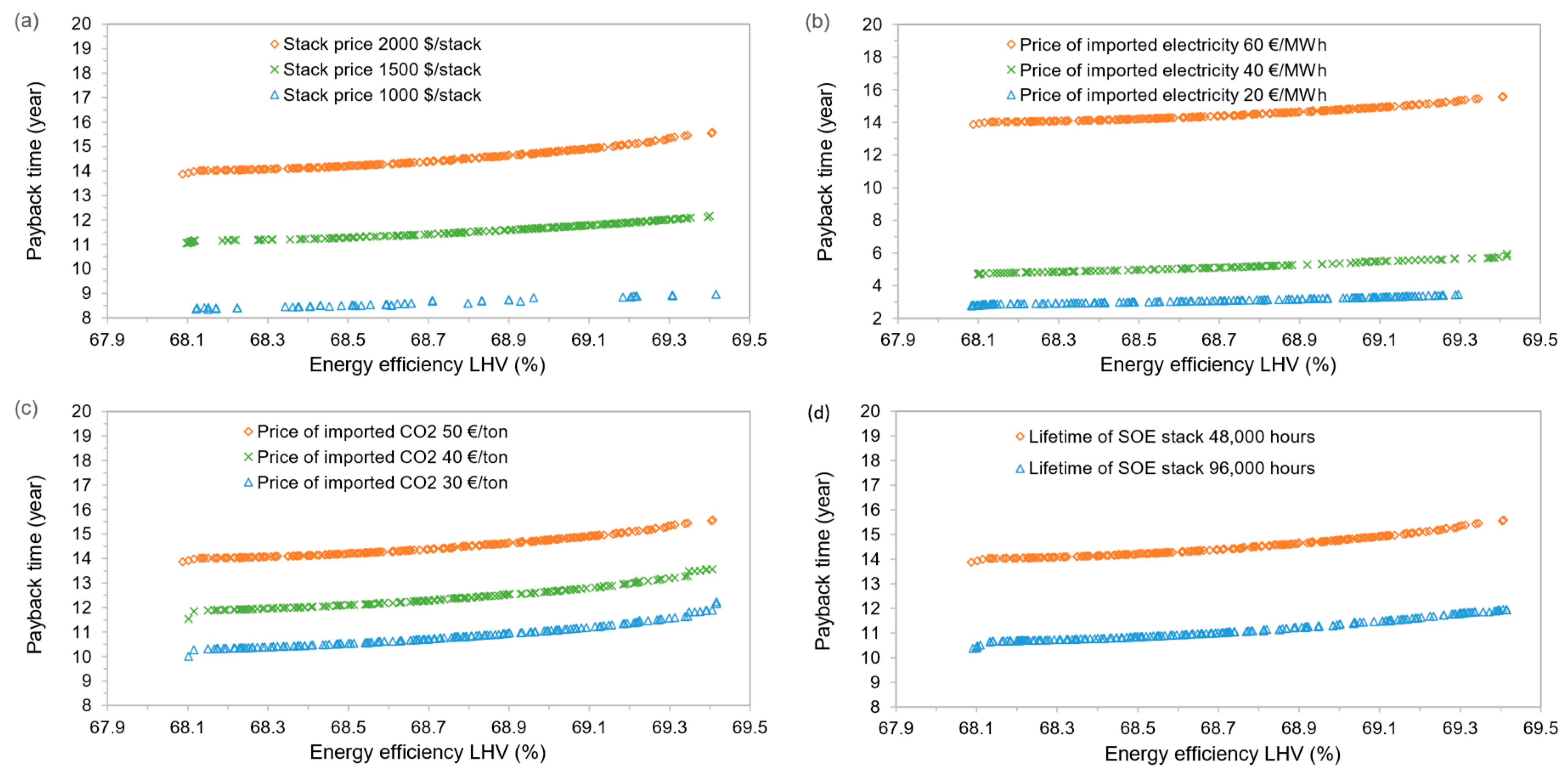
4.4. Sensitivity Analysis
5. Conclusions
- There is a trade-off between the system efficiency and methanol production cost. Increasing system efficiency will slightly increase the cost. The operating window of solid-oxide stack is rather narrow due to the high heat requirement of steam generation and methanol upgrading. The optimized system is with a system efficiency of above 68% with annual utilization of carbon dioxide 150 kton.
- The bottleneck of the heat integration comes from steam generation and distillation column. The SOE needs to operate at highly exothermic mode to drive the whole system and avoid heat transfer from/to the stack. High-pressure operation of the SOE stack is also preferred to avoid the work by hydrogen compression. The steam generation is driven mainly by the heat from the SOE outlet and the waste boiler. Almost no electrical heating is required for all designs.
- The economic performance is dominated by SOE stack, the electricity price, and the product sale revenue. Given the current market assumptions on these factors, the concept is not economically feasible with a payback time over 13 years. However, if reducing the stack to 1000 $/stack and electricity price down to 20 €/MWh, which is available in some countries, the payback time can be reduced to even less than 3 years, indicating its competitiveness for specific economic conditions.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Abbreviations | |
| AE | Alkaline electrolyzer |
| AMPL | A mathematical programming language |
| AOH | Annual operating hours |
| CCS | Carbon capture storage |
| CCU | Carbon capture and utilization |
| CCUS | Carbon capture, utilization and storage |
| EES | Electrical energy storage |
| HEN | Heat exchanger network |
| HHV | Higher heating value |
| IEA | International energy agency |
| IRENA | International renewable energy agency |
| LCI | Lifecycle indicator |
| LHV | Lower heating value |
| MCP | Minimum cost point |
| MEP | Maximum efficiency point |
| MILP | Mixed integer linear programming |
| MSS | Methanol synthesis system |
| MOO | Multi-objective optimizer |
| PEC | Purchased equipment cost |
| PEME | Polymer electrolyte membrane electrolyzer |
| PtH | Power-to-hydrogen |
| SE | Steam electrolysis |
| SOE | Solid-oxide electrolysis |
| USD | United states dollar |
| Greek Symbols | |
| η | Energy efficiency |
| τ | Payback time |
| Mathematical Symbols | |
| CAPEX | Capital expenditure |
| Cdep | Depreciation cost |
| Cinv | Investment cost |
| Copt | Operational cost |
| Methanol revenue | |
| Byproduct revenue | |
| CMeOH | Methanol production cost |
| Electric power input | |
| ∆H298K | Standard enthalpy of formation |
| i | Annual interest rate |
| M | Syngas modular |
| Mass flow of obtained methanol | |
| n | Number of electrons exchanged in the reaction |
| OPEX | Operational expenditure |
| PMeOH | Methanol production |
| T | Temperature |
| Subscripts | |
| n | Project lifetime |
References
- Bukhtiyarova, M.; Lunkenbein, T.; Kähler, K.; Schlögl, R. Methanol Synthesis from Industrial CO2 Sources: A Contribution to Chemical Energy Conversion. Catal. Lett. 2017, 147, 416–427. [Google Scholar] [CrossRef]
- Olah, G.A. Beyond oil and gas: The methanol economy. Angew. Chem. Int. Ed. 2005, 44, 2636–2639. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Jin, H.; Gao, L.; Han, W. Techno-economic evaluation of coal-based polygeneration systems of synthetic fuel and power with CO2 recovery. Energy Convers. Manag. 2011, 52, 274–283. [Google Scholar] [CrossRef]
- Pérez-Fortes, M.; Schöneberger, J.C.; Boulamanti, A.; Tzimas, E. Methanol synthesis using captured CO2 as raw material: Techno-economic and environmental assessment. Appl. Energy 2016, 161, 718–732. [Google Scholar] [CrossRef]
- Coal Industry Advisory Board (CIAB) to the International Energy Agency (IEA). An International Commitment to CCS: Policies and Incentives to Enable a Low-Carbon Energy Future; IEA: Paris, France, 2016. [Google Scholar]
- Peters, M.; Kler, B.; Kuckshinrichs, W.; Leitner, W.; Markewitz, P.; Müller, T. Chemical technologies for exploiting and recycling carbon dioxide into the value chain. ChemSusChem 2011, 4, 1216–1240. [Google Scholar] [CrossRef] [PubMed]
- Huber, G.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Swanson, R.; Statrio, J.; Brown, R. Techno-Economic Analysis of Biofuels Production Based on Gasification; Technical Report; NREL: Golden, CO, USA, 2010. [Google Scholar]
- Olah, G.A.; Goeppert, A.; Prakash, G.S. Chemical Recycling of Carbon Dioxide to Methanol and Dimethyl Ether: From Greenhouse Gas to Renewable, Environmentally Carbon Neutral Fuels and Synthetic Hydrocarbons. J. Org. Chem. 2009, 74, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Kung, H. Methanol Production and Use; Marcel Dekker: New York, NY, USA, 1994. [Google Scholar]
- Botta, G.; Solimeo, M.; Leone, P.; Aravind, P.V. Thermodynamic Analysis of Coupling a SOEC in Co-Electrolysis Mode with the Dimethyl Ether Synthesis. Fuel Cells 2015, 15, 669–681. [Google Scholar] [CrossRef]
- Moradi, G.; Yaripour, F.; Abbasian, H.; Rahmanzadeh, M. Intrinsic reaction rate and the effects of operating conditions in dimethyl ether synthesis from methanol dehydration. Korean J. Chem. Eng. 2010, 27, 1435–1440. [Google Scholar] [CrossRef]
- Specht, M.; Staiss, F.; Bandi, A.; Weimer, T. Comparison of the renewable transportation fuels, liquid hydrogen and methanol, with gasoline—Energetic and economic aspects. Int. J. Hydrogen Energy 1998, 23, 387–396. [Google Scholar] [CrossRef]
- Kuliyev, S.A.; Aksongur, S.; Mat, M.D.; Ibrahimoğlud, B.; Kozlue, M.D. Direct Methanol Solid Oxide Fuel Cell. J. Electrochem. Soc. 2009, 25, 1093–1098. [Google Scholar]
- Andika, R.; Nandiyanto, A.B.D.; Putra, Z.A.; Bilad, M.R.; Kim, Y.; Yun, C.M.; Lee, M. Co-electrolysis for power-to-methanol applications. Renew. Sustain. Energy Rev. 2018, 95, 227–241. [Google Scholar] [CrossRef]
- Ye, J.; Johnson, J. Catalytic hydrogenation of CO2 to methanol in a Lewis pair functionalized MOF. Catal. Sci. Technol. 2016, 6, 8392–8405. [Google Scholar] [CrossRef]
- Huff, C.A.; Sanford, M.S. Cascade catalysis for the homogeneous hydrogenation of CO2 to methanol. J. Am. Chem. Soc. 2011, 133, 18122–18125. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Himeda, Y.; Muckermann, J.T.; Manbeck, G.F.; Fujita, E. CO2 hydrogenation to formate and methanol as an alternative to photo- and electrochemical CO2 reduction. Chem. Rev. 2015, 115, 12936–12973. [Google Scholar] [CrossRef] [PubMed]
- Everton, S.; Chakib, B. Design and simulation of a methanol production plant from CO2 hydrogenation. J. Clean. Prod. 2013, 57, 38–45. [Google Scholar]
- Goeppert, A.; Czaun, M.; Jones, J.P.; Prakash, G.S.; Olah, G.A. Recycling of carbon dioxide to methanol and derived products—Closing the loop. Chem. Soc. Rev. 2014, 43, 7957–8194. [Google Scholar] [CrossRef]
- Nieminen, H.; Laari, A.; Koiranen, T. CO2 Hydrogenation to Methanol by a Liquid-Phase Process with Alcoholic Solvents: A Techno-Economic Analysis. Processes 2019, 7, 405. [Google Scholar] [CrossRef]
- Arakawa, H.; Dubois, J.; Sayama, K. Selective conversion of CO2 to methanol by catalytic hydrogenation over promoted copper catalyst. Energy Convers. Manag. 1992, 33, 521–528. [Google Scholar] [CrossRef]
- Masahiro, F.; Hisanori, A.; Mutsuo, T.; Yoshie, S. Hydrogenation of carbon dioxide over Cu-Zn-Cr oxide catalysts. Chem. Soc. Jpn. 1994, 67, 546–550. [Google Scholar]
- Graves, C.; Ebbesen, S.D.; Mogensen, M.; Lackner, K.S. Sustainable hydrocarbon fuels by recycling CO2 and H2O with renewable or nuclear energy. Renew. Sustain. Energy Rev. 2011, 15, 1–23. [Google Scholar] [CrossRef]
- Tremel, A.; Wasserscheid, P.; Baldauf, M.; Hammer, T. Techno-economic analysis for the synthesis of liquid and gaseous fuels based on hydrogen production via electrolysis. Int. J. Hydrogen Energy 2015, 40, 11457–11464. [Google Scholar] [CrossRef]
- Götz, M.; Lefebvre, J.; Mörs, F.; Koch, A.M.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef]
- Gahleitner, G. Hydrogen from renewable electricity: An international review of power-to-gas pilot plants for stationary applications. Hydrog. Energy 2013, 38, 2039–2061. [Google Scholar] [CrossRef]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future cost and performance of water electrolysis: An expert elicitation study. Hydrog. Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.; Leung, D. Technological development of hydrogen production by solid oxide electrolyzer cell (SOEC). Hydrog. Energy 2008, 33, 2337–2354. [Google Scholar] [CrossRef]
- Leonzio, G. Design and feasibility analysis of a Power-to-Gas plant in Germany. J Clean. Prod. 2017, 162, 609–623. [Google Scholar] [CrossRef]
- Sterner, M.; Stadler, I. Energiespeicher-Bedarf, Technologien, Integration; Springer-Verlag: Berlin, Germany, 2014. [Google Scholar]
- Pozzo, M.; Lanzini, A.; Santarelli, M. Enhanced biomass-to-liquid (BTL) conversion process through high temperature co-electrolysis in a solid oxide electrolysis cell (SOEC). Fuel 2015, 145, 39–49. [Google Scholar] [CrossRef]
- Wang, L.; Pérez-Fortes, M.; Madi, H.; Diethelm, S.; Van herle, J.; Maréchal, F. Optimal design of solid-oxide electrolyzer based power-to-methane systems: A comprehensive comparison between steam electrolysis and co-electrolysis. Appl. Energy 2018, 211, 1060–1079. [Google Scholar] [CrossRef]
- Wang, L.; Megha, R.; Stefan, D.; Tzu-En, L.; Hanfei, Z.; Maréchal, F. Power-to-methane via co-electrolysis of H2O and CO2: Process modeling, pressurized operation and internal methanation. Appl. Energy 2019, 250, 1432–1445. [Google Scholar] [CrossRef]
- Wang, L.; Johannes, D.; Francois, M.; Van herle, J. Trade-off designs and comparative exergy evaluation of solid-oxide electrolyzer based power-to-methane plants. Int. J. Hydrogen Energy 2018, 44, 9529–9543. [Google Scholar] [CrossRef]
- Wang, L.; Chen, M.; Kungas, R.; Tzu-En, L.; Stefan, D.; Maréchal, F.; Van herle, J. Power-to-fuels via solid-oxide electrolyzer: Operating window and techno-economics. Renew. Sustain. Energy Rev. 2019, 110, 174–187. [Google Scholar] [CrossRef]
- Turton, R.; Bailie, R.C.; Whiting, W.B.; Shaeiwitz, J.A. Analysis, Synthesis and Design of Chemical Processes; Pearson Education: London, UK, 2008. [Google Scholar]
- Kauw, M.; Benders, R.M.J.; Visser, C. Green methanol from hydrogen and carbon dioxide using geothermal energy and/or hydropower in Iceland or excess renewable electricity in Germany. Energy 2015, 90, 208–217. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Z.; Sharma, S.; Mian, A.; Lin, T.E.; Tsatsaronis, G.; Maréchal, F.; Yang, Y. A review of evaluation, optimization and synthesis of energy systems: Methodology and application to thermal power plants. Energies 2019, 12, 73. [Google Scholar] [CrossRef]
- Wallerand, A.S.; Kermani, M.; Kantor, I.D.; Maréchal, F. Optimal heat pump integration in industrial processes. Appl. Energy 2018, 219, 68–92. [Google Scholar] [CrossRef]
- Kermani, M.; Wallerand, A.S.; Kantor, I.D.; Maréchal, F. Generic superstructure synthesis of organic Rankine cycles for waste heat recovery in industrial processes. Appl. Energy 2018, 212, 1203–1225. [Google Scholar] [CrossRef]
- Peduzzi, E.; Boissonnet, G.; Haartemmer, G.; Marechal, F. Thermo-economic analysis and multi-objective optimisation of lignocellulosic biomass conversion to Fischer–Tropsch fuels. Sustain. Energy Fuels 2018, 2, 1069–1084. [Google Scholar] [CrossRef]
- Hamelinck, C.N.; Faaji, A.P.C.; den Uil, H.; Borrigter, H. Production of FT transportation fuels from biomass; technical options, process analysis and optimisation, and development potential. Energy 2004, 29, 1743–1771. [Google Scholar] [CrossRef]
- Tan, E.C.; Talmadge, M.; Dutta, A.; Hensley, J.; Schaldie, J.; Biddy, M.; Humbird, D.; Snowden-Swan, L.; Ross, J.; Sexton, D.; et al. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbons via Indirect Liquefaction, Thermochemical Research Pathway to High-Octane Gasoline Blendstock through Methanol/dimethyl Ether Intermediates; Technical Report: NREL/TP-5100-62402; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2015. [Google Scholar]
- Regis, A.; David, C.; Annabelle, B.; Mathieu, M. Bottom-up cost evaluation of SOEC systems in the range of 10e100 MW. Int. J. Hydrogen Energy 2018, 43, 20309–20322. [Google Scholar]
- Albarelli, J.Q.; Onorati, S.; Caliandro, P.; Peduzzi, E.; Meireles, M.A.; Marechal, F.; Ensinas, A.V. Multi-objective optimization of a sugarcane biorefinery for integrated ethanol and methanol production. Energy 2017, 138, 1281–1290. [Google Scholar] [CrossRef]
- Gerber, L. Integration of Life Cycle Assessment in the Conceptual Design of Renewable Energy Conversion Systems. Ph.D. Thesis, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 2012. [Google Scholar]
- Tock, L. Thermo-Environomic Optimisation of Fuel Decarbonisation Alternative Processes for Hydrogen and Power Production. Ph.D. Thesis, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 2013. [Google Scholar]
- Mian, A. Optimal Design Methods Applied to Solar-Assisted Hydrothermal Gasification Plants. Ph.D. Thesis, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 2016. [Google Scholar]
- Exchange Rate. Available online: www.x-rates.com/historical/?from=USD&amount=1&date=2018-05-08 (accessed on 5 August 2018).
- European Commission. Quarterly Report on European Electricity Markets, Fourth Quarter of 2017; European Commission, Directorate-General for Energy, Market Observatory for Energy: Brussels, Belgium, 2018. [Google Scholar]
- Asif, M.; Gao, X.; Lv, H.; Xi, X.; Dong, P. Catalytic hydrogenation of CO2 from 600 MW supercritical coal power plant to produce methanol: A techno-economic analysis. Int. J. Hydrogen Energy 2018, 43, 2726–2741. [Google Scholar] [CrossRef]
- Guilera, J.; Morante, J.R.; Andreu, T. Economic viability of SNG production from power and CO2. Energy Convers. Manag. 2018, 162, 218–224. [Google Scholar] [CrossRef]


| Variable | Lower Bound | Upper Bound | Unit |
|---|---|---|---|
| SOE operating pressure | 1.1 | 78 | bar |
| SOE steam utilization | 0.3 | 0.8 | - |
| Steam feed flowrate | 0.5 | 15 | sccm/cm2 |
| Sweep-gas feed flowrate | 0.1 | 36 | sccm/cm2 |
| Unit | Base Unit | Base Year | α | Ref. | ||
|---|---|---|---|---|---|---|
| SOE single stack a | 2 × 10−3 | - | - | - | - | - |
| Methanol reactor | 3.5 | 44.28 | ton/d methanol | 2011 | 0.8 | [44] |
| Steam turbine system | 5.9 | 10.3 | MWh | 2002 | 0.65 | [43] |
| Economic Parameter | Value | Unit | Ref. |
|---|---|---|---|
| Project lifetime | 25 | year | [46] |
| Loan interest rate | 10 | % | [46] |
| Annual operating hours (AOH) | 7200 | hours/year | - |
| Exchange rate (€ to $) | 1.18 | - | [50] |
| Electricity price (import) | 73.16 | $/MWh | [51] |
| Methanol price | 504 | $/ton | [52] |
| Catalyst (methanol reactor) | 21.36 | $/kg | [44] |
| Catalyst lifetime (methanol reactor) | 4 | year | [44] |
| Oxygen price | 177 | $/ton | [53] |
| Carbon dioxide captured | 59 | $/ton | [53] |
| Carbon dioxide trading | 5.9 | $/ton | [53] |
| Process water price | 0.4756 | $/ton | [52] |
| Operator salary | 52,900 | $/year | [37] |
| Design | (%) | SOE Electricity Consumption (MW) | Import Electricity (MW) | Investment Costs (M$) | Optimal Costs ($/Year) | Levelized MeOH Cost ($/Ton) | Payback Time (Years) |
|---|---|---|---|---|---|---|---|
| MEP | 69.4 | 107 | 110 | 171 | 68.3 | 582 | 15.6 |
| MCP | 68.0 | 108 | 113 | 134 | 69.4 | 557 | 13.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Wang, L.; Van herle, J.; Maréchal, F.; Desideri, U. Techno-Economic Optimization of CO2-to-Methanol with Solid-Oxide Electrolyzer. Energies 2019, 12, 3742. https://doi.org/10.3390/en12193742
Zhang H, Wang L, Van herle J, Maréchal F, Desideri U. Techno-Economic Optimization of CO2-to-Methanol with Solid-Oxide Electrolyzer. Energies. 2019; 12(19):3742. https://doi.org/10.3390/en12193742
Chicago/Turabian StyleZhang, Hanfei, Ligang Wang, Jan Van herle, François Maréchal, and Umberto Desideri. 2019. "Techno-Economic Optimization of CO2-to-Methanol with Solid-Oxide Electrolyzer" Energies 12, no. 19: 3742. https://doi.org/10.3390/en12193742
APA StyleZhang, H., Wang, L., Van herle, J., Maréchal, F., & Desideri, U. (2019). Techno-Economic Optimization of CO2-to-Methanol with Solid-Oxide Electrolyzer. Energies, 12(19), 3742. https://doi.org/10.3390/en12193742







