Lithium Hydroxide Reaction for Low Temperature Chemical Heat Storage: Hydration and Dehydration Reaction
Abstract
1. Introduction
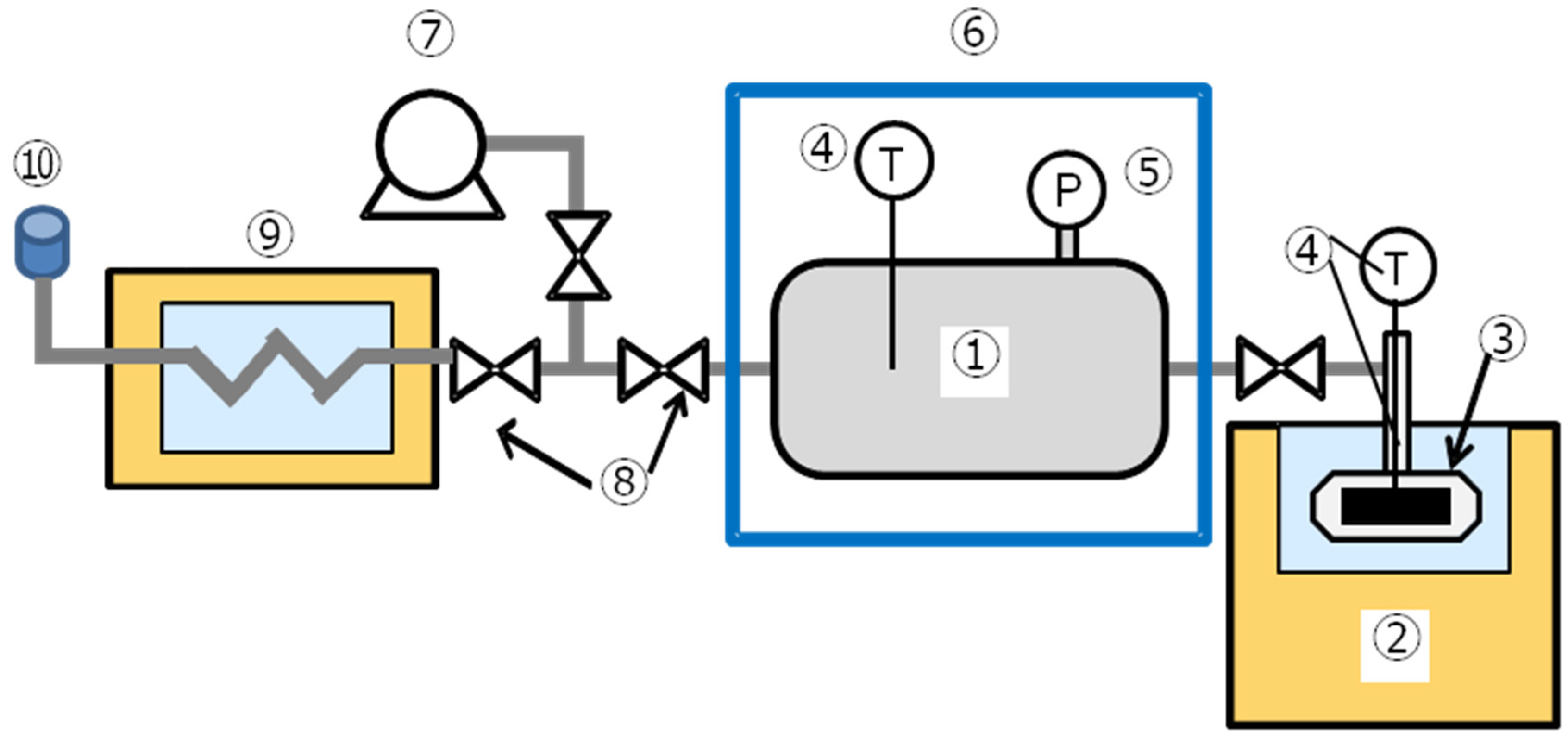
2. Experimental Apparatus and Method
2.1. The Constant Volume Method
2.2. Hydration Reaction Procedure
2.3. Dehydration Reaction Procedure
3. Discussion
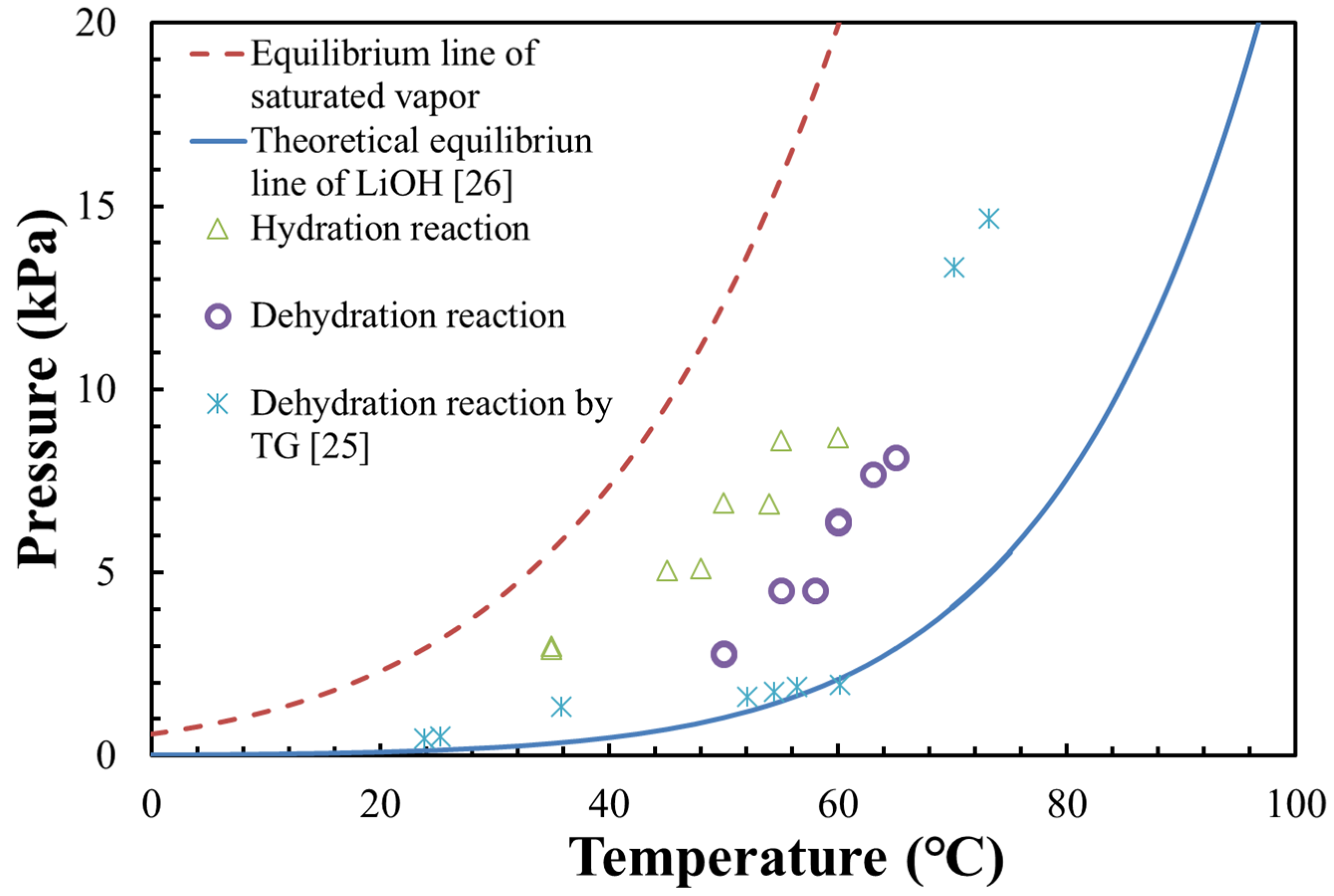
3.1. Pressure–Temperature Diagram of LiOH
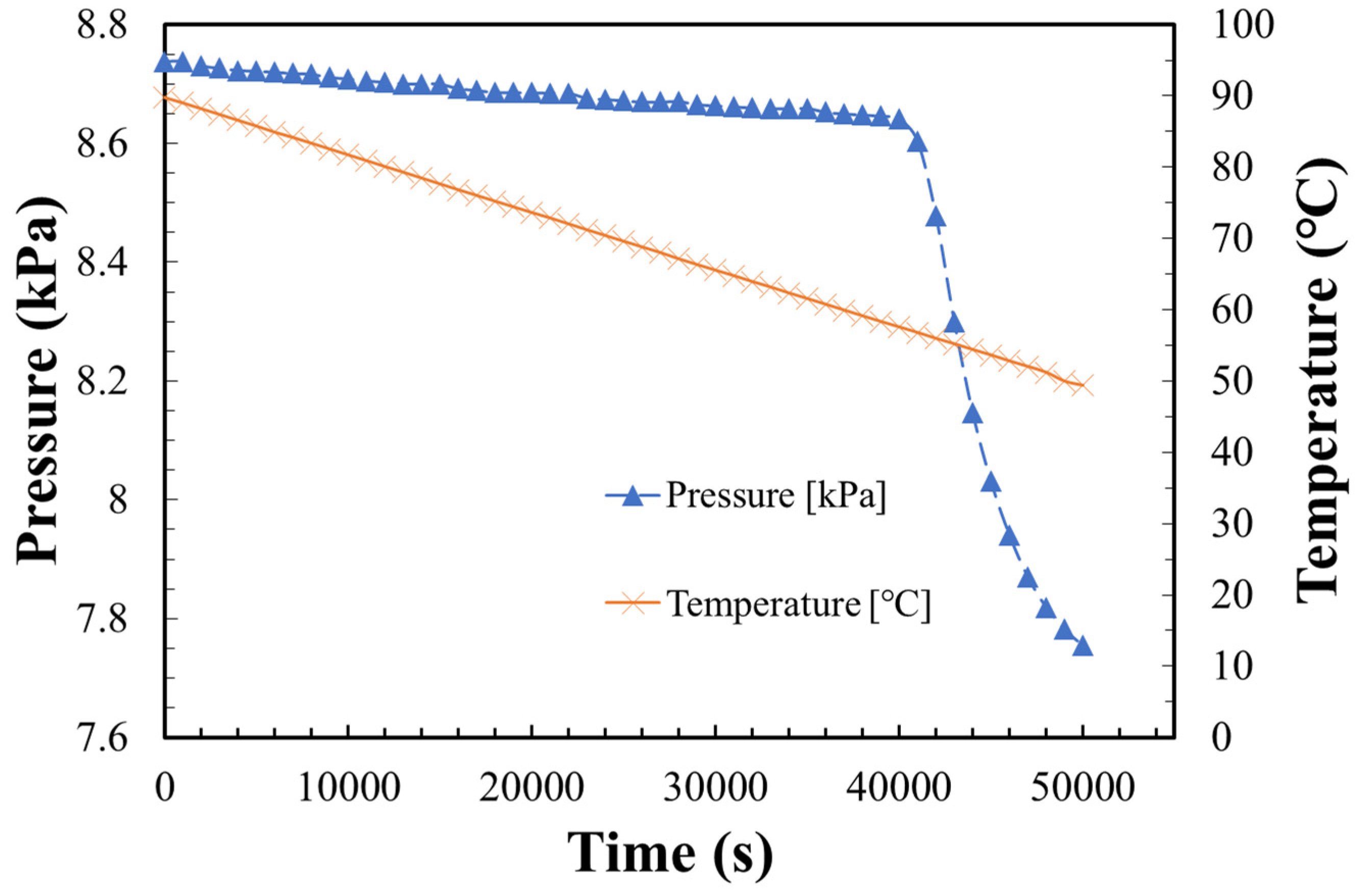
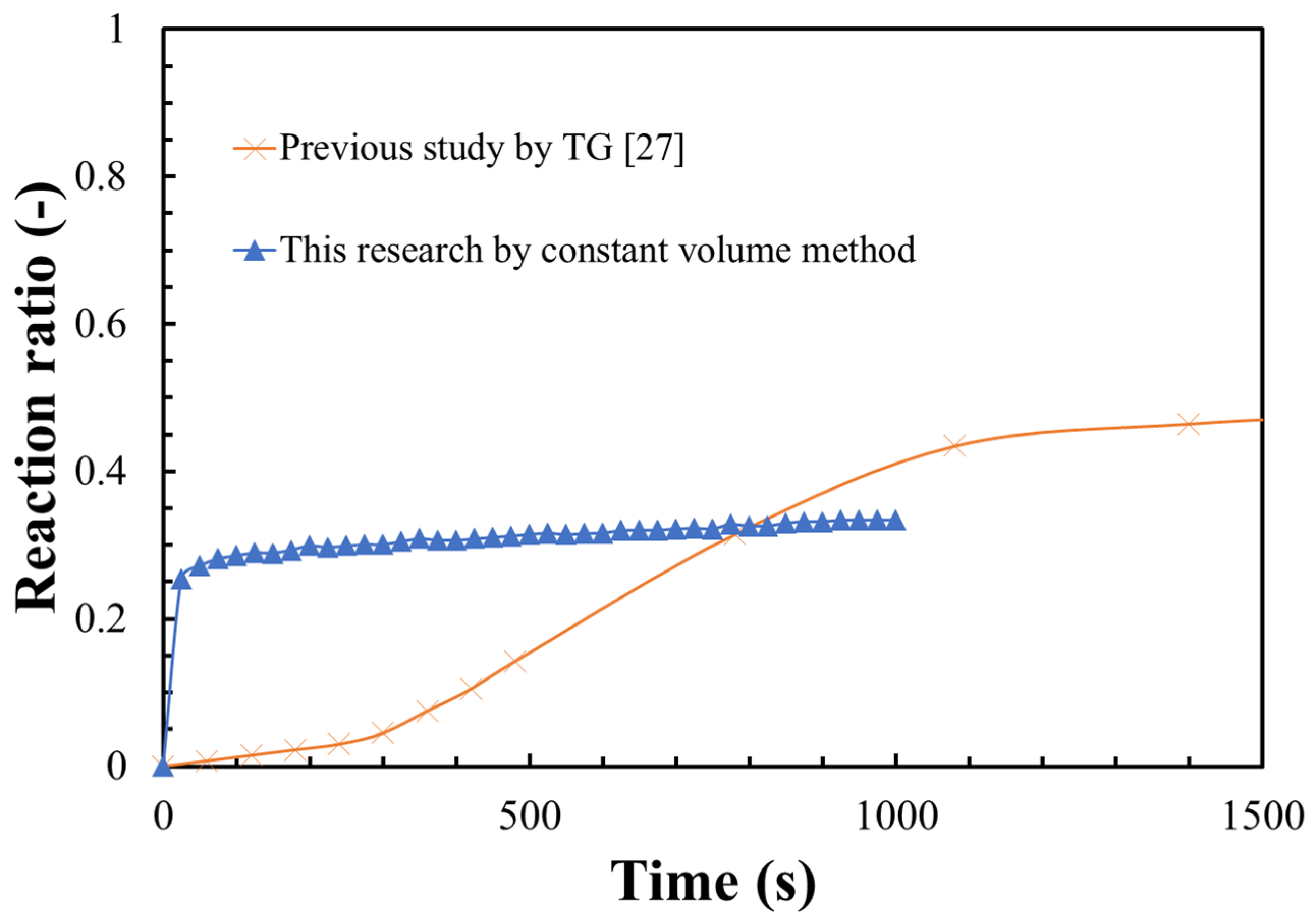
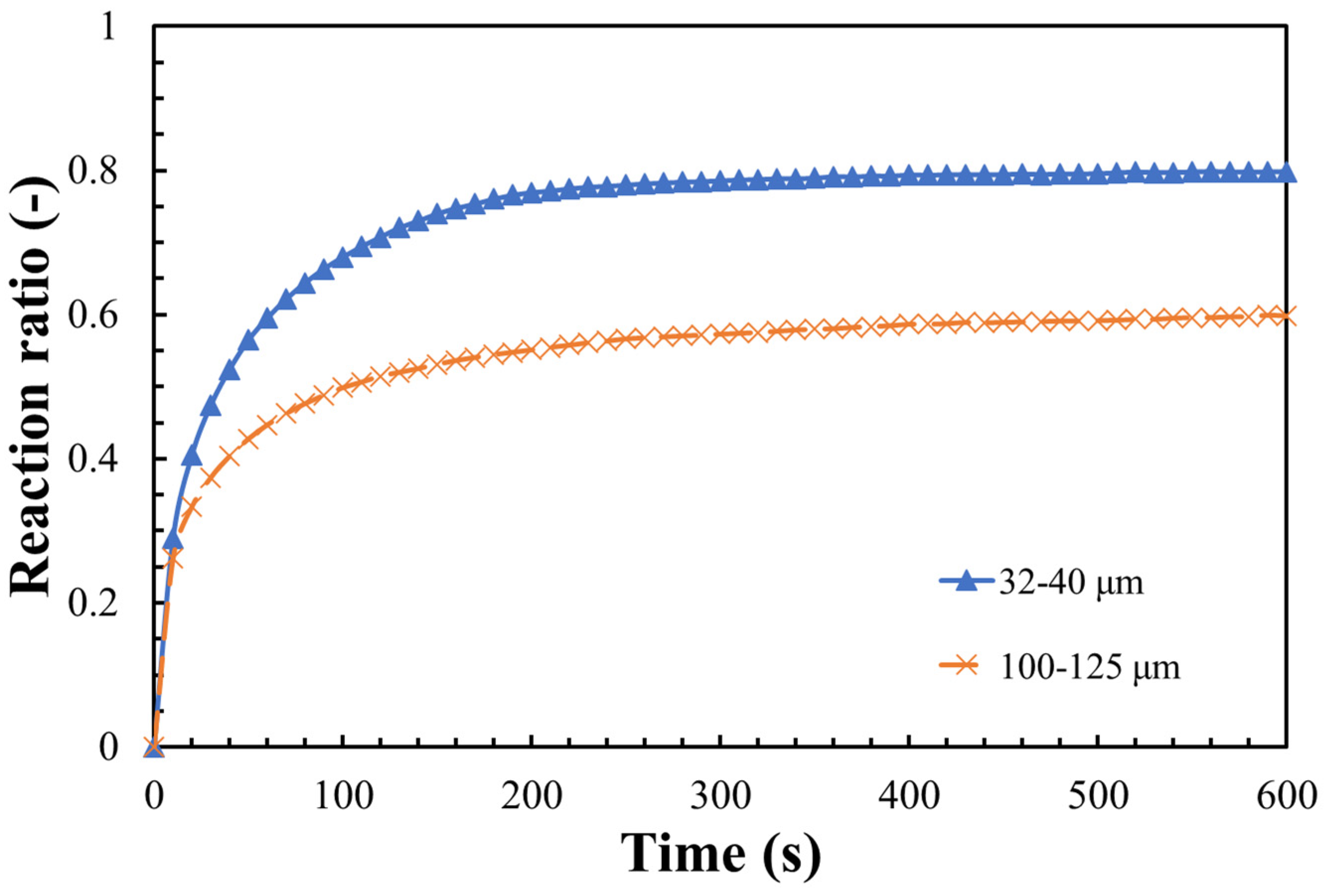
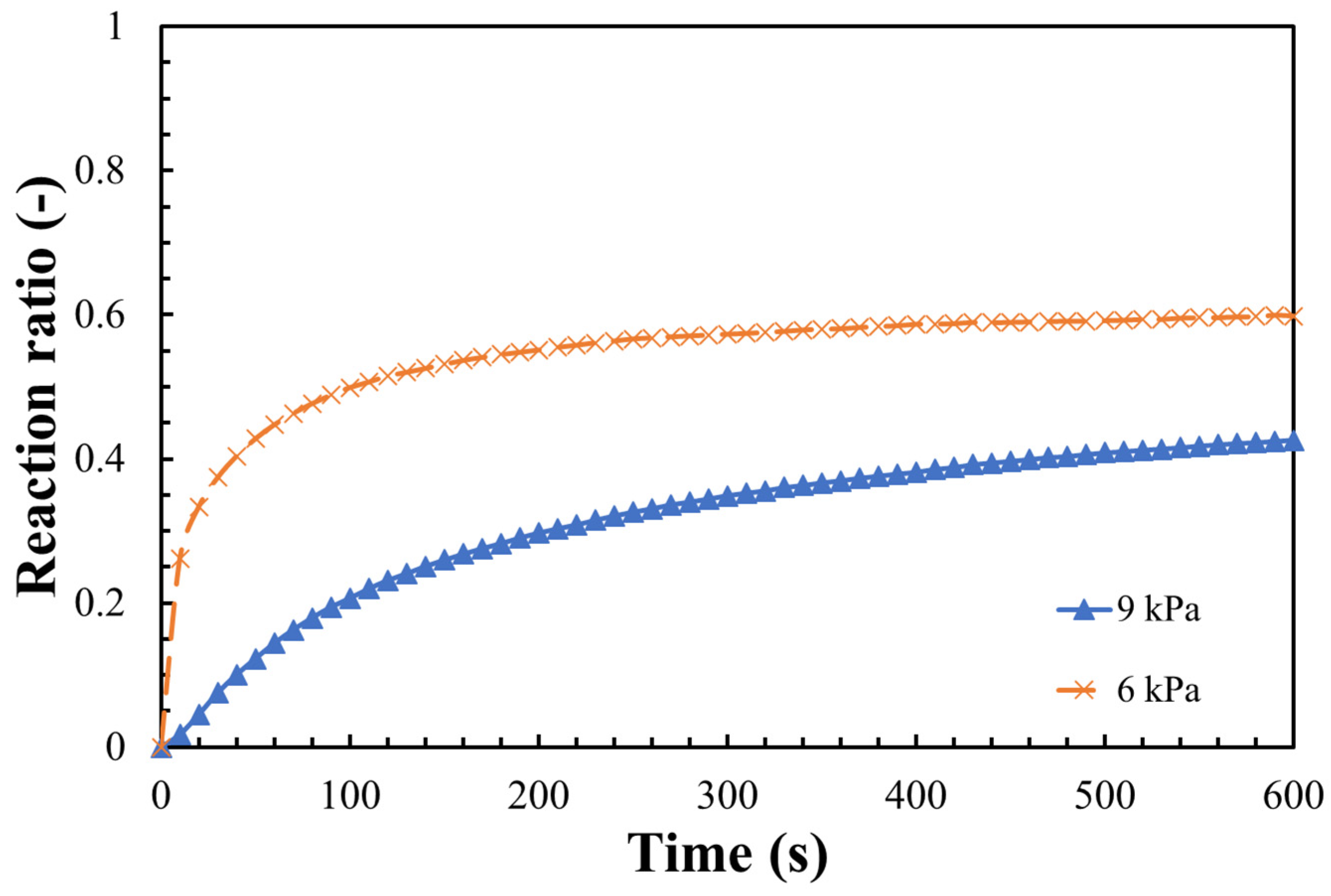
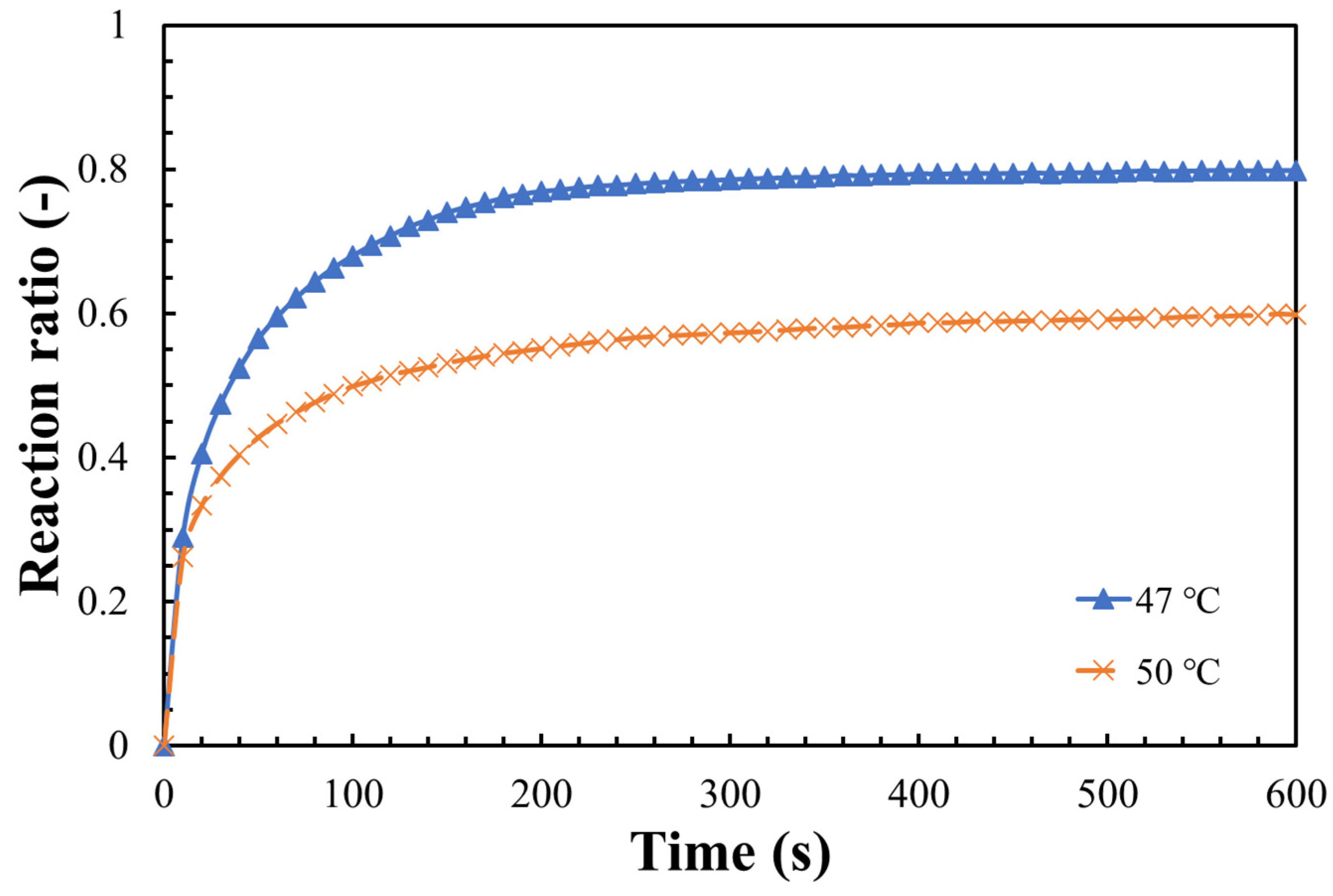
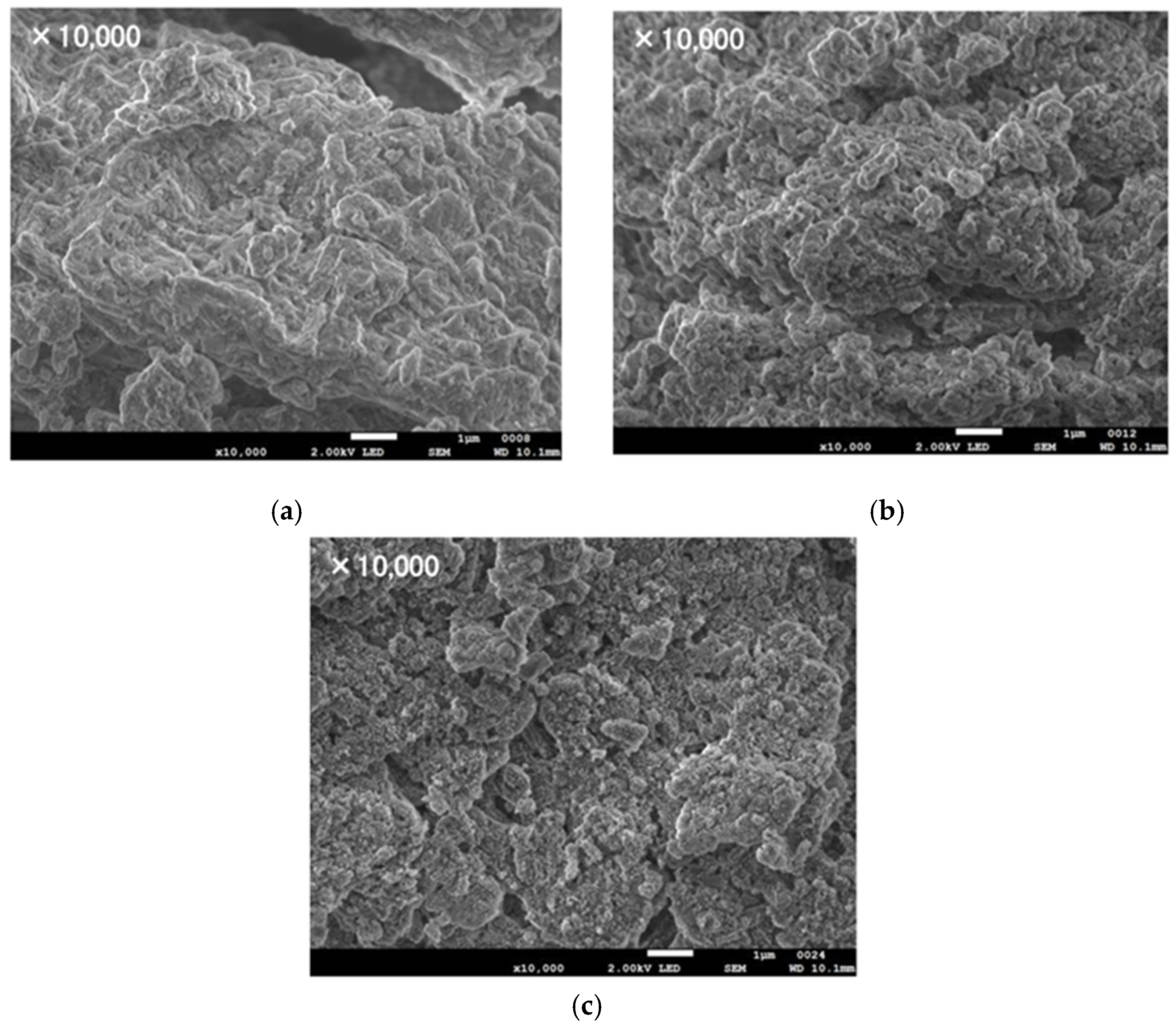
3.2. Hydration Reaction Rate of LiOH
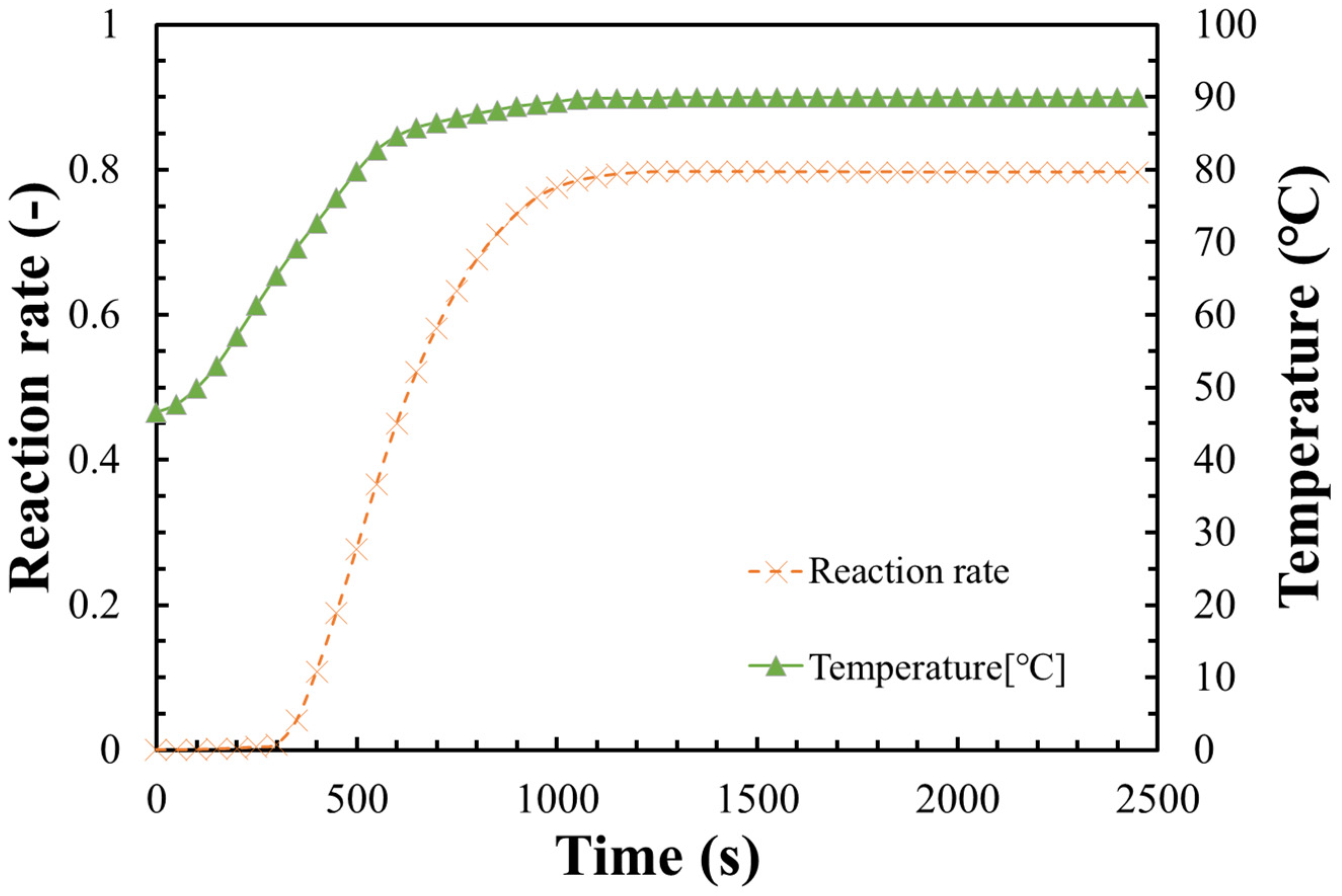
3.3. Dehydration Reaction Rate of LiOH
4. Conclusions
- The measurements of the dehydration reaction were carried out under vacuum condition. The dehydrated vapor could be easily escaped, leading to a lower dehydration temperature compared with the theoretical equilibrium line at atmosphere air condition;
- The steam diffusion could be greatly enhanced at vacuum condition. A thin layer of LiOH was uniformly dispersed in the reactor, which could greatly increase the heat transfer between the LiOH material and reactor, leading to a higher hydration reaction rate of LiOH;
- The steam pressure, reaction temperature, and the particle size of LiOH could greatly influence the hydration reaction. A maximum hydration reaction rate of 80% was obtained under the conditions of 47 °C, steam pressure of 9 kPa, and particle size 32–40 μm;
- LiOH exhibited a different reaction property with pure steam at vacuum condition. The store and reaction conditions of LiOH should be carefully considered in air conditions when applying LiOH as a heat storage material at low temperature.
Author Contributions
Funding
Conflicts of Interest
References
- Li, G. Sensible heat thermal storage energy and exergy performance evaluations. Renew. Sustain. Energy Rev. 2016, 53, 897–923. [Google Scholar] [CrossRef]
- Zeinelabdein, R.; Omer, S.; Gan, G. Critical review of latent heat storage systems for free cooling in buildings. Renew. Sustain. Energy Rev. 2018, 82, 2843–2868. [Google Scholar] [CrossRef]
- Yan, T.; Wang, R.; Li, T.; Wang, L.; Fred, I. A review of promising candidate reactions for chemical heat storage. Renew. Sustain. Energy Rev. 2015, 43, 13–31. [Google Scholar] [CrossRef]
- Saha, B.; Uddin, K.; Pal, A.; Thu, K. Emerging sorption pairs for heat pump applications: An overview. JMST Adv. 2019, 1, 161–180. [Google Scholar] [CrossRef]
- Alva, G.; Lin, Y.; Fang, G. An overview of thermal energy storage systems. Energy 2018, 144, 341–378. [Google Scholar] [CrossRef]
- Alva, G.; Lin, Y.; Huang, X.; Fang, G. Thermal energy storage materials and systems for solar energy applications. Renew. Sustain. Energy Rev. 2017, 68, 693–706. [Google Scholar] [CrossRef]
- Yu, N.; Wang, R.; Wang, L. Sorption thermal storage for solar energy. Prog. Energy Combust. Sci. 2013, 39, 489–514. [Google Scholar] [CrossRef]
- Scapino, L.; Zondag, H.; Bael, J.; Diriken, J.; Rindt, C. Energy density and storage capacity cost comparison of conceptual solid and liquid sorption seasonal heat storage systems for low-temperature space heating. Renew. Sustain. Energy Rev. 2017, 75, 1314–1331. [Google Scholar] [CrossRef]
- Li, T.; Wang, R.; Kiplagat, J. A target-oriented solid-gas thermochemical sorption heat transformer for integrated energy storage and energy upgrade. AIChE J. 2013, 59, 1334–1347. [Google Scholar] [CrossRef]
- Donkers, P.; Sögütoglu, L.; Huinink, H.; Fischer, H.; Adan, O. A review of salt hydrates for seasonal heat storage in domestic applications. Appl. Energy 2017, 45–68. [Google Scholar] [CrossRef]
- Van Essen, V.M.; Zondag, H.; Gores, J.C.; Bleijendaal, L.; Bakker, M.; Schuitema, R.; van Helden, W.G.J.; He, Z.; Rindt, C.C.M. Characterization of MgSO4 hydrate for thermochemical seasonal heat storage. J. Sol. Energy Eng. 2009, 131, 041014. [Google Scholar] [CrossRef]
- Boer, R.; Haije, W.; Veldhuis, J. Determination of structural, thermodynamic and phase properties in the Na2S–H2O system for application in a chemical heat pump. Thermochim. Acta 2002, 395, 3–49. [Google Scholar] [CrossRef]
- Lahmidi, H.; Mauran, S.; Goetz, V. Definition, test and simulation of a thermochemical storage process adapted to solar thermal systems. Sol. Energy 2006, 80, 883–893. [Google Scholar] [CrossRef]
- Zondag, H.; Kikkert, B.; Smeding, S.; Boer, R.; Bakker, M. Prototype thermochemical heat storage with open reactor system. Appl. Energy 2013, 109, 360–365. [Google Scholar] [CrossRef]
- Ferchaud, J. Experimental Study of Salt Hydrates for Thermochemical Seasonal Heat Storage. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, Netherlands, April 2016. [Google Scholar]
- Kubota, M.; Matsumoto, S.; Matsuda, H. Enhancement of hydration rate of LiOH by combining mesoporous carbon for Low-temperature chemical heat storage. Appl. Therm. Eng. 2019, 150, 858–863. [Google Scholar] [CrossRef]
- Yan, T.; Wang, R.; Li, T. Experimental investigation on thermochemical heat storage using manganese chloride/ammonia. Energy 2018, 143, 562–574. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, E. Study of ammoniated salts based thermochemical energy storage system with heat up-gradation: A thermodynamic approach. Energy 2017, 141, 1705–1716. [Google Scholar] [CrossRef]
- Stitou, D.; Mazet, N.; Mauran, S. Experimental investigation of a solid/gas thermochemical storage process for solar air-conditioning. Energy 2012, 41, 261–270. [Google Scholar] [CrossRef]
- Zhong, Y.; Critoph, R.; Thorpe, R.; Tamainot-Telto, Z.; Aristov, Y. Isothermal sorption characteristics of the BaCl2–NH3 pair in a vermiculite host matrix. Appl. Therm. Eng. 2007, 27, 2455–2462. [Google Scholar] [CrossRef]
- Li, S.; Huang, H.; Yang, X.; Bai, Y.; Li, J.; Kobayashi, N.; Kubota, M. Hydrophilic substance assisted low temperature LiOH•H2O based composite thermochemical materials for thermal energy storage. Appl. Therm. Eng. 2018, 706–711. [Google Scholar] [CrossRef]
- Li, S.; Huang, H.; Li, J.; Kobayashi, N.; Osaka, Y.; He, Z.; Yuan, H. The effect of 3D carbon nanoadditives on lithium hydroxide monohydrate based composite materials for highly efficient low temperature thermochemical heat storage. RSC Adv. 2018, 8, 8199–8208. [Google Scholar] [CrossRef]
- Kurosawa, R.; Ryu, J. Effect of LiOH addition on dehydration reaction of Mg(OH)2. J. Chem. Eng. Japan 2019, 52, 152–158. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Huang, H.; Li, J.; Kobayashi, N.; Kubota, M. Effect of carbon nanoadditives on lithium hydroxide monohydrate-based composite materials for low temperature chemical heat storage. Energies 2017, 52, 644. [Google Scholar] [CrossRef]
- Williams, D.; Miller, R. The Effect Water Vapor on the LiOH-CO2 Reaction. Part 1. Dynamic Isothermal System; Naval Research Lab: Washington DC, USA, 29 October 1969. [Google Scholar]
- Kubota, M. Novel Techniques of Latent Heat Thermal Storage, Chemical Thermal Storage and Latent Heat Transportation; CMC Publishing: Tokyo, Japan, 2016; pp. 109–116. (In Japanese) [Google Scholar]
- Pablo, J.; Aadersson, J.; Azoulay, M. Kinetic investigation of the sorption of water by lithium hydroxide. Thermochim. Acta 1987, 113, 87–94. [Google Scholar] [CrossRef]
- Williams, D.; Miller, R. Effect of water vapor on the LiOH-CO2 reaction. Dynamic isothermal system. Ind. Eng. Chem. Fundam. 1970, 9, 454–457. [Google Scholar] [CrossRef]


| Reference | Reaction Pair * | Description | Operation Conditions | Heat Storage Density (kJ/kg) | Heat Storage Density (kJ/L) |
|---|---|---|---|---|---|
| Van Essen et al. [11] | MgSO4/H2O (7–0.5) | Experimental, Open system, TG a and DSC b | Charging: 150 °C | 2498 | 3326 |
| Boer et al. [12] | Na2S/H2O (5–0.5) | Experimental | Charging: 83 °C | 3841 | 3564 |
| Lahmidi et al. [13] | SrBr2/H2O (6–1) | Experimental, Consolidated SrBr2 with expanded graphite | Charging: 80 °C | 900 | 630 |
| Zondag et al. [14] | MgCl2/H2O (6–2) | Experimental, DSC | Charging: 130 °C | 1717 | 2002 |
| Ferchaud [15] | Li2SO4/H2O (0–1) | Experimental | Charging: 103 °C | 457 | 943 |
| Kubota el al. [16] | LiOH/H2O (1–0) | Experimental | Charging: 64 °C | 1440 | 950 |
| Li et al. [9] | NH4Cl/NH3 (3–0) | Theoretical without sensible heat charging at 1167 kPa | Charging: 48 °C | 1652 | 1264 |
| - | NaBr/NH3 (5.25–0) | - | Charging: 51 °C | 1804 | 2887 |
| - | BaCl2/NH3 (8–0) | - | Charging: 56 °C | 1469 | 2833 |
| - | SrCl2/NH3 (8–1) | - | Charging: 96 °C | 1832 | 2794 |
| - | CaCl2/NH3 (8–1) | - | Charging: 99 °C | 2243 | 2423 |
| - | MnCl2/NH3 (6–2) | - | Charging: 152 °C | 1508 | 2246 |
| - | FeCl2/NH3 (6–2) | - | Charging: 186 °C | 1620 | 2560 |
| - | NiCl2/NH3 (6–2) | - | Charging: 259 °C | 1829 | 1757 |
| Group | Temperature (°C) | Particle Size (μm) | Steam Pressure (kPa) |
|---|---|---|---|
| A | 47 | 32–45 | 9 |
| B | 50 | 32–45 | 9 |
| C | 47 | 100–125 | 6 |
| D | 47 | 100–125 | 9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zeng, T.; Kobayashi, N.; Xu, H.; Bai, Y.; Deng, L.; He, Z.; Huang, H. Lithium Hydroxide Reaction for Low Temperature Chemical Heat Storage: Hydration and Dehydration Reaction. Energies 2019, 12, 3741. https://doi.org/10.3390/en12193741
Li J, Zeng T, Kobayashi N, Xu H, Bai Y, Deng L, He Z, Huang H. Lithium Hydroxide Reaction for Low Temperature Chemical Heat Storage: Hydration and Dehydration Reaction. Energies. 2019; 12(19):3741. https://doi.org/10.3390/en12193741
Chicago/Turabian StyleLi, Jun, Tao Zeng, Noriyuki Kobayashi, Haotai Xu, Yu Bai, Lisheng Deng, Zhaohong He, and Hongyu Huang. 2019. "Lithium Hydroxide Reaction for Low Temperature Chemical Heat Storage: Hydration and Dehydration Reaction" Energies 12, no. 19: 3741. https://doi.org/10.3390/en12193741
APA StyleLi, J., Zeng, T., Kobayashi, N., Xu, H., Bai, Y., Deng, L., He, Z., & Huang, H. (2019). Lithium Hydroxide Reaction for Low Temperature Chemical Heat Storage: Hydration and Dehydration Reaction. Energies, 12(19), 3741. https://doi.org/10.3390/en12193741





