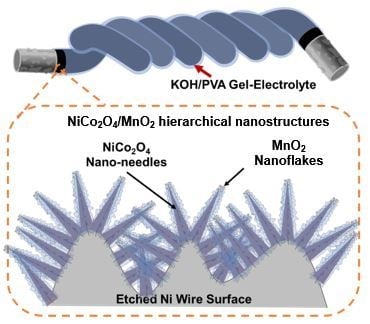
Fiber-Shaped Supercapacitors Fabricated Using Hierarchical Nanostructures of NiCo2O4 Nanoneedles and MnO2 Nanoflakes on Roughened Ni Wire
Abstract
1. Introduction
2. Experimental Section
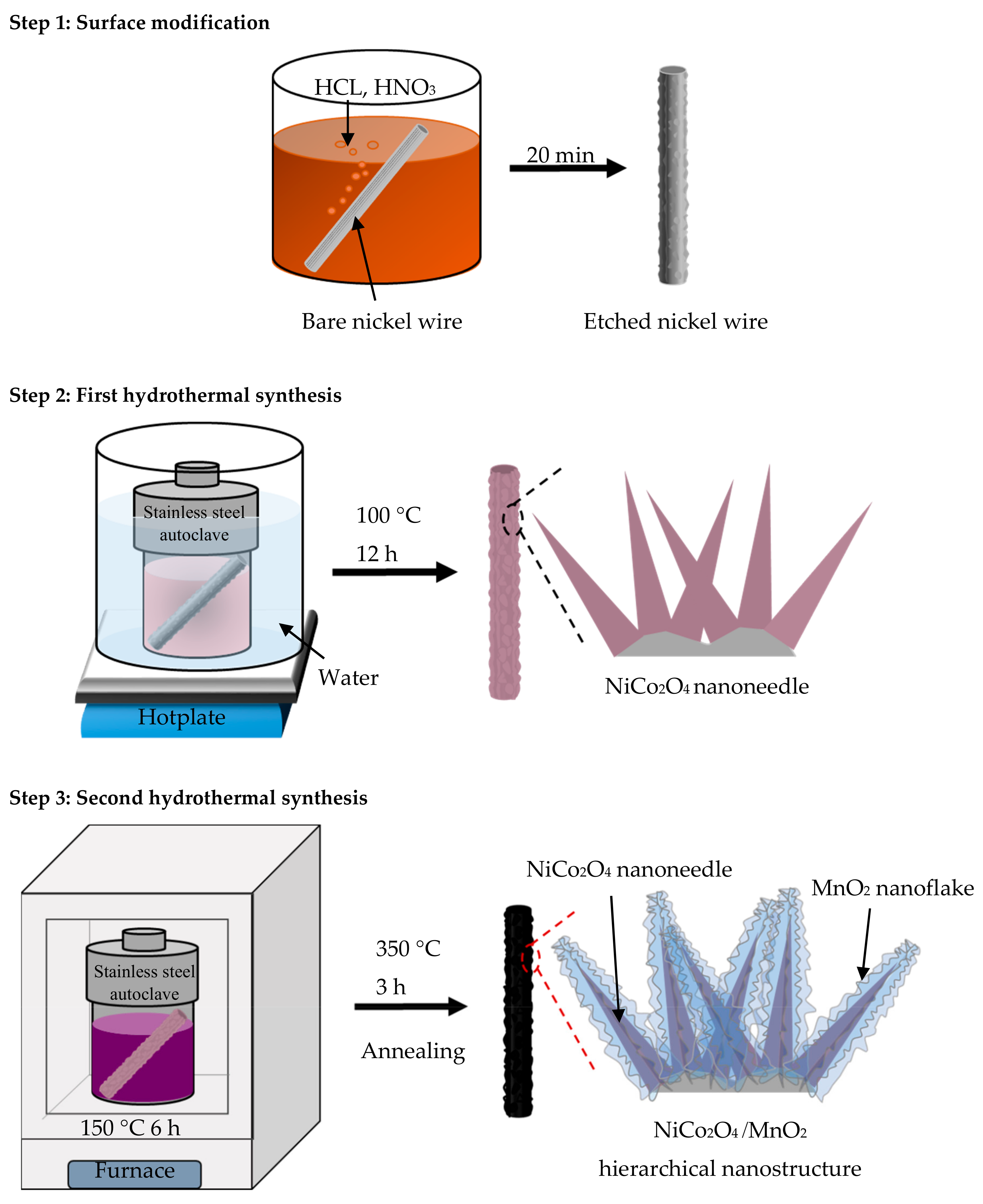
2.1. Synthesis of NiCo2O4/MnO2 Hierarchical Nanostructures
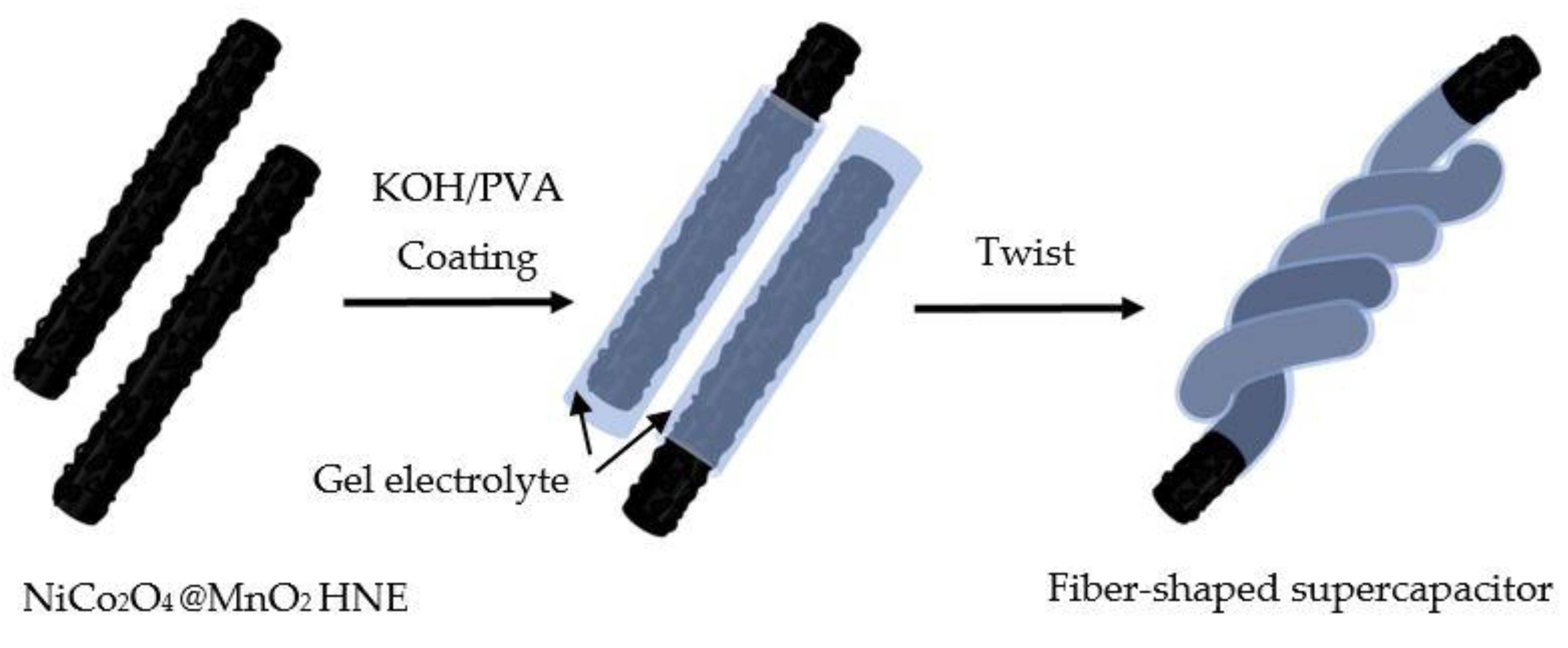
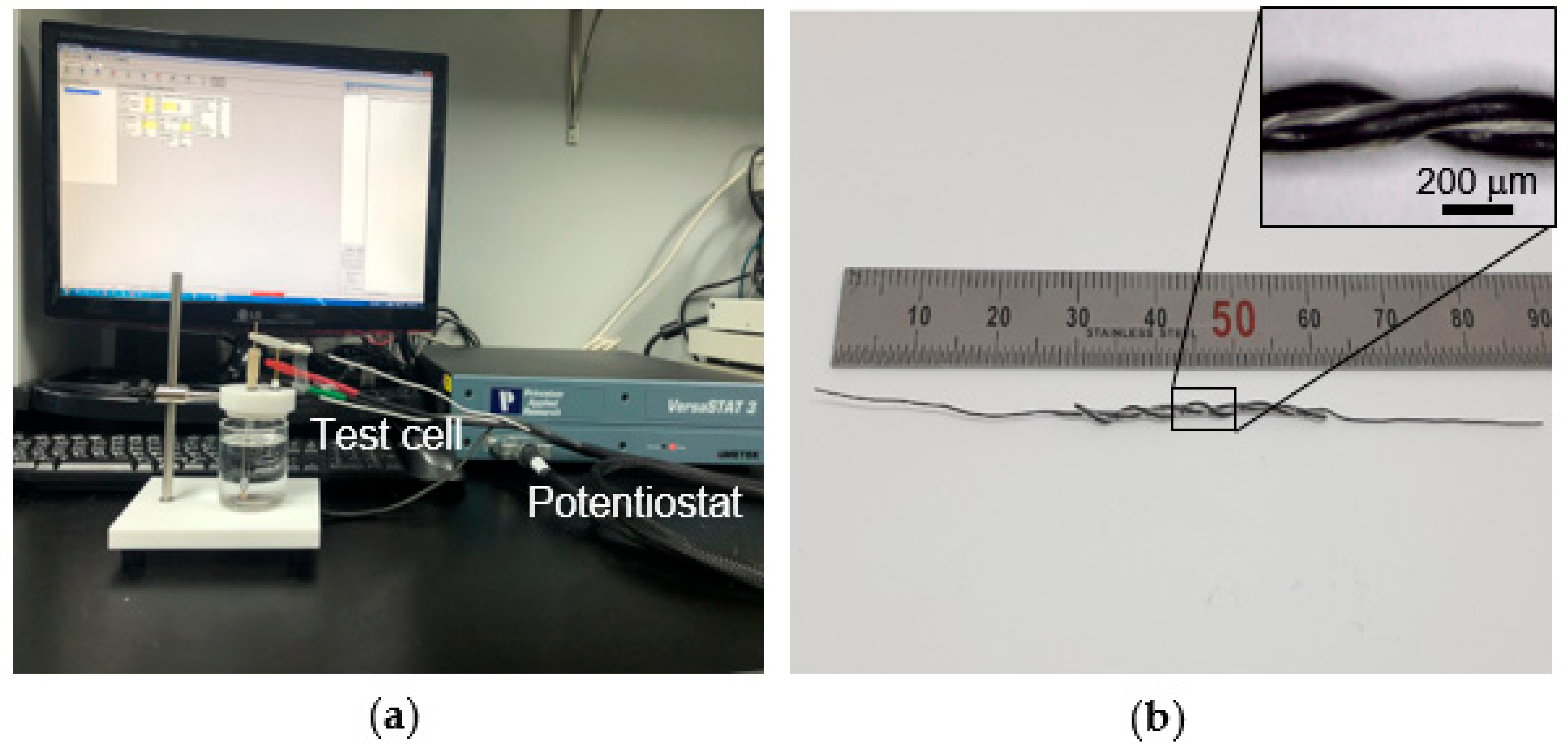
2.2. Fabrication of Fiber-Shaped Supercapacitor
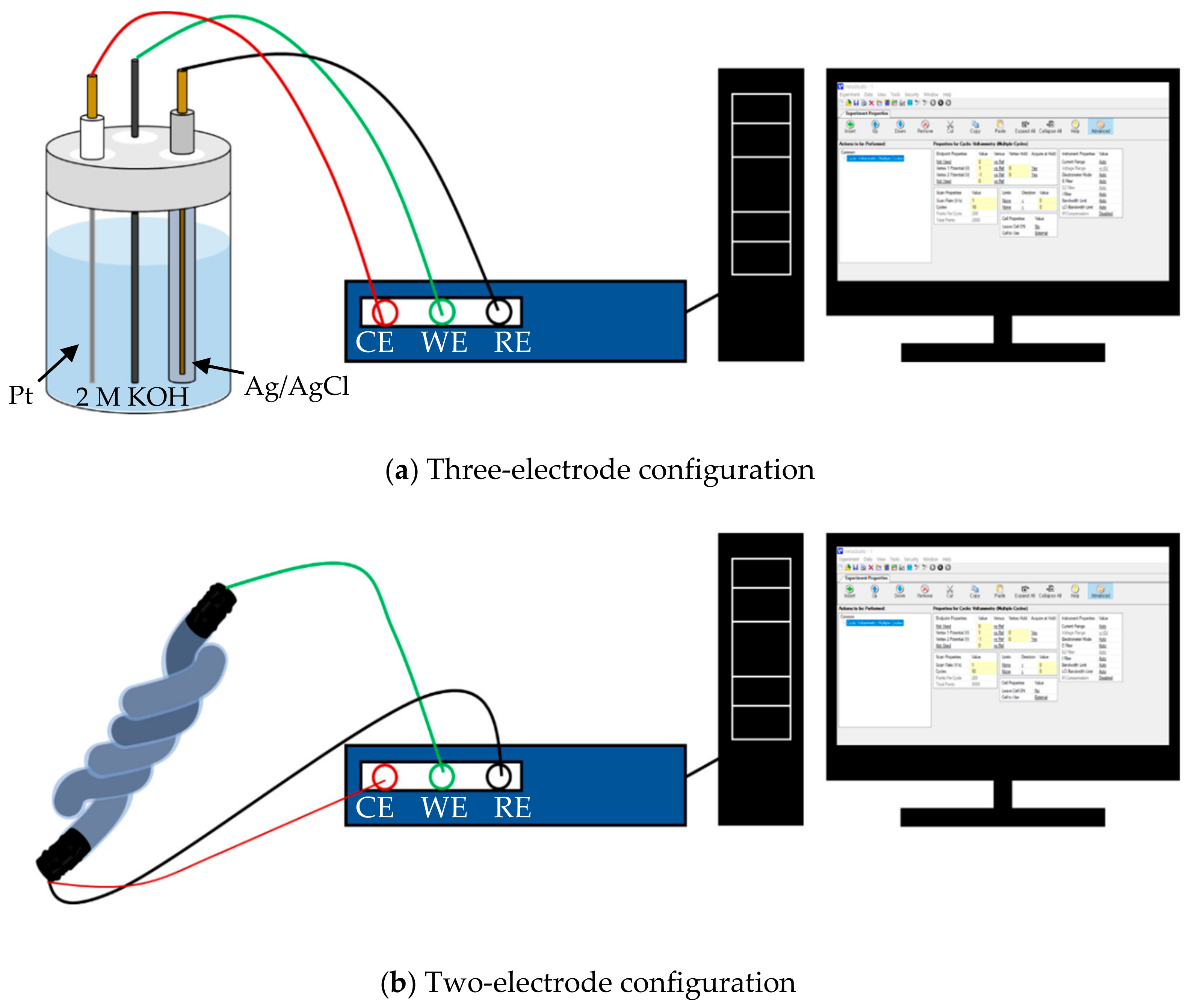
2.3. Characterization
3. Results and Discussion
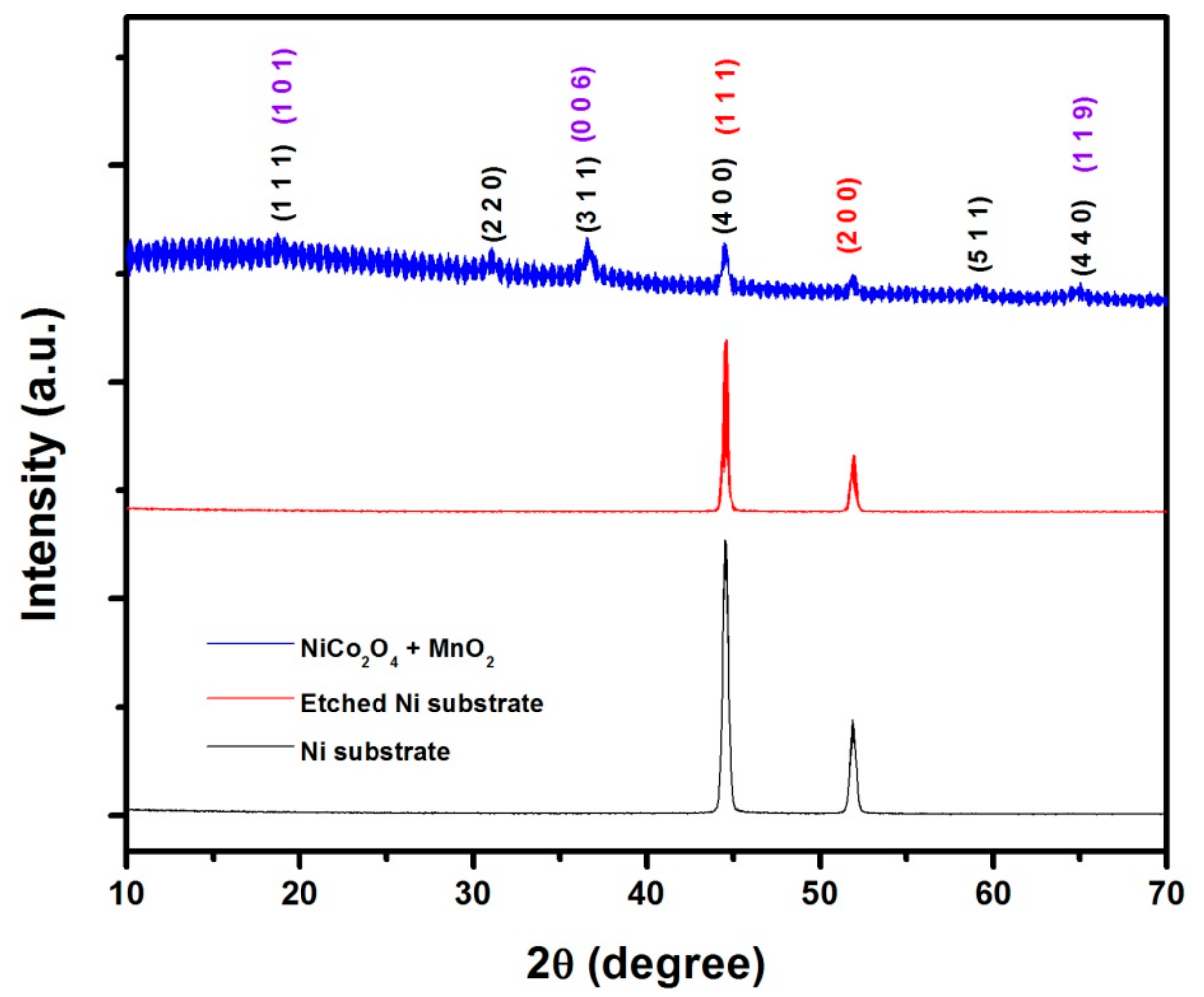
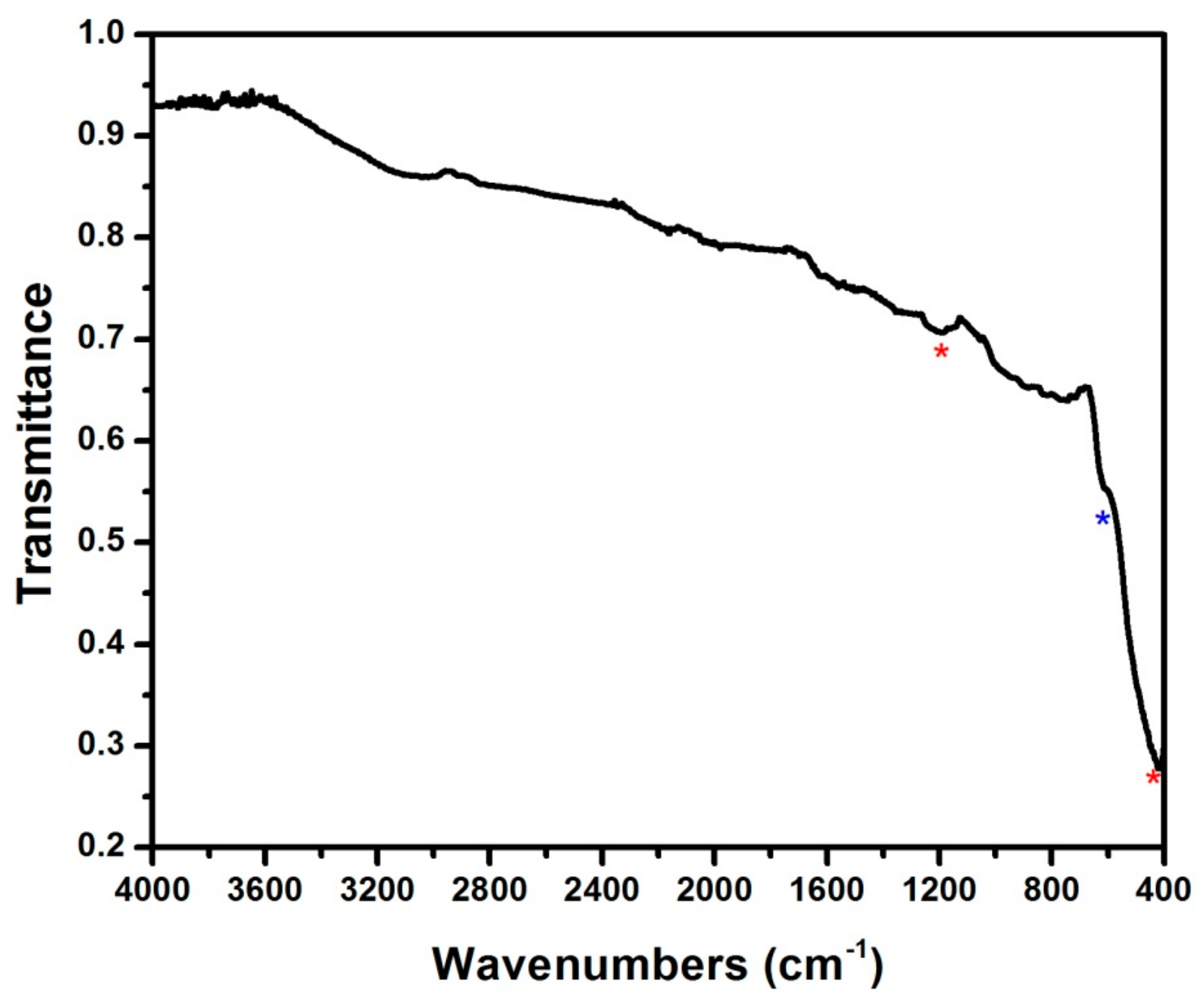
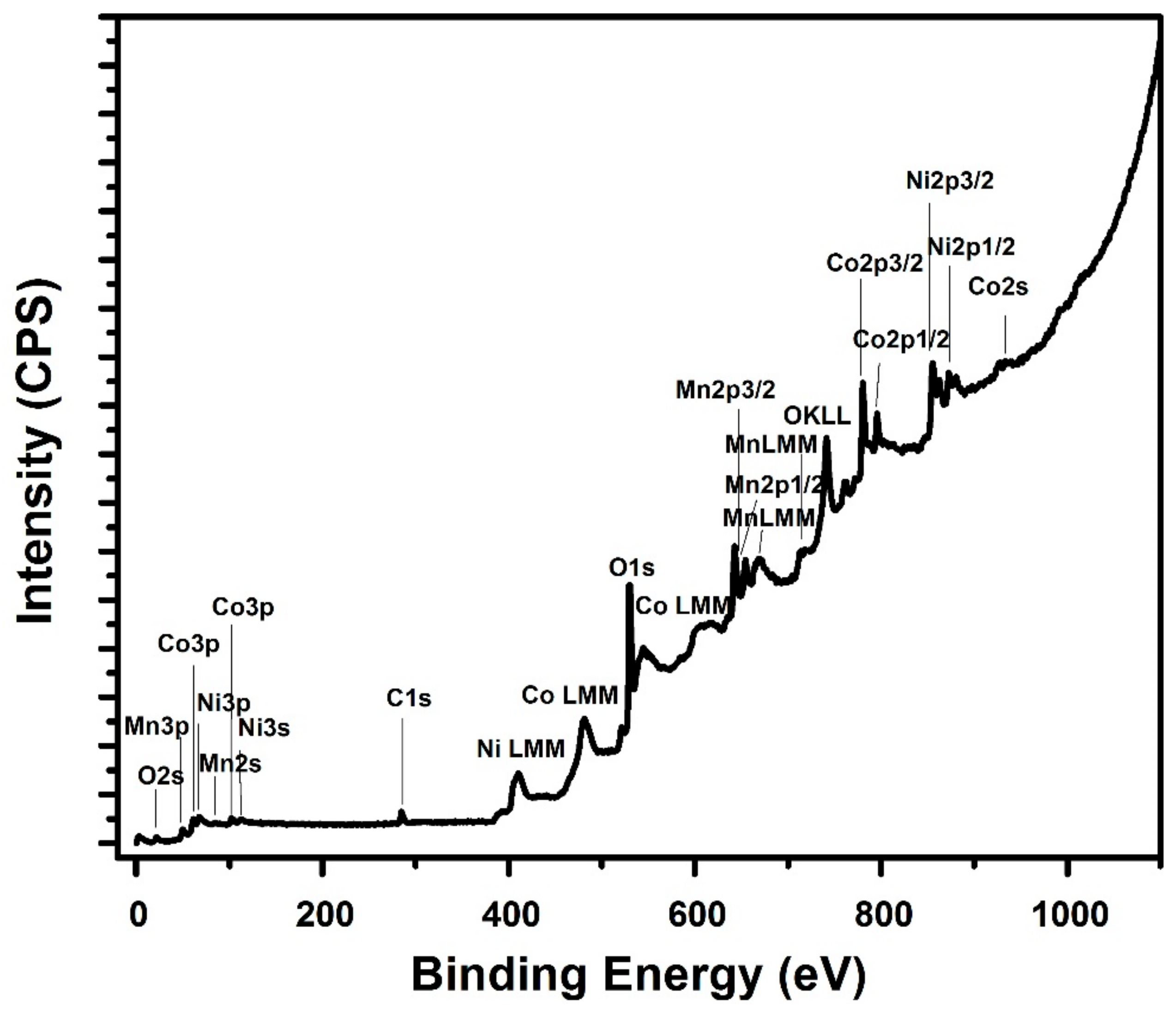
3.1. Structure and Elemental Composition of the Material
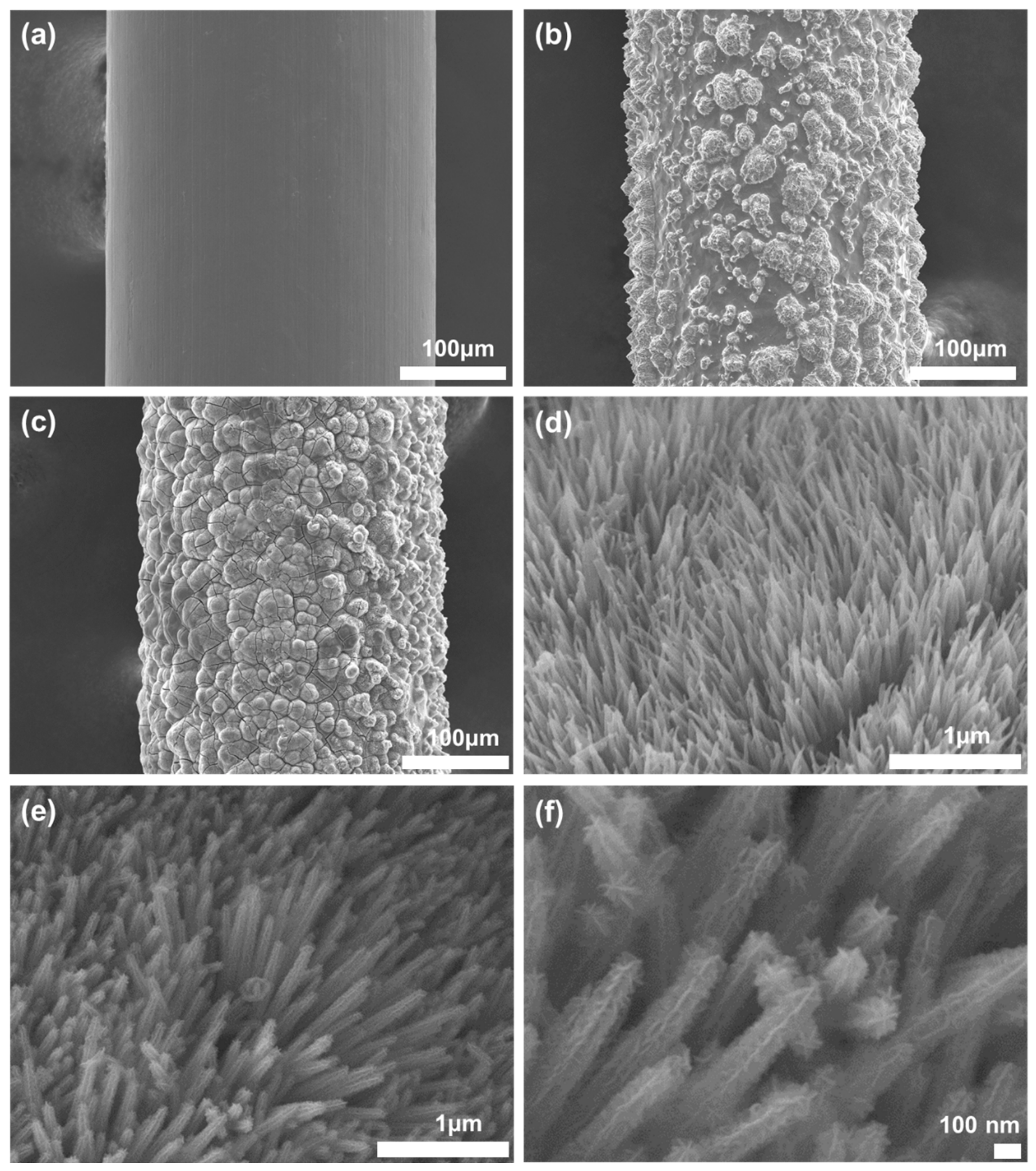
3.2. Fabrication Results
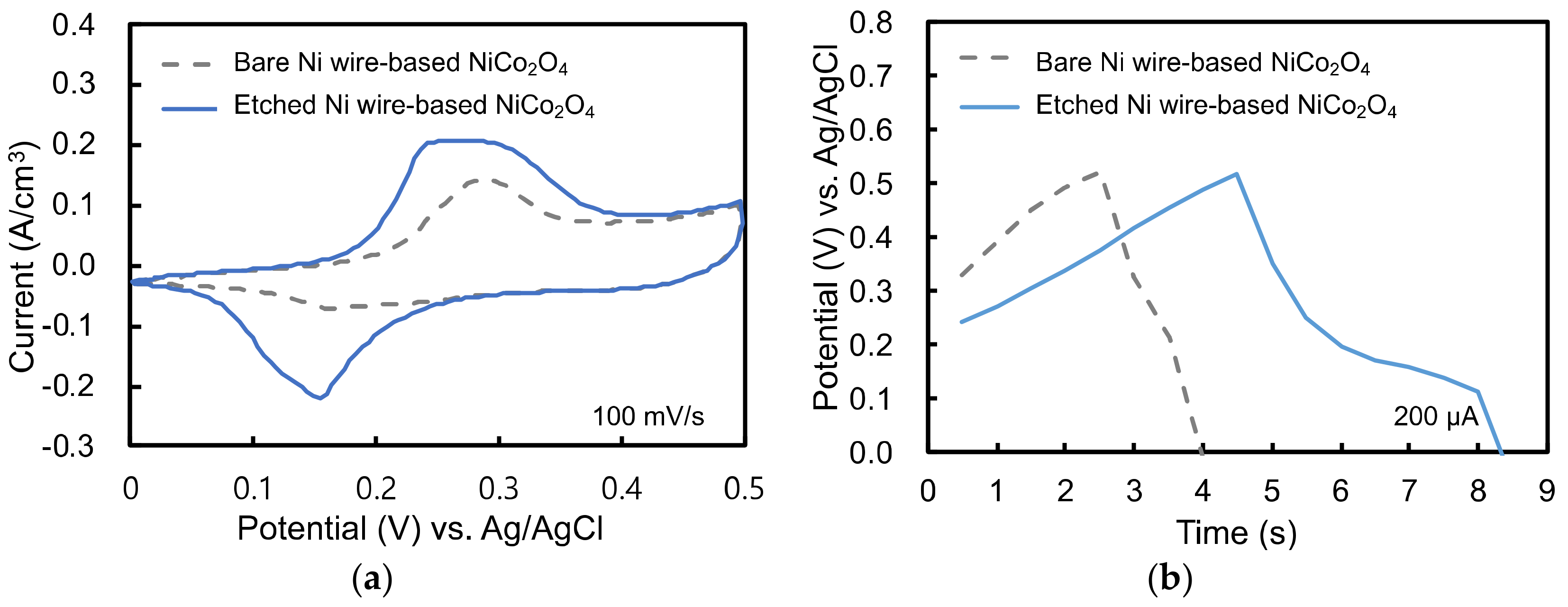
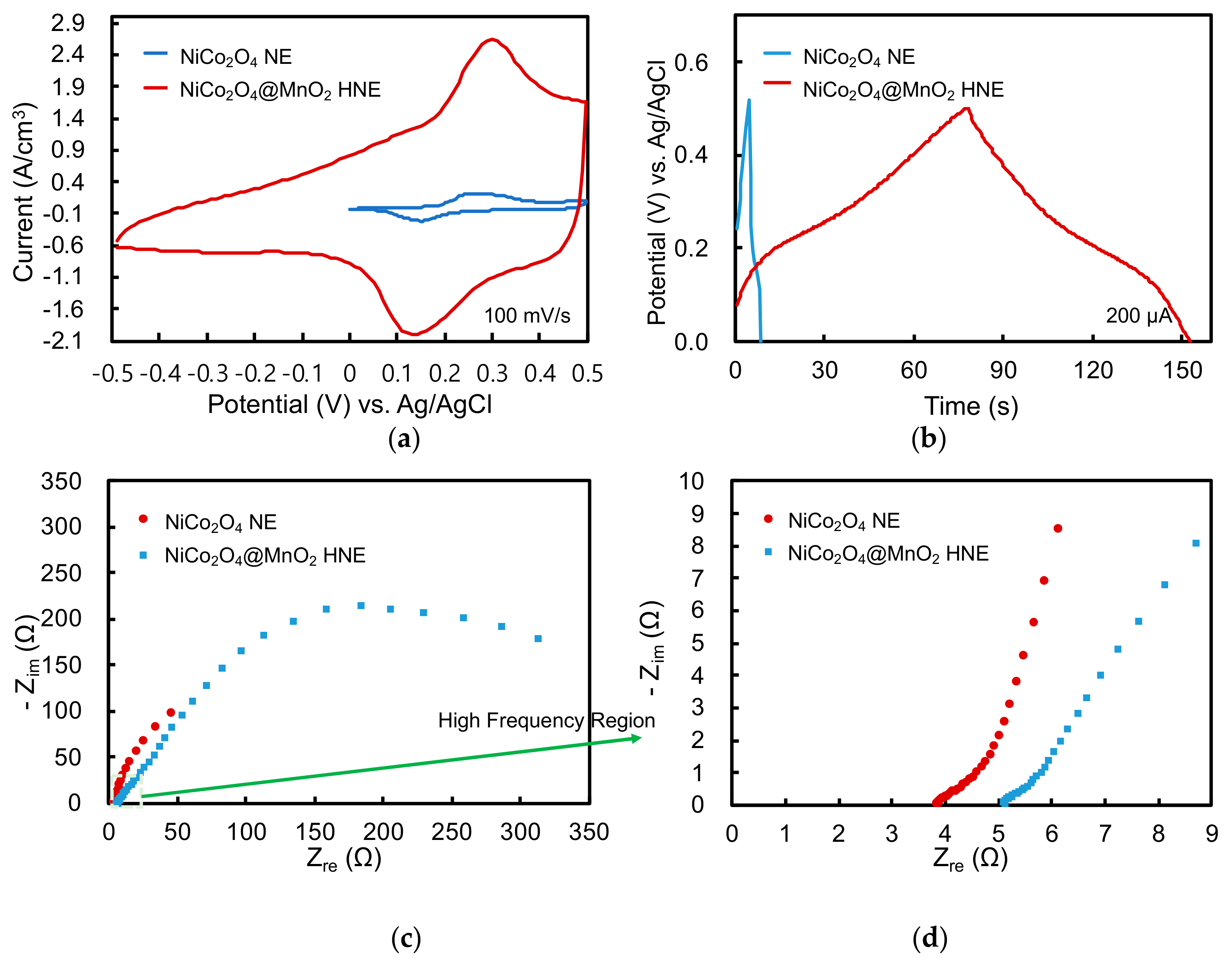
3.3. Electrochemical Analysis of Single Electrode
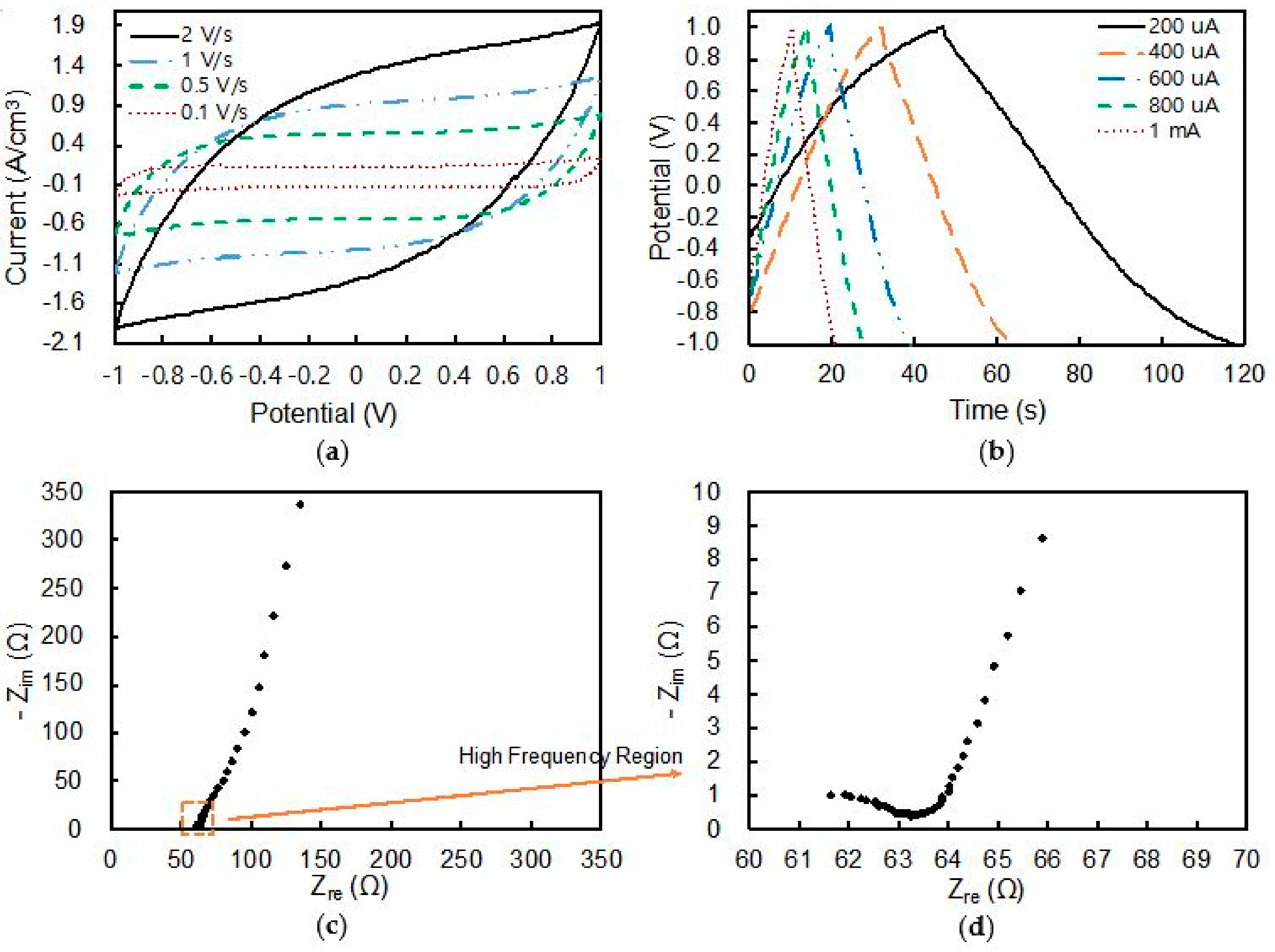
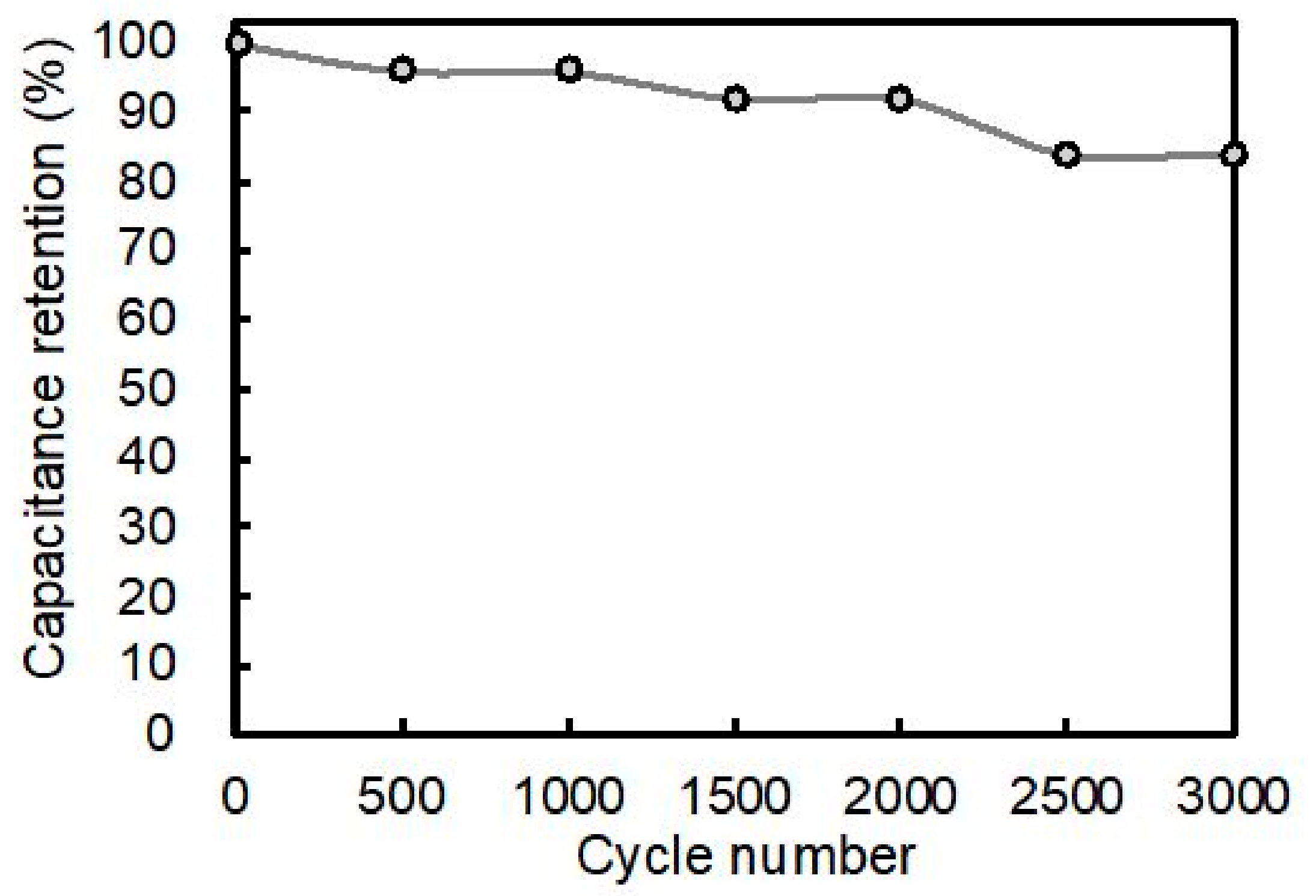
3.4. Performance of Fiber-Shaped Supercapacitor
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, X.; Sánchez, B.M.; Dobson, P.J.; Grant, P.S. The role of nanomaterials in redox-based supercapacitors for next generation energy storage devices. Nanoscale 2011, 3, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Tarascon, J.M. Key challenges in future Li-battery research. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 3227–3241. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Yang, P.; Mai, W. Flexible solid-state electrochemical supercapacitors. Nano Energy 2014, 8, 274–290. [Google Scholar] [CrossRef]
- Xia, H.; Meng, Y.S.; Yuan, G.; Cui, C.; Lu, L. A symmetric RuO2/RuO2 supercapacitor operating at 1.6 V by using a neutral aqueous electrolyte. Electrochem. Solid-State Lett. 2012, 15, A60–A63. [Google Scholar] [CrossRef]
- Xia, X.H.; Tu, J.P.; Mai, Y.J.; Wang, X.L.; Gu, C.D.; Zhao, X.B. Self-supported hydrothermal synthesized hollow Co3O4 nanowire arrays with high supercapacitor capacitance. J. Mater. Chem. 2011, 21, 9319–9325. [Google Scholar] [CrossRef]
- Lang, J.W.; Kong, L.B.; Wu, W.J.; Luo, Y.C.; Kang, L. Facile approach to prepare loose-packed NiO nano-flakes materials for supercapacitors. Chem. Commun. 2008, 35, 4213–4215. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Ryu, K.S.; Kim, K.M.; Park, N.G.; Park, Y.J.; Chang, S.H. Symmetric redox supercapacitor with conducting polyaniline electrodes. J. Power Sources 2002, 103, 305–309. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef]
- Wang, L.; Jiao, X.; Liu, P.; Ouyang, Y.; Xia, X.; Lei, W.; Hao, Q. Self-template synthesis of yolk-shelled NiCo2O4 spheres for enhanced hybrid supercapacitors. Appl. Surf. Sci. 2018, 427, 174–181. [Google Scholar] [CrossRef]
- Wang, X.; Fang, Y.; Shi, B.; Huang, F.; Rong, F.; Que, R. Three-dimensional NiCo2O4@NiCo2O4 core–shell nanocones arrays for high-performance supercapacitors. Chem. Eng. J. 2018, 344, 311–319. [Google Scholar] [CrossRef]
- Xu, J.; Sun, Y.; Lu, M.; Wang, L.; Zhang, J.; Qian, J.; Liu, X. Fabrication of hierarchical MnMoO4· H2O@MnO2 core-shell nanosheet arrays on nickel foam as an advanced electrode for asymmetric supercapacitors. Chem. Eng. J. 2018, 334, 1466–1476. [Google Scholar] [CrossRef]
- Xu, K.; Ren, Q.; Liu, Q.; Li, W.; Zou, R.; Hu, J. Design and synthesis of 3D hierarchical NiCo2S4@MnO2 core–shell nanosheet arrays for high-performance pseudocapacitors. Rsc Adv. 2015, 5, 44642–44647. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, X.; Wang, K.; Ma, G.; Cheng, H.; Xu, F. Preparation of NiCo2S4 flaky arrays on Ni foam as binder-free supercapacitor electrode. Appl. Surf. Sci. 2015, 347, 690–695. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, S.; Cao, D.; Wang, G.; Yin, J. Electrochemical capacitance of Co3O4 nanowire arrays supported on nickel foam. J. Power Sources 2010, 195, 1757–1760. [Google Scholar] [CrossRef]
- Xu, J.; Sun, Y.; Lu, M.; Wang, L.; Zhang, J.; Qian, J.; Kim, E.J. Fabrication of porous Mn2O3 microsheet arrays on nickel foam as high–rate electrodes for supercapacitors. J. Alloy. Compd. 2017, 717, 108–115. [Google Scholar] [CrossRef]
- Kong, W.; Lu, C.; Zhang, W.; Pu, J.; Wang, Z. Homogeneous core–shell NiCo2S4 nanostructures supported on nickel foam for supercapacitors. J. Mater. Chem. A 2015, 3, 12452–12460. [Google Scholar] [CrossRef]
- Yang, P.; Chao, D.; Zhu, C.; Xia, X.; Zhang, Y.; Wang, X.; Sun, P.; Tay, B.K.; Shen, Z.X.; Mai, W.; et al. Ultrafast-charging supercapacitors based on corn-like titanium nitride nanostructures. Adv. Sci. 2016, 3, 1500299. [Google Scholar] [CrossRef]
- Zhang, C.; Geng, X.; Tang, S.; Deng, M.; Du, Y. NiCo2O4@rGO hybrid nanostructures on Ni foam as high-performance supercapacitor electrodes. J. Mater. Chem. A 2017, 5, 5912–5919. [Google Scholar] [CrossRef]
- Li, J.; Shi, Q.; Shao, Y.; Hou, C.; Li, Y.; Zhang, Q.; Wang, H. Cladding nanostructured AgNWs-MoS2 electrode material for high-rate and long-life transparent in-plane micro-supercapacitor. Energy Storage Mater. 2019, 16, 212–219. [Google Scholar] [CrossRef]
- Meng, Y.; Zhao, Y.; Hu, C.; Cheng, H.; Hu, Y.; Zhang, Z.; Shi, G.; Qu, L. All-graphene core-sheath microfibers for all-solid-state, stretchable fibriform supercapacitors and wearable electronic textiles. Adv. Mater. 2013, 25, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xie, L.; Wen, Z.; Chen, C.; Chen, X.; Wei, A.; Cheng, P.; Xie, X.; Sun, X. Coaxial triboelectric nanogenerator and supercapacitor fiber-based self-charging power fabric. ACS Appl. Mater. Interfaces 2018, 10, 42356–42362. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Patil, B.; Yu, S.; Ahn, S.; Hwang, J.; Park, C.; Do, K.; Ahn, H. Flexible, Swiss roll, fiber-shaped, asymmetric supercapacitor using MnO2 and Fe2O3 on carbon fibers. Electrochim. Acta 2018, 269, 499–508. [Google Scholar] [CrossRef]
- An, K.H.; Kim, W.S.; Park, Y.S.; Choi, Y.C.; Lee, S.M.; Chung, D.C.; Bae, D.J.; Lim, S.C.; Lee, Y.H. Supercapacitors using single-walled carbon nanotube electrodes. Adv. Mater. 2001, 13, 497–500. [Google Scholar] [CrossRef]
- Xu, P.; Wei, B.; Cao, Z.; Zheng, J.; Gong, K.; Li, F.; Yu, J.; Li, Q.; Lu, W.; Byun, J.H.; et al. Stretchable wire-shaped asymmetric supercapacitors based on pristine and MnO2 coated carbon nanotube fibers. ACS Nano 2015, 9, 6088–6096. [Google Scholar] [CrossRef]
- Zhou, Q.; Jia, C.; Ye, X.; Tang, Z.; Wan, Z. A knittable fiber-shaped supercapacitor based on natural cotton thread for wearable electronics. J. Power Sources 2016, 327, 365–373. [Google Scholar] [CrossRef]
- Qu, G.; Cheng, J.; Li, X.; Yuan, D.; Chen, P.; Chen, X.; Wang, B.; Peng, H. A fiber supercapacitor with high energy density based on hollow graphene/conducting polymer fiber electrode. Adv. Mater. 2016, 28, 3646–3652. [Google Scholar] [CrossRef]
- Wu, X.; Meng, L.; Wang, Q.; Zhang, W.; Wang, Y. High flexibility and large energy density asymmetric fibered-supercapacitor based on unique NiCo2O4@MnO2 core-shell nanobrush arrays electrode. Electrochim. Acta 2019, 295, 532–539. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, J.; Pan, Z.; Zhang, J.; Zhao, J.; Wang, X.; Zhang, C.; Yao, Y.; Lu, W.; Li, Q.; et al. Stretchable fiber-shaped asymmetric supercapacitors with ultrahigh energy density. Nano Energy 2017, 39, 219–228. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, X.-L.; Wang, Z.-L.; Xu, J.-J.; Wang, H.-G.; Zhang, X.-B. Electrostatic Induced Stretch Growth of Homogeneous β-Ni (OH) 2 on Graphene with Enhanced High-Rate Cycling for Supercapacitors. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Ding, R.; Qi, L.; Wang, H. Porous NiCo2O4 as an anode material for 4.5 V hybrid Li-ion capacitors. Rsc Adv. 2013, 3, 12581–12584. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Wan, Y.; Liu, W.; Ma, Z.; Ji, S.; Wang, J.; Zhou, Y.; Hodgson, P.; Li, Y. Facile synthesis of NiCo2O4 nanorod arrays on Cu conductive substrates as superior anode materials for high-rate Li-ion batteries. Cryst. Eng. Commun. 2013, 15, 1578–1585. [Google Scholar] [CrossRef]
- Cui, B.; Lin, H.; Li, Y.Z.; Li, J.B.; Sun, P.; Zhao, X.C.; Liu, C.J. Photophysical and Photocatalytic properties of core-ring structured NiCo2O4 nanoparticles. J. Phys. Chem. C 2009, 113, 14083–14087. [Google Scholar] [CrossRef]
- Wu, P.; Cheng, S.; Yao, M.H.; Yang, L.F.; Zhu, Y.Y.; Liu, P.P.; Xing, O.; Zhou, J.; Wang, M.K.; Luo, H.W.; et al. Low-Cost, Self-Standing NiCo2O4@CNT/CNT Multilayer Electrode for Flexible Asymmetric Solid-State Supercapacitors. Adv. Funct. Mater. 2017, 27, 1702160. [Google Scholar] [CrossRef]
- Zhao, B.; Ke, X.K.; Bao, J.H.; Wang, C.L.; Dong, L.; Chen, Y.W.; Chen, H.L. Synthesis of Flower-Like NiO and Effects of Morphology on Its Catalytic Properties. J. Phys. Chem. C 2009, 113, 14440–14447. [Google Scholar] [CrossRef]
- Xu, K.; Li, W.; Liu, Q.; Li, B.; Liu, X.; An, L.; Chen, Z.; Zou, R.; Hu, J. Hierarchical mesoporous NiCo2O4@MnO2 core-shell nanowire arrays on nickel foam for aqueous asymmetric supercapacitors. J. Mater. Chem. A 2014, 2, 4795–4802. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, D.; Cai, S.; Zhang, L.; Huang, L.; Li, H.; Maitarad, P.; Shi, L.; Gao, R.; Zhang, J. Low-temperature selective catalytic reduction of NO with NH3 over nanoflaky MnOx on carbon nanotubes in situ prepared via a chemical bath deposition route. Nanoscale 2013, 5, 9199–9207. [Google Scholar] [CrossRef]
- Toupin, M.; Brousse, T.; Bélanger, D. Charge Storage Mechanism of MnO2 Electrode Used in Aqueous Electrochemical Capacitor. Chem. Mater. 2004, 16, 3184–3190. [Google Scholar] [CrossRef]
- Li, D.; Du, G.; Wang, J.; Guo, Z.; Chen, Z.; Liu, H. Microwave-assisted Synthesis of Flower-like Structure ε-MnO2 as Cathode for Lithium Ion Batteries. J. Chin. Chem. Soc. 2012, 59, 1211–1215. [Google Scholar] [CrossRef]
- Bi, Y.; Nautiyal, A.; Zhang, H.; Luo, J.; Zhang, X. One-pot microwave synthesis of NiO/MnO2 composite as a high-performance electrode material for supercapacitors. Electrochim. Acta 2018, 260, 952–958. [Google Scholar] [CrossRef]
- Yuan, C.; Li, J.; Hou, L.; Zhang, X.; Shen, L.; Lou, X.W.D. Ultrathin Mesoporous NiCo2O4 Nanosheets Supported on Ni Foam as Advanced Electrodes for Supercapacitors. Adv. Funct. Mater. 2012, 22, 4592–4597. [Google Scholar] [CrossRef]
- Jimenez, V.M.; Fernandez, A.; Espinos, J.P.; Gonzalez-Elipe, A.R. The state of the oxygen at the surface of polycrystalline cobalt oxide. J. Electron Spectrosc. Relat. Phenom. 1995, 71, 61–71. [Google Scholar] [CrossRef]
- Marco, J.F.; Gancedo, J.R.; Gracia, M.; Gautier, J.L.; Ríos, E.; Berry, F.J. Characterization of the Nickel Cobaltite, NiCo2O4, Prepared by Several Methods: An XRD, XANES, EXAFS, and XPS Study. J. Solid State Chem. 2000, 153, 74–81. [Google Scholar] [CrossRef]
- Li, Y.; Hasin, P.; Wu, Y. NixCo3−xO4 nanowire arrays for electrocatalytic oxygen evolution. Adv. Mater. 2010, 22, 1926–1929. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Koo, H.J.; Lee, A.; Braun, P.V. Selective Wetting-Induced Micro-Electrode Patterning for Flexible Micro-Supercapacitors. Adv. Mater. 2014, 26, 5108–5112. [Google Scholar] [CrossRef] [PubMed]
- Paleo, A.J.; Staiti, P.; Brigandì, A.; Ferreira, F.N.; Rocha, A.M.; Lufrano, F. Supercapacitors based on AC/MnO2 deposited onto dip-coated carbon nanofiber cotton fabric electrodes. Energy Storage Mater. 2018, 12, 204–215. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, R.; Fu, Y.; Wang, X.; Yu, X.; Li, J.; Zhu, Y.; Tan, S.; Wang, Z. Metal-organic framework derived Fe2O3 nanocubes on intertwined N-doped carbon nanowires for fiber-shaped supercapacitor. Mater. Lett. 2018, 228, 9–12. [Google Scholar] [CrossRef]
- Patil, B.; Ahn, S.; Yu, S.; Song, H.; Jeong, Y.; Kim, J.H.; Ahn, H. Electrochemical performance of a coaxial fiber-shaped asymmetric supercapacitor based on nanostructured MnO2/CNT-web paper and Fe2O3/carbon fiber electrodes. Carbon 2018, 134, 366–375. [Google Scholar] [CrossRef]
- Qi, J.; Liu, X.; Sui, Y.; He, Y.; Ren, Y.; Meng, Q.; Wei, F.; Zhang, X. High performance fiber-shaped all-solid-state symmetric supercapacitor based on mesoporous CuCo2S4 nanosheets. J. Mater. Sci. Mater. Electron. 2019, 30, 667–676. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, N.; Ge, Y.; Zhu, H.; Feng, X.; Liu, R.; Ma, Y.; Wang, L.; Hou, W. Flexible all-solid-state micro-supercapacitor based on Ni fiber electrode coated with MnO2 and reduced graphene oxide via electrochemical deposition. Sci. China Mater. 2018, 61, 243–253. [Google Scholar] [CrossRef]
- Wang, X.; Liu, B.; Liu, R.; Wang, Q.; Hou, X.; Chen, D.; Wang, R.; Shen, G. Fiber-based flexible all-solid-state asymmetric supercapacitors for integrated photodetecting system. Angew. Chem. Int. Ed. 2014, 53, 1849–1853. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Fan, R.; Xiao, R.; Cao, K.; Li, P.; Wang, W.; Lu, Y. NiO-bridged MnCo-hydroxides for flexible high-performance fiber-shaped energy storage device. Appl. Surf. Sci. 2019, 475, 1058–1064. [Google Scholar] [CrossRef]

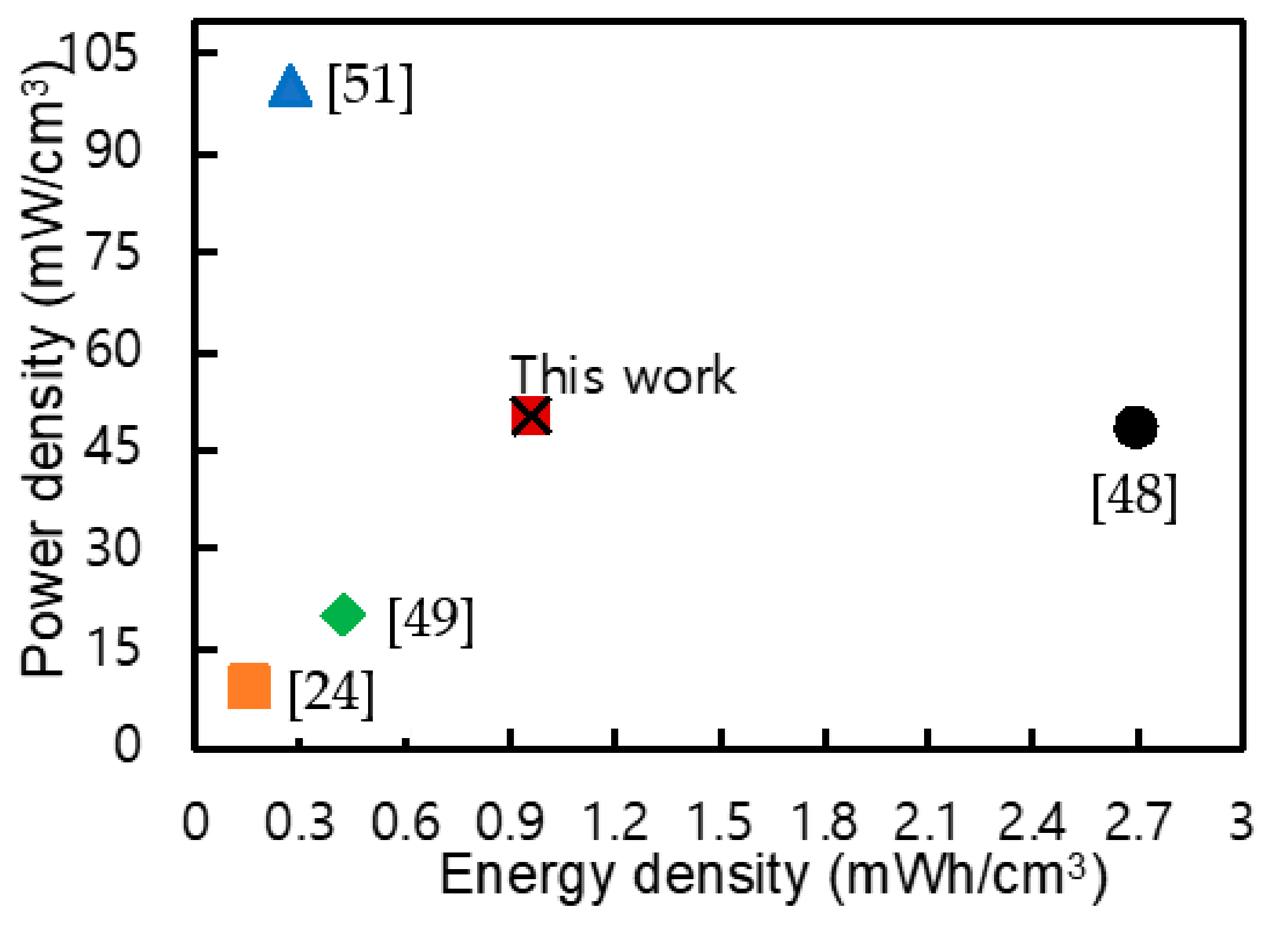
| Ref. | Device Components | Energy Density | Power Density |
|---|---|---|---|
| [24] | MnO2–carbon fiber Fe2O3–carbon fiber | 0.16 mWh/cm3 | 9 mW/cm3 |
| [29] | NiCo2O4/MnO2–carbon fiber | 1.983 mWh/cm2 | 1.72 mW/cm2 |
| [48] | Fe2O3/NCWs–carbon fiber MnO2/TiN–carbon fiber | 2.7 mWh/cm3 | 48.6 mW/cm3 |
| [49] | MnO2/CNT–web paper Fe2O3–carbon fiber | 0.43 mWh/cm3 | 20 mW/cm3 |
| [50] | CuCo2S4–Ni wire | 0.007 mWh/cm2 | 0.599 mW/cm2 |
| [51] | rGO/MnO2–Ni wire | 0.27 mWh/cm3 | 100 mW/cm3 |
| [52] | Co3O4–Ni fiber Graphene–carbon fiber | 0.62 mWh/cm3 | 1470 mW/cm3 |
| [53] | NiO/MnCo–LDH–Ni wire Active carbon–Ni wire | 0.0198 mWh/cm2 | 0.38 mW/cm2 |
| This work | NiCo2O4/MnO2–roughened Ni wire | 0.97 mWh/cm3 0.007 mWh/cm2 | 49.8 mW/cm3 0.36 mW/cm2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Shewale, P.S.; Yun, K.-S. Fiber-Shaped Supercapacitors Fabricated Using Hierarchical Nanostructures of NiCo2O4 Nanoneedles and MnO2 Nanoflakes on Roughened Ni Wire. Energies 2019, 12, 3127. https://doi.org/10.3390/en12163127
Zhang J, Shewale PS, Yun K-S. Fiber-Shaped Supercapacitors Fabricated Using Hierarchical Nanostructures of NiCo2O4 Nanoneedles and MnO2 Nanoflakes on Roughened Ni Wire. Energies. 2019; 12(16):3127. https://doi.org/10.3390/en12163127
Chicago/Turabian StyleZhang, Jing, Prashant S. Shewale, and Kwang-Seok Yun. 2019. "Fiber-Shaped Supercapacitors Fabricated Using Hierarchical Nanostructures of NiCo2O4 Nanoneedles and MnO2 Nanoflakes on Roughened Ni Wire" Energies 12, no. 16: 3127. https://doi.org/10.3390/en12163127
APA StyleZhang, J., Shewale, P. S., & Yun, K.-S. (2019). Fiber-Shaped Supercapacitors Fabricated Using Hierarchical Nanostructures of NiCo2O4 Nanoneedles and MnO2 Nanoflakes on Roughened Ni Wire. Energies, 12(16), 3127. https://doi.org/10.3390/en12163127