High-Pressure and Automatized System for Study of Natural Gas Hydrates
Abstract
1. Introduction
2. Materials and Methods
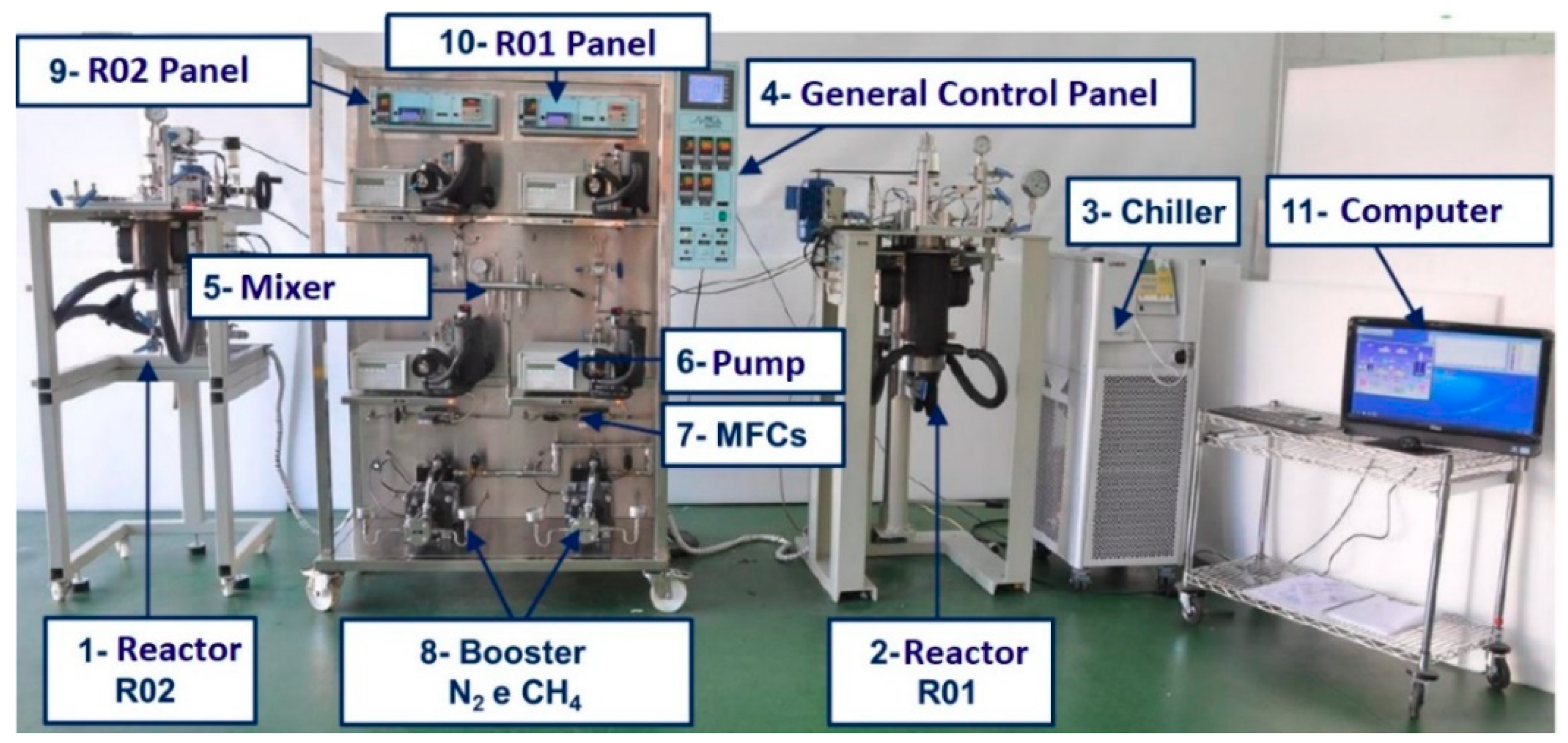
2.1. General Pilot Plant Description
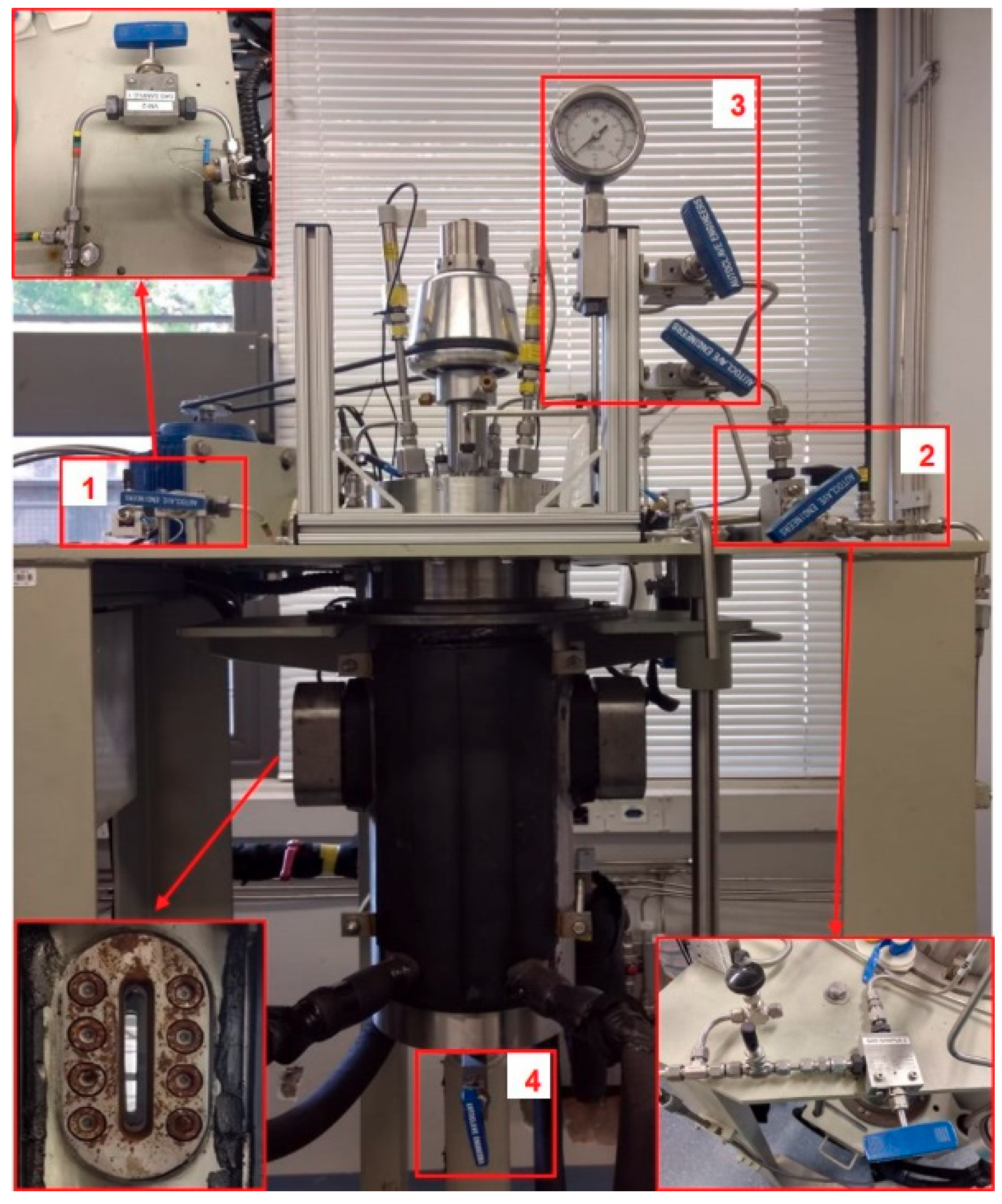
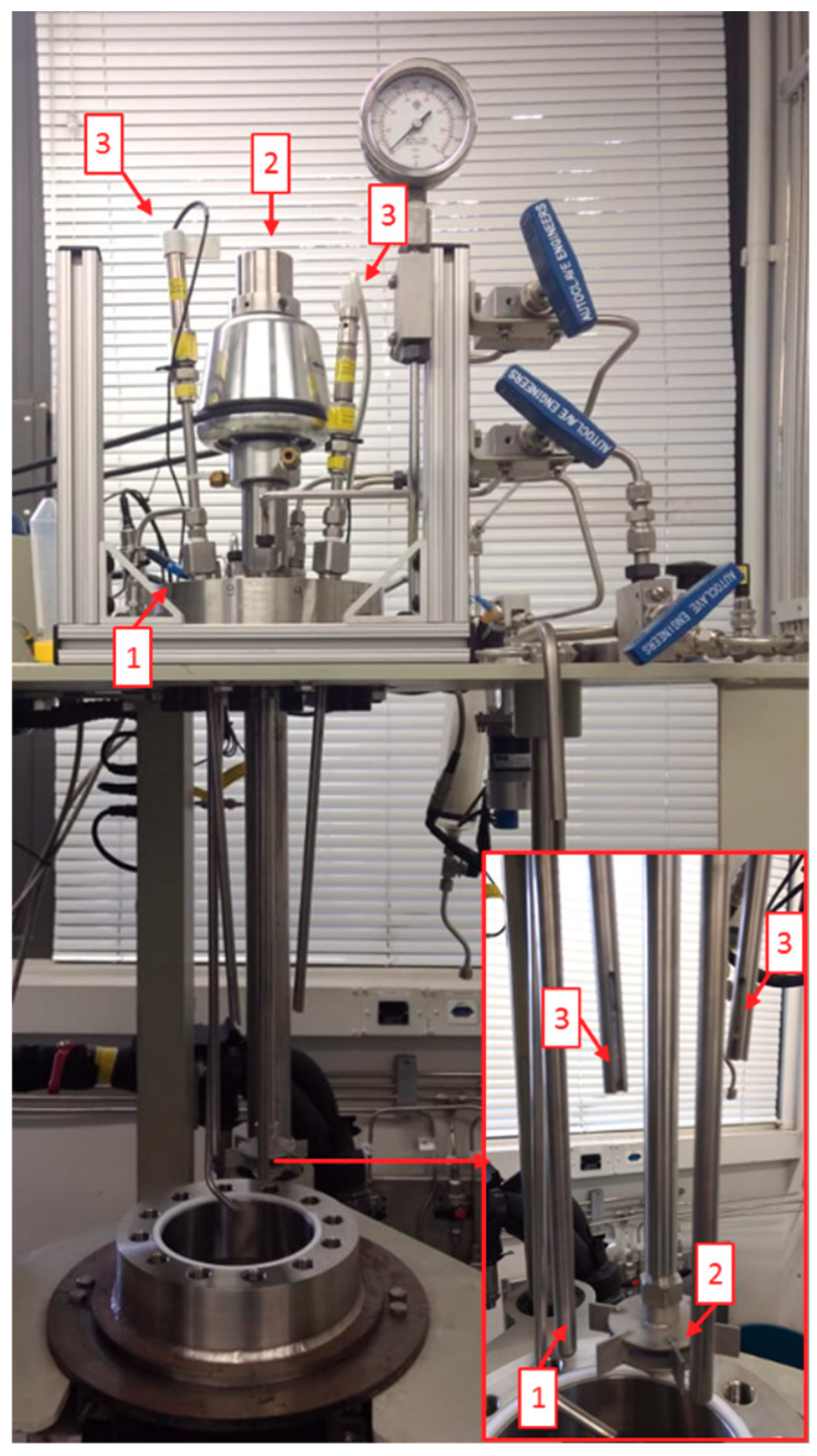
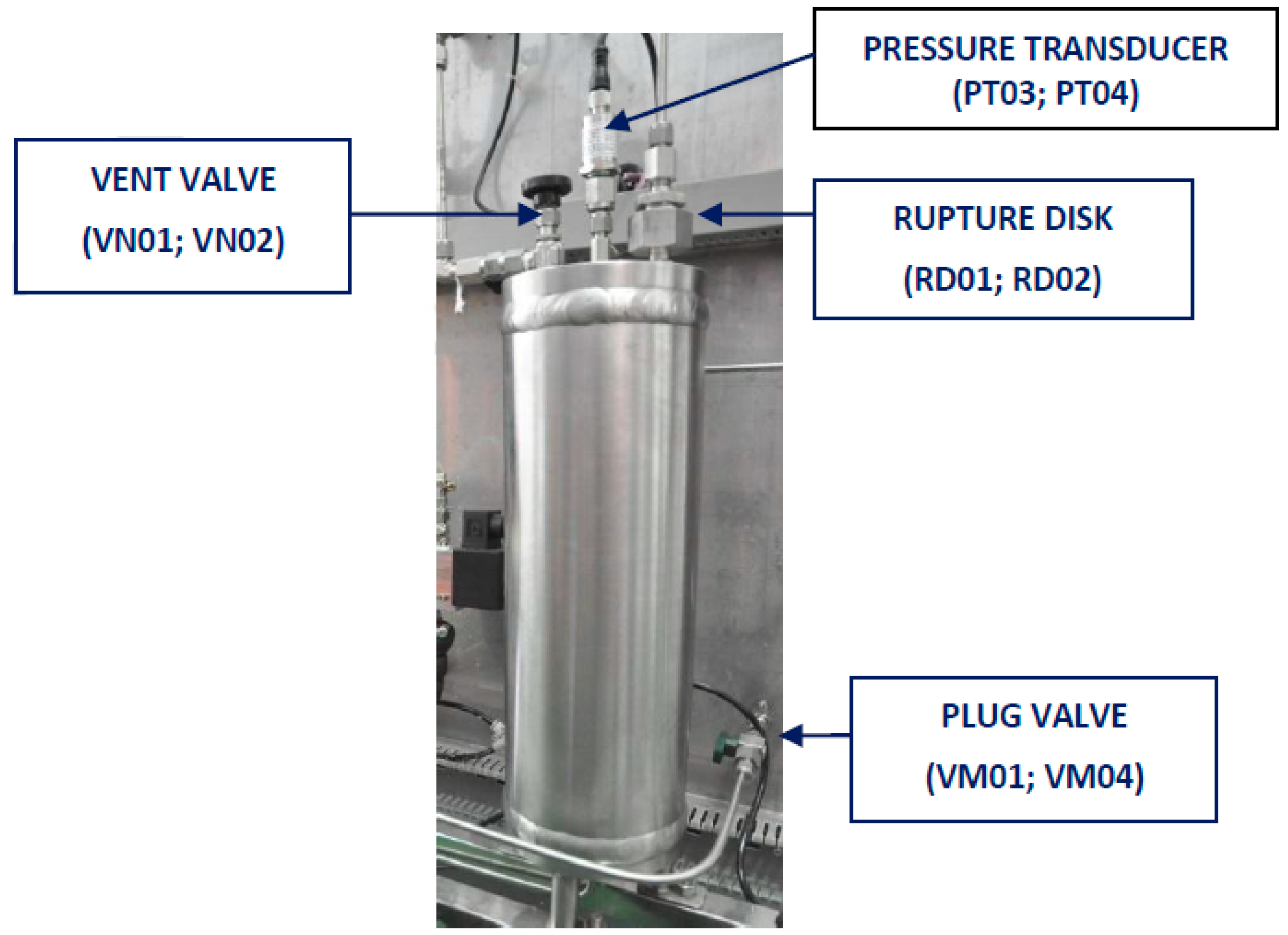
2.1.1. Characteristic of Reactors
2.1.2. Controls Parameters: Temperature, Pressure, Stirring Speed, and pH
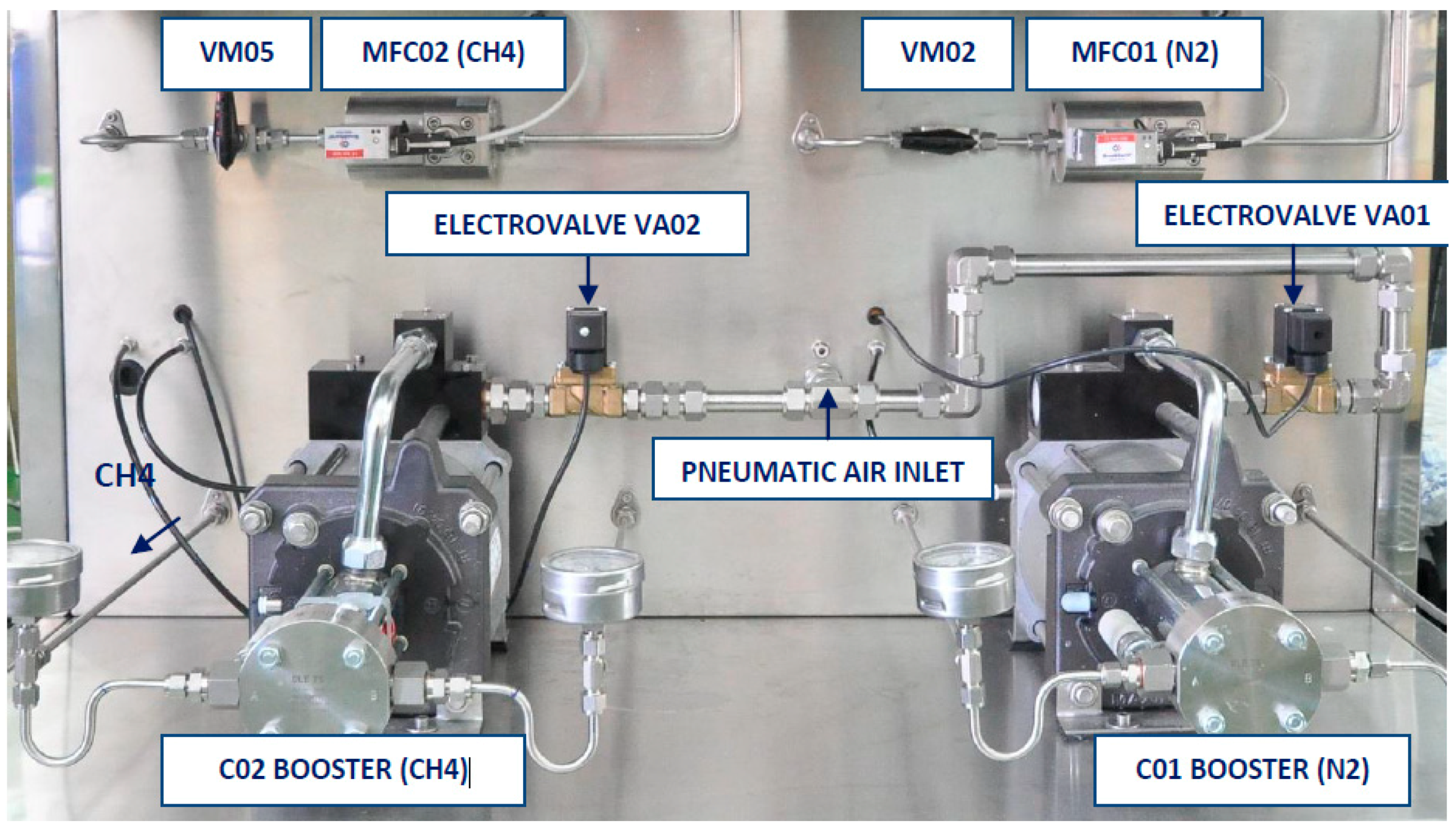
2.1.3. Gases System
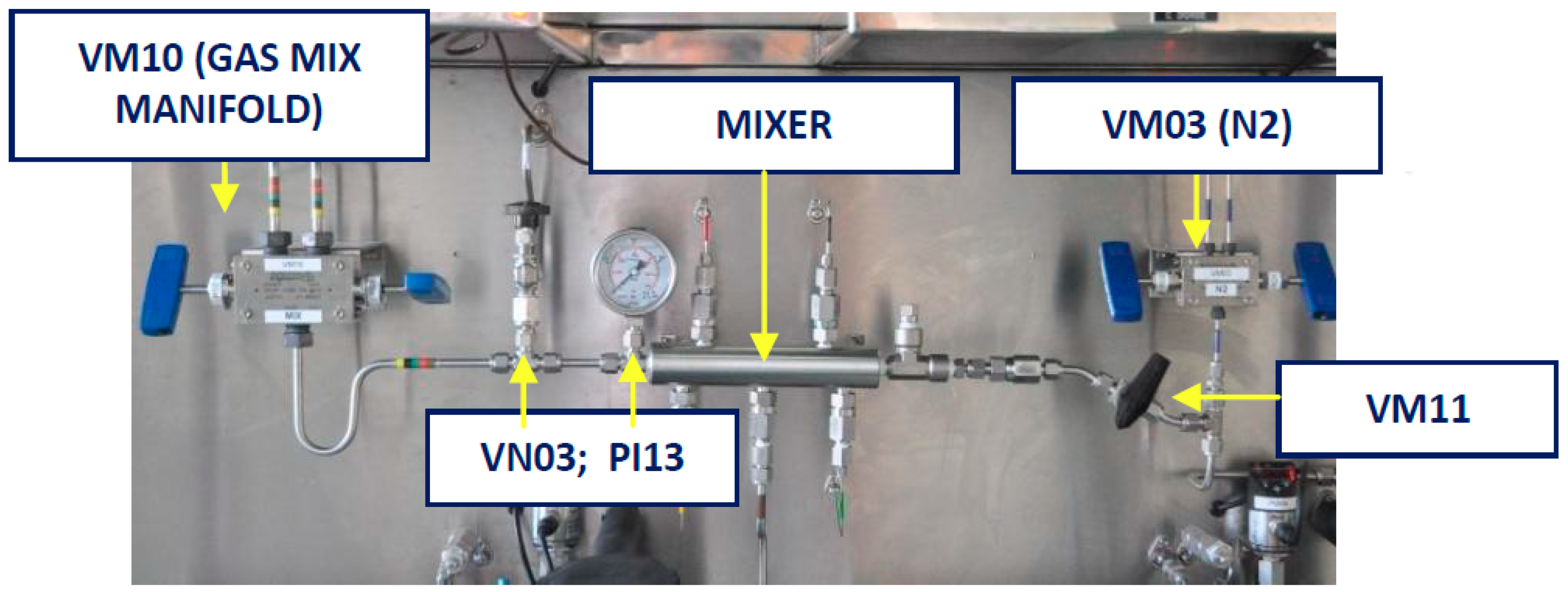
2.1.4. Gases Mixer System
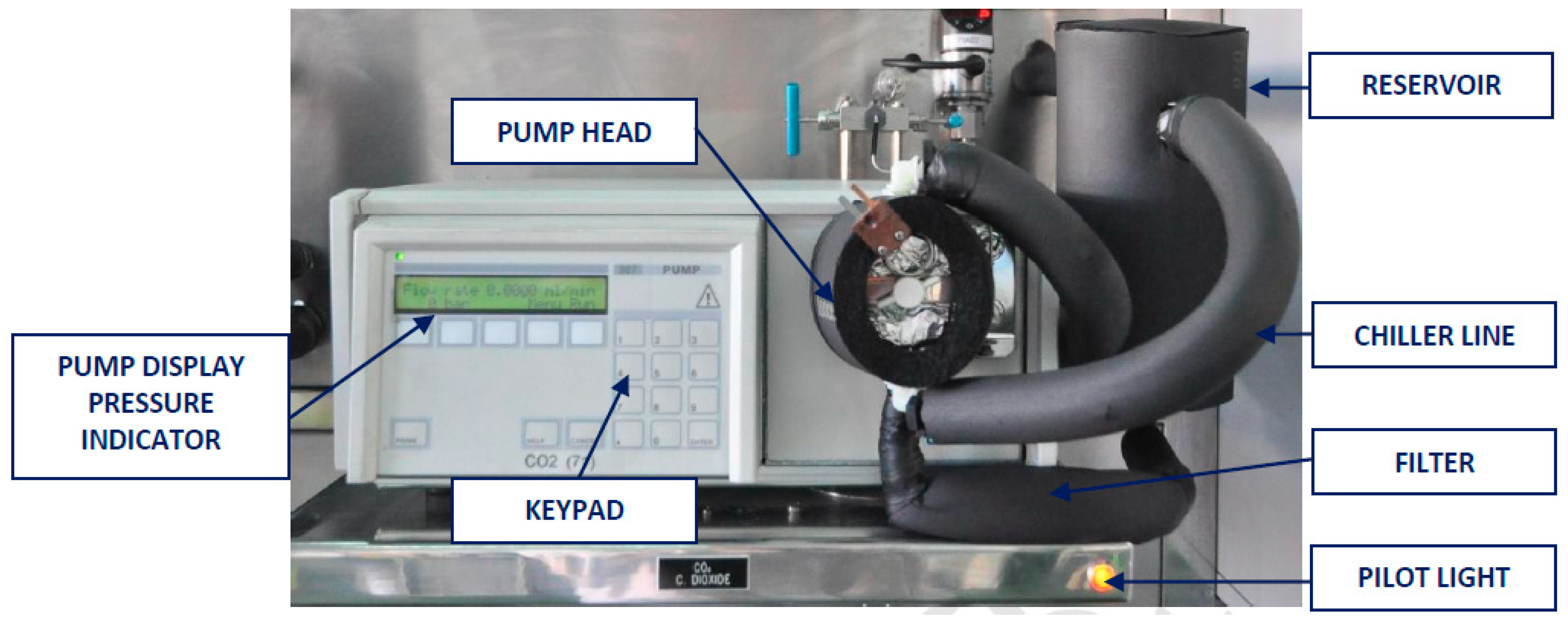
2.1.5. Gases Injection System
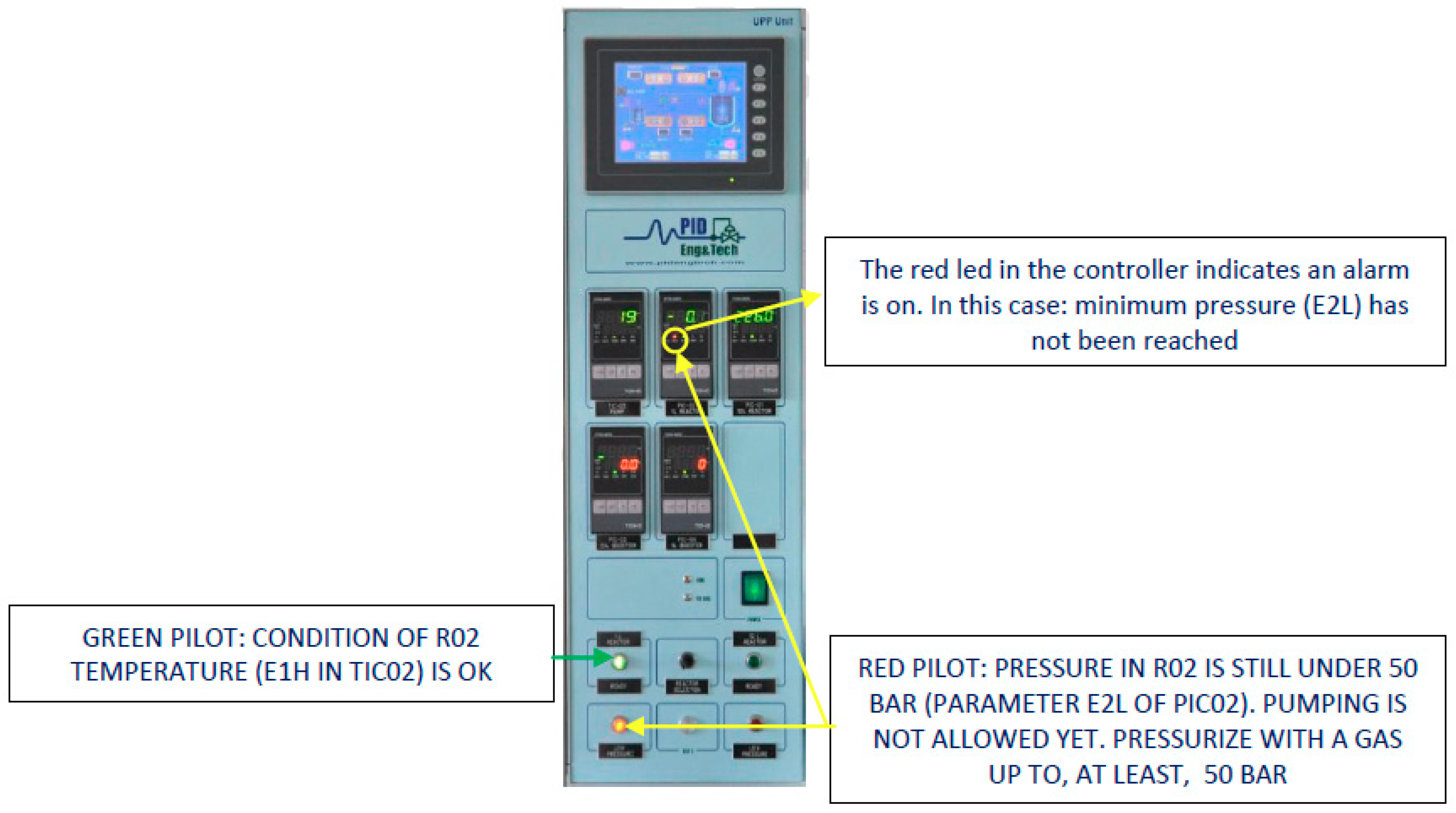
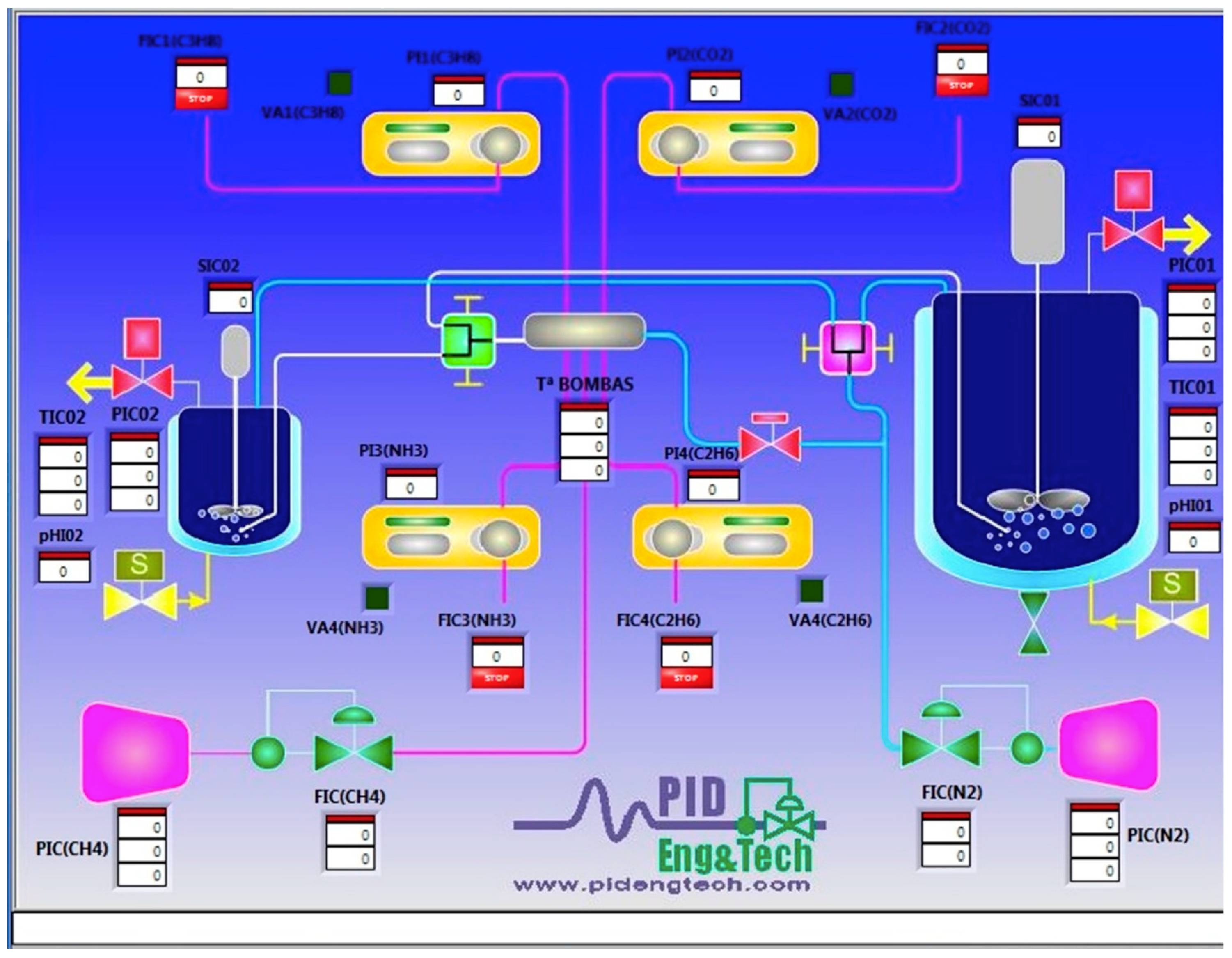
2.1.6. Panel Control System
2.1.7. Software for Plant Control
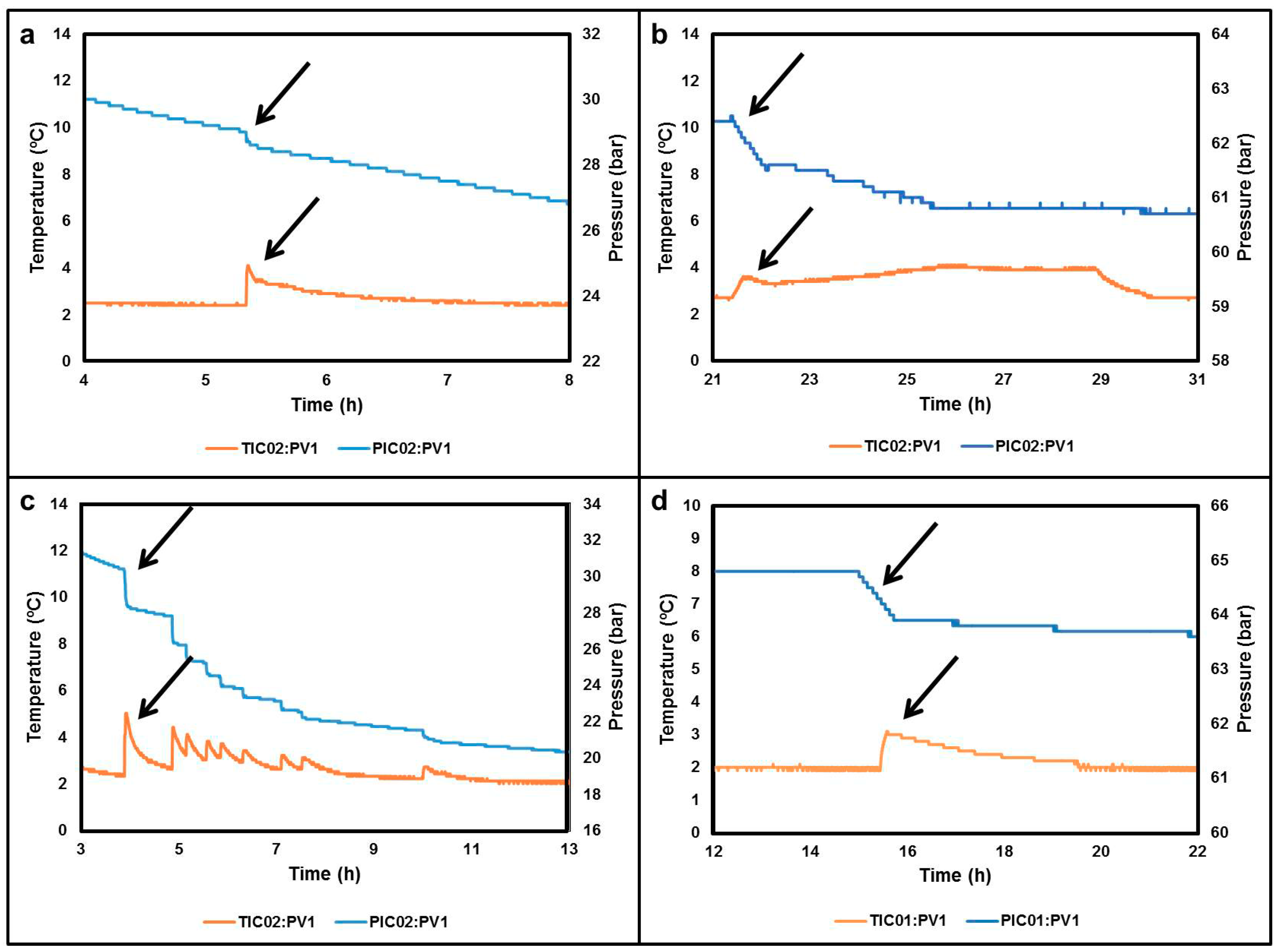
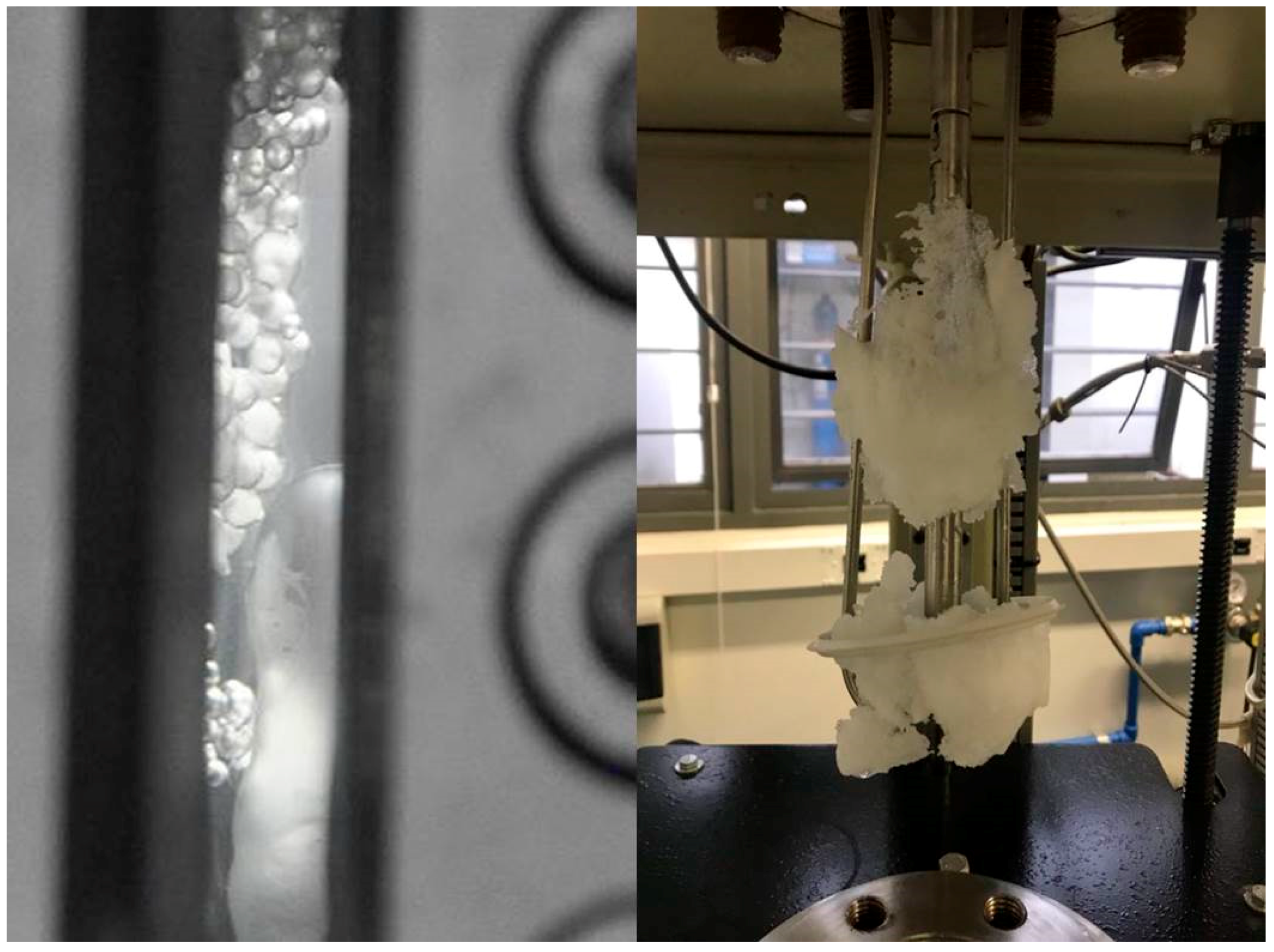
3. Experiments Validation for Gas Hydrate Formation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kvenvolden, K.A. Gas hydrates geological perspective and global change. Rev. Geophys. 1993, 31, 173–187. [Google Scholar] [CrossRef]
- Sloan, E.D., Jr. Clathrate Hydrates of Natural Gas, 2nd ed.; Marcel Dekker: New York, NY, USA, 1998; p. 703. [Google Scholar]
- Hunter, S.J.; Goldobin, D.S.; Haywood, A.M.; Ridgwell, A.; Rees, J.G. Sensitivity of the global submarine hydrate inventory to scenarios of future climate change. Earth Planet. Sci. Lett. 2013, 367, 105–115. [Google Scholar] [CrossRef]
- Pinero, E.; Marquardt, M.; Hensen, C.; Haeckel, M.; Wallmann, K. Estimation of the global inventory of methane hydrates in marine sediments using transfer functions. BGD 2013, 10, 959–975. [Google Scholar]
- Buffett, B.A. Natural Gas Hydrates. Ann. Rev. Earth Plan. Sci. 2000, 20, 1567–1570. [Google Scholar]
- Lee, H.; Lee, J.; Kim, D.Y.; Park, J.; Seo, Y.T.; Zeng, H.; Moudrakovski, I.L.; Ratcliffe, C.I.; Ripmeester, J.A. Tuning clathrate hydrates for hydrogen storage. Nature 2005, 434, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Ruppel, C.D. Methane Hydrates and Contemporary Climate Change. Nat. Educ. Knowl. 2011, 2, 12. [Google Scholar]
- Roosta, H.; Dashti, A.; Mazloumi, S.H.; Varaminian, F. Inhibition properties of new amino acids for prevention of hydrate formation in carbon dioxide–water system: Experimental and modeling investigations. J. Mol. Liq. 2016, 215, 656–663. [Google Scholar] [CrossRef]
- Prasad, P.S.; Kiran, B.S. Are the amino acids thermodynamic inhibitors or kinetic promoters for carbon dioxide hydrates? J. Nat. Gas Sci. Eng. 2018, 52, 461–466. [Google Scholar] [CrossRef]
- Ng, H.; Robinson, D.B. The measurement and prediction of hydrate formation in liquid hydrocarbon-water systems. Ind. Eng. Chem. Fundam. 1976, 15, 293–298. [Google Scholar] [CrossRef]
- Chen, T.S. A Molecular Dynamics Study of the Stability of Small Renucleation Water Clusters. Ph.D. Thesis, University Missouri-Rolla, Rolla, MO, USA, 1980. [Google Scholar]
- Hesse, R.; Harrison, W.E. Gas hydrates (clathrates) causing pore-water freshening and oxygen isotope fractionation in deep-water sedimentary sections of terrigenous continental margins. Earth Planet. Sci. Lett. 1981, 55, 453–462. [Google Scholar] [CrossRef]
- Rempel, A. Theorical and Experimental Investigations into the Formation and Accumulation of Gas Hydrates. Master’s Thesis, The University of British Columbia, Vancouver, BC, Canada, 1994. [Google Scholar]
- Lekvam, K.; Bishnoi, P.R. Dissolution of methane in water at low temperatures and intermediate pressures. Fluid Phase Equilib. 1997, 131, 297–309. [Google Scholar] [CrossRef]
- Fan, S.S.; Chen, G.J.; Ma, Q.L.; Guo, T.M. Experimental and modeling studies on the hydrate formation of CO2 and CO2-rich gas mixtures. Chem. Eng. J. 2000, 78, 173–178. [Google Scholar] [CrossRef]
- Lu, H.; Matsumoto, R. Preliminary experimental results of the stable PT conditions of methane hydrate in a nannofossil-rich claystone column. Geochem. J. 2002, 36, 21–30. [Google Scholar] [CrossRef]
- Ma, C.F.; Chen, G.J.; Wang, F.; Sun, C.Y.; Guo, T.M. Hydrate formation of (CH4 + C2H4) and (CH4 + C3H6) gas mixtures. Fluid Phase Equilib. 2001, 191, 41–47. [Google Scholar] [CrossRef]
- Kang, S.P.; Lee, H.; Lee, C.S.; Sung, W.M. Hydrate phase equilibria of the guest mixtures containing CO2, N2 and tetrahydrofuran. Fluid Phase Equilib. 2001, 185, 101–109. [Google Scholar] [CrossRef]
- Konno, H.O.; Narasimhan, S.; Song, F.; Smith, D.H. Synthesis of methane gas hydrate in porous sediments and its dissociation by depressurizing. Powder Technol. 2002, 122, 239–246. [Google Scholar] [CrossRef]
- Wang, L.K.; Chen, G.J.; Han, G.H.; Guo, X.Q.; Guo, T.M. Experimental study on the solubility of natural gas components in water with or without hydrate inhibitor. Fluid Phase Equilib. 2003, 207, 143–154. [Google Scholar] [CrossRef]
- Sugahara, T.; Murayama, S.; Hashimoto, S.; Ohgaki, K. Phase equilibria for H2 + CO2 + H2O system containing gas hydrates. Fluid Phase Equilib. 2005, 233, 190–193. [Google Scholar] [CrossRef]
- Pang, W.X.; Chen, G.J.; Dandekar, A.; Sun, C.Y.; Zhang, C.L. Experimental study on the scale-up effect of gas storage in the form of hydrate in a quiescent reactor. Chem. Eng. Sci. 2007, 62, 2198–2208. [Google Scholar] [CrossRef]
- Giavarini, C.; Maccioni, F.; Santarelli, M.L. Dissociation rate of THF-methane hydrates. Pet. Sci. Technol. 2008, 26, 2147–2158. [Google Scholar] [CrossRef]
- Madden, M.E.; Ulrich, S.; Szymcek, P.; McCallum, S.; Phelps, T. Experimental formation of massive hydrate deposits from accumulation of CH4 gas bubbles within synthetic and natural sediments. Mar. Pet. Geol. 2009, 26, 369–378. [Google Scholar] [CrossRef]
- Beltran, J.G.; Servio, P. Equilibrium studies for the system methane + carbon dioxide + neohexane + water. J. Chem. Eng. Data 2008, 53, 1745–1749. [Google Scholar] [CrossRef]
- Belandria, V.; Mohammadi, A.H.; Richon, D. Phase equilibria of clathrate hydrates of methane + carbon dioxide: New experimental data and predictions. Fluid Phase Equilib. 2010, 296, 60–65. [Google Scholar] [CrossRef]
- Belandria, V.; Eslamimanesh, A.; Mohammadi, A.H.; Richon, D. Gas hydrate formation in carbon dioxide + nitrogen + water system: Compositional analysis of equilibrium phases. Ind. Eng. Chem. Res. 2011, 50, 4722–4730. [Google Scholar] [CrossRef]
- Ke, W.; Svartaas, T.M. Effects of stirring and cooling on methane hydrate formation in a high-pressure isochoric cell. In Proceedings of the 7th International Conference on Gas Hydrates, Edinburgh, UK, 17–21 July 2011. [Google Scholar]
- Kim, S.M.; Lee, J.D.; Lee, H.J.; Lee, E.K.; Kim, Y. Gas hydrate formation method to capture the carbon dioxide for pre-combustion process in IGCC plant. Int. J. Hydrogen Energy 2011, 36, 1115–1121. [Google Scholar] [CrossRef]
- Lu, H.; Kawasaki, T.; Ukita, T.; Moudrakovski, I.; Fujii, T.; Noguchi, S.; Shimada, T.; Nakamizu, M.; Ripmeester, J.; Ratcliffe, C. Particle size effect on the saturation of methane hydrate in sediments e Constrained from experimental results. Mar. Pet. Geol. 2011, 28, 1801–1805. [Google Scholar] [CrossRef]
- Sfaxi, I.B.A.; Belandria, V.; Mohammadi, A.H.; Lugo, R.; Richon, D. Phase equilibria of CO2 + N2 and CO2 + CH4 clathrate hydrates: Experimental measurements and thermodynamic modelling. Chem. Eng. Sci. 2012, 84, 602–611. [Google Scholar] [CrossRef]
- Yang, S.O.; Cho, S.H.; Lee, H.; Lee, C.S. Measurement and prediction of phase equilibria for water + methane in hydrate forming conditions. Fluid Phase Equilib. 2001, 185, 53–63. [Google Scholar] [CrossRef]
- Ghavipour, M.; Chitsazan, M.; Najibi, S.H.; Ghidary, S.S. Experimental study of natural gas hydrates and a novel use of neural network to predict hydrate formation conditions. Chem. Eng. Res. Des. 2013, 91, 264–273. [Google Scholar] [CrossRef]
- Silva, V.S. Síntese e Caracterização de Hidratos de Metano. Master’s Thesis, Pontifical Catholic University of Rio Grande do Sul, Porto Alegre, Brazil, 2014. [Google Scholar]
- Li, X.S.; Xu, C.G.; Zhang, Y.; Ruan, X.K.; Li, G.; Wang, Y. Investigation into gas production from natural gas hydrate: A review. Appl. Energy 2016, 172, 286–322. [Google Scholar] [CrossRef]
- Ahn, Y.H.; Kang, H.; Koh, D.Y.; Park, Y.; Lee, H. Gas hydrate inhibition by 3-hydroxytetrahydrofuran: Spectroscopic identifications and hydrate phase equilibria. Fluid Phase Equilib. 2016, 413, 65–70. [Google Scholar] [CrossRef]
- Sun, Q.; Kang, Y.T. Review on CO2 hydrate formation/dissociation and its cold energy application. Renew. Sustain. Energy Rev. 2016, 62, 478–494. [Google Scholar] [CrossRef]
- Chen, L.T.; Li, N.; Sun, C.Y.; Chen, G.J.; Koh, C.A.; Sun, B.J. Hydrate formation in sediments from free gas using a one-dimensional visual simulator. Fuel 2017, 197, 298–309. [Google Scholar] [CrossRef]
- Buffett, B.A.; Zatsepina, O.Y. Formation of gas hydrate from dissolved gas in natural porous media. Mar. Geol. 2000, 164, 69–77. [Google Scholar] [CrossRef]
- Phelps, T.J.; Peters, D.J.; Marshall, S.L.; West, O.R.; Liang, L.Y.; Blencoe, J.G.; Alexiades, V.; Jacobs, G.K.; Naney, M.T.; Heck, J.L. A new experimental facility for investigating the formation and properties of gas hydrates under simulated seafloor conditions. Rev. Sci. Instrum. 2001, 72, 1514–1521. [Google Scholar] [CrossRef]
- Nagao, J. Development Methane Hydrate Production Method. Synthesiology 2012, 5, 88–95. [Google Scholar] [CrossRef]
- Link, D.D.; Ladner, E.P.; Elsen, H.A.; Taylor, C.E. Formation and dissociation studies for optimizing the uptake of methane by methane hydrates. Fluid Phase Equilib. 2003, 211, 1–10. [Google Scholar] [CrossRef]
- ZareNezhad, B.; Varaminian, F. A unified approach for description of gas hydrate formation kinetics in the presence of kinetic promoters in gas hydrate converters. Energy Convers. Manag. 2013, 73, 144–149. [Google Scholar] [CrossRef]
- Kondo, W.; Ohtsuka, K.; Ohmura, R.; Takeya, S.; Mori, Y.H. Clathrate-hydrate formation from a hydrocarbon gas mixture: Compositional evolution of formed hydrate during an isobaric semi-batch hydrate-forming operation. Appl. Energy 2014, 113, 864–871. [Google Scholar] [CrossRef]
- Mekala, P.; Busch, M.; Mech, D.; Patel, R.S.; Sangwai, J.S. Effect of silica sand size on the formation kinetics of CO2 hydrate in porous media in the presence of pure water and seawater relevant for CO2 sequestration. J. Pet. Sci. Eng. 2014, 122, 1–9. [Google Scholar] [CrossRef]
- Kamath, V.A.; Godbole, S.P. Evaluation of hot-brine stimulation technique for gas production from natural gas hydrates. J. Pet. Technol. 1987, 39, 1379–1388. [Google Scholar] [CrossRef]
- He, Z.; Gupta, K.M.; Linga, P.; Jiang, J. Molecular insights into the nucleation and growth of CH4 and CO2 mixed hydrates from microsecond simulations. J. Phys. Chem. C 2016, 120, 25225–25236. [Google Scholar] [CrossRef]
| Data | Reactor R01 | Reactor R02 |
|---|---|---|
| Internal diameter (inch) | 3.00 | 6.75 |
| External diameter (inch) | 6.19 | 8.38 |
| Total height—with external parts (inch) | 25.12 | 36.44 |
| Inside height (inch) | 8.87 | 17.38 |
| Stirrer and thermowell height (inch) | 7.68 | 16.34 |
| Window center position from the bottom (inch) | 6.87 | 12.75 |
| Windows width (inch) | 1.83 | 3.69 |
| Window quartz space (inch) | 0.50 | 0.50 |
| Total volume (L) | 1.0 | 9.5 |
| Maximum pressure (bar) | 221 | 221 |
| Minimum temperature (°C) | −29 | −29 |
| Maximum temperature (°C) | 177 | 177 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, L.F.; Ramos, A.; de Araujo, G.; Silveira, E.; Ketzer, M.; Lourega, R. High-Pressure and Automatized System for Study of Natural Gas Hydrates. Energies 2019, 12, 3064. https://doi.org/10.3390/en12163064
Rodrigues LF, Ramos A, de Araujo G, Silveira E, Ketzer M, Lourega R. High-Pressure and Automatized System for Study of Natural Gas Hydrates. Energies. 2019; 12(16):3064. https://doi.org/10.3390/en12163064
Chicago/Turabian StyleRodrigues, Luiz F., Alessandro Ramos, Gabriel de Araujo, Edson Silveira, Marcelo Ketzer, and Rogerio Lourega. 2019. "High-Pressure and Automatized System for Study of Natural Gas Hydrates" Energies 12, no. 16: 3064. https://doi.org/10.3390/en12163064
APA StyleRodrigues, L. F., Ramos, A., de Araujo, G., Silveira, E., Ketzer, M., & Lourega, R. (2019). High-Pressure and Automatized System for Study of Natural Gas Hydrates. Energies, 12(16), 3064. https://doi.org/10.3390/en12163064





