Effects of High Sludge Cycle Frequency on Performance and Syntrophic Metabolism of Anaerobic Membrane Bioreactor for Treating High-Lipid Kitchen Waste Slurry
Abstract
1. Introduction
2. Materials and Methods
2.1. Kitchen Waste Slurry Characterization
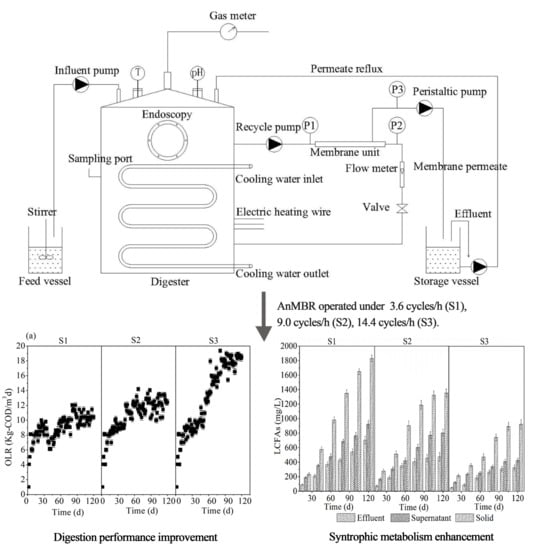
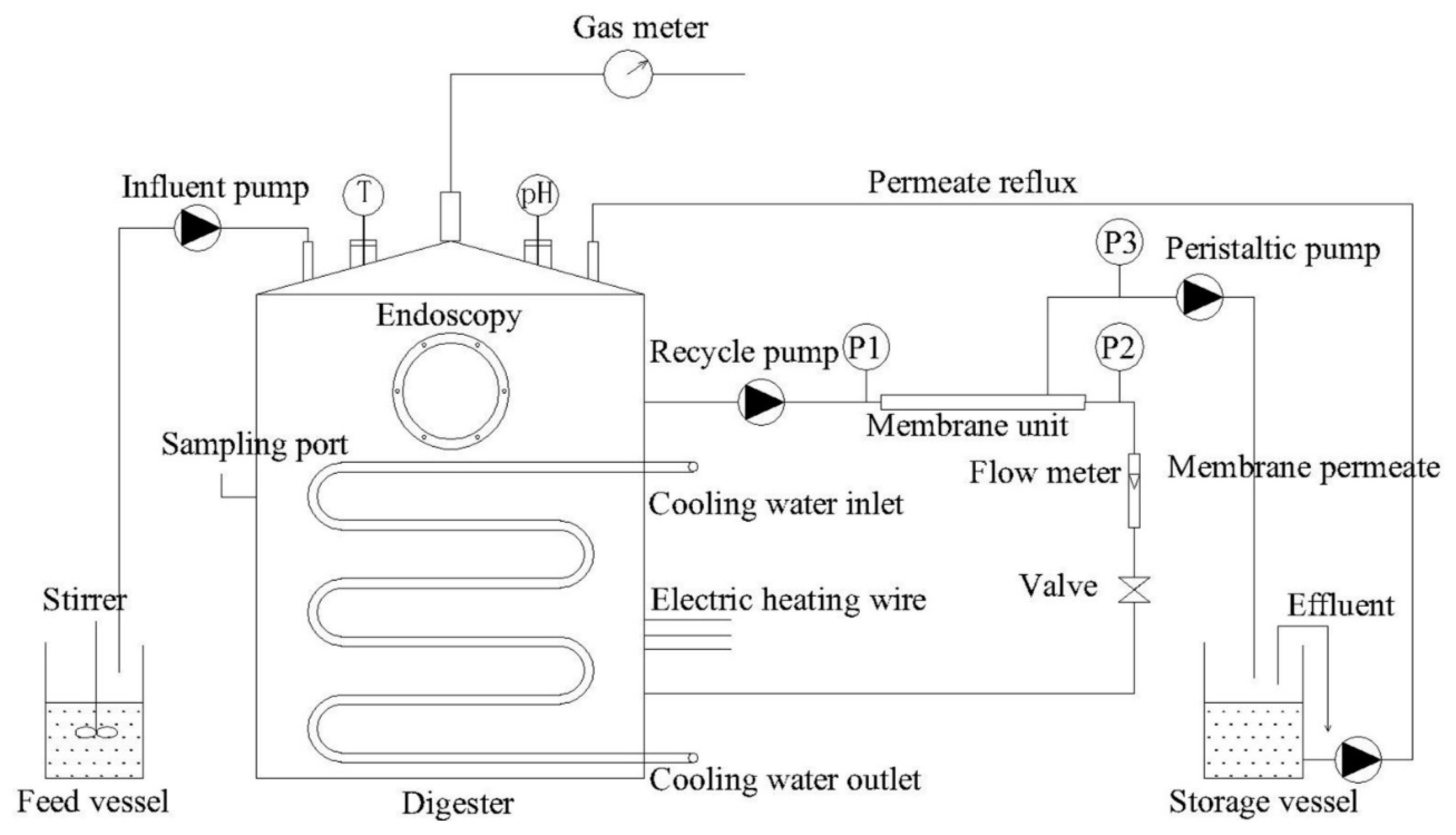
2.2. AnMBR Configuration and Parameters
2.3. Operation Strategy
2.4. Physicochemical Analysis
2.5. Microbial Community Analysis
3. Results and Discussion
3.1. Reactors Performance and Stability
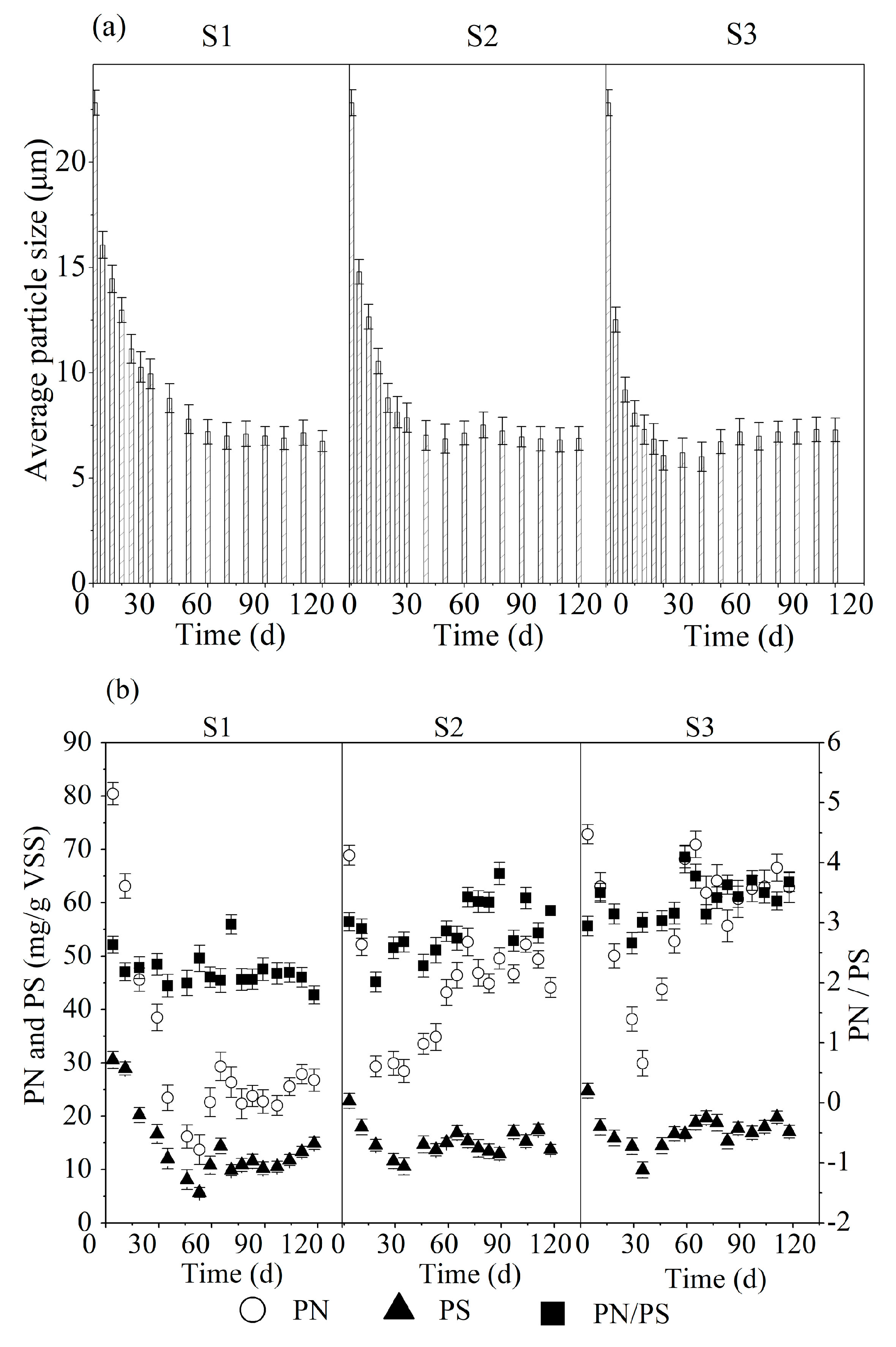
3.2. Sludge Characteristics
3.3. Syntrophic Metabolism of LCFAs
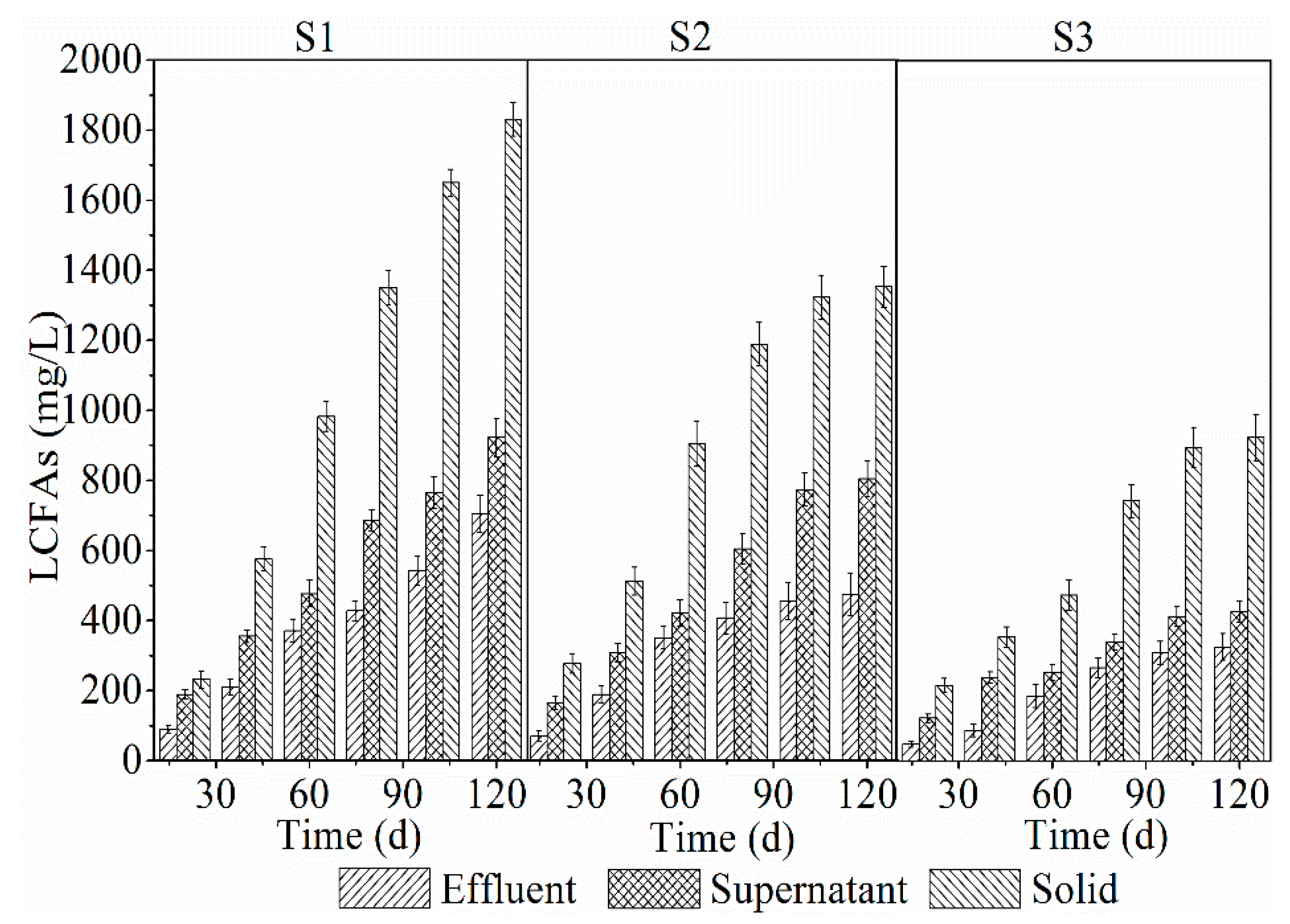
3.3.1. LCFAs Accumulation in the Reactor
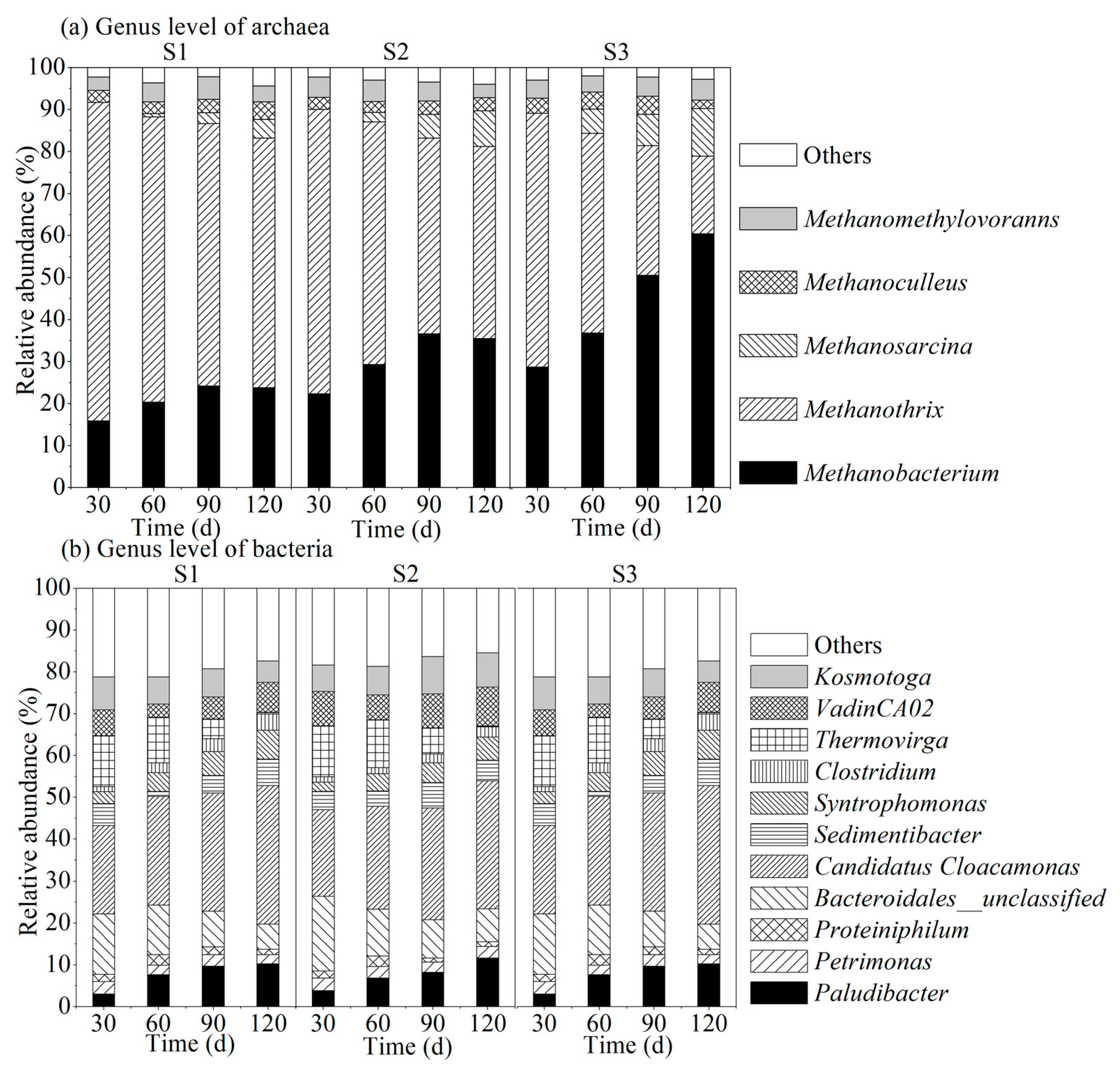
3.3.2. Microbial Community Structure
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abdurrahman, N.H.; Rosli, Y.M.; Azhari, N.H. Development of a membrane anaerobic system (MAS) for palm oil mill effluent (POME) treatment. Desalination 2011, 266, 208–212. [Google Scholar] [CrossRef]
- Dereli, R.K.; van der Zee, F.P.; Heffernan, B.; Grelot, A.; van Lier, J.B. Effect of sludge retention time on the biological performance of anaerobic membrane bioreactors treating corn-to-ethanol thin stillage with high lipid content. Water Res. 2014, 43, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, F.R.; Donnely, T.; Anderson, G.K. Comparative performance of anaerobic digesters operating on ice-cream wastewater. Water Res. 1995, 29, 525–533. [Google Scholar] [CrossRef]
- Siegrist, H.; Hunziker, W.; Hofer, H. Anaerobic digestion of slaughterhouse waste with UF-membrane separation and recycling of permeate after free ammonia stripping. Water Sci. Technol. 2005, 52, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Huang, Z.; Ruan, W.; Yan, L.; Miao, H.; Ren, H.; Zhao, M. Evaluation and characterization during the anaerobic digestion of high-strength kitchen waste slurry via a pilot-scale anaerobic membrane bioreactor. Bioresour. Technol. 2015, 193, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; El-Mashad, H.M.; Hartman, K.; Wang, F.; Liu, G.; Choate, C. Characterization of food waste as feedstock for anaerobic digestion. Bioresour. Technol. 2007, 98, 929–935. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, C.; He, W.; Zhu, H.; Huang, J.; Li, G. The content variation of fat, protein and starch in kitchen waste under microwave radiation. Proc. Environ. Sci. 2016, 31, 530–534. [Google Scholar] [CrossRef]
- Li, Y.Y.; Sasaki, H.; Yamashita, K.; Saki, K.; Kamigochi, I. High-rate methane fermentation of lipid-rich food wastes by a high-solids co-digestion process. Water Sci. Technol. 2002, 45, 143–150. [Google Scholar] [CrossRef]
- Pereira, M.A.; Cavaleiro, A.J.; Mota, M.; Mota, M.; Alves, M.M. Accumulation of long chain fatty acids onto anaerobic sludge under steady state and shock loading conditions: Effect on acetogenic and methanogenic activity. Water Sci. Technol. 2003, 48, 33–40. [Google Scholar] [CrossRef]
- Hanaki, K.; Matsuo, T.; Nagase, M. Mechanism of inhibition caused by long chain fatty acids in anaerobic digestion process. Biotechnol. Bioeng. 1981, 23, 1590–1610. [Google Scholar] [CrossRef]
- Koster, I.W.; Cramer, A. Inhibition of methanogenesis from acetate in granular sludge by long-chain fatty acids. Appl. Environ. Microbiol. 1987, 53, 403–409. [Google Scholar]
- Rinzema, A.; Boone, M.; Van Knippenberg, K.; Lettinga, G. Bactericidal effect of long chain fatty acids in anaerobic digestion. Water Environ. Res. 1994, 66, 40–49. [Google Scholar] [CrossRef]
- Hwu, S.H.; Lettinga, G. Acute toxicity of oleate to acetate-utilizing methanogens in mesophilic and thermophilic sludges. Enzyme Microb. Technol. 1997, 21, 297–301. [Google Scholar] [CrossRef]
- Xiao, X.; Shi, W.; Huang, Z.; Ruan, W.; Miao, H.; Ren, H.; Zhao, M. Process stability and microbial response of anaerobic membrane bioreactor treating high-strength kitchen waste slurry under different organic loading rates. Int. Biodeterior. Biodegrad. 2017, 121, 35–43. [Google Scholar] [CrossRef]
- Bohdziewicz, J.; Neczaj, E.; Kwarciak, A. Landfill leachate treatment by means of anaerobic membrane bioreactor. Desalination 2008, 221, 559–565. [Google Scholar] [CrossRef]
- Choo, K.H.; Kang, I.J.; Yoon, S.H.; Park, H.; Kim, J.H.; Adiya, S.; Lee, C.H. Approaches to membrane fouling control in anaerobic membrane bioreactors. Water Sci. Technol. 2000, 41, 363–371. [Google Scholar] [CrossRef]
- Gong, H.; Jin, Z.; Wang, X.; Wang, K. Membrane fouling controlled by coagulation/adsorption during direct sewage membrane filtration (DSMF) for organic matter concentration. J. Environ. Sci. 2015, 32, 1–7. [Google Scholar] [CrossRef]
- Lew, B.; Tarre, S.; Beliavski, M.; Dosoretz, C.; Green, M. Anaerobic membrane bioreactor (AnMBR) for domestic wastewater treatment. Desalination 2009, 243, 251–257. [Google Scholar] [CrossRef]
- Sun, J.; Rong, J.; Dai, L.; Liu, B.; Zhu, W. Control of membrane fouling during hyperhaline municipal wastewater treatment using a pilot-scale anoxic/aerobic-membrane bioreactor system. J. Environ. Sci. 2011, 23, 1619–1625. [Google Scholar] [CrossRef]
- Brockmann, M.; Seyfried, C.F. Sludge activity and cross-flow microfiltration- a non-beneficial relationship. Water Sci. Technol. 1996, 34, 205–213. [Google Scholar] [CrossRef]
- Cirnea, D.G.; Paloumeta, X.; Bjornssona, L.; Alvesb, M.M.; Mattiasson, B. Anaerobic digestion of lipid-rich waste-Effects of lipid concentration. Renew. Energy 2007, 32, 965–975. [Google Scholar] [CrossRef]
- Sousa, D.Z.; Pereira, A.A.; Smidt, H.; Stams, A.J.M.; Alves, M.M. Molecular assessment of complex microbial communities degrading long chain fatty acids in methanogenic bioreactors. FEMS Microbiol. Ecol. 2007, 60, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.N.; Jeris, J.S.S. Biochemical mechanism in the methane fermentation of glutamic and oleic acids. Water Res. 1976, 10, 9–11. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Yang, L.; Huang, Y.; Zhao, M.; Huang, Z.; Miao, H.; Xu, Z.; Ruan, W. Enhancing biogas generation performance from food wastes by high-solids thermophilic anaerobic digestion: Effect of pH adjustment. Int. Biodeterior. Biodegrad. 2015, 105, 153–159. [Google Scholar] [CrossRef]
- Huang, Z.; Yu, X.; Miao, H.; Ren, H.; Zhao, M.; Ruan, W. Enzymatic dynamics of microbial acid tolerance response (ATR) during the enhanced biohydrogen production process via anaerobic digestion. Int. J. Hydrogen Energy. 2012, 37, 10655–10662. [Google Scholar] [CrossRef]
- Thanakoses, P.; Black, A.S.; Holtzapple, M.T. Fermentation of corn stover to carboxylic acids. Biotechnol. Bioeng. 2003, 83, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Neves, L.; Pereira, M.A.; Mota, M.; Alves, M.M. Detection and quantification of long chain fatty acids in liquid and solid samples and its relevance to understand anaerobic digestion of lipids. Bioresour. Technol. 2009, 100, 91–96. [Google Scholar] [CrossRef]
- Chen, K.; Wang, X.; Li, X.; Qian, J.; Xiao, X. Impacts of sludge retention time on the performance of submerged membrane bioreactor with the addition of calcium ion. Sep. Purif. Technol. 2011, 82, 148–155. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Frølund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 1996, 30, 1749–1758. [Google Scholar] [CrossRef]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 2049–2618. [Google Scholar] [CrossRef] [PubMed]
- Poggi-Varaldo, H.M.; Oleszkiewicz, J.A. Anaerobic composting of municipal solid-waste and waste sludge at high total solids levels. Environ. Technol. 1992, 13, 409–421. [Google Scholar] [CrossRef]
- Callaghan, F.J.; Wase, D.A.J.; Thayanithy, K.; Forster, C.F. Continuous co-digestion of cattle slurry with fruit and vegetable wastes and chicken manure. Biomass Bioenergy 2002, 27, 71–77. [Google Scholar] [CrossRef]
- Schink, B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol. Mol. Biol. Rev. 1997, 61, 262–280. [Google Scholar] [PubMed]
- de Bok, F.A.M.; Plugge, C.M.; Stams, A.J.M. Interspecies Electron transfer in methanogenic propionate degrading consortia. Water. Res. 2004, 38, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Feng, X.; Zhu, N.; Chen, J. Role of extracellular protein in the formation and stability of aerobic granules. Enzyme Microb. Technol. 2007, 41, 551–557. [Google Scholar] [CrossRef]
- Pereira, M.A.; Mota, M.; Alves, M.M. Anaerobic biodegradation of oleic and palmitic acids: Evidence of mass transfer limitations caused by long chain fatty acid accumulation onto the anaerobic sludge. Biotechnol. Bioeng. 2005, 92, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Sousa, D.Z.; Smidt, H.; Alves, M.M.; Stams, A.J.M. Syntrophomonas zehnderi sp. nov., an anaerobe that degrades long chain fatty acids in co-culture with Methanobacterium formicicum. Int. J. Syst. Evol. Microbiol. 2007, 57, 609–615. [Google Scholar] [CrossRef]
- Ning, Z.F.; Zhang, H.; Li, W.W.; Zhang, R.H.; Liu, G.Q.; Chen, C. Anaerobic digestion of lipid-rich swine slaughterhouse waste: Methane production performance, long-chain fatty acids profile and predominant microorganisms. Bioresour. Technol. 2018, 269, 426–433. [Google Scholar] [CrossRef]
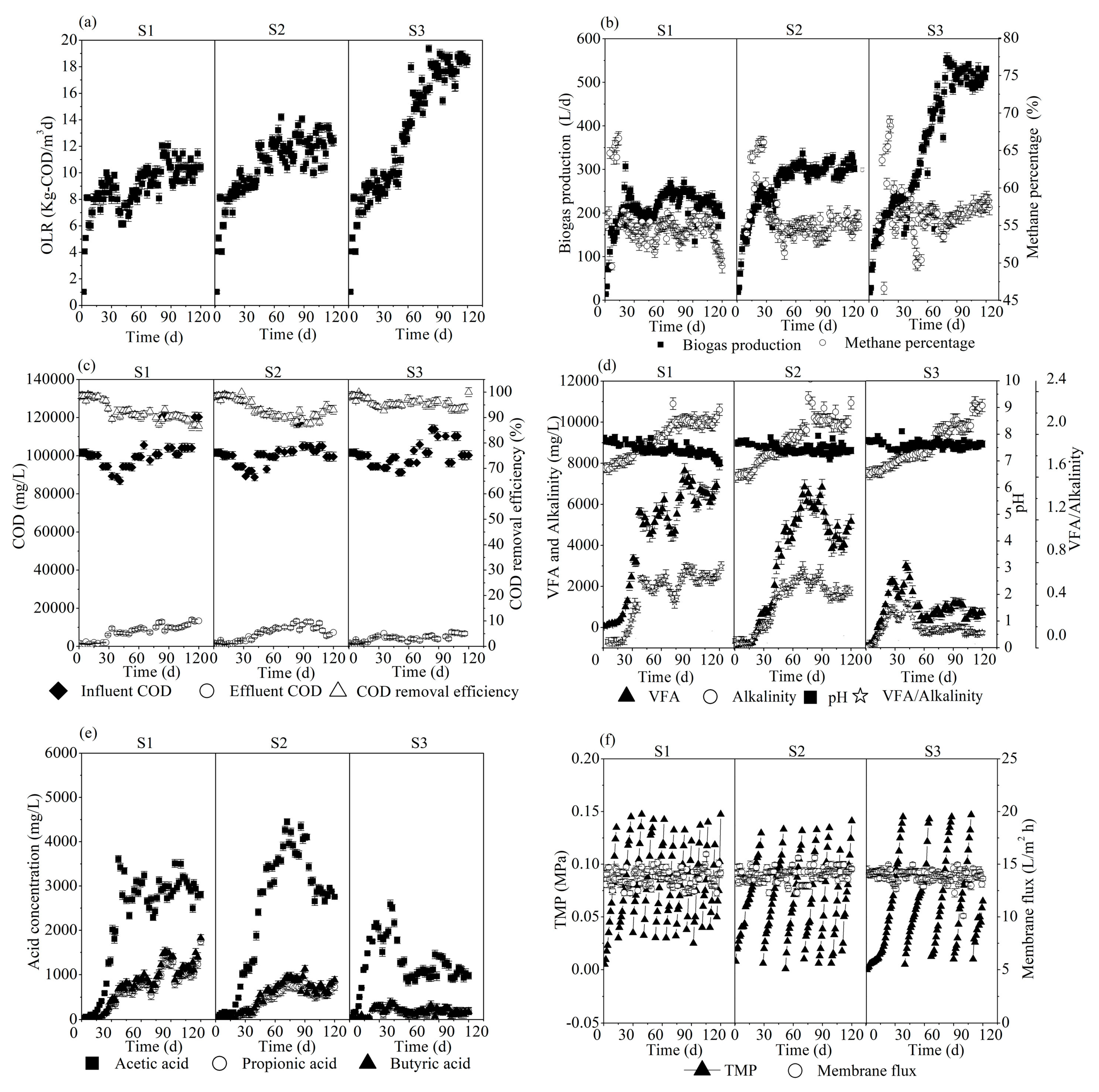
| Parameters | Unit | S1 | S2 | S3 |
|---|---|---|---|---|
| OLR | (kg COD/m3d) | 10.3 ± 0.7 | 12.4 ± 0.9 | 18.1 ± 0.5 |
| Permeate COD | (mg/L) | 13,400 ± 1370 | 7300 ± 690 | 5200 ± 540 |
| COD removal efficiency | (%) | 86.2 ± 0.8 | 90.4 ± 1.3 | 96.3 ± 0.6 |
| Biogas production | (L/d) | 190 ± 25 | 310 ± 31 | 520 ± 38 |
| Methane percentage | (%) | 50.3 ± 1.2 | 55.2 ± 0.8 | 58.4 ± 0.7 |
| VFAs | (mg/L) | 6860 ± 150 | 4780 ± 110 | 1690 ± 130 |
| VFAs/alkalinity | - | 0.68 ± 0.08 | 0.45 ± 0.03 | 0.17 ± 0.02 |
| pH | - | 6.9 ± 0.1 | 7.4 ± 0.1 | 7.6 ± 0.1 |
| Acetate | (mg/L) | 3100 ± 130 | 2800 ± 140 | 1140 ± 160 |
| Propionate | (mg/L) | 1150 ± 125 | 580 ± 88 | 172 ± 26 |
| Butyrate | (mg/L) | 1250 ± 90 | 620 ± 96 | 188 ± 35 |
| Parameters | Unit | S1 | S2 | S3 |
|---|---|---|---|---|
| average particle sizes | (μm) | 22.8 ± 0.6 a | 22.4 ± 0.8 a | 22.9 ± 0.7 a |
| 7.0 ± 0.4 b | 6.8 ± 0.2 c | 6.1 ± 0.3 d | ||
| 6.9 ± 0.3 e | 7.1 ± 0.1 e | 7.0 ± 0.2 e | ||
| PN | (mg/g VSS) | 31.3 ± 6.5 | 49.2 ± 5.4 | 64.5 ± 5.9 |
| PS | (mg/g VSS) | 15.2 ± 2.3 | 16.8 ± 2.1 | 17.1 ± 2.5 |
| PN/PS | -- | 2.0 ± 0.21 | 2.9 ± 0.30 | 3.8 ± 0.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, X.; Shi, W.; Ruan, W. Effects of High Sludge Cycle Frequency on Performance and Syntrophic Metabolism of Anaerobic Membrane Bioreactor for Treating High-Lipid Kitchen Waste Slurry. Energies 2019, 12, 2673. https://doi.org/10.3390/en12142673
Xiao X, Shi W, Ruan W. Effects of High Sludge Cycle Frequency on Performance and Syntrophic Metabolism of Anaerobic Membrane Bioreactor for Treating High-Lipid Kitchen Waste Slurry. Energies. 2019; 12(14):2673. https://doi.org/10.3390/en12142673
Chicago/Turabian StyleXiao, Xiaolan, Wansheng Shi, and Wenquan Ruan. 2019. "Effects of High Sludge Cycle Frequency on Performance and Syntrophic Metabolism of Anaerobic Membrane Bioreactor for Treating High-Lipid Kitchen Waste Slurry" Energies 12, no. 14: 2673. https://doi.org/10.3390/en12142673
APA StyleXiao, X., Shi, W., & Ruan, W. (2019). Effects of High Sludge Cycle Frequency on Performance and Syntrophic Metabolism of Anaerobic Membrane Bioreactor for Treating High-Lipid Kitchen Waste Slurry. Energies, 12(14), 2673. https://doi.org/10.3390/en12142673