Optimization of an Ultrasonic-Assisted Biodiesel Production Process from One Genotype of Rapeseed (TERI (OE) R-983) as a Novel Feedstock Using Response Surface Methodology
Abstract
1. Introduction
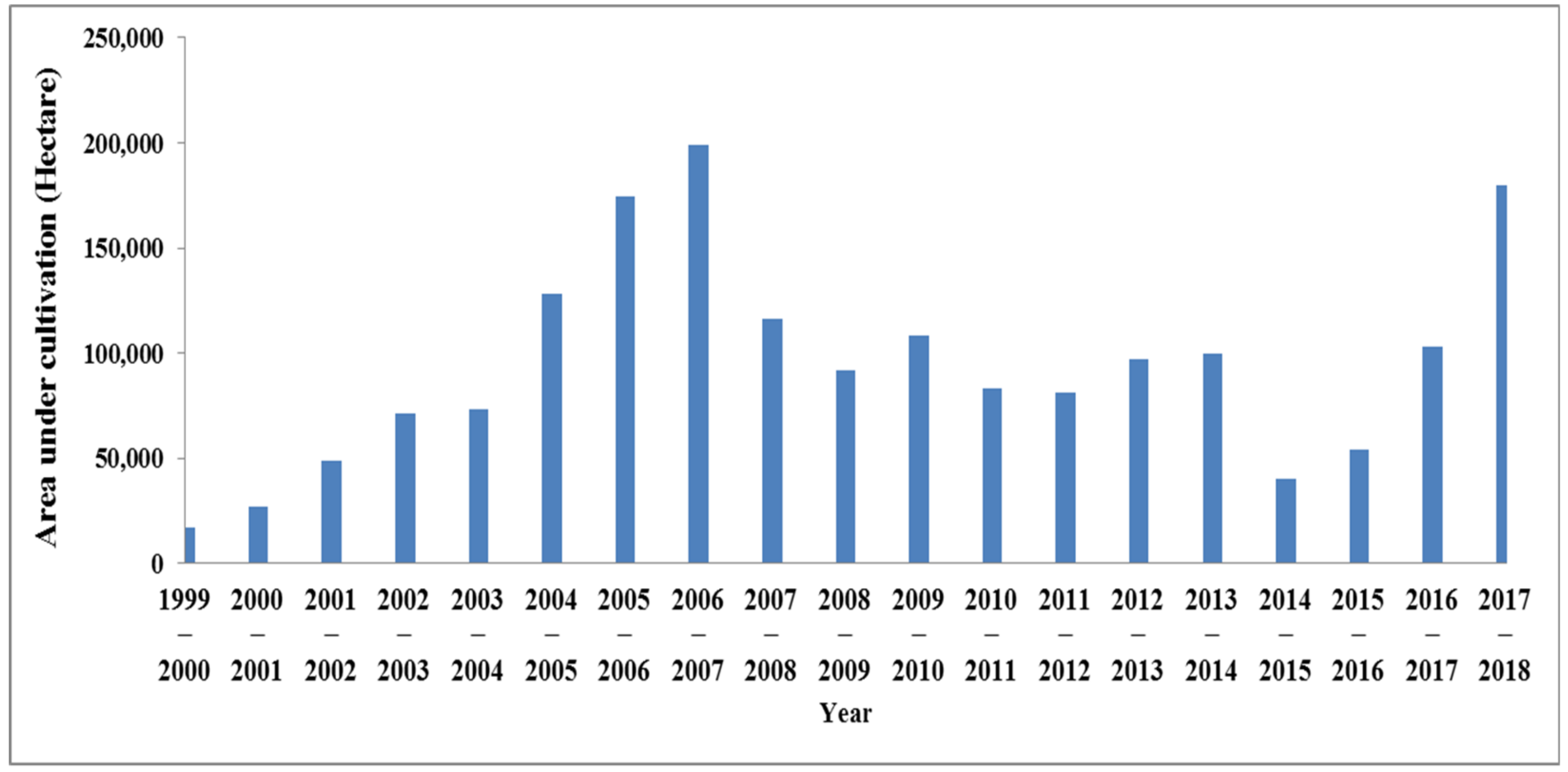
- In terms of canola cultivation, Iran is divided into four climates (the warm climates of the north and south of Iran, fairly cold, and cold). In cold climates, the winter cultivars of rapeseed are cultivated, and in the two warm climates, the spring cultivars are cultivated. So, all provinces of Iran have potential for cultivating rapeseed;
- Rapeseed requires low quantities of water and is appropriate for the climate of Iran;
- Due to its rotation with wheat, barley, and rice, rapeseed is called an economic plant;
- Due to the problems of drought and water scarcity and increasing desertification in Iran, according to the instructions of the Ministry of Agriculture in Iran, farmers must move towards strategic cultivation. One of the strategic products is the cultivation of rapeseed.
2. Material and Method
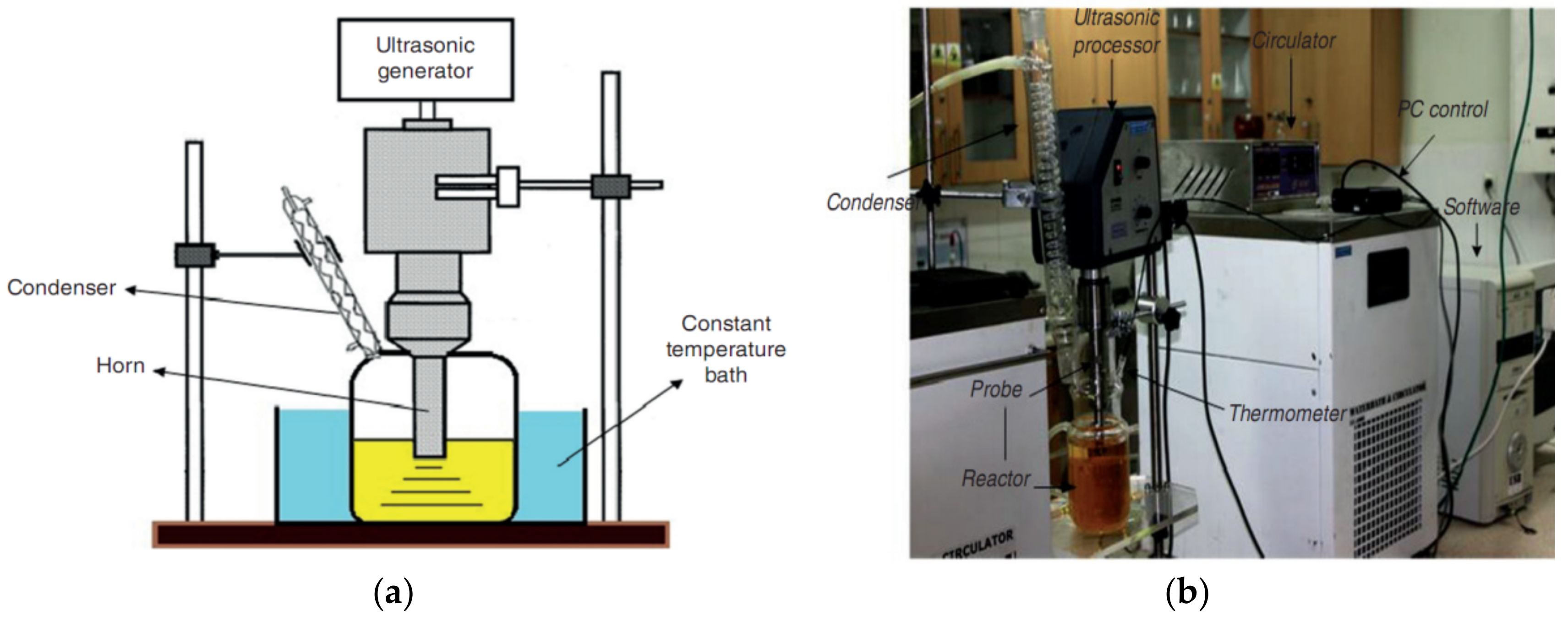
2.1. Equipment and Materials
2.2. Esterification of TERI (OE) R-983 Oil
2.3. Analysis of Fatty Acids Structure
2.4. Transesterification
2.5. FAME Yield Calculation and Statistical Analysis
3. Result and Discussion
3.1. Statistical Analysis
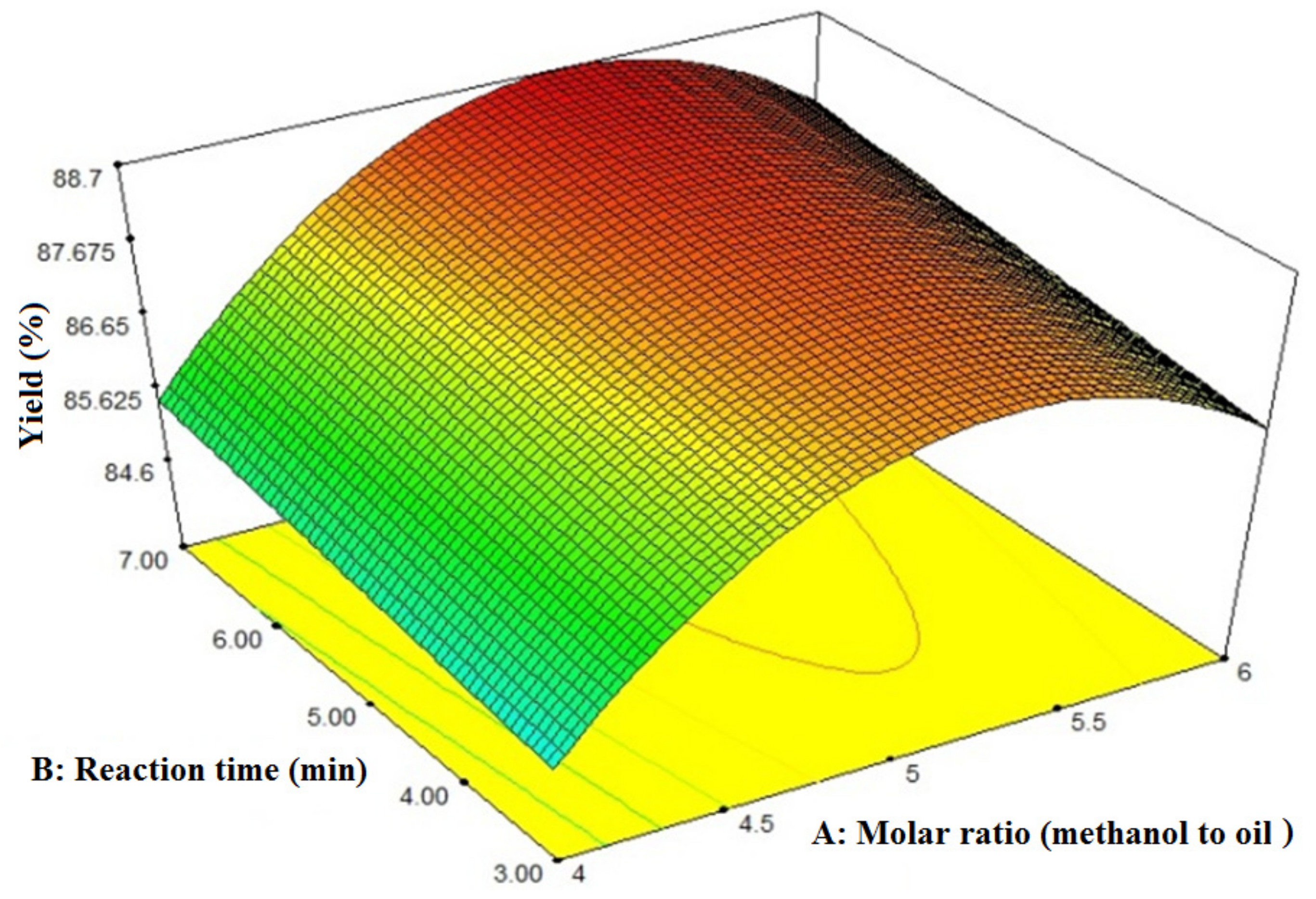
3.2. Effects of Molar Ratio and Reaction Time on FAME Yield
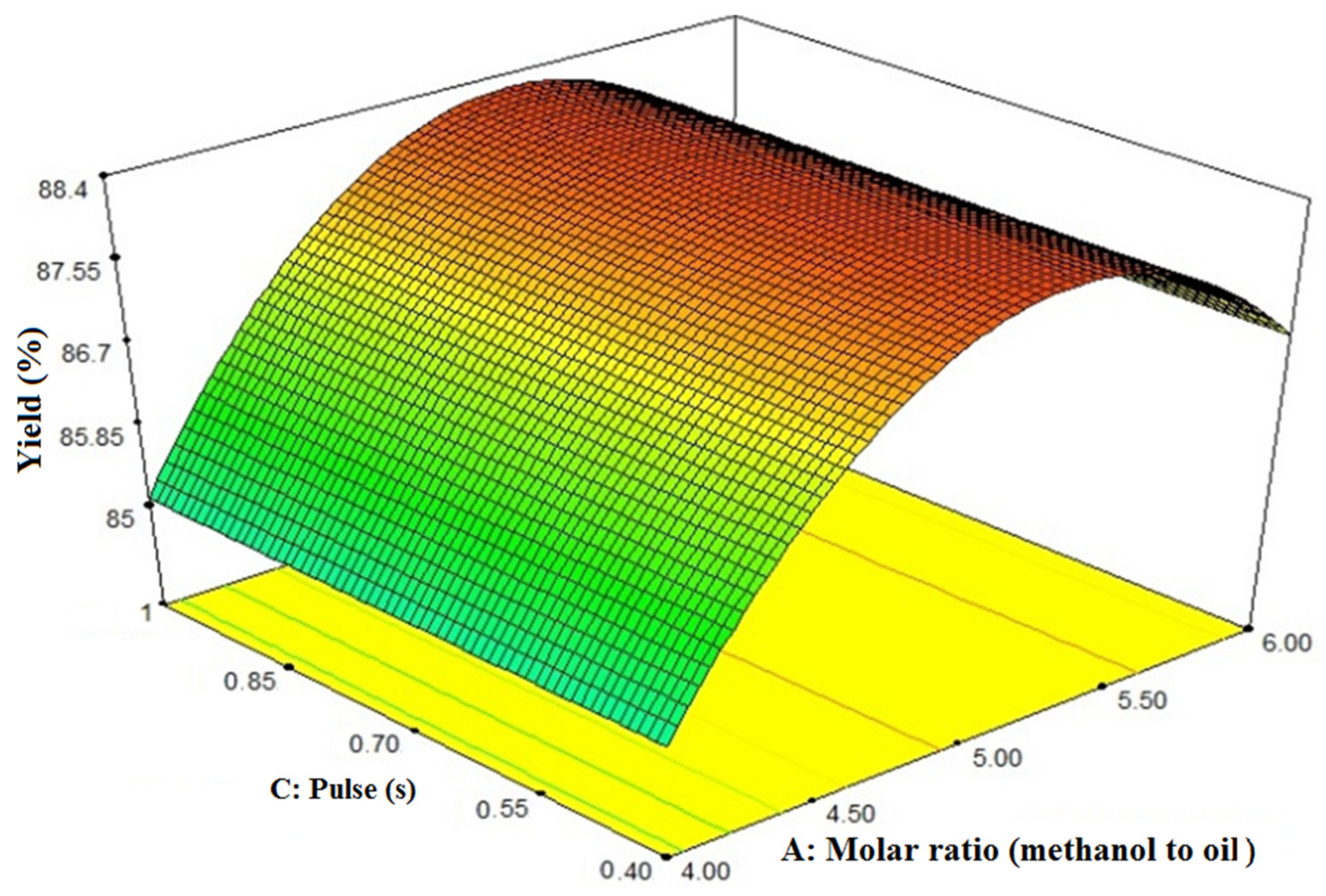
3.3. Interaction Effects of Pulse and Molar Ratio (Methanol-to-Oil Ratio) on FAME Yield
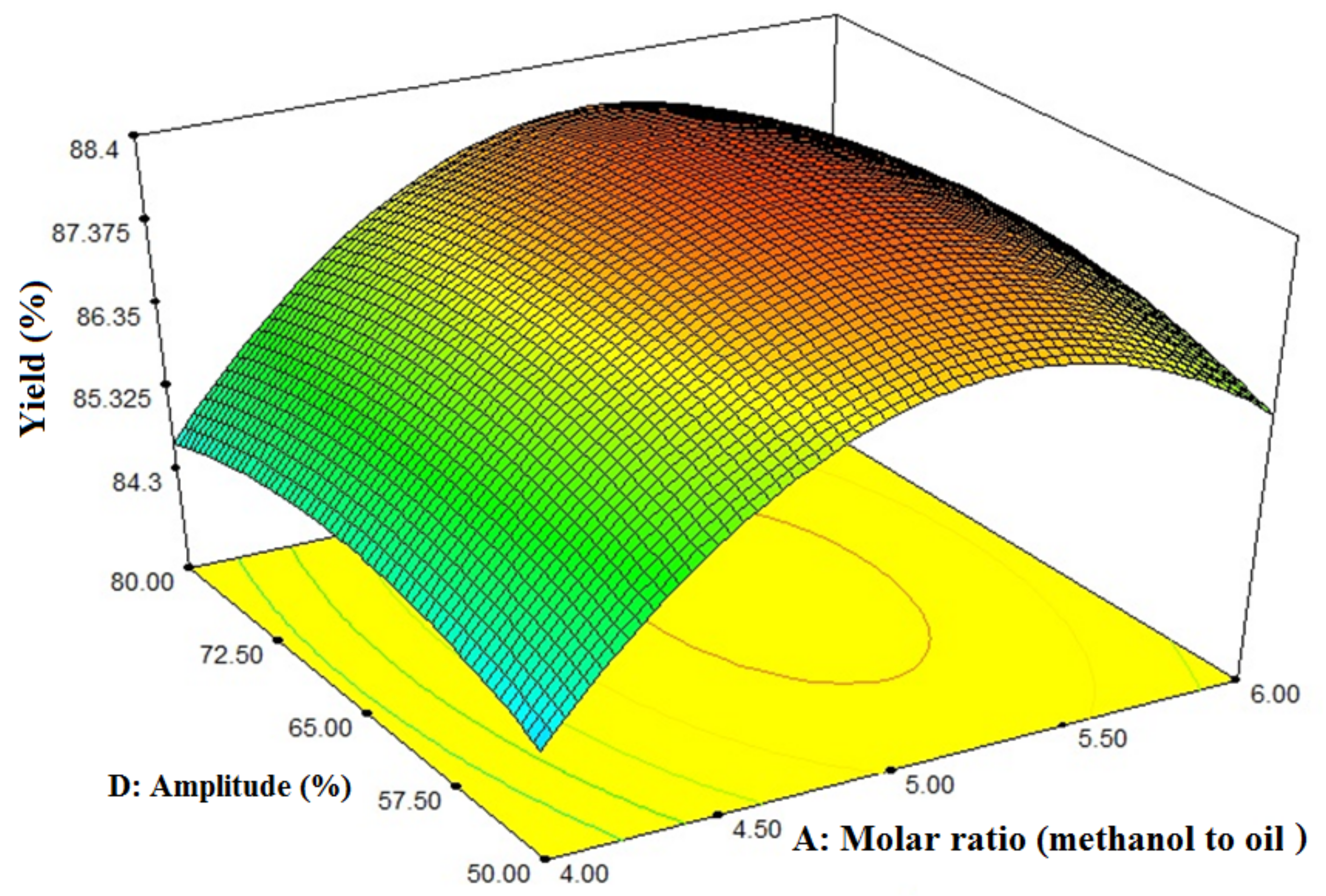
3.4. Interaction Effects of Amplitude and Molar Ratio (Methanol-to-Oil Ratio) on FAME Yield
3.5. Optimization of the Production Process Using Desirability Function
- (a)
- Molar ratio (methanol to oil) = 4.63:1;
- (b)
- Reaction time = 5.22 min;
- (c)
- Pulse = 0.4 s;
- (d)
- Amplitude = 56.50%.
3.6. Properties of the Produced Biodiesel
4. Comparison with the Literature
5. Conclusions
- The molar ratio of alcohol to oil has a positive effect on the transesterification reaction and, consequently, on biodiesel conversion. Increasing the molar ratio from 4:1 to 6:1 increased the FAME yield by approximately 4.5%;
- The optimal value for the FAME yield from TERI (OE) R-983 oil using ultrasound irradiation was 87.175%; the conditions that resulted in this value were: a 4.63:1 alcohol-to-oil molar ratio, 5.22 min reaction time, 0.40 s pulse, and 56.50% amplitude;
- The properties of the produced biodiesel being within the range of the standard-defined properties (ASTM D6751) highlights the potential of such a biodiesel to be used as an alternative fuel.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| References | [45] | [44] | [46] | [48] | [49] | [47] |
|---|---|---|---|---|---|---|
| Feedstock | Kusum | Waste cooking oil | Waste cooking oil | Rapeseed, soybean, coconut, and palm | Waste cooking oil | Canola |
| Production technology | Ultrasound | Ultrasound | Microwave | Ultrasound | Ultrasound | Ultrasound |
| Operating temperature (°C) | 50 | 60 | 60 | 25 | 30 | 25 |
| Run time (min) | 80 | 30 | 5 | − | 30 | 50 |
| Catalyst | Ba(OH)2 | Calcium triglyceride | NaOCH3 | KOH | KOH | KOH |
| Yield (%) | 96.8% | 93.5% | 98.87% | Rapeseed (95.03%), soybean (94.66%), palm (93.08%), coconut (81.37%) | 90% | >99% |
| Pour point (°C) | −2 | −1 | −19 | – | – | – |
| Flash point (°C) | 152 | 164 | 185 | Rapeseed (175.5), soybean (169), coconut (108), palm (131.5) | 150 | 184 |
| Acid value (mgKOH/g) | 0.42 | 0.27 | Rapeseed (0.41), soybean (0.51), coconut (0.29), palm (0.43) | _ | _ | |
| Viscosity (mm2/s, 40 °C) | 5.34 | 3.84 | 2.35 | Rapeseed (4.62), soybean (4.7), coconut (3.2), palm (5.63) | 4.08 | 4.51 |
| Density at 15 °C (mg/m−3) | 857 | 875 | 888 | Rapeseed (882), soybean (883), coconut (874), palm (874) | 891.1 | 884 |
| Oxidation stability (h) | 0.86 | _ | _ | _ | _ | _ |
| Water content (ppm) | _ | _ | _ | Rapeseed (303.21), soybean (412.2), coconut (485.17), palm (463.32) | _ | 390 |
References
- Kajitani, S.; Oguma, M.; Mori, T. DME Fuel Blends for Low-Emission, Direct-Injection Diesel Engines; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2000. [Google Scholar]
- Wang, J. Hybrid robust air-path control for diesel engines operating conventional and low temperature combustion modes. IEEE Trans. Control Syst. Technol. 2008, 16, 1138–1151. [Google Scholar] [CrossRef]
- Ediger, V.Ş.; Kentel, E. Renewable energy potential as an alternative to fossil fuels in Turkey. Energy Conv. Manag. 1999, 40, 743–755. [Google Scholar] [CrossRef]
- Barbir, F.; Veziroǧlu, T.; Plass, H. Environmental damage due to fossil fuels use. Int. J. Hydrog. Energy 1990, 15, 739–749. [Google Scholar] [CrossRef]
- Carraretto, C.; Macor, A.; Mirandola, A.; Stoppato, A.; Tonon, S. Biodiesel as alternative fuel: Experimental analysis and energetic evaluations. Energy 2004, 29, 2195–2211. [Google Scholar] [CrossRef]
- Huang, D.; Zhou, H.; Lin, L. Biodiesel: An alternative to conventional fuel. Energy Procedia 2012, 16, 1874–1885. [Google Scholar] [CrossRef]
- Gaurav, N.; Sivasankari, S.; Kiran, G.; Ninawe, A.; Selvin, J. Utilization of bioresources for sustainable biofuels: A Review. Renew. Sustain. Energy Rev. 2017, 73, 205–214. [Google Scholar] [CrossRef]
- Patil, P.D.; Deng, S. Optimization of biodiesel production from edible and non-edible vegetable oils. Fuel 2009, 88, 1302–1306. [Google Scholar] [CrossRef]
- Bilal, S.; Nuhu, M.; Kasim, S. Production of biolubricant from Jatropha curcas seed oil. J. Chem Eng. Mater. Sci. 2013, 4, 72–79. [Google Scholar]
- Sajjadi, B.; Raman, A.A.A.; Arandiyan, H. A comprehensive review on properties of edible and non-edible vegetable oil-based biodiesel: Composition, specifications and prediction models. Renew. Sustain. Energy Rev. 2016, 63, 62–92. [Google Scholar] [CrossRef]
- Annamalai, K. The status of biodiesel as an alternative fuel for diesel engine—An overview. J. Sustain. Energy Environ. 2011, 2, 71–75. [Google Scholar]
- Datta, A.; Mandal, B.K. A comprehensive review of biodiesel as an alternative fuel for compression ignition engine. Renew. Sustain. Energy Rev. 2016, 57, 799–821. [Google Scholar] [CrossRef]
- Hoseini, S.; Najafi, G.; Ghobadian, B.; Yusaf, T.; Ebadi, M. The effect of combustion management on diesel engine emissions fueled with biodiesel-diesel blends. Renew. Sustain. Energy Rev. 2017, 73, 307–331. [Google Scholar] [CrossRef]
- Hoseini, S.; Najafi, G.; Ghobadian, B.; Yusaf, T.; Ebadi, M. The Effects of Camelina “Soheil” as a Novel Biodiesel Fuel on the Performance and Emission Characteristics of Diesel Engine. Appl. Sci. 2018, 8, 1010. [Google Scholar] [CrossRef]
- Abbaszaadeh, A.; Ghobadian, B.; Omidkhah, M.R.; Najafi, G. Current biodiesel production technologies: A comparative review. Energy Conv. Manag. 2012, 63, 138–148. [Google Scholar] [CrossRef]
- Amelio, A.; Van de Voorde, T.; Creemers, C.; Degrève, J.; Darvishmanesh, S.; Luis, P.; Van der Bruggen, B. Comparison between exergy and energy analysis for biodiesel production. Energy 2016, 98, 135–145. [Google Scholar] [CrossRef]
- Naderloo, L.; Javadikia, H.; Mostafaei, M. Modeling the energy ratio and productivity of biodiesel with different reactor dimensions and ultrasonic power using ANFIS. Renew. Sustain. Energy Rev. 2017, 70, 56–64. [Google Scholar] [CrossRef]
- Samani, B.H.; Zareiforoush, H.; Lorigooini, Z.; Ghobadian, B.; Rostami, S.; Fayyazi, E. Ultrasonic-assisted production of biodiesel from Pistacia atlantica Desf. oil. Fuel 2016, 168, 22–26. [Google Scholar] [CrossRef]
- Hoseini, S.S.; Najafi, G.; Ghobadian, B.; Mamat, R.; Ebadi, M.T.; Yusaf, T. Ailanthus altissima (tree of heaven) seed oil: Characterisation and optimisation of ultrasonication-assisted biodiesel production. Fuel 2018, 220, 621–630. [Google Scholar] [CrossRef]
- Teixeira, L.S.G.; Assis, J.C.R.; Mendonça, D.R.; Santos, I.T.V.; Guimarães, P.R.B.; Pontes, L.A.M.; Teixeira, J.S.R. Comparison between conventional and ultrasonic preparation of beef tallow biodiesel. Fuel Proc. Technol. 2009, 90, 1164–1166. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, G.; Poonam; Singh, C.P. Fast, easy ethanolysis of coconut oil for biodiesel production assisted by ultrasonication. Ultrason. Sonochem. 2010, 17, 555–559. [Google Scholar] [CrossRef]
- Van Manh, D.; Chen, Y.-H.; Chang, C.-C.; Chang, M.-C.; Chang, C.-Y. Biodiesel production from Tung oil and blended oil via ultrasonic transesterification process. J. Taiwan Inst. Chem. Eng. 2011, 42, 640–644. [Google Scholar] [CrossRef]
- Tamilarasan, S.; Sahadevan, R. Ultrasonic assisted acid base transesterification of algal oil from marine macroalgae Caulerpa peltata: Optimization and characterization studies. Fuel 2014, 128, 347–355. [Google Scholar] [CrossRef]
- Maghami, M.; Sadrameli, S.M.; Ghobadian, B. Production of biodiesel from fishmeal plant waste oil using ultrasonic and conventional methods. Appl. Therm. Eng. 2015, 75, 575–579. [Google Scholar] [CrossRef]
- Bhangu, S.K.; Gupta, S.; Ashokkumar, M. Ultrasonic enhancement of lipase-catalysed transesterification for biodiesel synthesis. Ultrason. Sonochem. 2017, 34, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Mazanov, S.V.; Gabitova, A.R.; Usmanov, R.A.; Gumerov, F.M.; Labidi, S.; Amar, M.B.; Passarello, J.-P.; Kanaev, A.; Volle, F.; Neindre, B.L. Continuous production of biodiesel from rapeseed oil by ultrasonic assist transesterification in supercritical ethanol. J. Supercrit. Fluids 2016, 118, 107–118. [Google Scholar] [CrossRef]
- Choedkiatsakul, I.; Ngaosuwan, K.; Cravotto, G.; Assabumrungrat, S. Biodiesel production from palm oil using combined mechanical stirred and ultrasonic reactor. Ultrason. Sonochem. 2014, 21, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Shan, R.; Shi, J.; Yan, B. Ultrasonic-assisted production of biodiesel from transesterification of palm oil over ostrich eggshell-derived CaO catalysts. Bioresour. Technol. 2014, 171, 428–432. [Google Scholar] [CrossRef]
- Lee, S.B.; Lee, J.D.; Hong, I.K. Ultrasonic energy effect on vegetable oil based biodiesel synthetic process. J. Ind. Eng. Chem. 2011, 17, 138–143. [Google Scholar] [CrossRef]
- Takase, M.; Chen, Y.; Liu, H.; Zhao, T.; Yang, L.; Wu, X. Biodiesel production from non-edible Silybum marianum oil using heterogeneous solid base catalyst under ultrasonication. Ultrason. Sonochem. 2014, 21, 1752–1762. [Google Scholar] [CrossRef]
- Manh, D.-V.; Chen, Y.-H.; Chang, C.-C.; Chang, C.-Y.; Hanh, H.-D.; Chau, N.-H.; Tuyen, T.-V.; Long, P.-Q.; Minh, C.-V. Effects of blending composition of tung oil and ultrasonic irradiation intensity on the biodiesel production. Energy 2012, 48, 519–524. [Google Scholar] [CrossRef]
- Ji, J.; Wang, J.; Li, Y.; Yu, Y.; Xu, Z. Preparation of biodiesel with the help of ultrasonic and hydrodynamic cavitation. Ultrasonics 2006, 44, e411–e414. [Google Scholar] [CrossRef] [PubMed]
- Hanh, H.D.; Dong, N.T.; Okitsu, K.; Nishimura, R.; Maeda, Y. Biodiesel production through transesterification of triolein with various alcohols in an ultrasonic field. Renew. Energy 2009, 34, 766–768. [Google Scholar] [CrossRef]
- Yu, D.; Tian, L.; Wu, H.; Wang, S.; Wang, Y.; Ma, D.; Fang, X. Ultrasonic irradiation with vibration for biodiesel production from soybean oil by Novozym 435. Process Biochem. 2010, 45, 519–525. [Google Scholar] [CrossRef]
- Maneechakr, P.; Samerjit, J.; Uppakarnrod, S.; Karnjanakom, S. Experimental design and kinetic study of ultrasonic assisted transesterification of waste cooking oil over sulfonated carbon catalyst derived from cyclodextrin. J. Ind. Eng. Chem. 2015, 32, 128–136. [Google Scholar] [CrossRef]
- Fayyazi, E.; Ghobadian, B.; Najafi, G.; Hosseinzadeh, B.; Mamat, R.; Hosseinzadeh, J. An ultrasound-assisted system for the optimization of biodiesel production from chicken fat oil using a genetic algorithm and response surface methodology. Ultrason. Sonochem. 2015, 26, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Jain, S. 17 The production of biodiesel using Karanja (Pongamia pinnata) and Jatropha (Jatropha curcas) Oil. In Biomass, Biopolymer-Based Materials, and Bioenergy; Verma, D., Fortunati, E., Jain, S., Zhang, X., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 397–408. [Google Scholar]
- Metcalfe, L.; Schmitz, A.A.; Pelka, J. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal. Chem. 1966, 38, 514–515. [Google Scholar] [CrossRef]
- Hingu, S.M.; Gogate, P.R.; Rathod, V.K. Synthesis of biodiesel from waste cooking oil using sonochemical reactors. Ultrason. Sonochem. 2010, 17, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Mostafaei, M.; Ghobadian, B.; Barzegar, M.; Banakar, A. Optimization of ultrasonic assisted continuous production of biodiesel using response surface methodology. Ultrason. Sonochem. 2015, 27, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Mohamad, M.; Ngadi, N.; Wong, S.L.; Jusoh, M.; Yahya, N.Y. Prediction of biodiesel yield during transesterification process using response surface methodology. Fuel 2017, 190, 104–112. [Google Scholar] [CrossRef]
- Özgür, C.; Tosun, E. Prediction of density and kinematic viscosity of biodiesel by artificial neural networks. Energy Sour. Part A Recov. Util. Environ. Eff. 2017, 39, 985–991. [Google Scholar] [CrossRef]
- Gupta, A.R.; Yadav, S.V.; Rathod, V.K. Enhancement in biodiesel production using waste cooking oil and calcium diglyceroxide as a heterogeneous catalyst in presence of ultrasound. Fuel 2015, 158, 800–806. [Google Scholar] [CrossRef]
- Sarve, A.N.; Varma, M.N.; Sonawane, S.S. Ultrasound assisted two-stage biodiesel synthesis from non-edible Schleichera triguga oil using heterogeneous catalyst: Kinetics and thermodynamic analysis. Ultrason. Sonochem. 2016, 29, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Azcan, N.; Yilmaz, O. Microwave assisted transesterification of waste frying oil and concentrate methyl ester content of biodiesel by molecular distillation. Fuel 2013, 104, 614–619. [Google Scholar] [CrossRef]
- Thanh, L.T.; Okitsu, K.; Sadanaga, Y.; Takenaka, N.; Maeda, Y.; Bandow, H. Ultrasound-assisted production of biodiesel fuel from vegetable oils in a small scale circulation process. Bioresour. Technol. 2010, 101, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Bastante, J.; Pinzi, S.; Arzamendi, G.; Luque de Castro, M.D.; Priego-Capote, F.; Dorado, M.P. Influence of vegetable oil fatty acid composition on ultrasound-assisted synthesis of biodiesel. Fuel 2014, 125, 183–191. [Google Scholar] [CrossRef]
- Babajide, O.; Petrik, L.; Amigun, B.; Ameer, F. Low-cost feedstock conversion to biodiesel via ultrasound technology. Energies 2010, 3, 1691–1703. [Google Scholar] [CrossRef]
| Properties | Unit | Amount |
|---|---|---|
| Palmitic (C16:0) | wt.% | 4.30 |
| Palmitoleic (C16:1) | wt.% | 0.19 |
| Stearic (C18:0) | wt.% | 2.21 |
| Oleic (C18:1) | wt.% | 65.94 |
| Linoleic (C18:2) | wt.% | 17.32 |
| Linolenic (C18:3) | wt.% | 6.05 |
| Other fatty acids | wt.% | 3.99 |
| Mean molecular weight of used oil | gr/mol | 882.92 |
| Acid value | mg KOH/g oil | 0.57 |
| Independent Variables | Units | Symbols | Level of Each Factor | ||
|---|---|---|---|---|---|
| Coded Values | |||||
| −1 | 0 | 1 | |||
| Molar Ratio | - | X1 | 4 | 5 | 6 |
| Reaction Time | min | X2 | 3 | 5 | 7 |
| Pulse | s | X3 | 0.4 | 0.7 | 1 |
| Amplitude | % | X4 | 50 | 65 | 80 |
| Random | Run | Molar Ratio | Reaction Time | Pulse | Amplitude | Reposes (Yield) |
|---|---|---|---|---|---|---|
| 16 | 1 | 5:1 | 7 | 1 | 65 | 84.47 |
| 17 | 2 | 4:1 | 5 | 0.4 | 65 | 84.77 |
| 26 | 3 | 5:1 | 5 | 0.7 | 65 | 85.5 |
| 3 | 4 | 4:1 | 7 | 0.7 | 65 | 86.04 |
| 6 | 5 | 5:1 | 5 | 1 | 50 | 88.62 |
| 22 | 6 | 5:1 | 7 | 0.7 | 50 | 87 |
| 15 | 7 | 5:1 | 3 | 1 | 65 | 86.69 |
| 27 | 8 | 5:1 | 5 | 0.7 | 65 | 88.34 |
| 18 | 9 | 6:1 | 5 | 0.4 | 65 | 86.6 |
| 4 | 10 | 6:1 | 7 | 0.7 | 65 | 88.49 |
| 9 | 11 | 4:1 | 5 | 0.7 | 50 | 83.8 |
| 8 | 12 | 5:1 | 5 | 1 | 80 | 87.6 |
| 23 | 13 | 5:1 | 3 | 0.7 | 80 | 88.07 |
| 14 | 14 | 5:1 | 7 | 0.4 | 65 | 88.53 |
| 7 | 15 | 5:1 | 5 | 0.4 | 80 | 88.19 |
| 12 | 16 | 6:1 | 5 | 0.7 | 80 | 86.51 |
| 10 | 17 | 6:1 | 5 | 0.7 | 50 | 86.21 |
| 29 | 18 | 5:1 | 5 | 0.7 | 65 | 88.33 |
| 19 | 19 | 4:1 | 5 | 1 | 65 | 86.42 |
| 11 | 20 | 4:1 | 5 | 0.7 | 80 | 84.46 |
| 21 | 21 | 5:1 | 3 | 0.7 | 50 | 87.51 |
| 20 | 22 | 6:1 | 5 | 1 | 65 | 87.12 |
| 1 | 23 | 4:1 | 3 | 0.7 | 65 | 84.5 |
| 28 | 24 | 5:1 | 5 | 0.7 | 65 | 87.42 |
| 24 | 25 | 5:1 | 7 | 70 | 80 | 87.24 |
| 13 | 26 | 5:1 | 3 | 0.4 | 65 | 87.67 |
| 25 | 27 | 5:1 | 5 | 0.7 | 65 | 87.7 |
| 2 | 28 | 6:1 | 3 | 0.7 | 65 | 86.29 |
| 5 | 29 | 5:1 | 5 | 0.4 | 50 | 87.92 |
| Statistical Model | Std. Dev. | CV *% | R2 | Adjusted R2 | Predicted R2 | PRESS | Result of Model |
|---|---|---|---|---|---|---|---|
| Linear | 3.81 | 4.63 | 0.6836 | 0.6309 | 0.5501 | 495.97 | |
| 2FI ** | 3.73 | 4.53 | 0.7732 | 0.6472 | 0.4557 | 600.00 | |
| Quadratic | 2.67 | 3.24 | 0.9096 | 0.8692 | 0.5040 | 546.77 | Suggested |
| Cubic | 1.72 | 2.09 | 0.9839 | 0.9251 | −0.4745 | 1625.41 | Aliased |
| Source | Sum of Squares | df | Mean Square | F Value | p Value Prob > F |
|---|---|---|---|---|---|
| Model | 46.55496 | 14 | 3.325354 | 4.7904466 | 0.0030 |
| Molar ratio (A) | 11.90818 | 1 | 11.90818 | 17.154709 | 0.0010 |
| Reaction time (B) | 2.1168 | 1 | 2.1168 | 3.0494248 | 0.1027 |
| Pulse (C) | 0.128133 | 1 | 0.128133 | 0.1845866 | 0.6740 |
| Amplitude (D) | 0.250563 | 1 | 0.250563 | 0.3609566 | 0.5576 |
| A–B interaction effects | 0.1089 | 1 | 0.1089 | 0.1568794 | 0.6980 |
| A–C interaction effects | 0.319225 | 1 | 0.319225 | 0.4598699 | 0.5087 |
| A–D interaction effects | 0.293764 | 1 | 0.293764 | 0.4231913 | 0.5259 |
| B–C interaction effects | 0.2116 | 1 | 0.2116 | 0.3048272 | 0.5896 |
| B–D interaction effects | 0.0256 | 1 | 0.0256 | 0.0368789 | 0.8505 |
| C–D interaction effects | 0.416025 | 1 | 0.416025 | 0.5993183 | 0.4517 |
| A2 | 27.29715 | 1 | 27.29715 | 39.323791 | <0.0001 |
| B2 | 0.016001 | 1 | 0.016001 | 0.0230504 | 0.8815 |
| C2 | 0.293825 | 1 | 0.293825 | 0.4232792 | 0.5258 |
| D2 | 2.044467 | 1 | 2.044467 | 2.9452233 | 0.1082 |
| Residual | 9.718292 | 14 | 0.694164 | ||
| Lack of Fit | 8.834212 | 10 | 0.883421 | 3.9970191 | 0.0970 |
| Pure Error | 0.88408 | 4 | 0.22102 | ||
| Cor Total | 56.27325 | 28 |
| Properties | Test Method | Limits | Units | Measured Properties |
|---|---|---|---|---|
| Water and sediment | ASTM D2709 | Max 0.05 | % volume | <0.004 |
| Density at 15 °C | ASTM D4052 | 0.86–0.90 | kg/cm3 | 0.88 |
| Kinematic viscosity at 40 °C | ASTM D445 | 1.9–6.0 | mm2/s | 4.65 |
| Oxidation stability | EN 14112 | Min 3 | h | 3.2 |
| Flash point, closed cup | ASTM D93 | Min 130 | oC | 213 |
| Pour point | ASTM D97 | −15 to 10 | oC | −9 |
| Acid number | ASTM D664 | Max 0.50 | mg KOH/g | 0.29 |
| Feedstocks | Density at 15 °C (kg/cm3) | Kinematic Viscosity at 40 °C (mm2/s) | Pour Point (°C) | Flash Point (°C) | Acid Value (mg KOH/g) | Oxidation Stability (h) |
|---|---|---|---|---|---|---|
| Current study (TERI (OE) R-983) | 0.8822 | 4.6481 | −9 | 213 | 0.29 | 3.20 |
| Waste cooking oil [44] | 0.875 | 3.84 | −1 | 164 | 0.27 | - |
| Kusum oil [45] | 0.857 | 5.34 | −2 | 152 | 0.42 | 0.86 |
| Waste cooking oil [46] | 0.888 | 2.35 | −19 | 185 | - | |
| Canola oil [47] | 0.884 | 4.51 | 184 | - | ||
| Rapeseed oil [48] | 0.882 | 4.62 | 175.5 | 0.41 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almasi, S.; Ghobadian, B.; Najafi, G.H.; Yusaf, T.; Dehghani Soufi, M.; Hoseini, S.S. Optimization of an Ultrasonic-Assisted Biodiesel Production Process from One Genotype of Rapeseed (TERI (OE) R-983) as a Novel Feedstock Using Response Surface Methodology. Energies 2019, 12, 2656. https://doi.org/10.3390/en12142656
Almasi S, Ghobadian B, Najafi GH, Yusaf T, Dehghani Soufi M, Hoseini SS. Optimization of an Ultrasonic-Assisted Biodiesel Production Process from One Genotype of Rapeseed (TERI (OE) R-983) as a Novel Feedstock Using Response Surface Methodology. Energies. 2019; 12(14):2656. https://doi.org/10.3390/en12142656
Chicago/Turabian StyleAlmasi, Sara, Barat Ghobadian, Gholam Hassan Najafi, Talal Yusaf, Masoud Dehghani Soufi, and Seyed Salar Hoseini. 2019. "Optimization of an Ultrasonic-Assisted Biodiesel Production Process from One Genotype of Rapeseed (TERI (OE) R-983) as a Novel Feedstock Using Response Surface Methodology" Energies 12, no. 14: 2656. https://doi.org/10.3390/en12142656
APA StyleAlmasi, S., Ghobadian, B., Najafi, G. H., Yusaf, T., Dehghani Soufi, M., & Hoseini, S. S. (2019). Optimization of an Ultrasonic-Assisted Biodiesel Production Process from One Genotype of Rapeseed (TERI (OE) R-983) as a Novel Feedstock Using Response Surface Methodology. Energies, 12(14), 2656. https://doi.org/10.3390/en12142656






