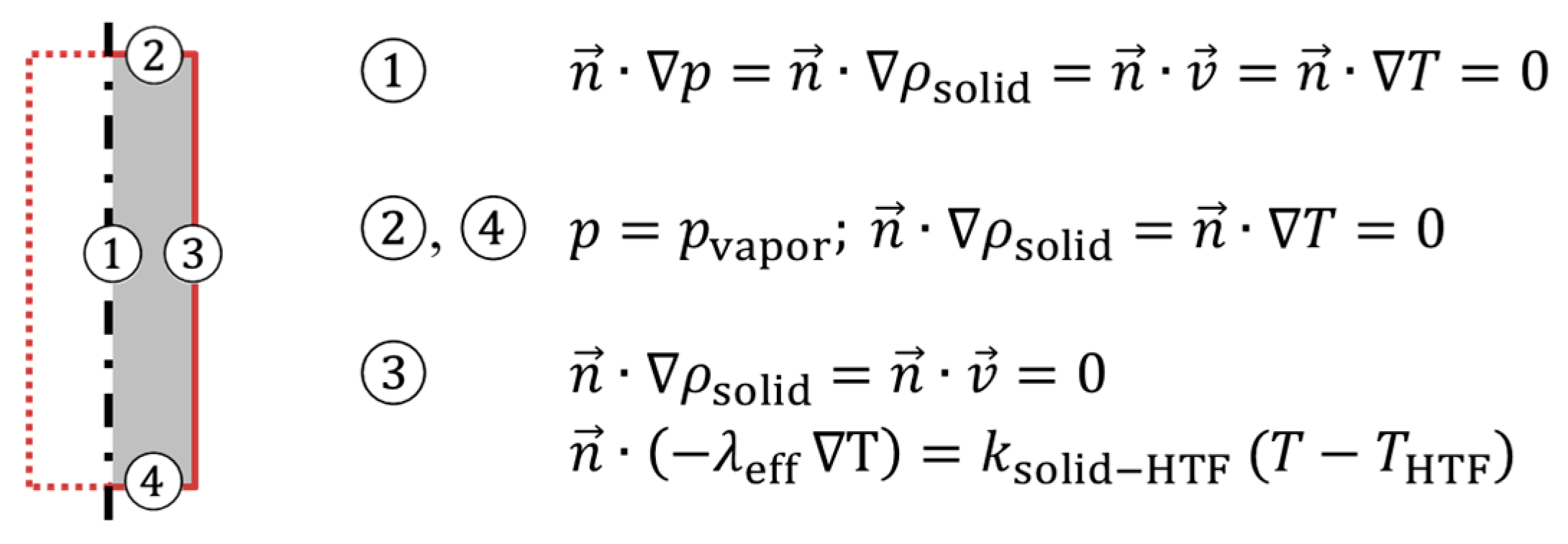1. Introduction
Thermochemical reactions, for example reversible reactions between a gas and a solid, have been widely discussed in literature in the context of thermal energy storage. Compared to latent or sensible energy storage technologies, thermochemical systems offer significantly higher energy storage densities [
1]. Another key feature of thermochemical energy storage is the possibility to control the charge and discharge temperatures by adjusting the concentration of the reactants: if the gas pressure is increased in a gas–solid reaction system, the reaction temperature rises. This effect can be used to transform thermal energy from a lower temperature level to a higher temperature level, the so-called heat transformation or chemical heat pump [
2,
3]. Different thermodynamic heat transformation and chemical heat pump processes have been discussed in the literature, and a number of studies have been published that clearly highlight the potential of heat transformers for the reduction of low-enthalpy waste heat in industrial processes [
4,
5]. Although there are several lab-scale setups proposed with different gas–solid working pairs, no technical implementation of a thermochemical heat transformer on an industrial scale has been reported [
6,
7,
8]. Regarding suitable gas–solid reactive couples, there is a broad spectrum of chemical reactions discussed, e.g., ammonia-, hydrogen- or steam-based working pairs, covering a wide range of operating temperatures [
9].
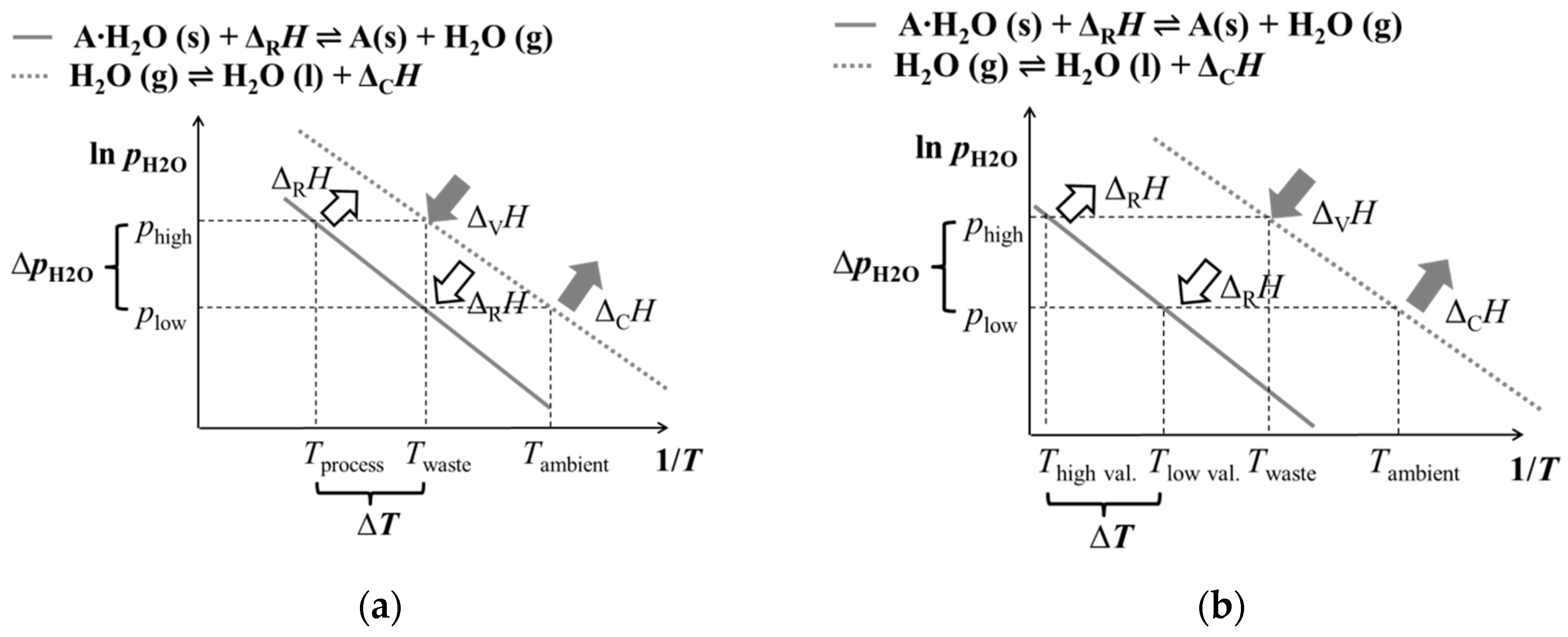
The basic concept of heat transformation generally requires three temperature levels and two pressure stages (see
Figure 1a, with water vapor being the gaseous reactant). For providing the reaction enthalpy (Δ
RH) of the endothermic reaction as well as for supplying the evaporation enthalpy of the gaseous component (Δ
VH), waste heat at a temperature of
Twaste is used. During the charging process, the emerging vapor is condensed at ambient temperature (
Tambient). In our work, we follow a different approach to achieve thermal upgrade at a higher absolute temperature level. As depicted in
Figure 1b, the heat transformation process described in this work requires four temperature stages: the two upper ones are related to the later application process, and the two lower ones are used for thermal compression of the gaseous reactant. The lowest temperature level (condensation at ambient temperature
Tambient) is needed for vapor removal from the reaction chamber in order for the endothermic reaction to reach complete turnover at a low pressure (
plow). Hence, the chemical reaction takes place at a low reaction temperature (
Tlow val.), and the thermochemical storage is charged by low-value process heat. During the exothermic discharging process, low-temperature waste heat is used for providing steam at a higher pressure (
phigh) for running the chemical reaction. The available waste heat temperature defines the vapor pressure and, thus, determines the maximum thermal upgrade of the released high-value process heat.
With this approach, we are able to re-use low-temperature waste heat as a driving source for high-temperature heat pump processes. Depending on the chosen gas–solid working pair, the operating temperatures of the heat pump process can be adapted to a specific application.
In a first study, we identified the hydration and dehydration reaction of strontium bromide (SrBr
2) as a potential candidate for thermochemical heat transformation in the temperature range above 150 °C [
10]. The non-toxic inorganic salt forms anhydrous, monohydrate, and hexahydrate phases and, prior to our study, it has been discussed in literature exclusively in the context of low-temperature energy storage for seasonal storage applications [
11,
12]. For achieving low temperatures required in seasonal storage applications, the chemical reaction is limited to the phase change from the monohydrate phase to the hexahydrate phase, which occurs at temperatures of 50 °C to 80 °C. The reaction temperatures can be increased significantly by limiting the phase change from the anhydrous to the monohydrous phase, which is the focus of our work:
This reversible gas–solid reaction takes place at temperatures of 150 °C or higher, which is very interesting for industrial heat transformation applications as it exceeds the working range of conventional heat pumps. Although the reaction is chemically reversible and cycle stability was experimentally demonstrated for 10 dehydration/re-hydration reaction cycles in thermogravimetric analysis (TGA) experiments, a thermal hysteresis of 22 K was reported in TGA measurements conducted at a water vapor partial pressure of 5 kPa [
10].
The van’t Hoff equation provides a first assumption of the correlation between the vapor pressure and reaction temperature based on the standard molar enthalpy and entropy of reaction:
with
R being the universal gas constant, and the reference pressure
p+ = 10³ hPa. From standard molar enthalpy and entropy of formation given in the NBS Tables [
13], the reaction enthalpy and entropy is calculated as Δ
RH = 71.98 kJ/mol and Δ
RS = 143.93 J/(mol∙K), respectively.
For confirming the
p(
T)-correlation, we developed a method for obtaining data from experiments on a 1 kg scale [
14]. In the experimental study, we observed that the exothermic reaction from the anhydrous phase to the monohydrate takes place at around 229 °C when water vapor is supplied at a pressure of 70 kPa. This value is higher than the reaction temperature expected from the van’t Hoff Equation (2), which returns a temperature of 217 °C for the same vapor pressure. During the dehydration process at a vapor pressure of 6.5 kPa, a minimum temperature of 190 °C was found. Calculated from Equation (2), this vapor pressure corresponds to a reaction temperature of 159 °C. Evidently, the van’t Hoff Equation (2) does not give an exact estimation of the correlation between vapor pressure and reaction temperature. However, this correlation is required for further investigations of the heat transformation operation conditions with the SrBr
2/H
2O working pair, and therefore needs to be experimentally determined.
When opening the reaction chamber after having conducted 11 dehydration/re-hydration cycles with different reaction conditions, we detected agglomeration effects in the bulk. Even though this was observed consistently during the series of experiments, the changes in the bulk structure did not affect the reaction dynamics of the thermochemical reactor. Based on the results of these investigations, we see very high potential in the SrBr2/H2O working pair for achieving a thermal upgrade of 50 K or more in a single-stage heat transformation process with only one working pair. Therefore, our current work is focused on the investigation of experimental operation conditions and reaction chamber designs that allow for heat transformation in a temperature range of 180 °C to 250 °C at high thermal powers.
2. Materials and Methods
To charge the storage system, thermal energy is transferred indirectly to the solid bulk material via a heat transfer fluid. In the course of the endothermic chemical reaction, the gaseous reactant escapes from the powder bulk and must be separated from the solid phase: the emerging steam is condensed in a separate heat exchanger in order to keep the pressure low and thus to reach complete reaction turnover. In this work, we focus on the thermal discharge process, which starts when water vapor is introduced into the reaction chamber. The thermal energy released by the subsequent chemical reaction is transferred from the powder bulk to the heat transfer fluid. For vapor generation, we use a tube bundle heat exchanger. In order to calculate the reaction conversion, it is equipped with a level indicator measuring the amount of water being consumed during the chemical reaction.
In general, there are three main processes that affect the thermal performance of a thermochemical reactor: vapor mass transfer into the solid bulk phase, heat transfer from the solid bulk phase to the heat transfer fluid, and the rate of the chemical reaction at the given operation conditions. To qualify how the physical and chemical properties of the reactive material affect the thermal performance of a thermochemical reactor and to quantify these local effects in order to identify potential bottlenecks, we included the relevant physical processes during the chemical reaction in a model based on the finite element method (FEM). For numerical calculations, we used solvers from the Comsol Multiphysics® simulation software (Stockholm, Sweden). The validity of the simulation studies is verified by comparison with experimental data from a packed bed thermochemical reactor.
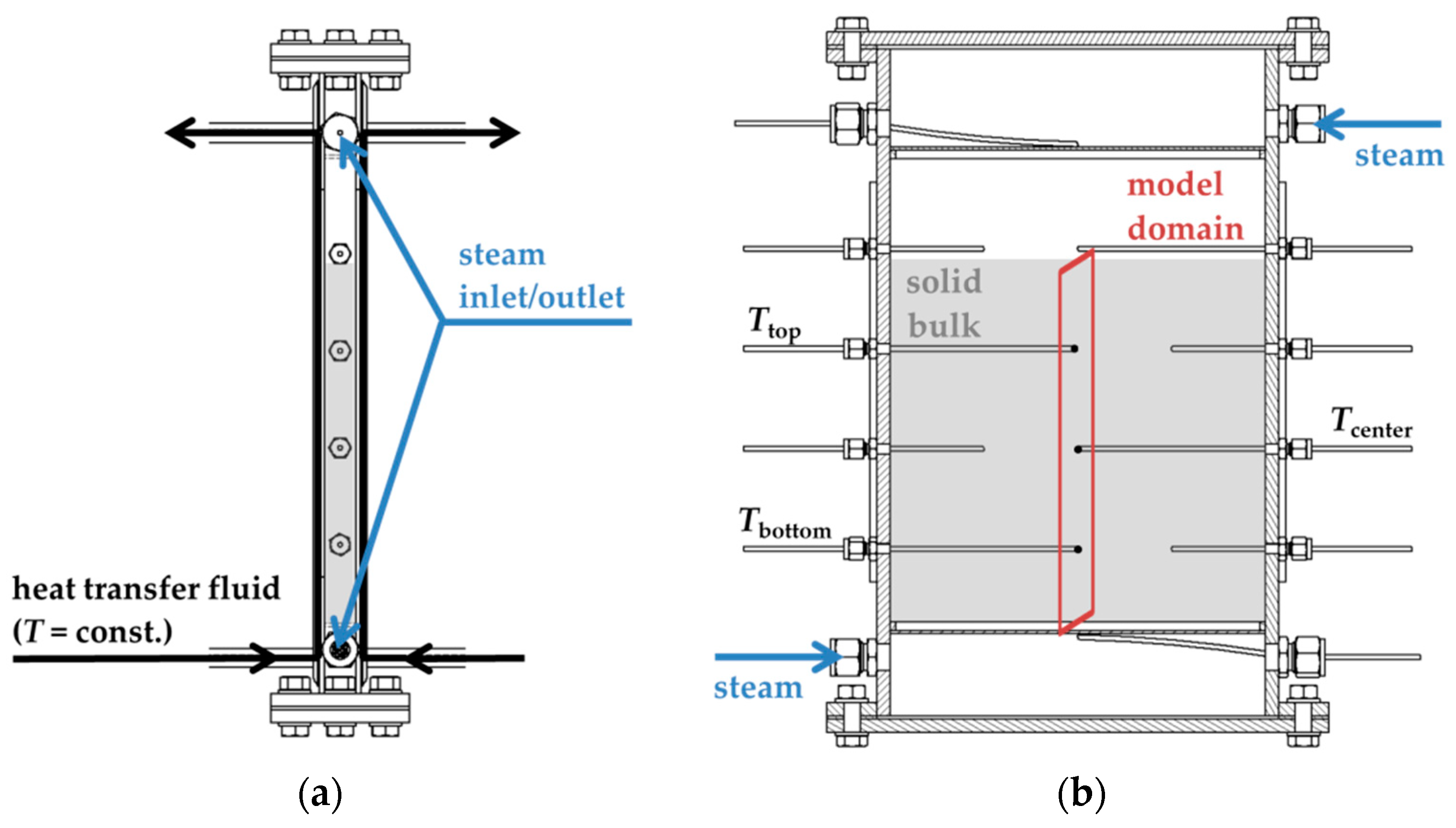
For the validation reactor, a rectangular reaction chamber configuration with indirect heat transfer was chosen. The heat exchanger consisted of two single-embossed pillow plates that were mounted back to back and were equally flowed through by heat transfer fluid (HTF, Purity™ FG Heat Transfer Fluid (Petro-Canada Lubricants Inc., Mississauga, Ontario, Kanada)) at a constant rate of 2 kg/min. The heat exchanger plates formed a 290 mm × 225mm × 20 mm volume filled with 1,050 g of SrBr
2∙H
2O (resulting packed bed height: 205 mm). From the top and the bottom of the reaction chamber, water vapor was introduced into the reactive bulk. Metal filters with a mesh size of 5 μm kept the packed bed in position and minimized undesired release of powder into the pipework of the test setup. The thermochemical reactor was equipped with a pressure sensor and several temperature probes that monitored the solid bulk’s temperature at different positions within the fixed bed. During the experiment, the temperature of the heat transfer fluid was kept at a constant value. A schematic drawing of the setup is depicted in
Figure 2.
As 2D-model domain, we chose a cross-section through the center of the solid bulk volume. For model implementation, the solid phase was assumed to be a homogenous porous medium. Material properties were assessed by the means of literature data or own measurements and calculations. A summary is given in
Table 1.
Movement of the solid is neglected, and the porosity is set to a constant value during the reaction. Furthermore, a change in bulk volume or bulk permeability is not yet considered. The gas phase, which consists of pure water vapor with ideal gas properties, penetrates the porous media according to Darcy’s law. Local thermal equilibrium is assumed between the gas and the solid phase. As the temperature differences within the observed volume are very small, heat radiation does not play a significant role and is therefore neglected.
Boundary conditions concerning heat and mass transfer in the relevant model domain are depicted in
Figure 3. It is assumed that the overall heat transfer coefficient
ksolid-HTF from the powder bed to the heat transfer fluid is determined by the heat transfer coefficient on the HTF side of the pillow plate heat exchanger. This value is calculated from the correlations on forced convection in planar gaps given in the VDI Heat Atlas [
17] for laminar fluid flow. Assuming the pillow plates as planar gap with 1.5 mm gap width and a HTF flow rate of 2 kg/min result in
ksolid-HTF = 365 W/(m²∙K).
The exothermic hydration reaction is implemented as a heat source with a first order rate law,
with
X being the reaction conversion and
p the water vapor (partial) pressure. The effective rate coefficient
keff and the pressure term exponent
n given in
Table 1 were fitted to isothermal TGA experiments (temperature range 186 °C to 212 °C) at a partial vapor pressure of 68.8 kPa.
In these measurements, it is observed that the effective reaction rate decelerates with increasing temperature, as the equilibrium pressure increases with increasing temperature, thus shifting the system closer to the thermodynamic equilibrium. This rate-diminishing effect is modelled by the last term in Equation (3), and generally prevents any thermal runaway. The temperature-dependent water vapor pressure pR,Hyd(T) of the hydration reaction can be obtained from thermodynamic data on the reaction enthalpy and reaction entropy (van’t Hoff line), as described by Equation (2), or from experimental correlations. Extending the reaction rate model to larger temperature and pressure ranges will be part of our future work.
3. Results
We conducted several sets of experiments. Within the series, the vapor pressure and, accordingly, the temperature of the heat transfer fluid varied: as strontium bromide forms a hexahydrate phase (SrBr
2∙6H
2O) at high vapor pressures or low temperatures, respectively, the preheat temperature of the anhydrous solid must be raised for high vapor pressures in order to ensure the exclusive formation of SrBr
2∙H
2O. Up to 15 dehydration/re-hydration cycles were performed in a row with one batch of reactive material. Afterwards, the reactor was refilled with a fresh batch of SrBr
2∙H
2O. The vapor pressure was varied from 18 kPa up to 145 kPa, and the discharge temperature was set in a range of 132 °C to 210 °C. The maximum temperature measured at the central position in the packed bed during the hydration reaction is plotted against the set vapor pressure in
Figure 4. All experiments considered, the highest maximum temperature was 256 °C at 144 kP vapor pressure.
The data points shown in
Figure 4 reveal that the measured temperature values clearly exceed the temperatures derived from thermodynamic data, especially for high pressures. For this reason, a linear regression of the experimental data points was calculated to model the temperature dependency of the water vapor pressure
pR,Hyd of the hydration reaction in the FEM simulation:
This correlation was also used in the reaction rate model described in Equation (3).
The simulation study results are compared with the results of the prior described experiments, with the measured pressure curve being defined as input variable for the simulation. In
Figure 5a, the temperature progression at different positions within the solid bulk is shown for a discharge temperature of 209.5 °C and a vapor pressure of 70 kPa (corresponding to 90 °C evaporation temperature). The graph includes the results of two simulation studies with varying bulk thermal conductivity λ
eff. The increase in temperature indicates that the exothermic reaction is in progress. As the reaction approaches complete conversion to the monohydrate, the temperature in the solid bulk begins to decrease. Evidently, temperature progression in the experimental setup is not homogenous within the bulk phase: at the lowest temperature measuring point (
Tbottom), a lower maximum temperature is observed. We assume that this effect is caused by macroscopic inhomogeneities within the porous medium, such as cracks, which may affect the temperature measurement. In the simulation study, the temperatures trends at the three positions proceeded identically, which is why only the temperature at the central position of the reactor (
Tsim) is depicted in
Figure 5a. The jumps occurring the temperature curves (e.g., at 1.3 h experimental time) are caused by a sudden increase in pressure due to condensation/evaporation in the setup. The pressure increase leads to a higher reaction temperature in the solid bulk. As the measured pressure curve was used as input parameter for the simulation study, the same effect occurs in the numerical study. In the case of the lower bulk thermal conductivity (λ
eff = 0.2 W/(m∙K)), maximum temperatures in the simulation were about 7 K lower than in the experiment. In addition, the temperature in the simulation study dropped to its initial value after 3.5 h, whereas in the experiment it was not possible to measure a complete temperature drop even after four hours. In the study with increased bulk thermal conductivity (λ
eff = 0.6 W/(m∙K)), temperatures dropped to the initial value after two hours.
In
Figure 5b, the reaction conversion is depicted for both the simulation as well as the experiment, where the turnover is calculated from the fluid level measurement. In the initial study with λ
eff = 0.2 W/(m∙K), the experimental yield is higher than the simulation result up to a turnover of roughly 85%. Afterwards, the progress of the experimental turnover decelerates for high reaction yields, and full turnover is achieved about 20 min later in the experiment than in the corresponding simulation study. Increasing the thermal conductivity of the bulk phase to λ
eff = 0.6 W/(m∙K) leads to significantly shorter discharging times.
4. Discussion
Although the model does not reproduce the experiments quantitatively due to the uncertainty of the input parameters (in particular, the parameters of the reaction rate model and macroscopic inhomogeneities), it allows for the qualitative observation and identification of the factors having the greatest influence on the progression of the hydration reaction and thus on the discharge of the storage module.
From the observation that the simulated temperature curves perfectly overlap for the three different positions within the fixed bed, the conclusion can be drawn that mass transport within the bulk material does not lead to a limitation for the given hydration reaction conditions. This is an important finding for the design of suitable reactor geometries. Still, this effect should be further analyzed regarding the lower absolute pressures and gas densities during the charging process of the thermochemical reactor.
The influence of low bulk thermal conductivities on the reaction turnover curve and, hence, the reactor’s overall performance was numerically studied. Increasing the effective thermal conductivity from 0.2 to 0.6 W/(m∙K) (f.e. by adding highly conductive additives such as aluminum) reduces the total discharging time by 40% in the simulation study (3.5 h versus 2.1 h for 99% reaction conversion). This is equivalent to a 1.7 times higher average thermal power. According to the experimental results of the generic reactor geometry presented in this work, we recommend a maximum heat transport distance of 10 mm for the design of high-power thermochemical reactors based on SrBr2/H2O. Furthermore, the effective thermal conductivity of the bulk could be increased by adding highly conductive inert additives or by the integration of heat conducting structures.
The FEM simulation developed in this work can be used as design tool for the layout of high-power reactor geometries. We assume that the following modifications of the first model can improve the prediction quality of the simulation: firstly, the mathematical model of the reaction rate over-estimates the rate at higher yields. Therefore, the development of an advanced reaction rate model is a subject of our ongoing work. Secondly, the solid bulk is not a homogenous porous medium; therefore, slower reaction progress can occur locally, which is not considered in the model. Moreover, in further experimental work, we found that the macroscopic and the microscopic properties of the solid bulk material change considerably due to the chemical reaction: the primary particles agglomerated, which was already observed in the experiments described above, and the overall volume of the porous bulk increased after several dehydration/re-hydration cycles [
18]. The observed structural changes could also affect the bulk thermal conductivity and thus have an effect on the long-term thermal performance of the storage reactor.
From the thermodynamic point of view, a discharging temperature of 250 °C is feasible with the reaction system SrBr2/H2O. To achieve this high reaction temperature, steam must be supplied with a pressure of approx. 140 kPa or higher. This requires waste heat temperatures of at least 110 °C for vapor generation. At the same time, the discharging temperature must not be too low: as strontium bromide forms a hexahydrate phase (SrBr2∙6H2O) at high vapor pressures or low temperatures, respectively, the minimum discharge temperature must be raised for high vapor pressures in order to ensure the exclusive formation of SrBr2∙H2O.
Taking the reaction kinetics into account, a minimal temperature difference of 10 K or more between the reaction temperature and the temperature of the heat transfer fluid could be required in case the chemical reaction decelerates considerably close to the thermodynamic equilibrium. This would significantly decrease the thermal power of the storage reactor, or limit the maximum possible discharge temperature. Quantifying the required temperature difference for high reaction rates is part of our ongoing work.
5. Conclusions
In a previous study based on thermogravimetric experiments performed on a mg scale, we identified strontium bromide (SrBr
2) as a promising candidate for thermochemical heat transformation [
10]. The work presented here is focused on the investigation of the exothermic hydration reaction from the anhydrous SrBr
2 to its monohydrate phase on a 1 kg scale. Our objective is the identification of suitable operation conditions that allow for heat transformation with output temperatures in the range of 180 °C to 250 °C. Our key findings are as follows:
The working pair SrBr2/H2O allows discharging temperatures up to 250 °C when water vapor is supplied at 110 °C (f.e. driven by low-temperature waste heat);
Vapor mass transfer in the porous bulk phase does not limit the thermal discharging performance in the analyzed reactor setup;
The low effective thermal conductivity of the fixed reactive bed is a potential bottleneck for high-power thermochemical storage and heat transformation modules.
Based on the experimental results and the conclusions of our simulation studies presented here, we are currently developing a reactor setup that allows for high specific thermal powers, and which is easily scalable for industrial applications. To increase the effective thermal conductivity of the bulk phase, we are using heat conducting structures made from aluminum. Further design considerations include a minimum pressure loss on the vapor side and a reaction chamber design that is robust with respect to changes of the bulk’s macroscopic structure. With this design, we expect to achieve specific thermal powers of minimum 0.25 kW per kg of SrBr2. Future work will include investigations with our new test facility, allowing for heat transformation with thermal powers up to 5 kW and output temperatures of up to 320 °C. In particular, quantifying the required temperature difference between the solid’s reaction temperature and the discharge temperature, ensuring high reaction rates and, thus, high thermal powers, will be part of our future work.