Fundamental Investigation of the Effects of Modified Starch, Carboxymethylcellulose Sodium, and Xanthan Gum on Hydrate Formation under Different Driving Forces
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials and Instruments
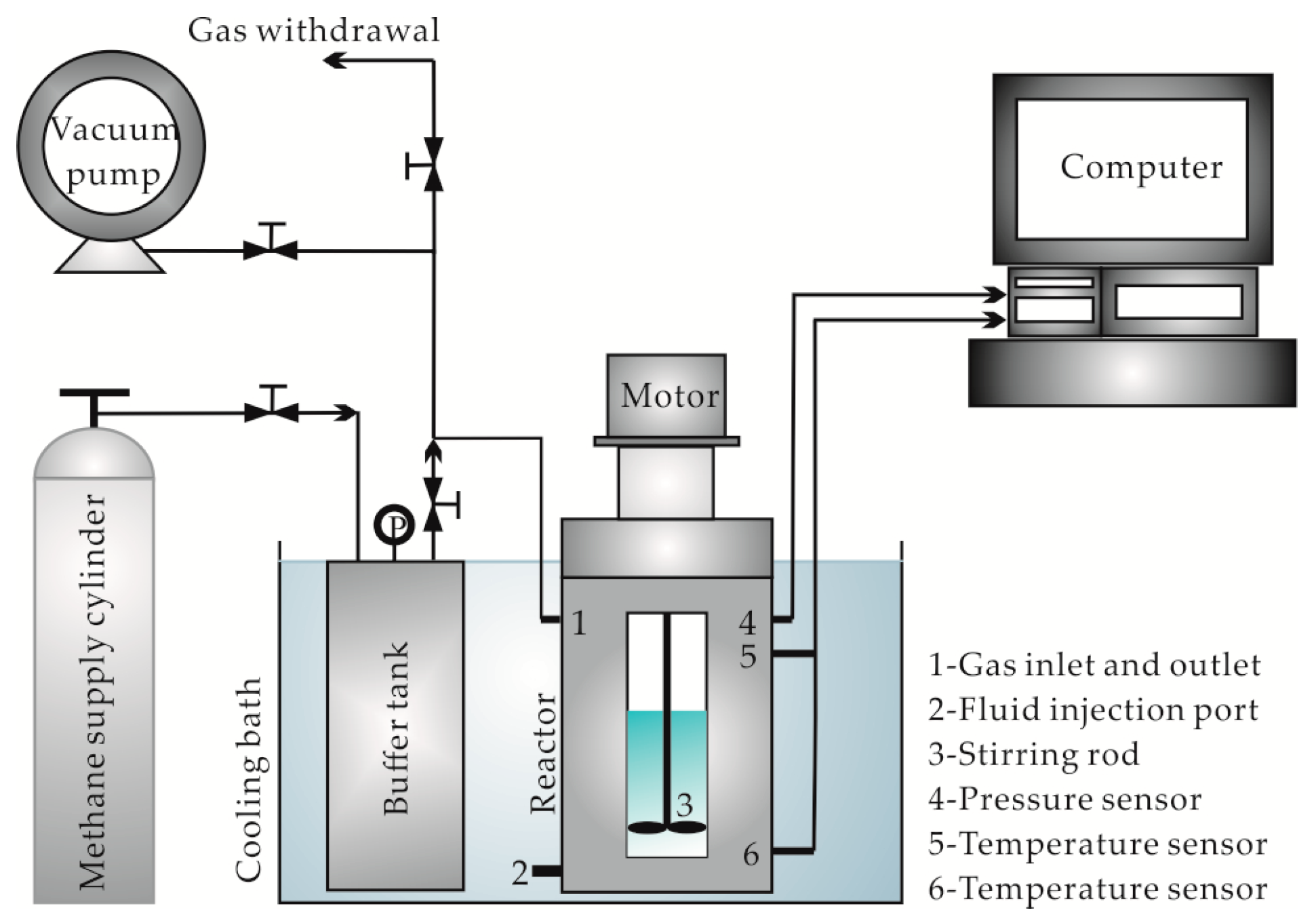
2.2. Experimental Methods and Processes
2.2.1. Simulated Experiment for Hydrate Formation
- (1)
- The reactor was cleaned and the air tightness checked.
- (2)
- Next, 250 mL of the sample was injected into the 650 mL reactor and then the reactor was continuously vacuumized for 30 min.
- (3)
- CH4 gas was continuously injected into the buffer tank until the pressure reached 20.0 MPa, and then the temperature control system was turned on.
- (4)
- When the temperature of the experimental system decreased and stabilized at 5.0 °C, the inlet of the reactor was switched on to let the CH4 gas in the buffer tank enter the reactor, which allowed the pressure to reach the designed value (5.0 MPa or 12.0 MPa).
- (5)
- The monitoring software was turned on to collect the temperature/pressure data in the reactor (at a collection frequency of one sample per 2 s) and the mechanical stirring apparatus was turned on with a stirring rate of 600 rev/min.
2.2.2. Mesostructure Observation Experiment
- (1)
- A small amount of solution was dripped onto a new cleavage mica sheet and immerse the specimen in liquid nitrogen for 2 min.
- (2)
- The frozen sample was placed into a freeze-drier and lyophilized for 12 h.
- (3)
- The specimen was observed with SEM.
2.3. Determination of Experimental Parameters
2.3.1. Data Processing of the Simulated Experiments
2.3.2. Mesostructural Observation of Freeze-Dried Solution Samples
3. Results
3.1. Simulated Experiments
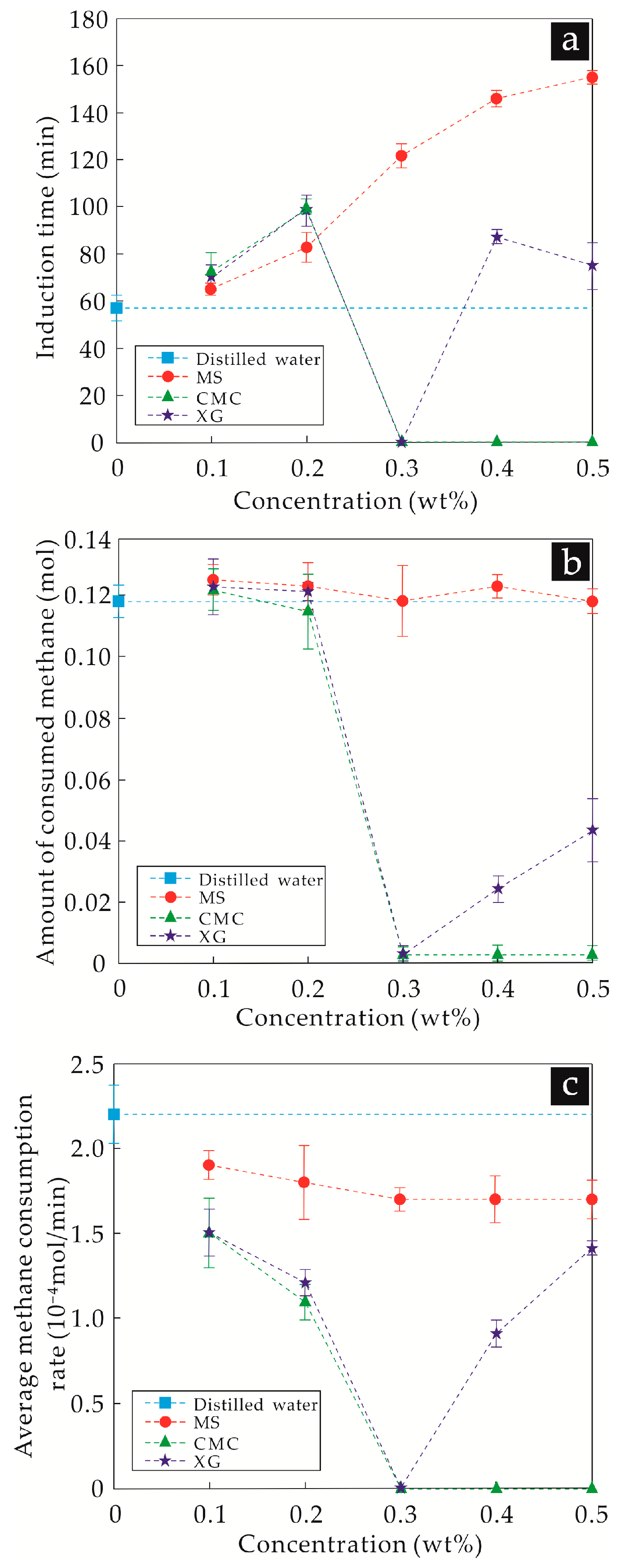
3.1.1. Initial Temperature–Pressure Conditions of Experiments at 5.0 °C and 5.0 MPa
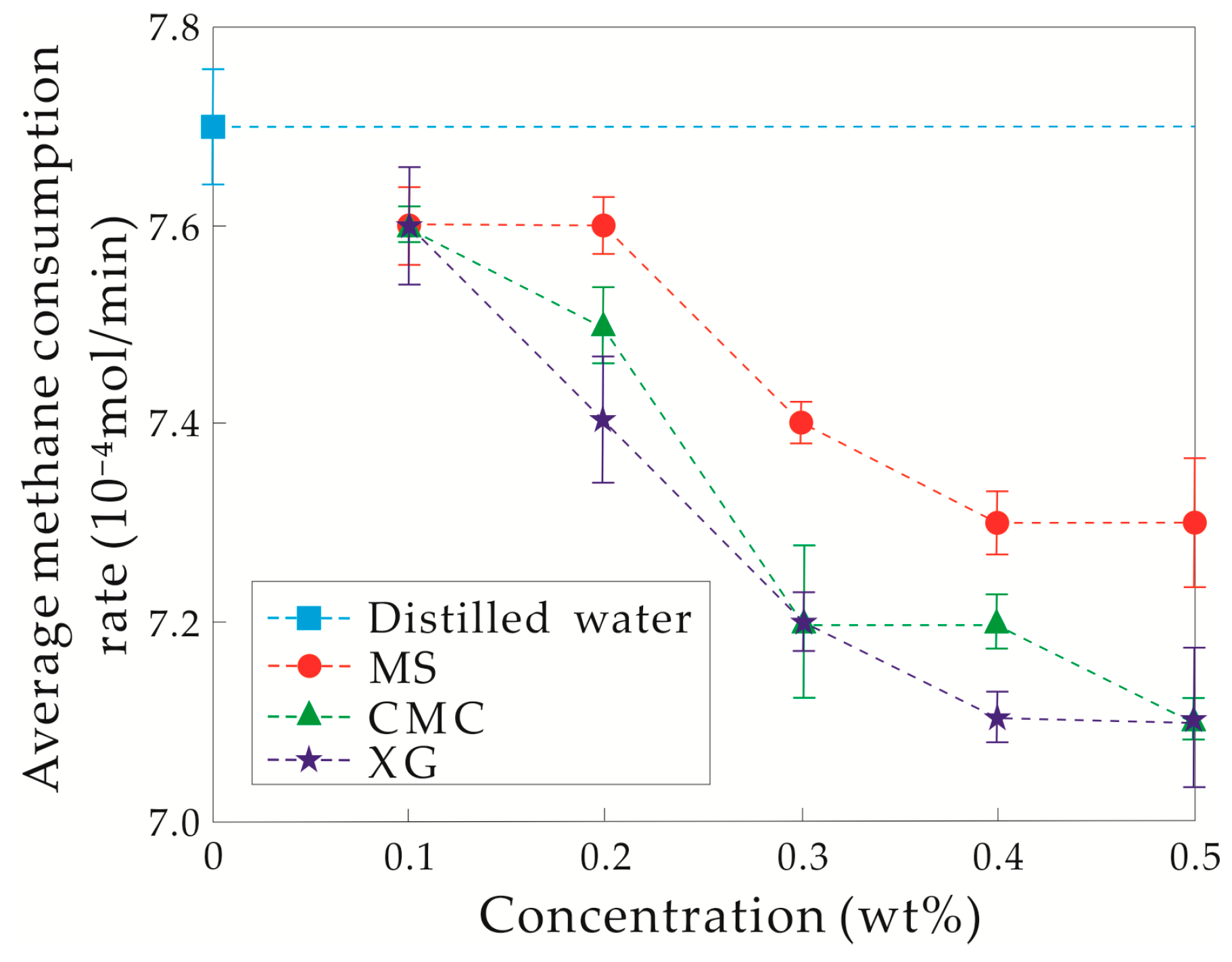
3.1.2. Initial Temperature–Pressure Conditions of Experiments at 5.0 °C and 12.0 MPa
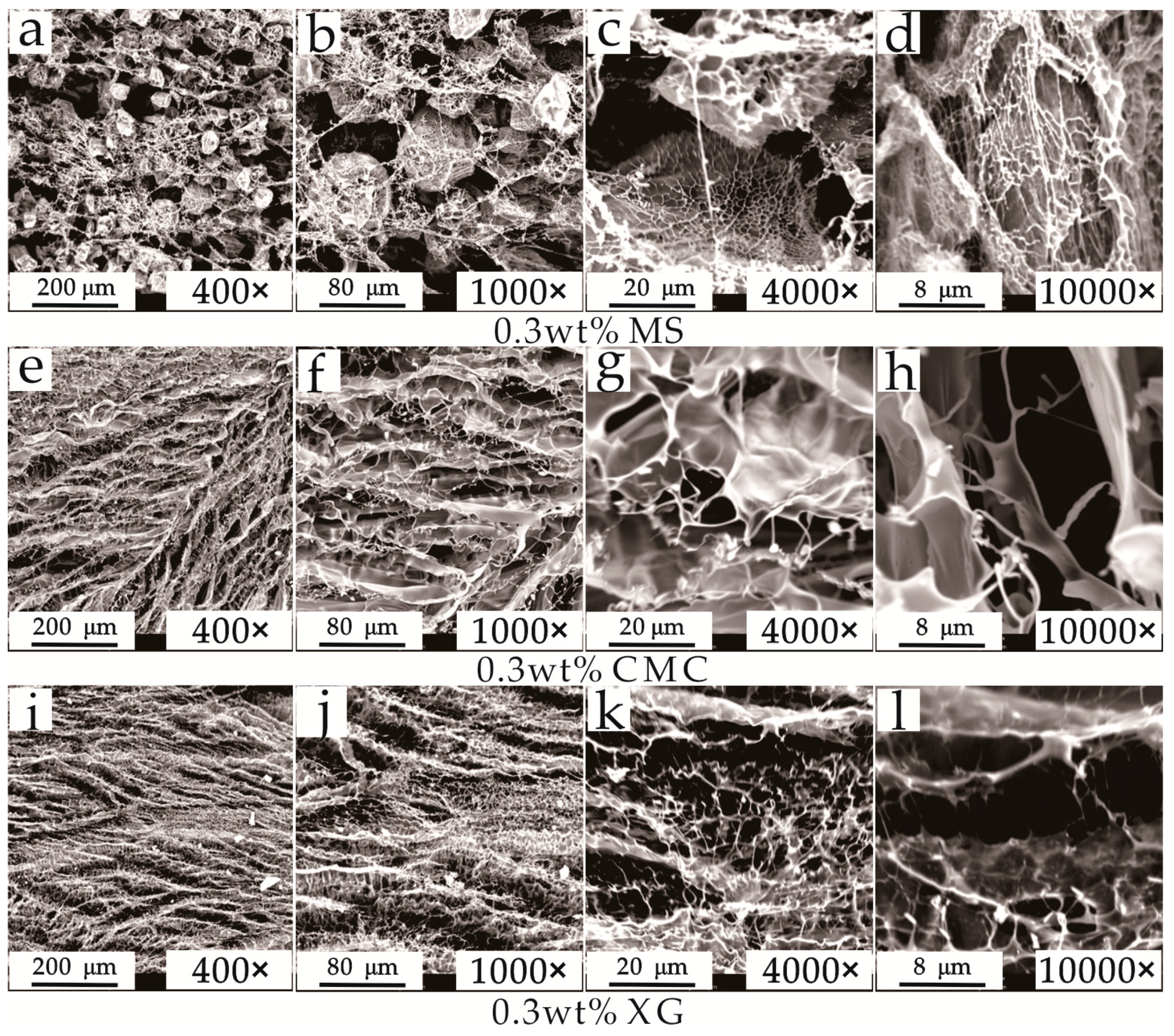
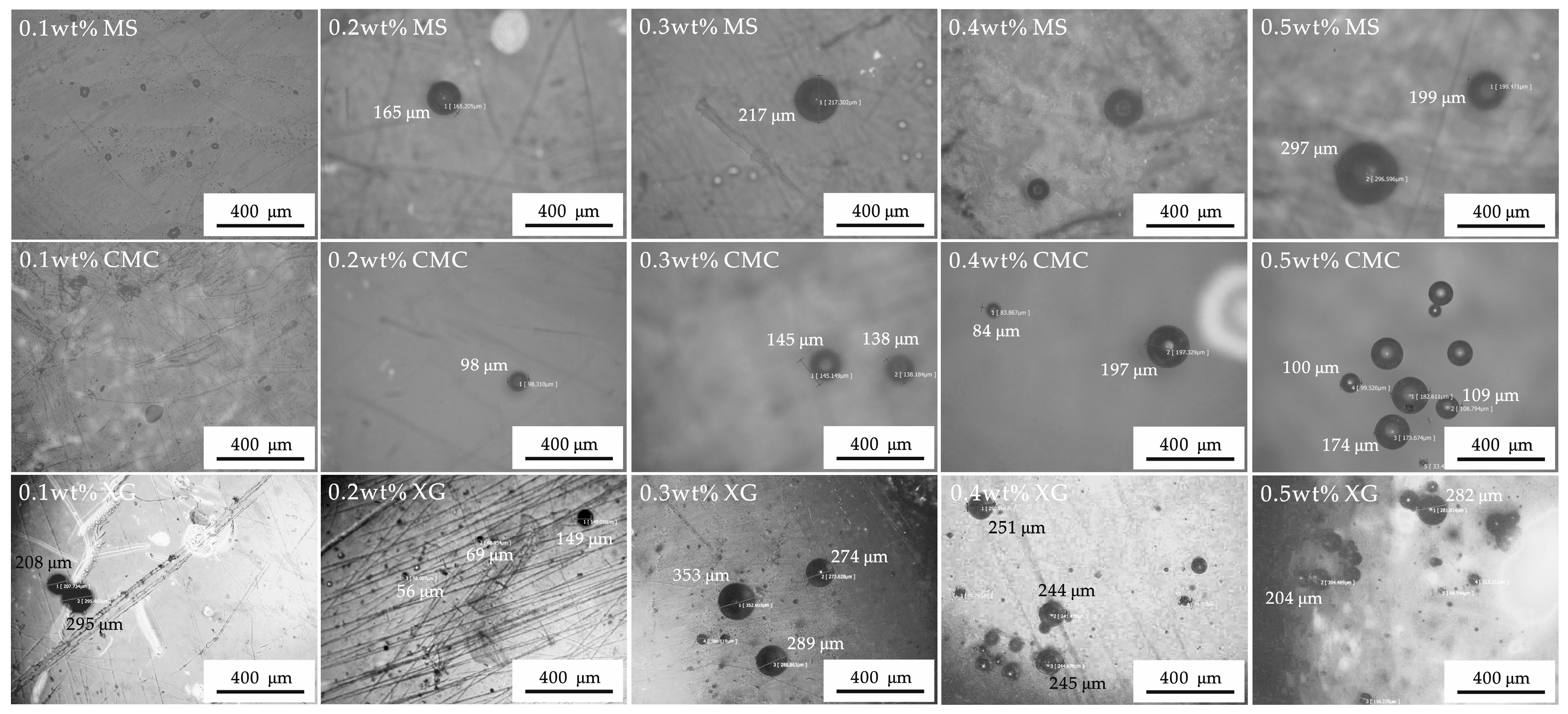
3.2. Mesostructure Experiments
4. Discussion
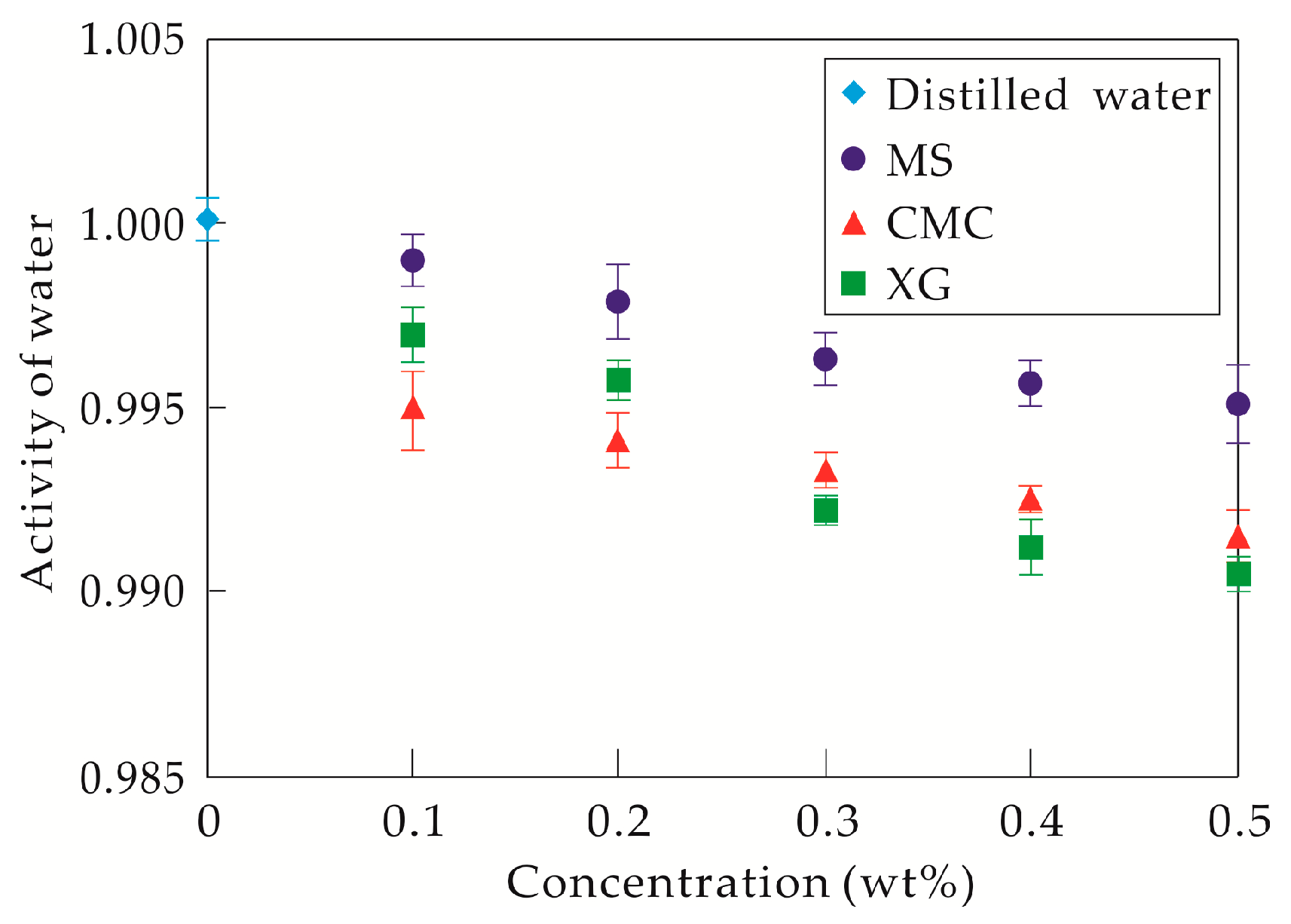
4.1. Hydrate Inhibition and Inhibitory Mechanism Analysis of the Three Thickeners
4.2. Applicability Analysis of the Three Thickeners in Different Drilling Fluid Systems
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sloan, E.D. Fundamental principles and applications of natural gas hydrates. Nature 2003, 426, 353–363. [Google Scholar] [CrossRef]
- Walsh, M.R.; Koh, C.A.; Sloan, E.D.; Sum, A.K.; Wu, D.T. Microsecond simulations of spontaneous methane hydrate nucleation and growth. Science 2009, 326, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, L.C.; Hujo, W.; Molinero, V. Amorphous precursors in the nucleation of clathrate hydrates. J. Am. Chem. Soc. 2010, 132, 11806–11811. [Google Scholar] [CrossRef]
- Hammerschmidt, E.G. Formation of gas hydrates in natural gas transmission lines. Ind. Eng. Chem. 1934, 26, 851–855. [Google Scholar] [CrossRef]
- Makogon, Y.F. Natural gas hydrates—A promising source of energy. J. Nat. Gas Sci. Eng. 2010, 2, 49–59. [Google Scholar] [CrossRef]
- Kvenvolden, K.A. Gas hydrates—Geological perspective and global change. Rev. Geophys. 1993, 31, 173–187. [Google Scholar] [CrossRef]
- Feng, J.C.; Wang, Y.; Li, X.S. Dissociation characteristics of water-saturated methane hydrate induced by huff and puff method. Appl. Energy 2018, 211, 1171–1178. [Google Scholar] [CrossRef]
- Collett, T.S. Energy resource potential of natural gas hydrates. AAPG Bull. 2002, 86, 1971–1992. [Google Scholar]
- Sloan, E.D. Introductory overview: Hydrate knowledge development. Am. Mineral. 2004, 89, 1155–1161. [Google Scholar] [CrossRef]
- Liu, C.L.; Meng, Q.G.; He, X.L.; Li, C.F.; Ye, Y.G.; Lu, Z.Q.; Zhu, Y.H.; Li, Y.H.; Liang, J.Q. Comparison of the characteristics for natural gas hydrate recovered from marine and terrestrial areas in China. J. Geochem. Explor. 2015, 152, 67–74. [Google Scholar] [CrossRef]
- Mcconnell, D.R.; Zhang, Z.; Boswell, R. Review of progress in evaluating gas hydrate drilling hazards. Mar. Petrol. Geol. 2012, 34, 209–223. [Google Scholar] [CrossRef]
- Khabibullin, T.; Falcone, G.; Teodoriu, C. Drilling through gas-hydrate sediments, managing wellbore-stability risks. SPE Drill. Complet. 2011, 26, 287–294. [Google Scholar] [CrossRef]
- Ning, F.L.; Zhang, K.N.; Wu, N.Y.; Zhang, L.; Li, G.; Jiang, G.S.; Yu, Y.B.; Liu, L.; Qin, Y.H. Invasion of drilling mud into gas-hydrate-bearing sediments. Part I: Effect of drilling mud properties. Geophys. J. Int. 2013, 193, 1370–1384. [Google Scholar] [CrossRef]
- Ning, F.L.; Wu, N.Y.; Yu, Y.B.; Zhang, K.N.; Jiang, G.S.; Zhang, L.; Sun, J.X.; Zheng, M.M. Invasion of drilling mud into gas-hydrate-bearing sediments. Part II: Effects of geophysical properties of sediments. Geophys. J. Int. 2013, 193, 1385–1398. [Google Scholar] [CrossRef]
- Hege, E.; Majeed, Y.; Eirik, S. Hydrate control during deepwater drilling: Overview and new drilling-fluids formulations. SPE Drill. Complet. 2001, 16, 16–19. [Google Scholar]
- Zhang, L.; Zhang, C.; Hhuang, H.D.; Qi, D.M.; Zhang, Y.; Ren, S.R.; Wu, Z.M.; Fang, M.Z. Gas hydrate risks and prevention for deep water drilling and completion: A case study of well QDN-X in Qiongdongnan Basin, South China Sea. Petrol. Explor. Dev. 2014, 41, 755–762. [Google Scholar] [CrossRef]
- Jiang, G.S.; Liu, T.L.; Ning, F.L.; Tu, Y.Z.; Zhang, L.; Yu, Y.B.; Kuang, L.X. Polyethylene glycol drilling fluid for drilling in marine gas hydrates-bearing sediments: An experimental study. Energies 2011, 4, 140–150. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, H.C.; Han, B.; Peng, L.; Ning, F.L.; Jiang, G.S.; Chehotkin, V.F. Effect of shearing actions on the rheological properties and mesostructures of CMC, PVP and CMC+PVP aqueous solutions as simple water-based drilling fluids for gas hydrate drilling. J. Unconv. Oil Gas Resour. 2016, 14, 86–98. [Google Scholar] [CrossRef]
- Sun, X.; Mohanty, K.K. Kinetic simulation of methane hydrate formation and dissociation in porous media. Chem. Eng. Sci. 2006, 61, 3476–3495. [Google Scholar] [CrossRef]
- Kotkoskie, A.U.; Al-ubaidi, B.; Wildeman, T.R.; Sloan, E.D. Inhibition of gas hydrates in water-based drilling mud. SPE Drill. Eng. 1992, 7, 130–136. [Google Scholar] [CrossRef]
- Sun, B.J.; Liu, X.L.; Ren, S.R. Experiment on inhibiting of drilling fluid additives for natural gas hydrate formation. J. China Univ. Pet. 2008, 32, 56–59. [Google Scholar]
- Fan, Z.X.; Dong, L.S.; Dong, X.J.; He, Y.H.; Wang, T.F. Effect of chemical additives used in drilling fluid on hydrate formation. J. Fuel Chem. Technol. 2010, 38, 190–194. [Google Scholar]
- Liu, X.L.; Li, Z.H.; Zheng, Y.; Li, R.Q. Gas hydrate inhibition of drilling fluid additives. In Proceedings of the 7th International Conference on Gas Hydrates, Edinburgh, UK, 17–21 July 2011. [Google Scholar]
- Ishmuratov, F.G.; Rakhimova, N.T.; Ishmiyarov, E.R.; Voloshin, A.I.; Gusakov, V.N.; Tomilov, Y.V.; Nifant’yev, N.E.; Dokichev, V.A. New “Green” polysaccharidal inhibitor of gas hydrate formation on the basis of carboxymethylcellulose sodium salt. Russ. J. Appl. Chem. 2018, 91, 653–656. [Google Scholar] [CrossRef]
- Hu, Y.L.; Yue, Q.S.; Liu, S.J.; Fu, Z.J.; Liang, S. Research on deepwater synthetic drilling fluid and its low temperature rheological properties. Pet. Sci. 2011, 8, 485–489. [Google Scholar] [CrossRef]
- Caenn, R.; Chikkingar, G.V. Drilling fluids: State of the art. J. Petrol. Sci. Eng. 1996, 14, 221–230. [Google Scholar] [CrossRef]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Li, G.; Moridis, G.J.; Zhang, K.N.; Li, X.S. The use of huff and puff method in a single horizontal well in gas production from marine gas hydrate deposits in the Shenhu Area of South China Sea. J. Petrol. Sci. Eng. 2011, 77, 49–68. [Google Scholar] [CrossRef]
- Wang, R.; Liu, T.L.; Ning, F.L.; Ou, W.J.; Zheng, L.; Wang, Z.; Peng, L.; Sun, J.X.; Liu, Z.C.; Li, T.S.; et al. Effect of hydrophilic silica nanoparticles on hydrate formation: Insight from the experimental study. J. Energy Chem. 2018, 30, 90–100. [Google Scholar] [CrossRef]
- Farhang, F.; Nguyen, A.V.; Sewell, K.B. Fundamental investigation of the effects of hydrophobic fumed silica on the formation of carbon dioxide gas hydrates. Energy Fuels 2014, 28, 7025–7037. [Google Scholar] [CrossRef]
- Skovborg, P.; Ng, H.J. Measurement of induction times for the formation of methane and ethane gas hydrates. Chem. Eng. Sci. 1993, 48, 445–453. [Google Scholar] [CrossRef]
- Farhang, F.; Nguyen, A.V.; Hampton, M.A. Influence of sodium halides on the kinetics of CO2 hydrate formation. Energy Fuels 2014, 28, 1220–1229. [Google Scholar] [CrossRef]
- Ahmed, T.H. Hydrocarbon Phase Behavior; Guif Publishing Company: Houston, TX, USA, 1989. [Google Scholar]
- Wang, R.; Sun, H.C.; Xu, X.G.; Zhang, J.; Wang, J.H.; Yang, Z.; Zhang, Z.L.; Lai, X.Q.; Liu, T.L.; Jiang, G.S. Study of the mechanism of hydrate formation promoted by hydrophobic nano-SiO2. Energy Sources Part A 2018, 40, 2257–2264. [Google Scholar] [CrossRef]
- Wang, R.; Sun, H.C.; Sun, J.S.; Qu, Y.Z.; Zhang, J.; Shi, X.M.; Zhang, L.; Guo, D.D. Effect of dissolution and dispersion conditions of VC-713 on the hydrate inhibition. J. Chem. 2019. [Google Scholar] [CrossRef]
- Jiang, G.S.; Ning, F.L.; Zhang, L.; Tu, Y.Z. Effect of agents on hydrate formation and low-temperature rheology of polyalcohol drilling fluid. J. Earth Sci. 2011, 22, 652–657. [Google Scholar] [CrossRef]
- Furushima, Y.; Ishikiriyama, K.; Ueno, Y.; Sugayab, H. Analysis of the state of water in polyvinylpyrrolidone aqueous solutions using DSC method. Thermochim. Acta 2012, 538, 43–47. [Google Scholar] [CrossRef]
- Bai, D.S.; Chen, G.J.; Zhang, X.R.; Sum, A.K.; Wang, W.C. How properties of solid surfaces modulate the nucleation of gas hydrate. Sci. Rep. 2015, 5, 12747. [Google Scholar] [CrossRef]
- Li, H.J.; Stanwix, P.; Aman, Z.; Johns, M.; May, E.; Wang, L.G. Raman spectroscopic studies of clathrate hydrate formation in the presence of hydrophobized particles. J. Phys. Chem. A 2016, 120, 417–424. [Google Scholar] [CrossRef]
- Lederhos, J.P.; Long, J.P.; Sum, A.; Christiansen, R.L.; Sloan, E.D. Effective kinetic inhibitors for natural gas hydrates. Chem. Eng. Sci. 1996, 51, 1221–1229. [Google Scholar] [CrossRef]
- Kang, S.P.; Shin, J.Y.; Lim, J.S.; Lee, S.Y. Experimental measurement of the induction time of natural gas hydrate and its prediction with polymeric kinetic inhibitor. Chem. Eng. Sci. 2014, 116, 817–823. [Google Scholar] [CrossRef]
- Sheng, Y.J.; Lu, S.X.; Xu, M.J.; Wu, X.J.; Li, C.H. Effect of Xanthan gum on the performance of aqueous film-forming foam. J. Disper. Sci. Technol. 2016, 37, 1664–1670. [Google Scholar] [CrossRef]
- Wang, R.; Ning, F.L.; Liu, T.L.; Zhang, L.; Sun, H.C.; Peng, L.; Guo, D.D.; Jiang, G.S. Dynamic simulation of hydrate formed from free methane gas in borehole. Acta Petrol. Sin. 2017, 38, 963–972. [Google Scholar]
- Tan, G.X.; Cui, Y.D.; Yi, G.B.; Zhou, J.H. Influence of different states of water in hydrogels on tensile properties. J. Chem. Ind. Eng. 2005, 56, 2019–2023. [Google Scholar]
| Kind of Thickener | Concentration (wt%) | Apparent Viscosity (mPa·s) | Plastic Viscosity (mPa·s) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 °C | 10 °C | 15 °C | 20 °C | 5 °C | 10 °C | 15 °C | 20 °C | ||
| MS | 0.1 | 2.00 | 1.75 | 1.60 | 1.50 | 1.90 | 1.80 | 1.70 | 1.60 |
| 0.2 | 2.50 | 2.25 | 2.05 | 1.95 | 2.40 | 2.30 | 2.20 | 2.10 | |
| 0.3 | 2.85 | 2.50 | 2.15 | 2.05 | 2.80 | 2.60 | 2.30 | 2.20 | |
| 0.4 | 3.45 | 3.05 | 2.80 | 2.45 | 3.70 | 3.20 | 3.00 | 2.60 | |
| 0.5 | 4.00 | 3.50 | 3.30 | 2.95 | 4.10 | 3.80 | 3.50 | 3.10 | |
| CMC | 0.1 | 7.45 | 6.80 | 6.10 | 5.70 | 5.90 | 5.50 | 5.10 | 4.80 |
| 0.2 | 14.65 | 13.55 | 12.35 | 11.20 | 10.80 | 10.20 | 9.60 | 8.60 | |
| 0.3 | 19.15 | 17.65 | 16.25 | 14.95 | 13.50 | 12.80 | 12.10 | 11.40 | |
| 0.4 | 23.55 | 22.05 | 20.25 | 18.35 | 16.20 | 16.00 | 14.90 | 13.80 | |
| 0.5 | 31.80 | 29.25 | 27.15 | 24.90 | 20.90 | 20.00 | 18.90 | 18.00 | |
| XG | 0.1 | 6.45 | 6.00 | 5.50 | 5.20 | 4.40 | 4.10 | 3.90 | 3.40 |
| 0.2 | 10.00 | 9.30 | 8.60 | 8.15 | 5.30 | 4.30 | 4.00 | 3.70 | |
| 0.3 | 12.05 | 11.30 | 10.60 | 9.95 | 6.00 | 5.50 | 5.10 | 4.80 | |
| 0.4 | 14.40 | 13.60 | 13.00 | 12.15 | 7.10 | 6.40 | 5.80 | 5.40 | |
| 0.5 | 16.70 | 15.80 | 15.00 | 14.30 | 8.40 | 7.80 | 7.30 | 6.90 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Sun, H.; Shi, X.; Xu, X.; Zhang, L.; Zhang, Z. Fundamental Investigation of the Effects of Modified Starch, Carboxymethylcellulose Sodium, and Xanthan Gum on Hydrate Formation under Different Driving Forces. Energies 2019, 12, 2026. https://doi.org/10.3390/en12102026
Wang R, Sun H, Shi X, Xu X, Zhang L, Zhang Z. Fundamental Investigation of the Effects of Modified Starch, Carboxymethylcellulose Sodium, and Xanthan Gum on Hydrate Formation under Different Driving Forces. Energies. 2019; 12(10):2026. https://doi.org/10.3390/en12102026
Chicago/Turabian StyleWang, Ren, Huicui Sun, Xiaomei Shi, Xianguang Xu, Ling Zhang, and Zhilei Zhang. 2019. "Fundamental Investigation of the Effects of Modified Starch, Carboxymethylcellulose Sodium, and Xanthan Gum on Hydrate Formation under Different Driving Forces" Energies 12, no. 10: 2026. https://doi.org/10.3390/en12102026
APA StyleWang, R., Sun, H., Shi, X., Xu, X., Zhang, L., & Zhang, Z. (2019). Fundamental Investigation of the Effects of Modified Starch, Carboxymethylcellulose Sodium, and Xanthan Gum on Hydrate Formation under Different Driving Forces. Energies, 12(10), 2026. https://doi.org/10.3390/en12102026




