Effect of Initial Wettability on Performance of Smart Water Flooding in Carbonate Reservoirs—An Experimental Investigation with IOR Implications
Abstract
1. Introduction
2. Materials and Methodology
2.1. Fluids
2.2. Carbonate Rock Samples
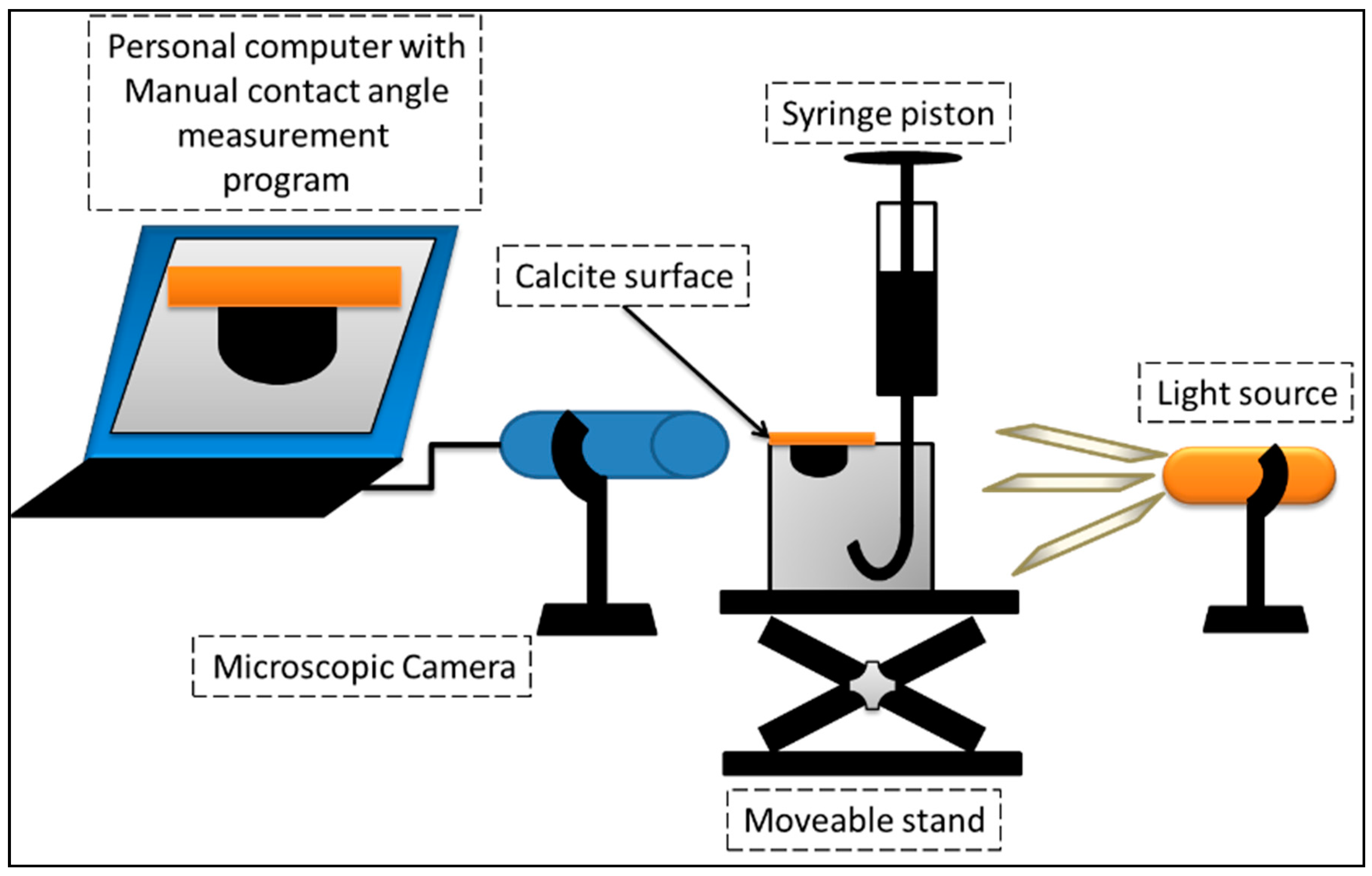
2.3. Contact Angle Measurements
2.4. Energy-Dispersive X-ray Spectroscopy
3. Results and Discussion
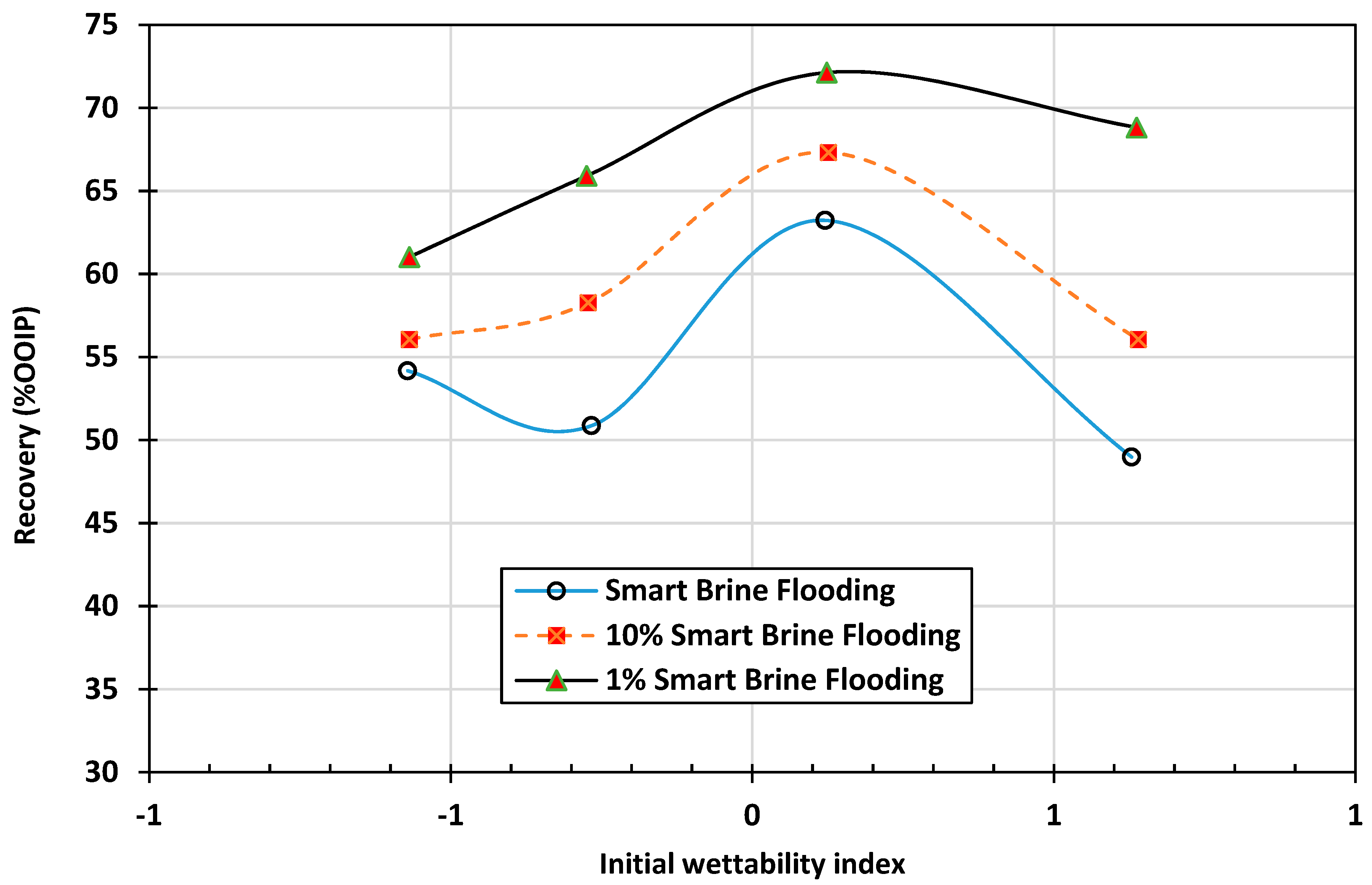
3.1. Effect of Initial Wettability on the Performance of the Designed Smart Waters
3.1.1. Strong (Case 1) and Preferentially Water-Wet (Case 2) Conditions
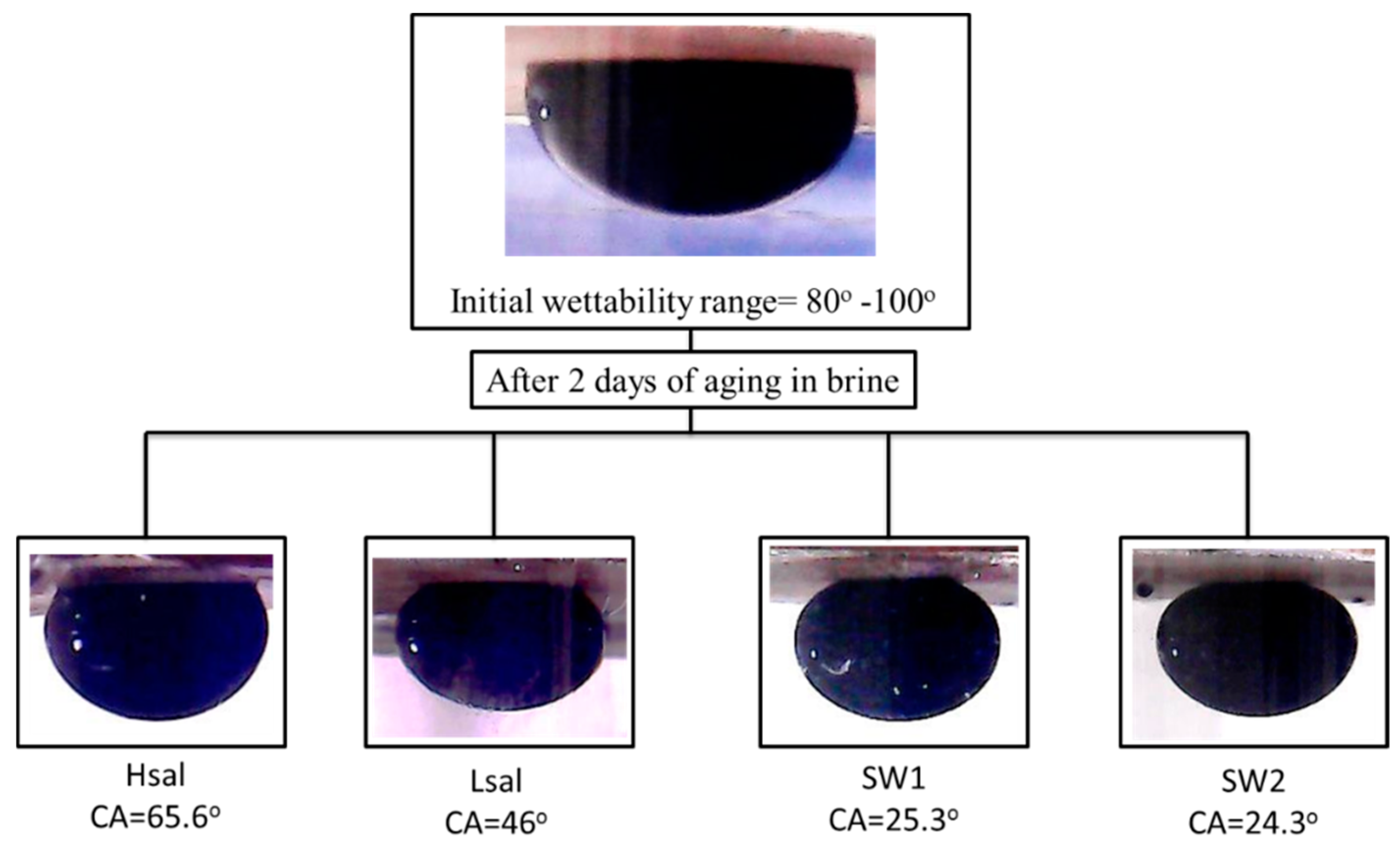
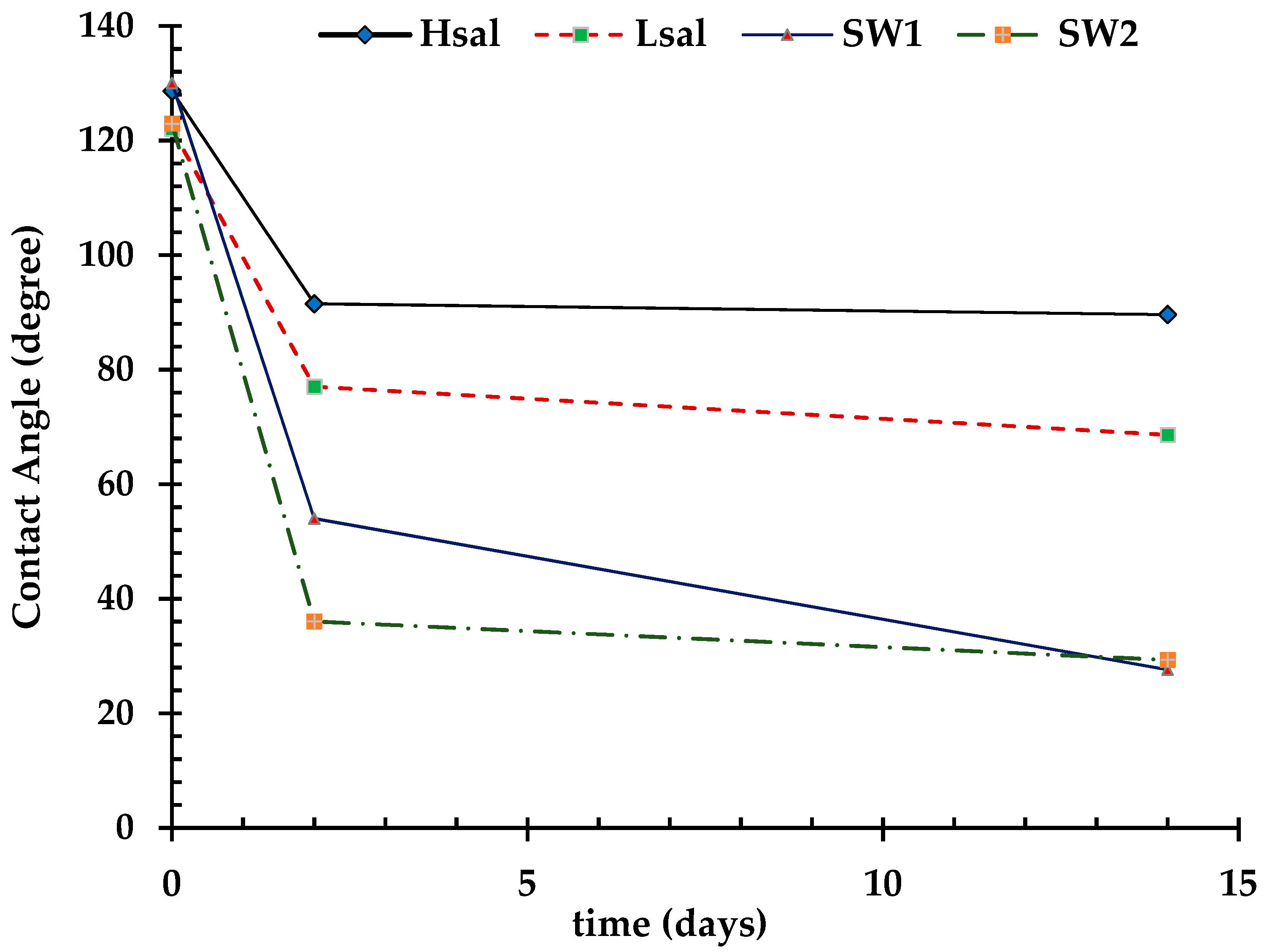
3.1.2. Neutral-Wet (Case 3), Oil-Wet (Case 4), and Strongly Oil-Wet (Case 5) Conditions
- is the aged in crude oil CA of the sample after aging in oil;
- is the CA after aging in different brines;
- is the CA after aging in brine at the start of the test.
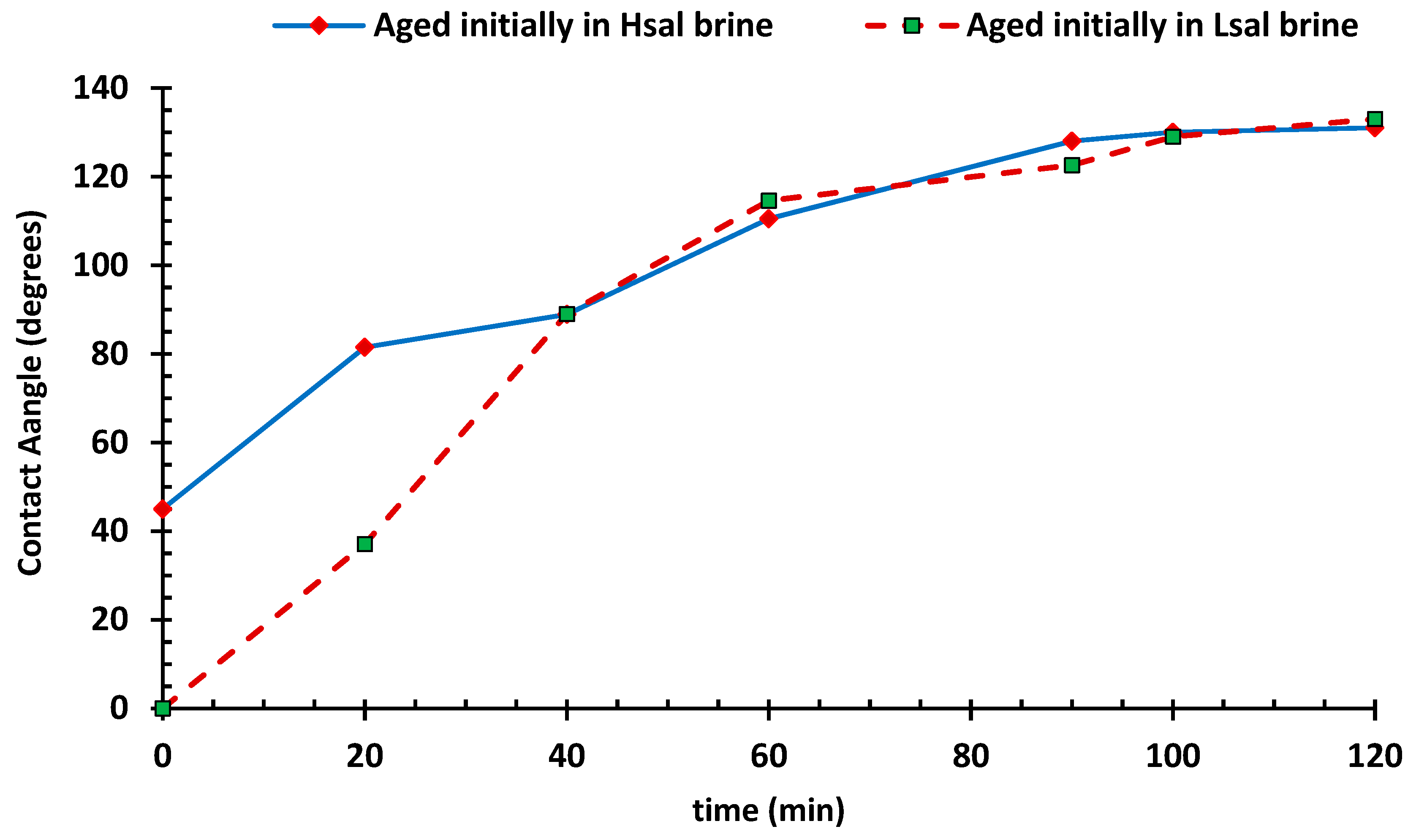
3.2. Effect of NaCl Aqueous Solutions (Hsal and Lsal) on the Wettability Alteration Process
- During the salting-out mechanism, the solubility of a nonelectrolyte substance in water decreases with increases in salt concentration in brine. Firstly, the non-electrolyte carboxylate molecule has lower solubility in brine with higher salt concentrations. As the salinity of the brine increases, the salting-out effect leads to a decrease in the solubility of carboxylic compounds, resulting in the deposition of more organic materials as the thin oil phase near the rock surface increases the chance of bonding between the positively charged carbonate surface and negatively charged carboxylic components. Conversely, a lower salt concentration increases the solubility of carboxylic groups. Active ions with negative charges tend to be attracted to the positive calcite surface and substitute for the calcium ion, which is bonded to the negative molecules of carboxylates; hence, the wettability of the surface becomes less oil-wet.
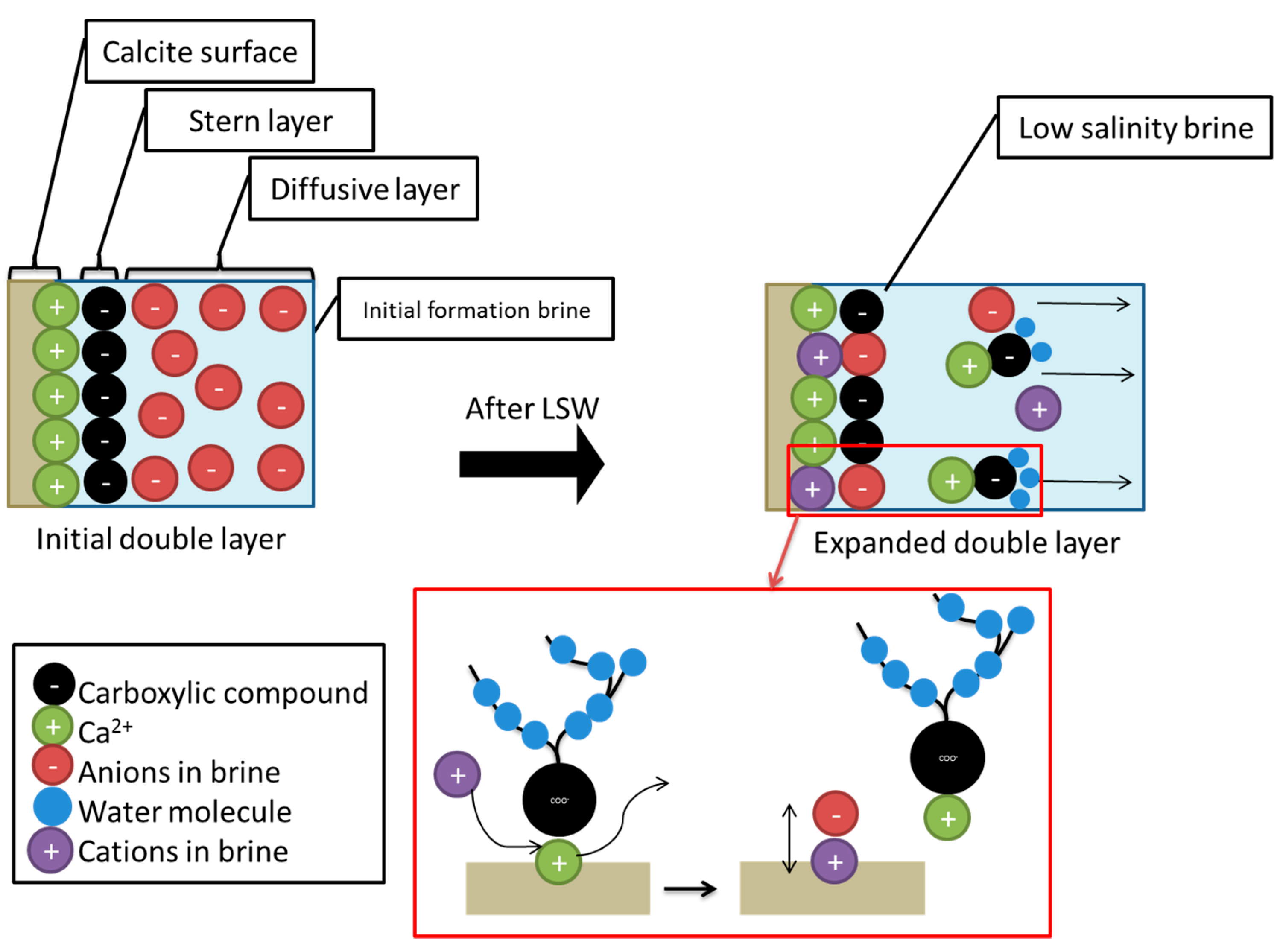
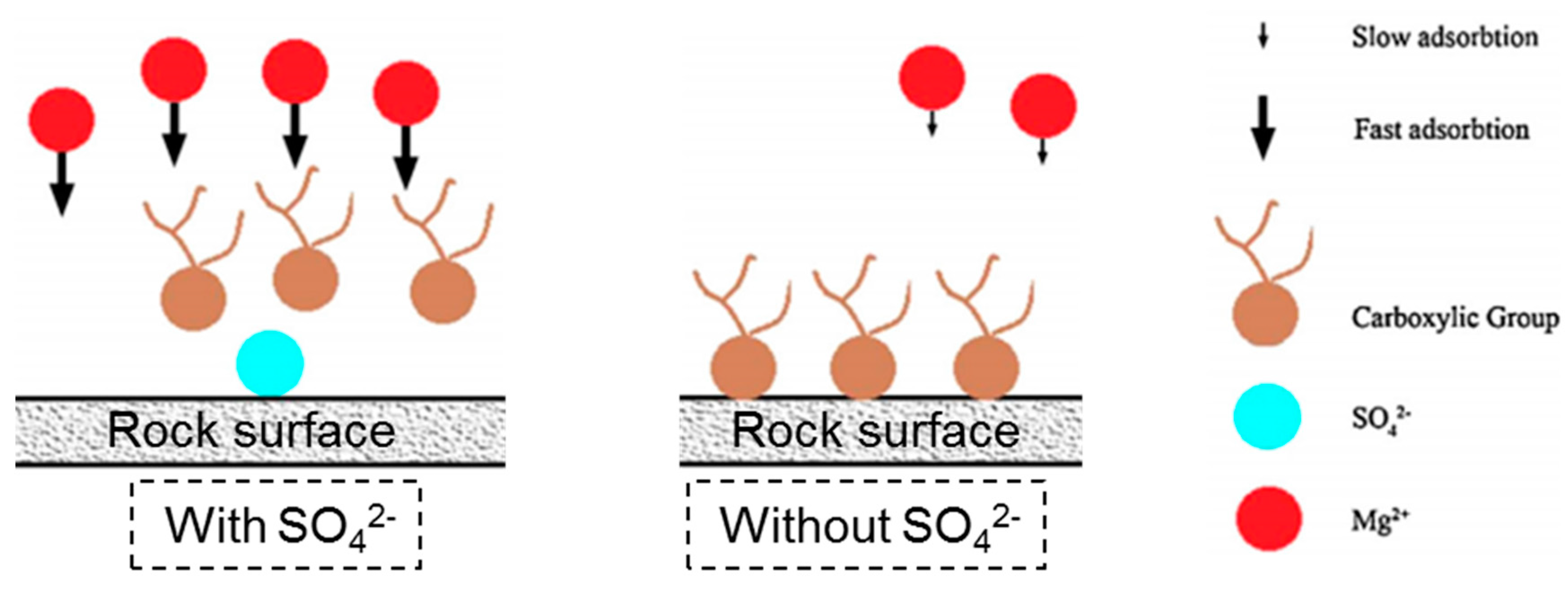
- Secondly, the term “double layer” refers to the bulk of the ions that are close to the surface, which form due to the interaction between the charged rock surface and the brine. The distribution of charges at the surface is composed of opposite charges (counter-ions) attracted to the surface and other equal charges (co-ions) repulsed from the surface in an aqueous medium. Because of this charge accumulation, an electrical layer is formed. During LSW in carbonate rocks, the surface tends to be less positive, due to the ionic exchange between cations in the aqueous brine and calcium ion. The substitution of calcium ions with the aid of a solvation process results in the detachment of adsorbed oil. This leads to double layer expansion, and ultimately results in the wettability alteration (i.e., more water wet) of the rock surface, as illustrated in Figure 14.
- The final step is the dissolution of calcite (CaCO3), which takes place as the low-salinity brine invades the carbonate surface. For the Hsal and Lsal samples, the lack of calcium ions promotes the dissolution of calcite, and thus oil is expelled from the surface.
3.3. Effect of Smart Waters (SW1 and SW2) on the Wettability Alteration Process
4. Conclusions
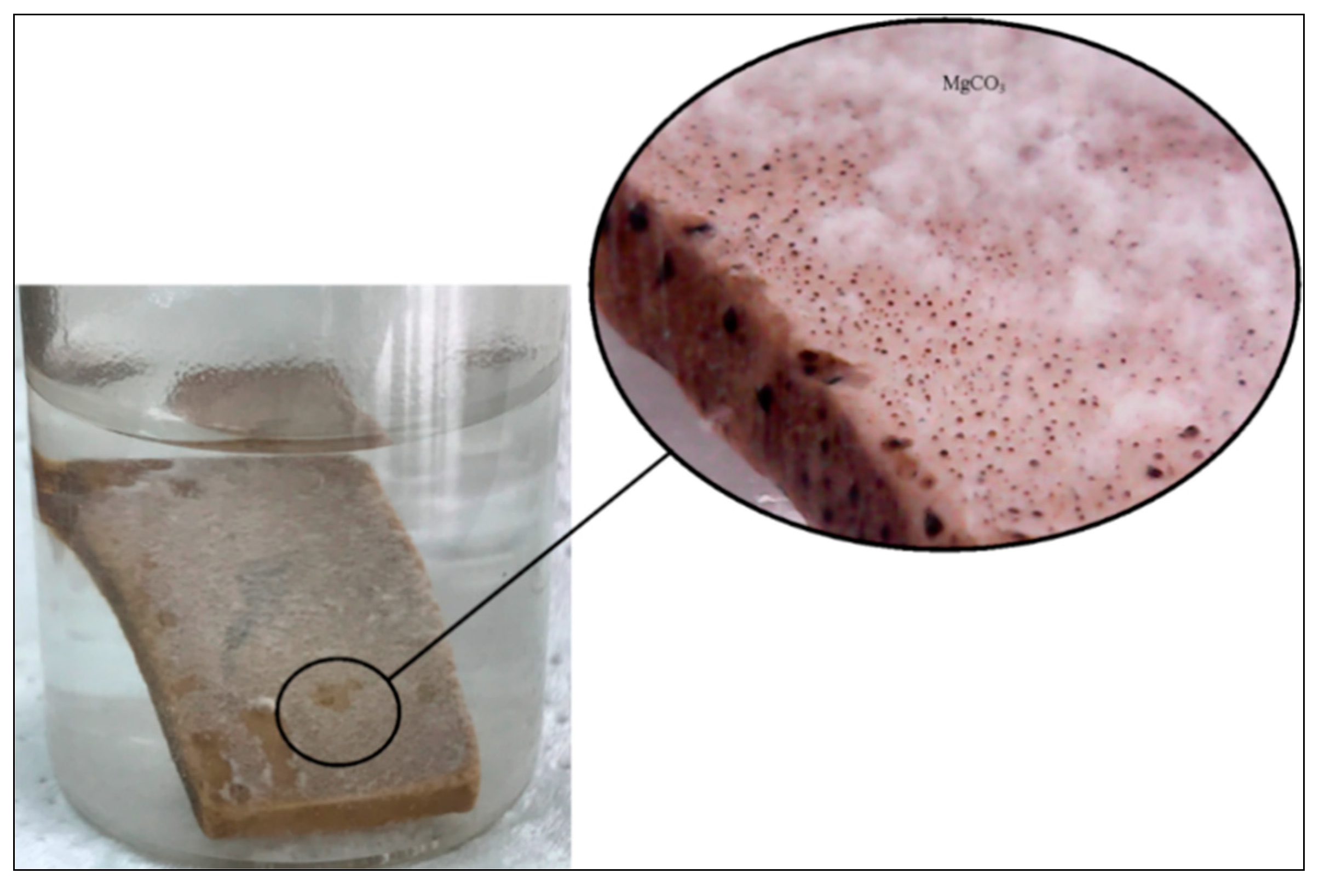
- Low-salinity brine (Lsal) was more effective than high-salinity brine (Hsal) for the wettability alteration of calcite surfaces at intermediate (neutral) or oil-wet conditions. The smart brine containing only the Mg2+ ion (SW1) was able to alter the wettability of calcite surfaces in intermediate or oil-wet states. The substitution of Ca2+ ions by Mg2+ was the most accepted mechanism as proved by Magnesite (MgCO3 mineral) precipitation during the experiments.
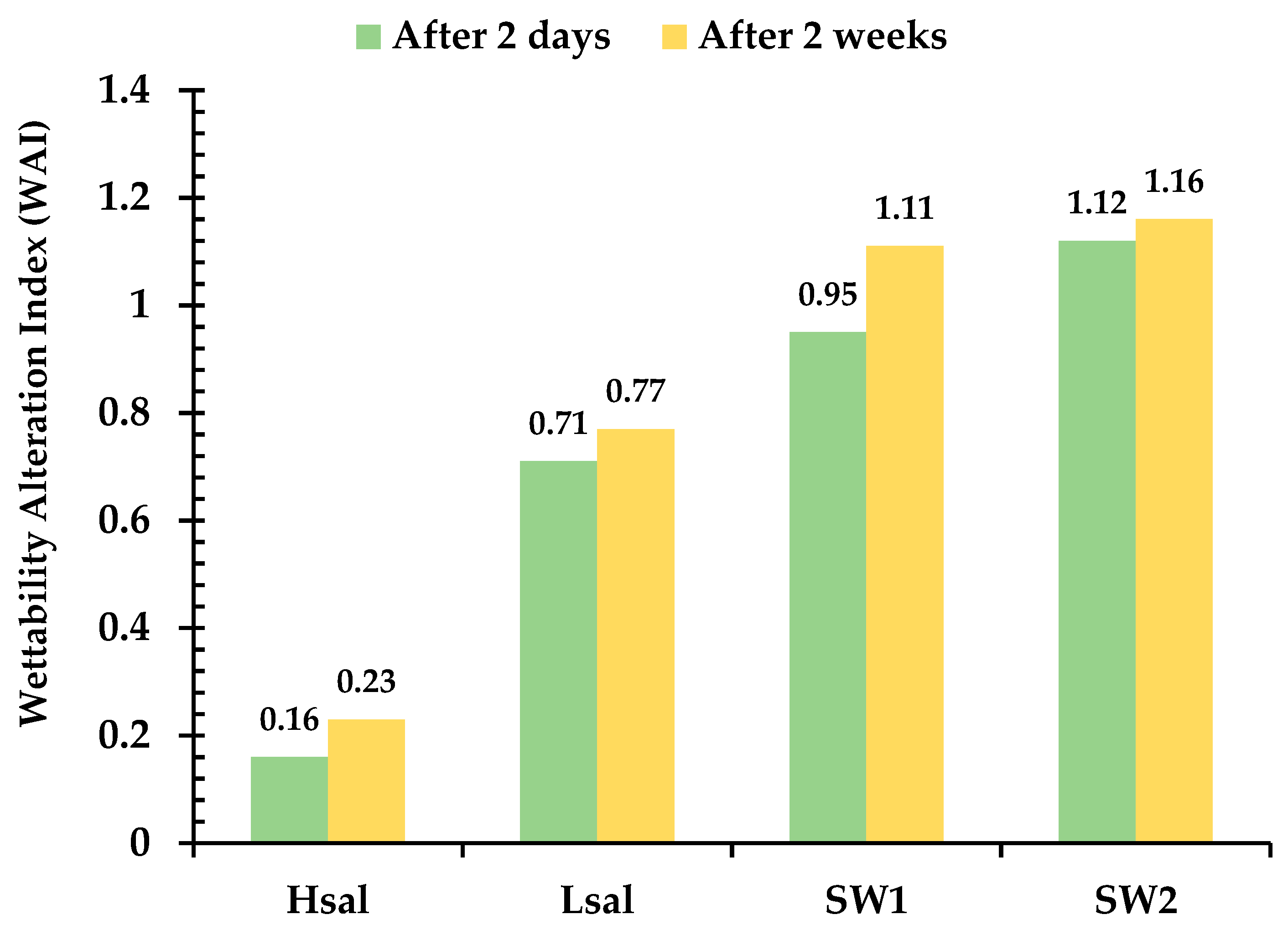
- The sulfate ion played a catalytic role in wettability alteration by the magnesium ion, and the process was faster, as indicated by higher wettability alteration index values. This tends to reduce the electrostatic repulsive force, meaning that Mg2+ is near the surface, and substitute Ca2+ ions bond to carboxylic compounds more rapidly.
- High-salinity brine (Hsal) is a good choice for design of water floods in reservoir rocks with initial wettability in the range of strongly water wet to neutral wet conditions.
- pH measurements showed an increase in Lsal and Hsal after the wettability reversal process. This was interpreted using a calcite dissolution reaction that results in an increase of the hydroxide ion concentration. The pH values of SW1 and SW2 decreased after the wettability alteration process, which can be attributed to the precipitation of Magnesite and CO2 production.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sheng, J. Enhanced Oil Recovery Field Case Studies, 1st ed.; Gulf Professional Publishing: Houston, TX, USA, 2013. [Google Scholar]
- Sheng, J. Critical review of low-salinity waterflooding. J. Petrol. Sci. Eng. 2014, 120, 216–224. [Google Scholar] [CrossRef]
- Zhou, X.; Morrow, N.R.; Ma, S. Interrelationship of wettability, initial water saturation, aging time and oil recovery by spontaneous imbibition and water-flooding. SPE J. 2000, 5, 199–207. [Google Scholar] [CrossRef]
- Yousef, A.A.; Liu, J.; Blanchard, G.; Al-Saleh, S.; Al-Zahrani, T.; Al-Zahrani, R.; Al-Tammar, H.; Al-Mulhim, N. Smart Water Flooding: Industry’s First Field Test in Carbonate Reservoirs. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 8–10 October 2012. [Google Scholar]
- Fathi, S.J.; Austad, T.; Strand, S. Water-based enhanced oil recovery (EOR) by “Smart Water”: Optimal ionic composition for EOR in carbonates. Energy Fuels 2011, 25, 5173–5179. [Google Scholar] [CrossRef]
- Hiorth, A.; Cathles, L.M.; Madland, M.V. Impact of pore water chemistry on carbonate surface charge and oil wettability. Transp. Porous Media 2010, 85, 1–21. [Google Scholar] [CrossRef]
- Hiorth, A.; Cathles, L.M.; Kolnes, J.; Vikane, O.; Lohne, A.; Madland, M.V. Chemical modelling of wettability change in carbonate rocks. In Proceedings of the 10th Wettability Conference, Abu Dhabi, UAE, 26–28 October 2008. [Google Scholar]
- Hiorth, A.; Cathles, L.M.; Kolnes, J.; Vikane, O.; Lohne, A.; Madland, M.V. A chemical model for the seawater-CO2-carbonate system aqueous and surface chemistry. In Proceedings of the 10th Wettability Conference, Abu Dhabi, UAE, 26–28 October 2008. [Google Scholar]
- Al-Attar, H.H.; Mahmoud, M.Y.; Zekri, A.Y.; Almehaideb, R.A.; Ghannam, M.T. Low Salinity Flooding in a Selected Carbonate Reservoir: Experimental Approach. In Proceedings of the EAGE Annual Conference & Exhibition, London, UK, 10–13 June 2013. Paper SPE 164788. [Google Scholar]
- Al-Shalabi, E.W.; Sepehrnoori, K.; Delshad, M. Mechanisms behind low salinity water injection in carbonate reservoirs. Fuel 2014, 121, 11–19. [Google Scholar] [CrossRef]
- Al-Shalabi, E.W.; Sepehrnoori, K.A. comprehensive review of low salinity/engineered water injections and their applications in sandstone and carbonate rocks. J. Petrol. Sci. Eng. 2016, 139, 137–161. [Google Scholar] [CrossRef]
- Webb, K.J.; Black, C.J.J.; Tjetland, G.A. Laboratory Study Investigating Methods for Improving Oil Recovery in Carbonates. In Proceedings of the SPE International Petroleum Technology Conference, Doha, Qatar, 21–23 November 2005. Paper SPE 10506. [Google Scholar]
- Hadia, N.J.; Ashraf, A.; Tweheyo, M.T.; Torsæter, O. Laboratory investigation on effects of initial wettabilities on performance of low salinity waterflooding. J. Petrol. Sci. Eng. 2013, 105, 18–25. [Google Scholar] [CrossRef]
- Yousef, A.A.; Al-Saleh, S.; Al-Kaabi, A.; Al-Jawfi, M. Laboratory investigation of the impact of injection-water salinity and ionic content on oil recovery from carbonate reservoirs. SPE Reserv. Eval. Eng. 2011, 14, 578–593. [Google Scholar] [CrossRef]
- Rashid, S.; Mousapour, M.S.; Ayatollahi, S.; Vossoughi, M.; Beigy, A.H. Wettability alteration in carbonates during “Smart Waterflood”: Underlying mechanisms and the effect of individual ions. Colloid Surf. A 2015, 487, 142–153. [Google Scholar] [CrossRef]
- Awolayo, A.; Sarma, H.; Al-Sumaiti, A.M. A laboratory study of ionic effect of smart water for enhancing oil recovery in carbonate reservoirs. Presented at the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 26–28 March 2014. Paper SPE 169662. [Google Scholar]
- Karimi, M.; Al-Maamari, R.S.; Ayatollahi, S.; Mehranbod, N. Impact of Sulfate Ions on Wettability Alteration of Oil-Wet Calcite in the Absence and Presence of Cationic Surfactant. Energy Fuels 2016, 30, 819–829. [Google Scholar] [CrossRef]
- Karimi, M.; Al-Maamari, R.S.; Ayatollahi, S.; Mehranbod, N. Wettability alteration and oil recovery by spontaneous imbibition of low salinity brine into carbonates: Impact of Mg2+, SO42− and cationic surfactant. J. Petrol. Sci. Eng. 2016, 147, 560–569. [Google Scholar] [CrossRef]
- Lashkarbolooki, M.; Ayatollahi, S.; Riazi, M. Mechanistical study of effect of ions in smart water injection into carbonate oil reservoir. Process Saf. Environ. Prot. 2017, 105, 361–372. [Google Scholar] [CrossRef]
- Amott, E. Observations relating to the wettability of porous rock. Pet. Trans. AIME 1959, 216, 156–162. [Google Scholar]
- Al-Harrasi, A.; Al-Maamari, R.S.; Masalmeh, S.K. Laboratory investigation of low salinity waterflooding for carbonate reservoirs. In Proceedings of the International Petroleum Conference and Exhibition, Abu Dhabi, UAE, 11–14 November 2012. [Google Scholar]
- Austad, T.; Shariatpanahi, S.F.; Strand, S.; Black, C.J.J.; Webb, K.J. Conditions for a low-salinity enhanced oil recovery (EOR) effect in carbonate oil reservoirs. Energy Fuels 2011, 26, 569–575. [Google Scholar] [CrossRef]
- Alameri, W.; Teklu, T.W.; Graves, R.M.; Kazemi, H.; Al-Sumaiti, A.M. Wettability alteration during low-salinity waterflooding in carbonate reservoir cores. In Proceedings of the SPE Asia Pacific Oil & Gas Conference and Exhibition, Adelaide, Australia, 14–16 October 2014. [Google Scholar]
- Buckman, J.O.; Polson, E.J.; Todd, A.C. Measuring wettability in the environmental scanning electron microscope: A preliminary study using microinjected oil for contact angle measurement and hysteresis. Microscopy and Analysis, September 2014; 13–16. [Google Scholar]
- Jabbar, M.Y.; Al-Hashim, H.S.; Abdallah, W. Effect of brine composition on wettability alteration of carbonate rocks in the presence of polar compounds. In Proceedings of the SPE Saudi Arabia Section Technical Symposium and Exhibition, Saudi Arabia, UAE, 8–11 April 2013. [Google Scholar]
- Tweheyo, M.T.; Zhang, P.; Austad, T. The Effects of Temperature and Potential Determining Ions Present in Seawater on Oil Recovery from Fractured Carbonates. In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 22–26 April 2006. [Google Scholar]
- Petrovich, R.; Hamouda, A.A. Dolomitization of Ekofisk oil field reservoir chalk by injected seawater. In Proceedings of the Ninth International Symposium on Water–Rock Interactions, Taupo, New Zealand, 30 March–3 April 1998. [Google Scholar]
- Gomari, K.R.; Hamouda, A.A. Effect of fatty acids, water composition and pH on the wettability alteration of calcite surface. J. Petrol. Sci. Eng. 2006, 50, 140–150. [Google Scholar] [CrossRef]

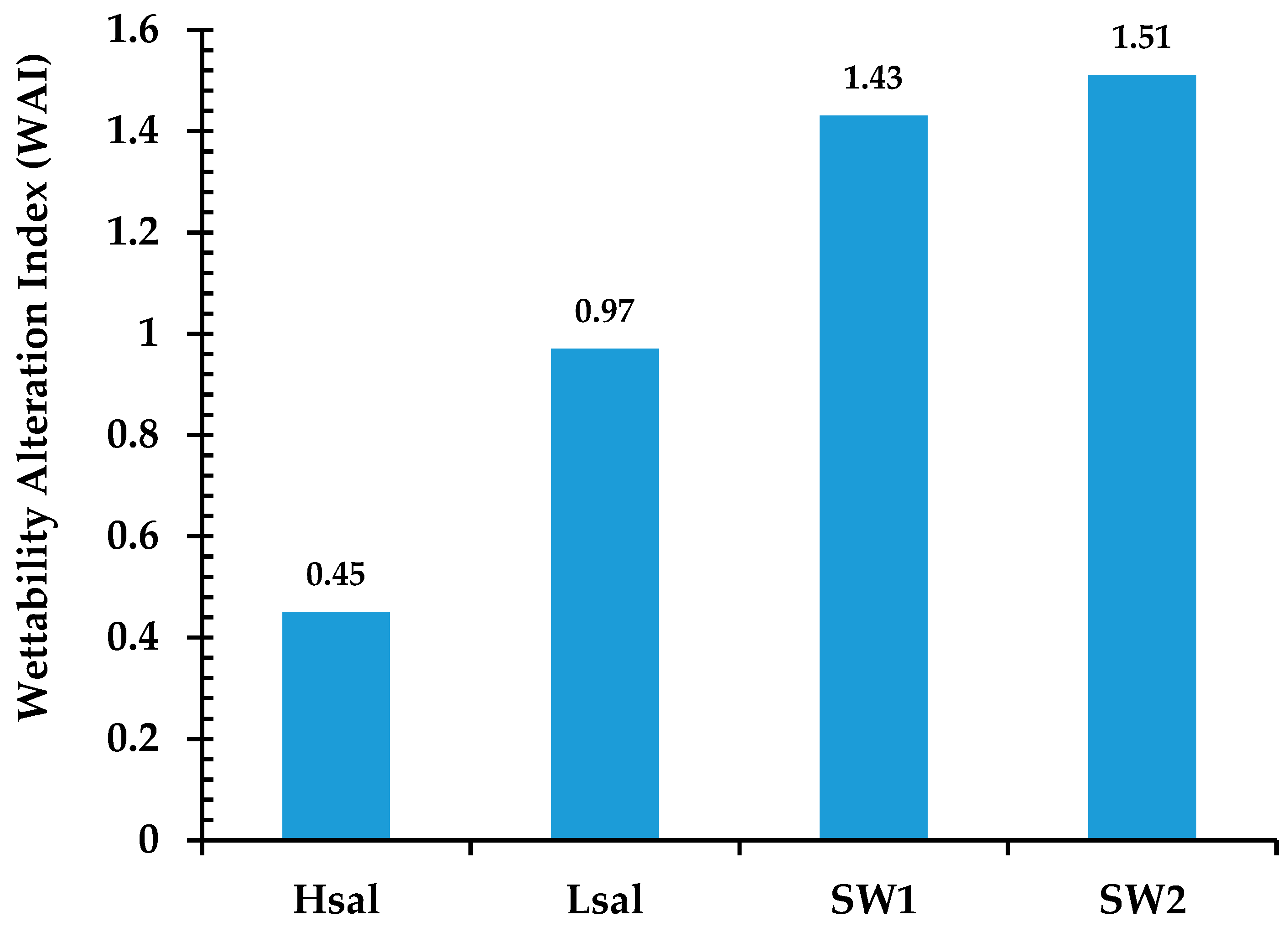
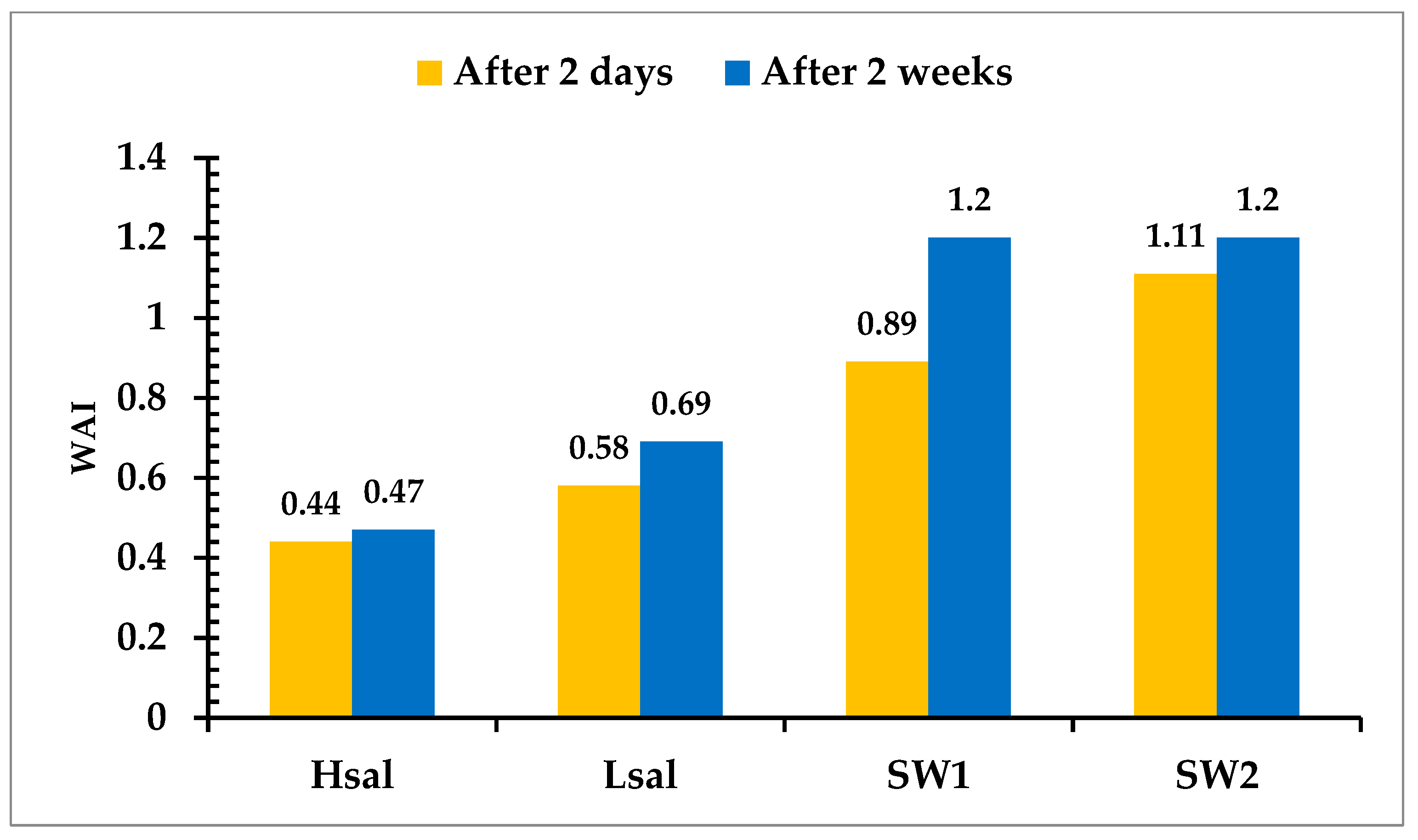

| Brine | Composition | mol/L | ppm | To Study the Effects of: | ||
|---|---|---|---|---|---|---|
| NaCl (g/L) | Na2SO4 (g/L) | MgCl2 (g/L) | ||||
| Hsal | 40 | 0 | 0 | 0.6845 | 40,000 | Normal water flooding |
| Lsal | 5 | 0 | 0 | 0.0856 | 5000 | Dilution only |
| SW1 | 0 | 0 | 9.521 | 0.1 | 9521 | Mg2+ |
| SW2 | 0 | 7.102 | 4.76 | 0.1 | 11,862 | SO42− and Mg2+ |
| Chemical Formula | wt. % | Crystal System |
|---|---|---|
| CaCO3 | 98.5 | Hexagonal (Rh) |
| SiO2 | 1.5 | Hexagonal |
| Case No. | Wettability Condition | Time | Expected Contact Angle (°) |
|---|---|---|---|
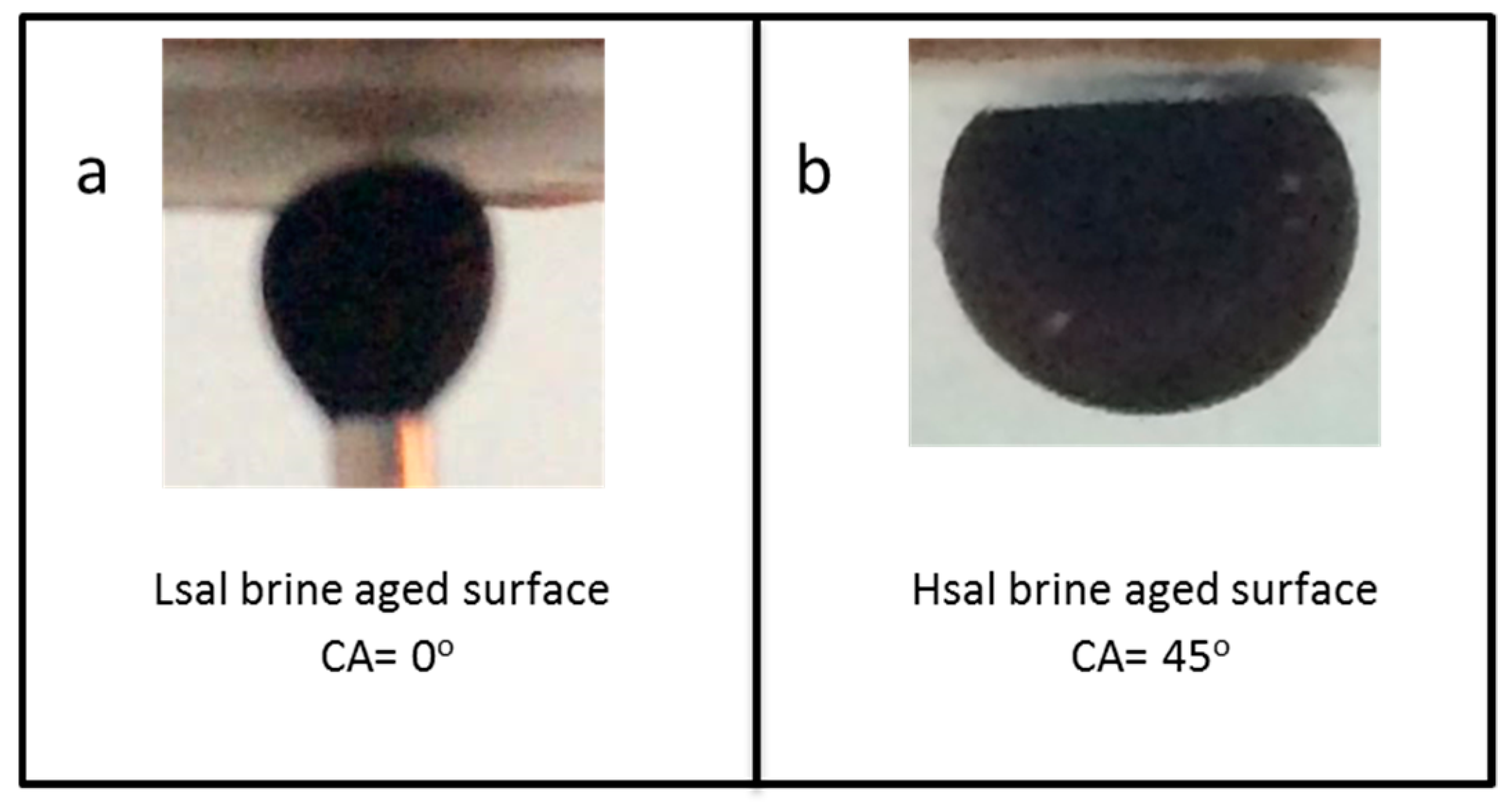
| Case 1 | Strongly water wet | Zero (initially aged in Lsal) | 0 |
| Case 2 | Preferentially water wet | Zero (initially aged in Hsal) | 45 |
| Case 3 | Neutral wet | 40 min | 80–100 |
| Case 4 | Oil wet | 2 days | 120–140 |
| Case 5 | Strongly oil wet | 1 month | Checking point |
| Initial CA | Final Contact Angle | |||
|---|---|---|---|---|
| Hsal | Lsal | SW1 | SW2 | |
| 0° | 31.3° | 0° | 0° | 0° |
| 45° | 45° | 0° | 0° | 0° |
| Hsal | Lsal | SW1 | SW2 | |
|---|---|---|---|---|
| Aged in crude oil CA | 82.6° | 87.7° | 90.8° | 85.4° |
| Final CA | 65.6° | 46° | 25.3° | 24.3° |
| Hsal | Lsal | SW1 | SW2 | |
|---|---|---|---|---|
| Aged in crude oil CA | 128.6° | 122° | 130° | 122.8° |
| CA (after two days) | 91.5° | 77° | 54° | 36° |
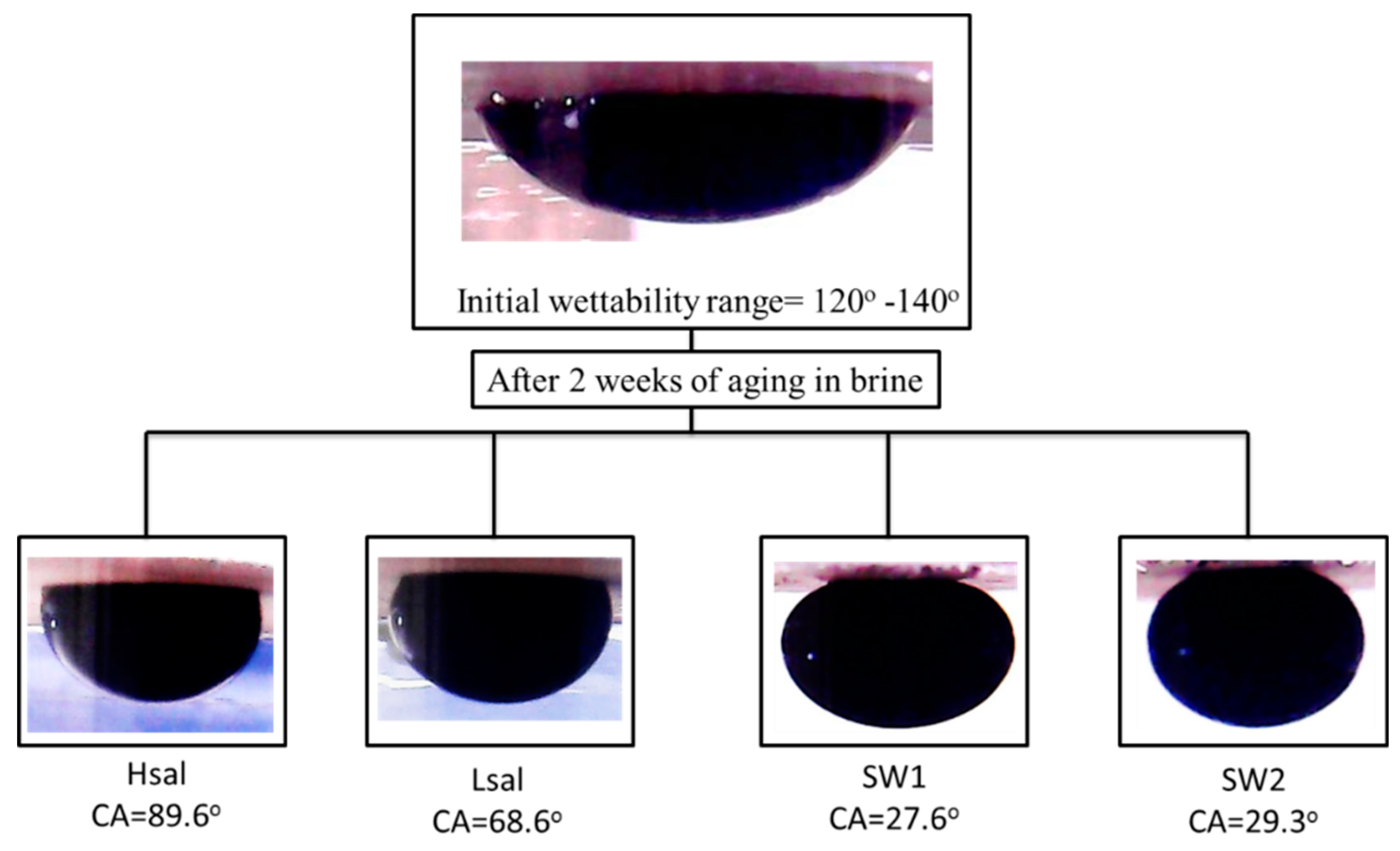
| CA (after two weeks) | 89.6° | 68.6° | 27.6° | 29.3° |
| Hsal | Lsal | SW1 | SW2 | |
|---|---|---|---|---|
| Aged in crude oil CA | 133.3° | 134.6° | 135.6° | 133.9° |
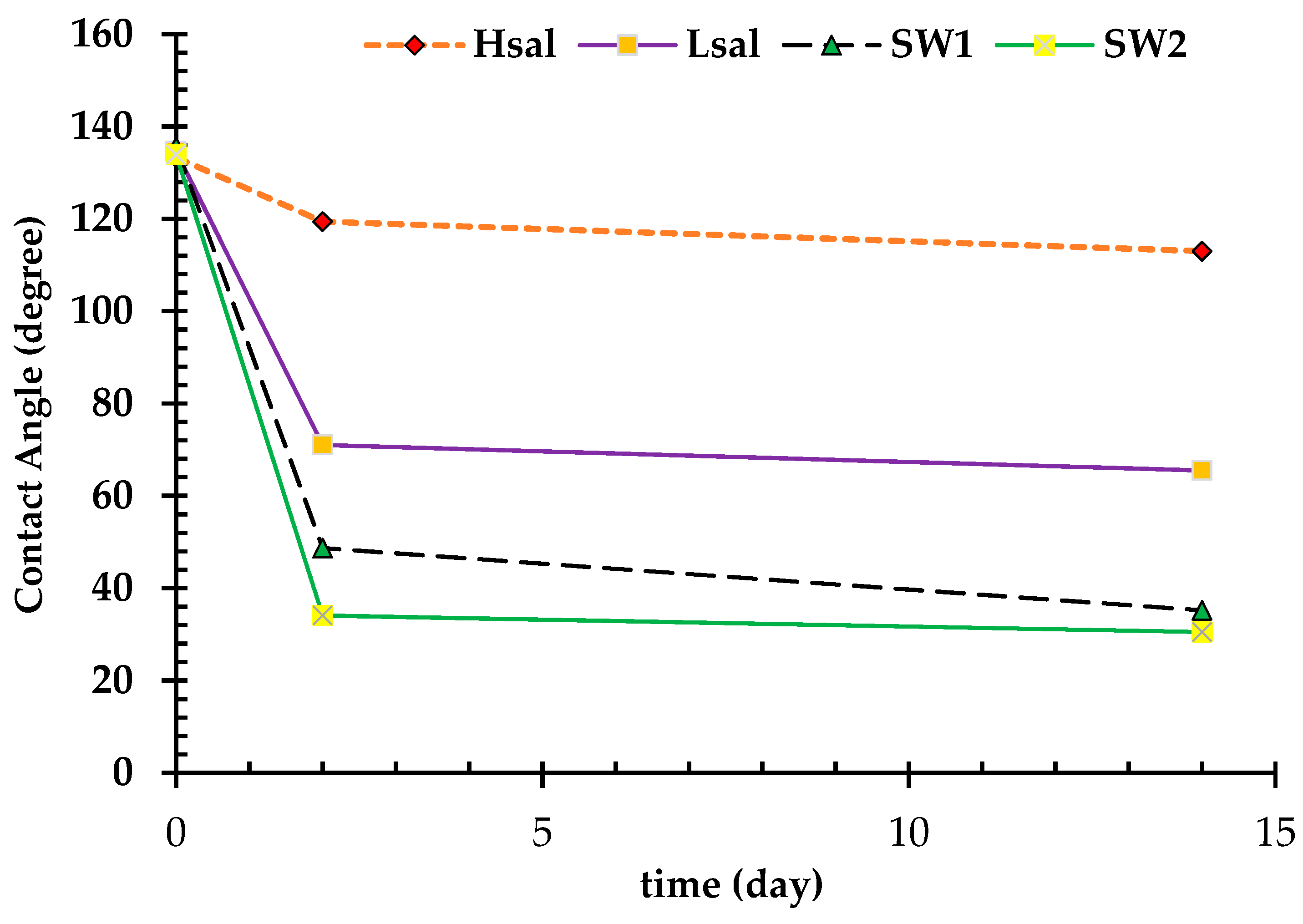
| CA (after two days) | 119.4° | 71° | 48.7° | 34.1° |
| CA (after two weeks) | 113° | 65.5° | 35.2° | 30.5° |
| Case No. | Brine Aging Time | Treating Brines | |||
|---|---|---|---|---|---|
| Hsal | Lsal | SW1 | SW2 | ||
| Initial pH | 5.55 | 5.71 | 8.92 | 9.92 | |
| Cases 1 and 2 | 2 days | 9.1 | 9.25 | 7.91 | 8.53 |
| Case 3 | 2 days | 9.17 | 9.39 | 7.97 | 8.28 |
| Case 4 | 2 days | 9.11 | 9.24 | 8.12 | 8.46 |
| 2 weeks | 8.99 | 9.27 | 7.74 | 8.38 | |
| Case 5 | 2 days | 9.03 | 9.03 | 8.14 | 8.48 |
| 2 weeks | 9.12 | 9.22 | 7.84 | 8.38 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Nofli, K.; Pourafshary, P.; Mosavat, N.; Shafiei, A. Effect of Initial Wettability on Performance of Smart Water Flooding in Carbonate Reservoirs—An Experimental Investigation with IOR Implications. Energies 2018, 11, 1394. https://doi.org/10.3390/en11061394
Al-Nofli K, Pourafshary P, Mosavat N, Shafiei A. Effect of Initial Wettability on Performance of Smart Water Flooding in Carbonate Reservoirs—An Experimental Investigation with IOR Implications. Energies. 2018; 11(6):1394. https://doi.org/10.3390/en11061394
Chicago/Turabian StyleAl-Nofli, Kholood, Peyman Pourafshary, Nader Mosavat, and Ali Shafiei. 2018. "Effect of Initial Wettability on Performance of Smart Water Flooding in Carbonate Reservoirs—An Experimental Investigation with IOR Implications" Energies 11, no. 6: 1394. https://doi.org/10.3390/en11061394
APA StyleAl-Nofli, K., Pourafshary, P., Mosavat, N., & Shafiei, A. (2018). Effect of Initial Wettability on Performance of Smart Water Flooding in Carbonate Reservoirs—An Experimental Investigation with IOR Implications. Energies, 11(6), 1394. https://doi.org/10.3390/en11061394





