Abstract
In this paper, the effects of salinity and active ions on wettability alteration in carbonate reservoirs with different initial wettability conditions with implications in smart water flood design, optimization, and performance analysis are experimentally investigated. Contact angle measurement was used as the main tool to study the alteration in wettability. Other analytical techniques such as pH measurements along with energy-dispersive X-ray spectroscopy (EDS) were used to support the analysis. Initial wettability of the tested carbonate samples ranges from strongly water wet to preferentially water wet, neutral wet, oil wet, and strongly oil wet (5 cases or groups) condition. Four different synthetic brines, namely high salinity (Hsal), low salinity (Lsal), and smart waters 1 and 2 (SW1 = a Mg brine, and SW2 = a Mg and sulfate brine) were prepared and used by adjusting the salinity and ion concentration to study their effects on wettability alteration. Low-salinity brine (Lsal) proved to be more effective than high-salinity brine (Hsal) for the wettability alteration of calcite surfaces at intermediate (neutral) or oil-wet conditions. The smart brine containing only the Mg2+ ion (SW1) was able to alter the wettability of calcite surfaces in intermediate or oil-wet states. The sulfate ion played a catalytic role in wettability alteration by the magnesium ion, and the process was faster, as indicated by higher wettability alteration index values. High-salinity brine (Hsal) is a good choice for design of water floods in reservoir rocks with initial wettability in the range of strongly water wet to neutral wet conditions. In the wettability alteration process of oil-wet samples, brine with a high magnesium ion concentration was slower than brine containing high concentrations of both magnesium and sulfate ions. This can be attributed to the catalytic role of the sulfate ion compared to that of the magnesium ion. Finally, the results showed that the initial wettability of the reservoir rock plays a major role in design of a proper water flood to maximize oil recovery from carbonate reservoirs. The results obtained from this research work suggests that some effective smart water flooding scenarios can be developed and executed incorporating different smart brines to manage the reservoir rock wettability and maximize the oil recovery from carbonate oil reservoirs.
1. Introduction
In any crude oil/brine/rock (CBR) system, the flow of a two-phase fluid is strongly affected by the wetting characteristics, which determine the relative permeabilities of oil and water and the capillary pressure of the porous medium. Several laboratory studies have demonstrated the importance of the initial wettability of the rock in the efficiency of improved oil recovery techniques [1,2].
Zhou et al. [3] reported that modifying the chemistry of the injected brine affects oil recovery mainly controlled by properties of the CBR system and the composition of the brine. If the injected brine has a different composition from the formation brine, the chemical equilibrium of the CBR system can be disturbed. During this process, the new equilibrium changes the wetting properties, which can enhance the recovery. In other words, the efficiency of the water flooding process in carbonate formations can be enhanced by modifying the chemistry of the injected water, which alters the wettability to a desired state and improves the oil recovery. The concept of “smart water flooding” involves the injection of brine that has been chemically optimized in terms of its ionic composition and salinity into the reservoir, rather than the water that is normally injected [4].
Several procedures have been put forward to explain the additional oil recovery using smart water/low-salinity water (LSW) flooding in carbonate formations. Fathi et al. [5] proposed the theory of multi-component ionic exchange (MIE) mechanism for carbonate rocks. LSW injection into carbonate reservoir can result in changes in wettability by using water containing sulfate ions and calcium and/or magnesium at a high temperature (>90 °C). It was suggested that the adsorption of sulfate ions onto the carbonate rock surface increases as the temperature increases. Simultaneously, the affinity of calcium ions increases as the original positive charge of the rock is reduced. Thus, an excess amount of Ca2+ is found near the surface, which results in release of some carboxylic material through a reaction. Furthermore, Mg2+ tends to be more active at high temperatures, substituting for Ca2+, and SO42− therefore tends to be less active due to its reaction with Mg2+. Hiorth et al. [6,7,8] also suggested that rock dissolution is an alternative mechanism for incremental oil recovery via smart water flooding.
Several researchers have observed that the salinity is the most important parameter controlling the recovery in carbonate formations; it has been reported that by decreasing the salinity of the injection water, oil production has improved [9,10,11,12,13,14,15]. For example, Yousef et al. [14] observed an increase in the recovery of oil of up to 18% when stepwise dilutions of seawater were injected in tertiary mode into carbonate core samples. Many experimental works have confirmed the role of potential determining ions (SO42−, Ca2+ and/or Mg2+) in the efficiency of LSW flooding. Awolayo et al. [16] carried out a series of experiments on carbonate core plugs to determine the effects of injection of solutions of seawater that varied in terms of their sulfate ion ratios (0.5, 1, 2, 4, 8) on the recovery of oil. The results showed that increasing the sulfate concentration in seawater alters the wettability of the CBR system toward a less oil-wet state. The same behavior was observed by Karimi et al. [17,18] and Rashid et al. [15].
The concentrations of Ca2+ and Mg2+ ions also affect alterations in wettability by smart water; the presence of these cations together with sulfate ions improves recovery [19]. Karimi et al. [17] performed contact angle measurements on oil-wet calcite surface samples, using different synthetic solutions: formation brine, formation brine diluted by 100 times, and diluted brine spiked with sulfate ions, or magnesium ions, or both. The results showed that both Mg2+ and SO42− ions can act as agents altering the wettability of the calcite surface. The contact angle was lowered significantly when both ions were added to the diluted brine. The authors concluded that the performance of the surface treatment increases when both magnesium and sulfate ions are present. Hence, to achieve successful smart water flooding, the concentration of active ions needs to be adjusted.
Another parameter that should be considered is the initial condition of the reservoir rock (i.e., original wettability). Wettability is considered to be a significant factor affecting the efficiency of water flooding and oil recovery, and large differences in ultimate oil recovery are seen depending on the rock wettability. Amott [20] conducted several water flooding tests to correlate oil recovery with wettability, and observed that oil recovery was low in very strong water-wet cores and very strong oil-wet cores, while the highest oil recovery was achieved in the range of neutral wettability. Zhou et al. [3] performed 50 water flooding tests to examine the correlation between oil recovery and wettability. Oil recovery was highest for cores with neutral wetting conditions.
Hadia et al. [13] investigated the effects of initial wettability on oil recovery during low-salinity water flooding as a secondary process. They performed core flooding experiments on four Berea outcrop core samples under ambient conditions, with four different initial wettability conditions ranging from water-wet to oil-wet. They concluded that the cores with an initial neutral-wet condition showed the highest recovery compared to the other conditions. Oil recovery decreased as wettability changed from neutral-wet to oil-wet, as shown in Figure 1. Generally, the wettability of the rock is an essential parameter that affects the recovery of oil during any process involving CBR interactions such as low-salinity water flooding. Rashid et al. [15] also used contact angle measurements and spontaneous imbibition tests for carbonate rock to show that the initial wettability of the rock plays a significant role in smart water optimization.
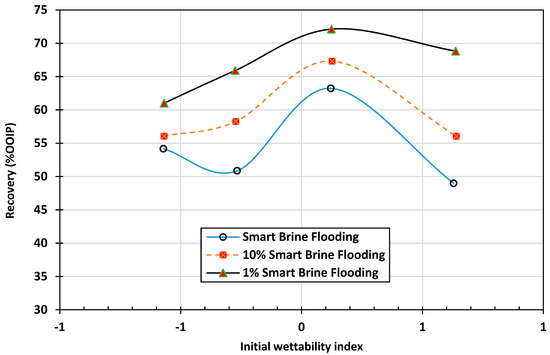
Figure 1.
Relationship between initial core wettability and secondary water flood oil recovery with injection of high- and low-salinity brines (Hadia et al., 2013) [13]. SB = Smart Brine. Recovery is higher in the range of neutral wettability in all cases and smart water flood (1% SB) is superior compared with the other two water floods.
As discussed above, sandstone reservoirs were the focus of several studies on the effect of initial wettability on oil recovery. However, very few studies have been reported on carbonates [21,22,23], and the investigation process has yet to give rise to a definitive conclusion regarding carbonate reservoirs. The potential of LSW in carbonate reservoirs has been proven and it was observed that wettability alteration is the main mechanism of LSW/smart water injection.
In this research work, we focus on the relationship between the initial wettability of carbonate rock and oil recovery by LSW, and the ways in which initial wettability affects the performance of the LSW technique. An experimental investigation was carried out using contact angle measurements and Zeta potential measurements for carbonate samples with different initial wettability conditions ranging from strongly water wet to preferentially water wet, neutral wet, oil wet, and strongly oil wet. Four synthetic brines with different salinities and ion concentrations were also used to study the effect of initial wettability on the performance of the designed brines. pH measurements and EDS analysis were also used to examine and analyze the mechanism underlying the wettability alteration. Some mechanism(s) were also proposed to justify the wettability alteration process in each case. Results obtained from this study can be used in design, execution, and optimization of smart water flood processes to maximize oil recovery from carbonate reservoirs.
2. Materials and Methodology
2.1. Fluids
Four different synthetic brines were used in this study, including a base brine (HSal) containing 40 (g/L) NaCl and a diluted base brine (LSal) with 5 (g/L) NaCl. The other two brines contained 0.1 mol/L of Mg2+ in one, and 0.1 mol/L of Mg2+ and SO42− in the other. Na2SO4 and MgCl2 salts were the ion suppliers for SO42− and Mg2+, respectively, as shown in Table 1. The main idea was to investigate the effects of salinity and active ions on wettability alteration for different initial wettability conditions. Deionized Water (DIW) was used for brine preparation and dilution.

Table 1.
Salt concentration in the brines used in the experiments. Hsal = high-salinity brine, Lsal = low-salinity brine, SW1 = Smart Water 1, SW2 = Smart Water 2.
The pH values of the brine solutions were measured before and after the treatment of the rock surfaces. A SevenMulti S47 dual pH/conductivity meter (Mettler-Toledo, Columbus, OH, USA) was used for this purpose (accuracy = ±0.002). Crude oil was obtained from an Omani carbonate reservoir. The initial pressure and temperature of the reservoir were 13,600 kPa and 90 °C, respectively. The API of the oil was 28°, with a viscosity of 7.99 cP.
2.2. Carbonate Rock Samples
Thin polished slices from the carbonate reservoir in Oman were prepared for contact angle measurements. Table 2 presents the X-ray powder diffraction (XRD) analysis of the rock samples.

Table 2.
X-ray powder diffraction (XRD) analysis of calcite slices.
The XRD also shows that most of the carbonate rock sample is composed of calcite.
2.3. Contact Angle Measurements
There are different methods widely used for determining wettability of a CBR system including: contact angle measurement, Amott wettability test, microinjection method [24], and US Bureau of Mines (USBM) technique. The contact angle method is perfect for our tests as we want to be able to compare the wettability condition before and after the treatment. The CA was measured by the system shown schematically in Figure 2. Prior to contact angle measurement, calcite surfaces were polished to remove the fluid from the surface to give a smooth and uniform surface. They were then washed using a mixture of toluene and methylcyclohexane. Finally, they were dried overnight at 60 °C. The calcite surface was then placed at the top of the test cell in contact with the water. A droplet of the oil was released from the bottom to move and was attached to the rock surface. Hence, we faced a CBR system at equilibrium. The shape of the oil droplet in the CBR system was monitored by a microscopic camera. The CA was measured for each sample at different points and the average CA was calculated using at least three measurements. All CA data reported in this paper are this average value for each case.
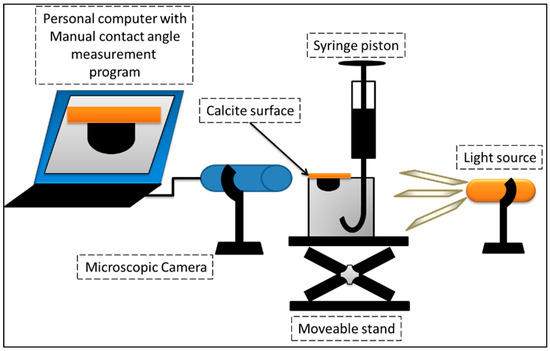
Figure 2.
Schematic diagram of the contact angle (CA) measurement system.
The investigation of the effect of initial wettability on the performance of LSW for carbonate rock was carried out using contact angle measurements and Zeta potential measurements. A primary test was done to detect the effect of aging time on calcite wettability. Two groups, each of ten calcite slices, were first aged in high-salinity (Hsal) and low-salinity (Lsal) brines for three days at 90 °C to mimic the conditions of the original rock surface in contact with the formation brine. Contact angle (CA) measurements were then carried out to determine the initial wettability of the surfaces after aging. Following this, all slices were aged in crude oil at 90 °C to restore the original wettability under the conditions in the reservoir. During the aging period, CA measurements were collected every 20 min to produce a graph of CA versus aging time in oil. Every 20 min, samples were taken out of the aging water. Fluid was removed from the surface to provide a smooth surface for measurement. CA was measured, and the samples were cleaned and put back to the aging cell.
Based on the results of the primary test, five aging periods were selected to examine five conditions of wettability (from water-wet to oil-wet). For each condition, four calcite slices were collected, giving 20 rock samples in five initial wettability categories (IWC). To investigate the effect of smart brines on mechanism of wettability alteration, four slices for each IWC were aged in four samples of brine, as shown in Table 1, for two days at 90 °C. Following this, the slices were washed with DIW and dried in air.
2.4. Energy-Dispersive X-ray Spectroscopy
Energy-dispersive X-ray spectroscopy (EDS) was utilized in the analysis of the elemental composition of the aged calcite surfaces in both brines and crude oil. For this test, the same procedures were carried out to treat the carbonate surface as for the contact angle measurement. A combination of EDS and scanning electron microscopy (SEM) (JEOL Model JSM-7600 F field-emission SEM system) was used for the EDS analysis. Before measurements were taken, all surfaces were coated with platinum to avoid charging, since the samples are non-conductive.
3. Results and Discussion
In this section, the effects of the initial wettability of the calcite samples on the performance of four different brines, three of them are smart considering the effects of salinity and the Mg2+ and SO42− ions are discussed. The mechanism of the wettability alteration process was investigated using measurements of brine properties before and after CA measurement and energy-dispersive X-ray spectroscopy (EDX) analysis. The technique used to measure CA was utilized to determine the wettability of calcite surfaces in presence of Hsal and three different smart brines, listed previously in Table 1 as Lsal, SW1, and SW2. The average initial CA of the calcite samples was 45°, indicating a strong water-wet state.
To prepare calcite samples with different initial wettability, samples were aged in Hsal and Lsal brines. They were then aged in crude oil to investigate the wettability modifying behavior. A graph of CA in degrees versus time in minutes is presented in Figure 3 and shows the progress of wettability alteration for the two groups. At the early stages, the CA of the group aged in Hsal brine ranged from 45–90°; while, the samples aged in Lsal brine had values ranging from 0° to 90°. This procedure was designed to prepare samples with complete water-wet conditions.
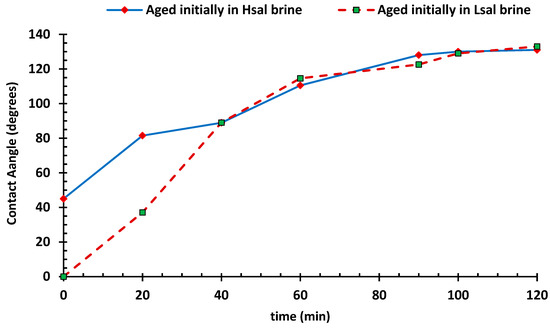
Figure 3.
Contact angle of the oil/brine/rock versus time for primary aged calcites in high-salinity brine (Hsal) and Low-salinity brine (Lsal).
The differences in the CA for the Hsal and Lsal brines can be explained by the mechanism of calcite dissolution illustrated in Figure 4. During the aging process in Hsal and Lsal brines, calcite (CaCO3) dissolution takes place. Consequently, Ca2+ ions are present as free ions in the solution, leaving CO32− as binding sites on the surface. For the Hsal case in Figure 4a, Na+ and Cl− ions are present in high concentrations, giving Na+ ions a chance to accumulate on the surface since they are attracted to CO3−. This process can also be considered to involve a strong cation exchange between Ca2+ at the surface and Na+ within the brine. This mechanism changes the surface charge to slightly positive, which increases the attraction of negative carboxylic groups. Since there is no difference in CA between the clean samples and the Hsal pre-aged samples, it can be speculated that aging in Hsal resembles the initial condition of the sample.
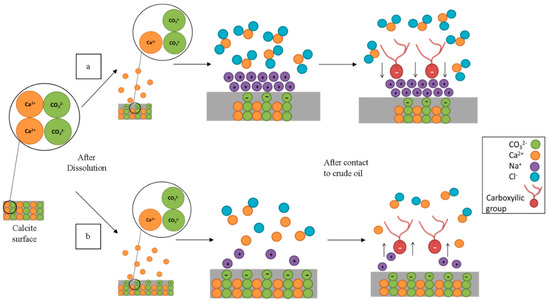
Figure 4.
Schematic model of the proposed mechanism for wettability alteration by (a) Hsal and (b) Lsal brines.
According to Lashkarbolooki et al. [19], calcite dissolution increases as the salinity of NaCl brines decreases; for the Lsal case shown in Figure 4b, the dissolution of calcium carbonate is larger and more negative sites are exposed on the surface, thus increasing the electrostatic repulsive force. Hence, negative carboxylic compounds are repulsed, which leads to a lower CA for Lsal-aged surfaces.
As the aging period in oil elapsed, the CA increased toward an oil-wetting condition. This behavior may be related to the strong adsorption of polar organic components of the crude oil on the calcite surfaces [17]. After 40 min of aging in oil, the CAs for the two groups became similar, showing a similar adsorption process of polar molecules. The wettability alteration in this case was relatively fast, due to the presence of long acidic chains of molecules in the oil sample. Jabbar et al. [25] emphasized the role of polar compounds in oil and acidity in the wettability alteration of carbonate rocks and found that more oil-wet conditions are achieved as the length of acid chain increases.
Various samples were selected, as shown in Table 3, to mimic water-wet, neutral-wet, and oil-wet conditions. Long-term aging was also added to investigate whether the CA changed further when the aging time was increased to one month.

Table 3.
Oil aging time for carbonate rock samples with 5 different initial wettability conditions.
EDS analyses were performed to study the initial wettability in more detail. Based on the data, the percentage of elements at the surface such as carbon, calcium, and oxygen were calculated. The highest adsorption of an organic compound was achieved in Case 5, as shown by a percentage carbon weight of 31.3 wt. % as compared with 19.7 wt. % in the strong water-wet state. The highest weight percentage of oxygen of 48 wt. % was observed in Case 1; this is evidence for the proposed calcite dissolution mechanism shown in Figure 5, where higher dissolution led to more CO32− ions being exposed at the surface as binding sites.
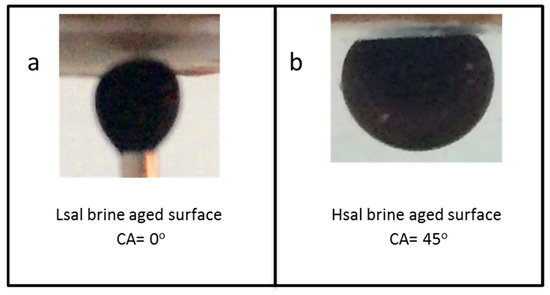
Figure 5.
Images of pendant oil drops during the contact angle measurements of non-aged calcite in oil. (a) Cases 1 = strongly water wet and (b) Case 2 = preferentially water wet.
3.1. Effect of Initial Wettability on the Performance of the Designed Smart Waters
Samples from all cases listed in Table 3 were aged in the treated brines for two days at 90 °C to investigate the effect of the brines on the mechanism of wettability alteration. Following this, the surfaces were collected and prepared for CA measurement.
3.1.1. Strong (Case 1) and Preferentially Water-Wet (Case 2) Conditions
The data in Table 4 show the results of the CA for calcite surfaces aged in the brines. The CA was initially 0° (Case 1) when the samples were aged in Lsal brine (Case 2), as shown in Figure 5a. These samples were treated as being representative of strong water-wet samples. After aging in the Hsal brine for two days, the CA increased from 0° to 31.3°, while this remained at zero after aging in the other brines (Lsal, SW1, and SW2). Lsal and other smart brines had no effect on strongly water-wet samples. When the surfaces were aged in the Hsal brine, the CA was initially around 45°, as depicted in Figure 5b, and remained at the same value after aging in Hsal brine for two days. The same quantity decreased from 45° to zero after aging the samples in Lsal, SW1, and SW2.

Table 4.
Contact Angle values of the non-aged calcite surfaces in oil (Case 1 = strongly water wet and Case 2 = preferentially water wet).
3.1.2. Neutral-Wet (Case 3), Oil-Wet (Case 4), and Strongly Oil-Wet (Case 5) Conditions
Table 5 shows CA values for the samples of Case 3 after aging in different brines for two days. The use of Hsal and Lsal brines altered the angle from 82.6° to 65.6° and from 87.7° to 46°, respectively. With smart waters (SW1 and SW2), the CA decreased to approximately similar values: 25.3° for the former and 24.3° for the latter. For these cases, the Wettability Alteration Index (WAI) is calculated as:
where

Table 5.
Contact Angle values of Case 3 (neutral wet) calcite surfaces aged in crude oil and treated for two days in brine.
- is the aged in crude oil CA of the sample after aging in oil;
- is the CA after aging in different brines;
- is the CA after aging in brine at the start of the test.
A WAI value of around zero indicates that no wettability alteration occurs because of brine, the larger the value means more alteration of wettability. The bar graph in Figure 6 shows values of WAI for different brines. Hsal brine has the lowest WAI value of 0.45 as it does not alter the CA by a large amount, while the highest values are shown for SW1 and SW2, which increase to 1.43 and 1.51 respectively. Figure 7 shows samples of oil drops during CA measurements for Case 3 (calcite samples).
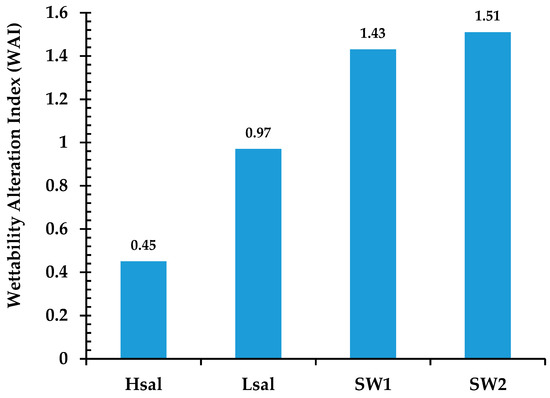
Figure 6.
Wettability Alteration Index for Case 3 (neutral wet) calcite surfaces affected by different brines.
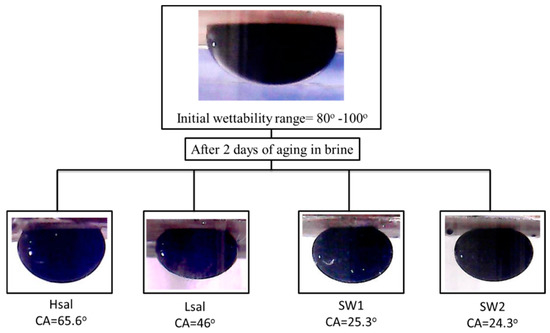
Figure 7.
Images of oil droplets during CA measurements of Case 3 (neutral wet) calcite samples treated with different brines.
Alterations in the CA of oil-wet samples are shown in Table 6 and Figure 8. The CA decreases to values of 91.5°, 77°, 54°, and 36° after aging the samples (Case 4) in Hsal, Lsal, SW1, and SW2 brines, respectively, for two days. The WAI values shown in Figure 9 indicate that the value for the SW2 brine is the highest. Hsal brine still gives the lowest value and has limited ability to alter wettability. After two weeks of aging, the CA results were approximately similar to those after two days, especially for the Hsal and Lsal brines. The WAI for SW1 increased from 0.89 after two days to 1.2 after two weeks, similarly to the value for SW2. Figure 10 and Figure 11 show sample images of oil drops during CA measurements for Case 4 calcite samples treated in different brines after two days and two weeks.

Table 6.
CA values of samples of Case 4 (oil wet) in contact with brines for two days and two weeks.
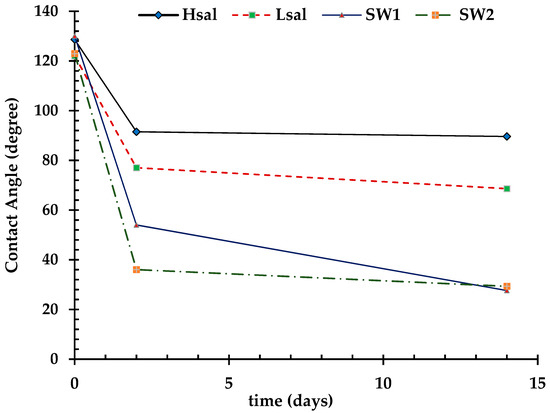
Figure 8.
Change in CA after treatment of Case 4 (oil wet) calcite surface by different brines for two days and two weeks.
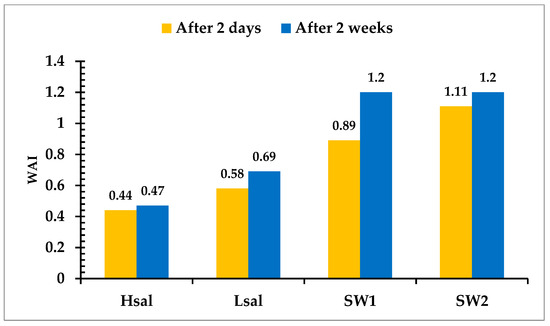
Figure 9.
Wettability Alteration Index for Case 4 (oil wet) calcite surface treated with different brines.
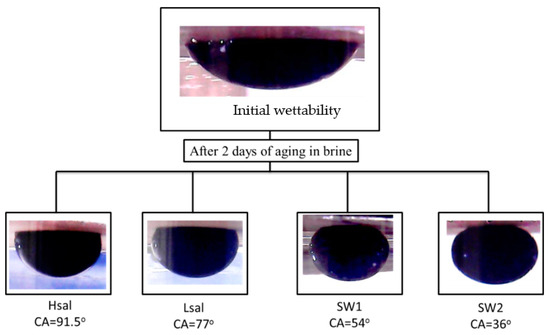
Figure 10.
Sample images of pendant oil drops during CA measurements of Case 4 (oil wet) calcite samples in crude oil and treated with different brines for two days.
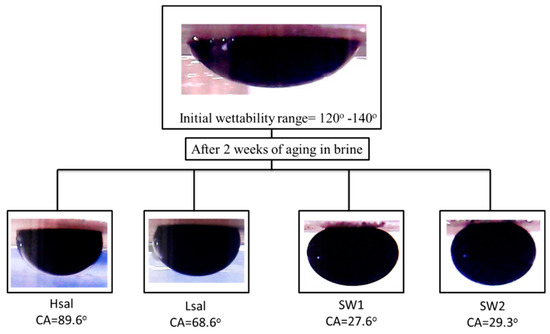
Figure 11.
Sample images of pendant oil drops during CA measurements of Case 4 (oil wet) calcite samples in crude oil and treated with different brines for two weeks.
For Case 5 (strongly oil wet), after one month of aging in crude oil, the CA reached the range of 120–140°, similarly to that after two days of aging, as shown in Table 7. After aging the samples in brines for two days, the Hsal brine did not show a great deal of wettability alteration; the CA decreased from 133.3° to 119.4° and the sample had a WAI value of 0.16, as shown in Figure 12 and Figure 13. On the other hand, CA decreased to values of 71°, 48.7°, and 34.1° after aging the samples in Lsal, SW1 and SW2, respectively. After two weeks, CA values were 113°, 65.5°, 35.2°, and 30.5° after aging the samples in Hsal, Lsal, SW1 and SW2 brines, respectively. It is obvious that the Hsal brine had a much lower ability alter the wettability even with a longer aging period, whereas the other brines changed the wettability to a more water-wet condition.

Table 7.
Contact Angle values of Case 5 (strongly oil wet) calcite surfaces in crude oil and treated with brines for two days and two weeks.
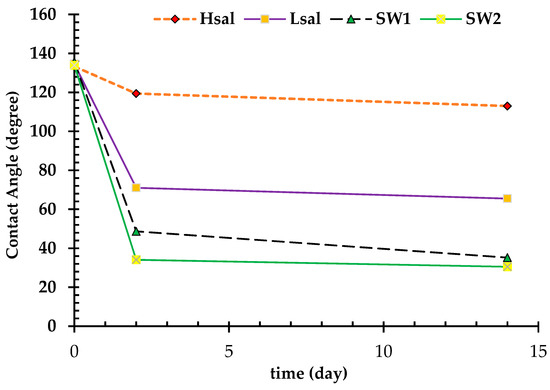
Figure 12.
Change in CA after treatment of Case 5 (strongly oil wet) calcite surface with different brines for two days and two weeks.
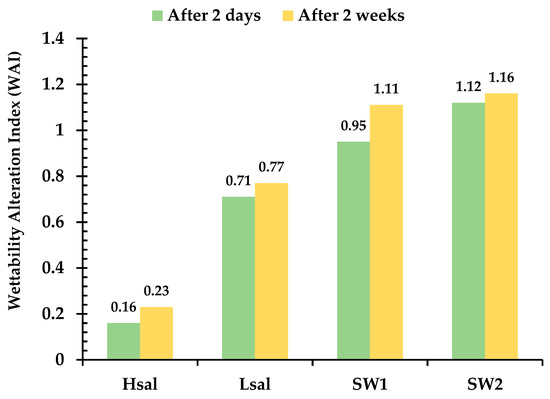
Figure 13.
WAI for Case 5 (strongly oil wet) calcite surface aged using different brines.
3.2. Effect of NaCl Aqueous Solutions (Hsal and Lsal) on the Wettability Alteration Process
Based on the results, the brine with high-salinity NaCl aqueous solution could only weakly alter the wettability of the sample, whereas the low-salinity NaCl brine had the ability to alter the wettability rather than a neutral wetting state or an oil-wet state to more water-wet state. Lashkarbolooki et al. [19] reported similar results, noticing that the CA decreased as the salinity of NaCl solutions decreased. They found that a brine of 45,000 ppm NaCl could change the CA from 138.8° to 98.1°, and a brine of 5000 ppm NaCl changed the CA from 148.4° to 35.6° after 10 days of aging.
Three mechanisms are proposed here for the wettability alteration process: the salting-out effect, double layer expansion, and microscopic dissolution of carbonate rock:
- During the salting-out mechanism, the solubility of a nonelectrolyte substance in water decreases with increases in salt concentration in brine. Firstly, the non-electrolyte carboxylate molecule has lower solubility in brine with higher salt concentrations. As the salinity of the brine increases, the salting-out effect leads to a decrease in the solubility of carboxylic compounds, resulting in the deposition of more organic materials as the thin oil phase near the rock surface increases the chance of bonding between the positively charged carbonate surface and negatively charged carboxylic components. Conversely, a lower salt concentration increases the solubility of carboxylic groups. Active ions with negative charges tend to be attracted to the positive calcite surface and substitute for the calcium ion, which is bonded to the negative molecules of carboxylates; hence, the wettability of the surface becomes less oil-wet.
- Secondly, the term “double layer” refers to the bulk of the ions that are close to the surface, which form due to the interaction between the charged rock surface and the brine. The distribution of charges at the surface is composed of opposite charges (counter-ions) attracted to the surface and other equal charges (co-ions) repulsed from the surface in an aqueous medium. Because of this charge accumulation, an electrical layer is formed. During LSW in carbonate rocks, the surface tends to be less positive, due to the ionic exchange between cations in the aqueous brine and calcium ion. The substitution of calcium ions with the aid of a solvation process results in the detachment of adsorbed oil. This leads to double layer expansion, and ultimately results in the wettability alteration (i.e., more water wet) of the rock surface, as illustrated in Figure 14.
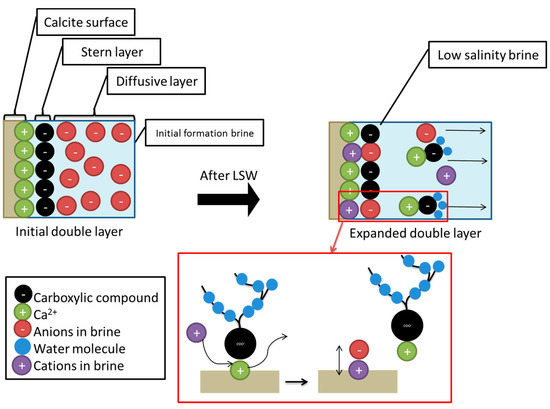 Figure 14. The mechanism of double layer expansion.
Figure 14. The mechanism of double layer expansion. - The final step is the dissolution of calcite (CaCO3), which takes place as the low-salinity brine invades the carbonate surface. For the Hsal and Lsal samples, the lack of calcium ions promotes the dissolution of calcite, and thus oil is expelled from the surface.
For Cases 4 (oil wet) and 5 (strongly oil wet), the values of the WAI for Lsal ranged from 0.58 to 0.77 after both two days and two weeks. This indicates that the process of wettability alteration is completed within about two days. It also shows that there is a limit for wettability alteration by Lsal brine, since this did not exceed a value of about 0.8. For Hsal brine, the WAI was approximately the same for Cases 3 (neutral wet) and 4 (oil wet), showing that the adsorption of carboxylic compounds did not reach wettability equilibrium although the initial wettability was different. It therefore appears that the initial wettability does not affect the wettability reversal by Hsal brine. All binding sites on the surface of calcite are fully filled after one month of aging in crude oil, even if the CA does not increase further after two days of aging in crude oil.
3.3. Effect of Smart Waters (SW1 and SW2) on the Wettability Alteration Process
The aqueous solution SW1 contains only Mg2+ as an active ion for wettability alteration, while SW2 contains both Mg2+ and SO42− as potential ions. As the CA was altered from 90.8° to 25.3° in Case 3 (neutral wet) and from 130° to 27.6° in Case 4 (oil wet) by SW1, this reveals that the magnesium ion can alter the wettability of the calcite surface in the absence of the sulfate ion. This result agrees with the work of Rashid et al. [15], who observed a reduction in CA from 148° to 13°. These authors concluded that the presence of the sulfate ion is not important for wettability alteration by Mg2+, although the presence of the SO42− ion increases the concentration of Mg2+ near the carbonate surface via a reduction in the electrostatic repulsive force. The pre-adsorbed carboxylate molecules decrease the electrostatic repulsive force, allowing Mg2+ to be sited near the surface. In the initial stages, the solubility equilibrium between solid calcite and its component ions, Ca2+ and CO32−, is established as expressed by the chemical equation:
CaCO3(s) ↔ Ca2+(aq) + CO32−(aq)
Then, the magnesium ion starts to disturb this equilibrium through its reaction with some of the CO32− ions to form MgCO3 precipitate. Consequently, the chemical reaction is shifted to the right. As a result, calcite dissolution is promoted, which generates an excess of calcium ions over the calcite surface. These calcium ions can react with adsorbed carboxylic groups and remove them from the surface [26]. Finally, the dissolution of calcite and desorption of organometallic complexes out of the calcite surface lead to a wettability alteration for the rock to a more water-wet state. Carbon dioxide gas may be produced in negligible amounts, causing only a slight reduction in pH.
The chemical reaction for this mechanism is suggested to be as follows:
MgCl2(aq) + CaCO3(s) ↔ MgCO3(s) + CaCl2(aq) + H2O(l) + CO2(g)
It is generally believed that magnesium ions can change the wettability of an oil-wet calcite surface. However, the correct mechanism(s) of wettability change is not completely understood. Petrovich and Hamouda [27] observed an increase in calcium ions and a decrease in magnesium ions in the produced water and linked this to the substitution of Ca2+ ions on the calcite surface by Mg2+ ions in the injected water. This observation can be accompanied with the chemical reaction in Equation (3), as Mg2+ ions react with CaCO3 and result in MgCO3 precipitate. Hence, calcium ions are detached by the reaction and remove carboxylic compounds from the surface, and the wettability is changed from oil-wet to water-wet.
SW2 has a significant effect on wettability alteration for all wettability ranges. As depicted in Figure 15, the electrostatic repulsive force is reduced by the adsorption of sulfate ions onto the positively charged sites of the calcite surface. This phenomenon leads to a higher magnesium ion concentration close to the surface. The adsorbed sulfate ions reduce the density of the positive charge on the surface, resulting in desorption of loosely adsorbed carboxylates. This mechanism means that the wettability alteration by magnesium and sulfate ions is faster than that of magnesium ions alone, and SW2 therefore shows better results in terms of wettability alteration than SW1.
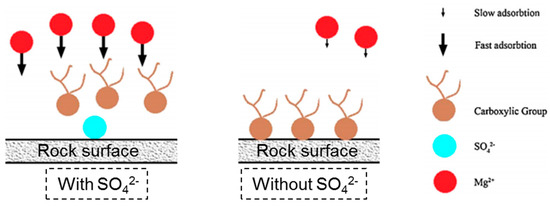
Figure 15.
Role of the sulfate ion in the wettability alteration process by “smart water” in carbonates.
It is clear that the WAI is approximately the same for SW2 after both two days and two weeks. This indicates that the wettability alteration process by SW2 is complete within two days. SW1 has a lower WAI after two days than after two weeks; this can be associated with an incomplete process of wettability reversal due to a lack of the sulfate ion, which has a catalytic role during the alteration of surface wettability by the magnesium ion. SW1 has a similar ability to alter the wettability to that of SW2, but it is slower, reaching a similar value to that of SW2 after two weeks.
In terms of the WAI for Case 3 (neutral wet) calcite samples, SW1 and SW2 had approximately similar values, which were higher than those for Cases 4 and 5 after aging for two days. This shows the effect of initial wettability on the success of smart brines. The oil aging period for Case 3 (neutral wet) is shorter than that for Cases 4 (oil wet) and 5 (strongly oil wet), and the adsorption of polar compounds is therefore lower, as reflected by the lower CA for Case 3 (neutral wet). This indicates that the wettability alteration process is faster when the initial wetting condition is intermediate or neutral.
pH measurements were conducted before and after the treatment of the calcite surfaces in different brine aqueous solutions. The initial and final pH values of the brines were recorded for all cases. pH values before and after aging in brine for one day at the start of the main test range from 5.55 to 9.13 and from 5.71 to 9.52 for Hsal and Lsal brines, respectively. There is a significant increase in the pH values, from a slightly acidic condition to a basic condition for both brines (Hsal and Lsal). The change in pH value was higher in the case of the Lsal brine. This increase can be related to the dissolution of calcite (CaCO3). Because of this mechanism, a large amount of hydroxide ions (OH−) may be released based on the chemical equations below, thus increasing the pH values. As the salinity of the solution decreases, the invasion of the calcite surface increases and the dissolution becomes larger.
CaCO3(s) ↔ Ca2+(aq) + CO32−(aq)
CO32−(aq) + H2O(l) ↔HCO3−(aq) + OH−(aq)
At low pH values, the calcite surface tends to be positively charged, due to the presence of calcium ions. In contrast, at a higher pH, the concentration of carbonate ions (CO32−) is high, which makes the charge of the surface become negative [28]. Hence, more repulsions occur between the surface and carboxylic groups as the pH increases. The same trend of an increase in pH was observed when calcite samples with different initial wettability were aged in the Hsal and the Lsal brines, as shown in Table 8.

Table 8.
Initial and final pH values (after treatment of calcite surfaces with different brines) of the treating brines.
The decrease in the pH values of SW1 and SW2 is due to the reaction expressed in Equations (3) and (6).
CO2 + H2O ↔ 2H+ + CO32−
The production of carbon dioxide results in reduction of pH values, even if this reduction is small. This can be justified by the small amount of CO2 produced by the equilibrium reaction. The precipitation of magnesium carbonate was recognized as white powder at the top of the calcite surface in the form of dendritic magnesite; the MgCO3 mineral (Figure 16).
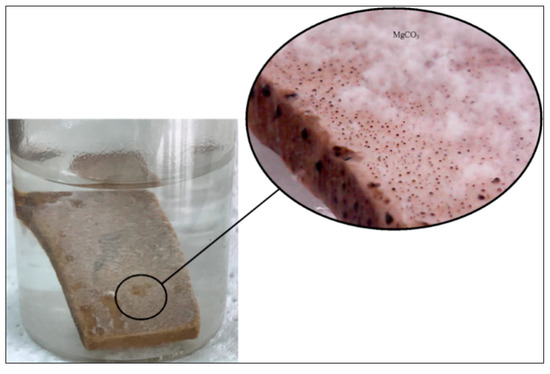
Figure 16.
Precipitation of magnesite during the experiments.
Our main objective here was to correlate the oil recovery under various synthetic brines with the initial wettability of the formation. As mentioned earlier, Figure 1 shows the relationship between the initial wettability effect and oil recovery during water flooding for the Berea sandstone. It is clear that the Recovery Factor (RF) increases as the initial wetting state changes from water-wet to neutral wet and decreases as the initial wettability changes from a neutral-wet to an oil-wet condition. This behavior can be associated with the results of the CA for the Case 1 samples (strongly water wet). It can be concluded that, it is not beneficial to use Lsal or other smart brines for wettability alteration process when the initial wetting state is water-wet as no change was observed in wettability during the experiments. In contrast, Hsal brine can alter the wettability back to a more neutral-wet state, which can be a good choice in water flooding experiments due to its higher recovery rate. For Case 2 (preferentially water wet), Hsal is better than Lsal and the smart brines (SW1 and SW2) as it can keep the samples at the same CA values. Therefore, for these two cases (Case 1 and Case 2 or strongly and preferentially water wet), the most favorable wettability alteration can be achieved by the injection of Hsal brine.
For Case 3 (Neutral wet) samples with intermediate or neutral wetting state, Hsal brine would be the best option from an economical point of view to keep the CA within a range similar to the initial wettability. Other brines tend to decrease the CA significantly, which can decrease the water permeability and cause water blockage leading to lower oil recovery. For the samples with initially oil-wet states in Cases 4 and 5, Lsal and the smart brines (SW1 and SW2) are the best options for incremental oil recovery. These brines tend to decrease the wettability of the reservoir from oil-wet to more neutral and water-wet conditions. In Case 5, Hsal brine is not a good choice for the water flooding of a strongly oil-wet reservoir. LSW is effective in oil-wet carbonate reservoirs, as wettability changes from an oil-wet state to a water-wet state. Ultimately, this reaches an unfavorable water-wet condition that decreases the recovery. Hence, it would be more beneficial to switch to high-salinity water flooding to move back to a natural state after the injection of LSW. In practice, based on the results obtained from this research work, some scenarios can be developed incorporating different smart brines to manage the reservoir rock wettability to facilitate the flow conditions for oil and maximize the oil recovery through smart water flooding in carbonate oil reservoirs.
4. Conclusions
To further our understanding of the effects of salinity and active ions on wettability alteration for different initial wettability conditions in carbonate reservoir a series of contact angle and pH measurements along with energy-dispersive X-ray spectroscopy were conducted on carbonate rock samples obtained from an oil field in Oman. The wettability of the prepared samples ranges from strongly water wet to preferentially water wet, neutral wet, oil wet, and strongly oil wet (5 cases or groups). The samples were tested using 4 different synthetic brines, namely high salinity (Hsal), low salinity (Lsal), and smart waters 1 and 2 (SW1 = a Mg brine, and SW2 = a Mg and sulfate brine). The following 4 main conclusions can be drawn from this experimental investigation:
- Low-salinity brine (Lsal) was more effective than high-salinity brine (Hsal) for the wettability alteration of calcite surfaces at intermediate (neutral) or oil-wet conditions. The smart brine containing only the Mg2+ ion (SW1) was able to alter the wettability of calcite surfaces in intermediate or oil-wet states. The substitution of Ca2+ ions by Mg2+ was the most accepted mechanism as proved by Magnesite (MgCO3 mineral) precipitation during the experiments.
- The sulfate ion played a catalytic role in wettability alteration by the magnesium ion, and the process was faster, as indicated by higher wettability alteration index values. This tends to reduce the electrostatic repulsive force, meaning that Mg2+ is near the surface, and substitute Ca2+ ions bond to carboxylic compounds more rapidly.
- High-salinity brine (Hsal) is a good choice for design of water floods in reservoir rocks with initial wettability in the range of strongly water wet to neutral wet conditions.
- pH measurements showed an increase in Lsal and Hsal after the wettability reversal process. This was interpreted using a calcite dissolution reaction that results in an increase of the hydroxide ion concentration. The pH values of SW1 and SW2 decreased after the wettability alteration process, which can be attributed to the precipitation of Magnesite and CO2 production.
Author Contributions
K.A.-N. conducted the experimental work and analyzed the results. P.P. was the main supervisor of the research, contributed in analysis of the results, and wrote the manuscript. N.M. contributed in experiments design and analysis of the results. A.S. contributed in analysis and discussion of the results and reviewing and editing of the manuscript.
Acknowledgments
We would like to thank two anonymous reviewers for providing critical but fair comments which helped us to improve the quality and clarity of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sheng, J. Enhanced Oil Recovery Field Case Studies, 1st ed.; Gulf Professional Publishing: Houston, TX, USA, 2013. [Google Scholar]
- Sheng, J. Critical review of low-salinity waterflooding. J. Petrol. Sci. Eng. 2014, 120, 216–224. [Google Scholar] [CrossRef]
- Zhou, X.; Morrow, N.R.; Ma, S. Interrelationship of wettability, initial water saturation, aging time and oil recovery by spontaneous imbibition and water-flooding. SPE J. 2000, 5, 199–207. [Google Scholar] [CrossRef]
- Yousef, A.A.; Liu, J.; Blanchard, G.; Al-Saleh, S.; Al-Zahrani, T.; Al-Zahrani, R.; Al-Tammar, H.; Al-Mulhim, N. Smart Water Flooding: Industry’s First Field Test in Carbonate Reservoirs. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 8–10 October 2012. [Google Scholar]
- Fathi, S.J.; Austad, T.; Strand, S. Water-based enhanced oil recovery (EOR) by “Smart Water”: Optimal ionic composition for EOR in carbonates. Energy Fuels 2011, 25, 5173–5179. [Google Scholar] [CrossRef]
- Hiorth, A.; Cathles, L.M.; Madland, M.V. Impact of pore water chemistry on carbonate surface charge and oil wettability. Transp. Porous Media 2010, 85, 1–21. [Google Scholar] [CrossRef]
- Hiorth, A.; Cathles, L.M.; Kolnes, J.; Vikane, O.; Lohne, A.; Madland, M.V. Chemical modelling of wettability change in carbonate rocks. In Proceedings of the 10th Wettability Conference, Abu Dhabi, UAE, 26–28 October 2008. [Google Scholar]
- Hiorth, A.; Cathles, L.M.; Kolnes, J.; Vikane, O.; Lohne, A.; Madland, M.V. A chemical model for the seawater-CO2-carbonate system aqueous and surface chemistry. In Proceedings of the 10th Wettability Conference, Abu Dhabi, UAE, 26–28 October 2008. [Google Scholar]
- Al-Attar, H.H.; Mahmoud, M.Y.; Zekri, A.Y.; Almehaideb, R.A.; Ghannam, M.T. Low Salinity Flooding in a Selected Carbonate Reservoir: Experimental Approach. In Proceedings of the EAGE Annual Conference & Exhibition, London, UK, 10–13 June 2013. Paper SPE 164788. [Google Scholar]
- Al-Shalabi, E.W.; Sepehrnoori, K.; Delshad, M. Mechanisms behind low salinity water injection in carbonate reservoirs. Fuel 2014, 121, 11–19. [Google Scholar] [CrossRef]
- Al-Shalabi, E.W.; Sepehrnoori, K.A. comprehensive review of low salinity/engineered water injections and their applications in sandstone and carbonate rocks. J. Petrol. Sci. Eng. 2016, 139, 137–161. [Google Scholar] [CrossRef]
- Webb, K.J.; Black, C.J.J.; Tjetland, G.A. Laboratory Study Investigating Methods for Improving Oil Recovery in Carbonates. In Proceedings of the SPE International Petroleum Technology Conference, Doha, Qatar, 21–23 November 2005. Paper SPE 10506. [Google Scholar]
- Hadia, N.J.; Ashraf, A.; Tweheyo, M.T.; Torsæter, O. Laboratory investigation on effects of initial wettabilities on performance of low salinity waterflooding. J. Petrol. Sci. Eng. 2013, 105, 18–25. [Google Scholar] [CrossRef]
- Yousef, A.A.; Al-Saleh, S.; Al-Kaabi, A.; Al-Jawfi, M. Laboratory investigation of the impact of injection-water salinity and ionic content on oil recovery from carbonate reservoirs. SPE Reserv. Eval. Eng. 2011, 14, 578–593. [Google Scholar] [CrossRef]
- Rashid, S.; Mousapour, M.S.; Ayatollahi, S.; Vossoughi, M.; Beigy, A.H. Wettability alteration in carbonates during “Smart Waterflood”: Underlying mechanisms and the effect of individual ions. Colloid Surf. A 2015, 487, 142–153. [Google Scholar] [CrossRef]
- Awolayo, A.; Sarma, H.; Al-Sumaiti, A.M. A laboratory study of ionic effect of smart water for enhancing oil recovery in carbonate reservoirs. Presented at the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 26–28 March 2014. Paper SPE 169662. [Google Scholar]
- Karimi, M.; Al-Maamari, R.S.; Ayatollahi, S.; Mehranbod, N. Impact of Sulfate Ions on Wettability Alteration of Oil-Wet Calcite in the Absence and Presence of Cationic Surfactant. Energy Fuels 2016, 30, 819–829. [Google Scholar] [CrossRef]
- Karimi, M.; Al-Maamari, R.S.; Ayatollahi, S.; Mehranbod, N. Wettability alteration and oil recovery by spontaneous imbibition of low salinity brine into carbonates: Impact of Mg2+, SO42− and cationic surfactant. J. Petrol. Sci. Eng. 2016, 147, 560–569. [Google Scholar] [CrossRef]
- Lashkarbolooki, M.; Ayatollahi, S.; Riazi, M. Mechanistical study of effect of ions in smart water injection into carbonate oil reservoir. Process Saf. Environ. Prot. 2017, 105, 361–372. [Google Scholar] [CrossRef]
- Amott, E. Observations relating to the wettability of porous rock. Pet. Trans. AIME 1959, 216, 156–162. [Google Scholar]
- Al-Harrasi, A.; Al-Maamari, R.S.; Masalmeh, S.K. Laboratory investigation of low salinity waterflooding for carbonate reservoirs. In Proceedings of the International Petroleum Conference and Exhibition, Abu Dhabi, UAE, 11–14 November 2012. [Google Scholar]
- Austad, T.; Shariatpanahi, S.F.; Strand, S.; Black, C.J.J.; Webb, K.J. Conditions for a low-salinity enhanced oil recovery (EOR) effect in carbonate oil reservoirs. Energy Fuels 2011, 26, 569–575. [Google Scholar] [CrossRef]
- Alameri, W.; Teklu, T.W.; Graves, R.M.; Kazemi, H.; Al-Sumaiti, A.M. Wettability alteration during low-salinity waterflooding in carbonate reservoir cores. In Proceedings of the SPE Asia Pacific Oil & Gas Conference and Exhibition, Adelaide, Australia, 14–16 October 2014. [Google Scholar]
- Buckman, J.O.; Polson, E.J.; Todd, A.C. Measuring wettability in the environmental scanning electron microscope: A preliminary study using microinjected oil for contact angle measurement and hysteresis. Microscopy and Analysis, September 2014; 13–16. [Google Scholar]
- Jabbar, M.Y.; Al-Hashim, H.S.; Abdallah, W. Effect of brine composition on wettability alteration of carbonate rocks in the presence of polar compounds. In Proceedings of the SPE Saudi Arabia Section Technical Symposium and Exhibition, Saudi Arabia, UAE, 8–11 April 2013. [Google Scholar]
- Tweheyo, M.T.; Zhang, P.; Austad, T. The Effects of Temperature and Potential Determining Ions Present in Seawater on Oil Recovery from Fractured Carbonates. In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 22–26 April 2006. [Google Scholar]
- Petrovich, R.; Hamouda, A.A. Dolomitization of Ekofisk oil field reservoir chalk by injected seawater. In Proceedings of the Ninth International Symposium on Water–Rock Interactions, Taupo, New Zealand, 30 March–3 April 1998. [Google Scholar]
- Gomari, K.R.; Hamouda, A.A. Effect of fatty acids, water composition and pH on the wettability alteration of calcite surface. J. Petrol. Sci. Eng. 2006, 50, 140–150. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).