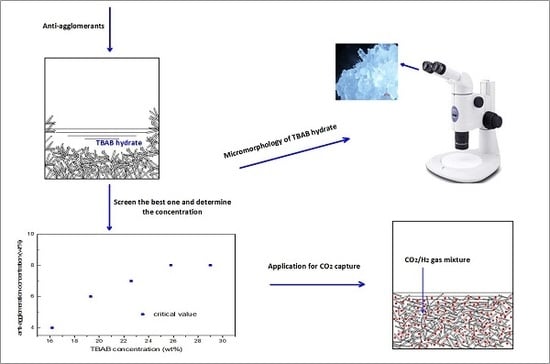
Anti-Agglomerator of Tetra-n-Butyl Ammonium Bromide Hydrate and Its Effect on Hydrate-Based CO2 Capture
Abstract
:1. Introduction
2. Results and Discussion
2.1. Anti-Agglomerant Screening
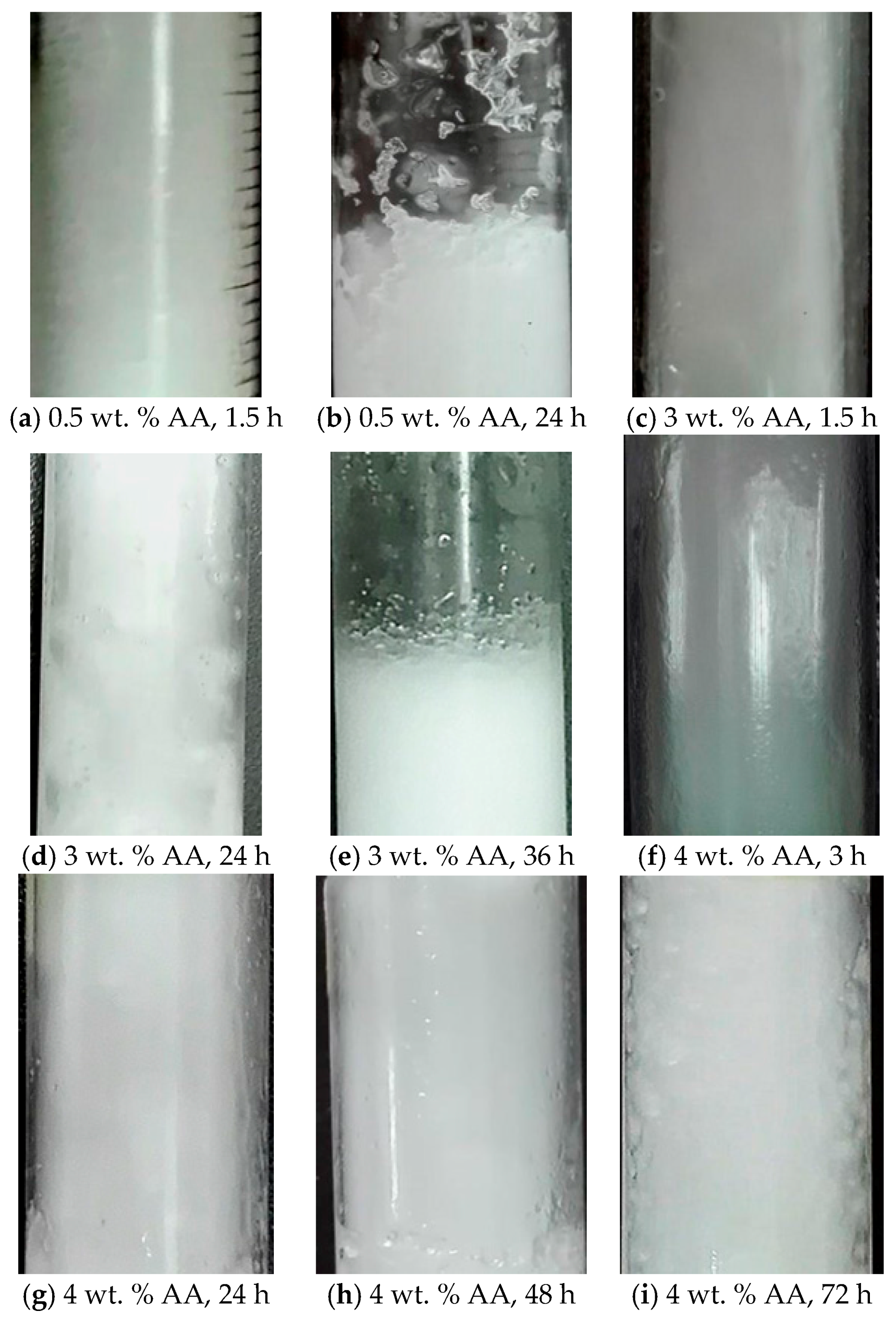
2.2. Effect of Anti-Agglomerant Dosage on Its Anti-Agglomeration Duration
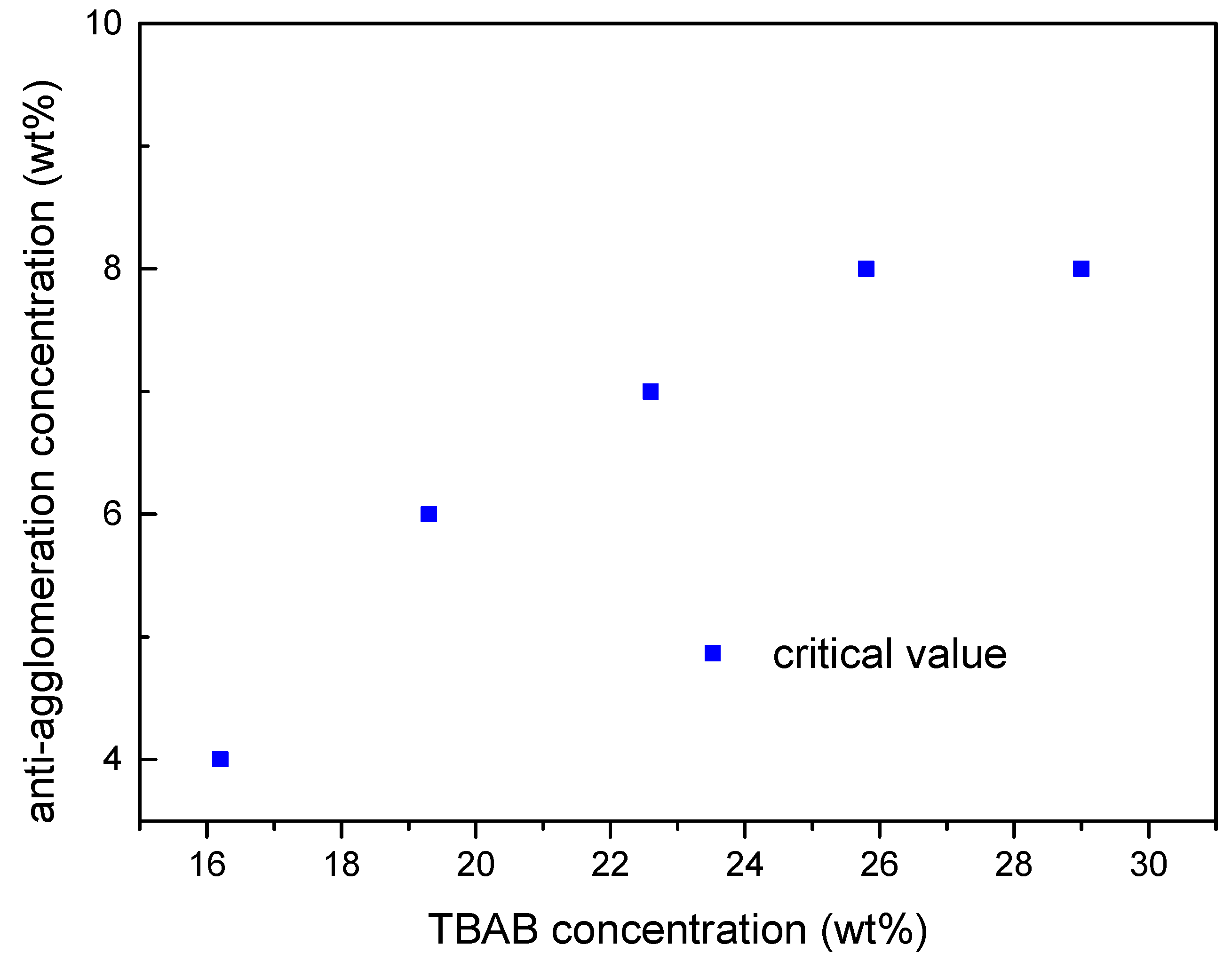
2.3. Effect of TBAB Concentration on AA′s Critical Concentration Needed
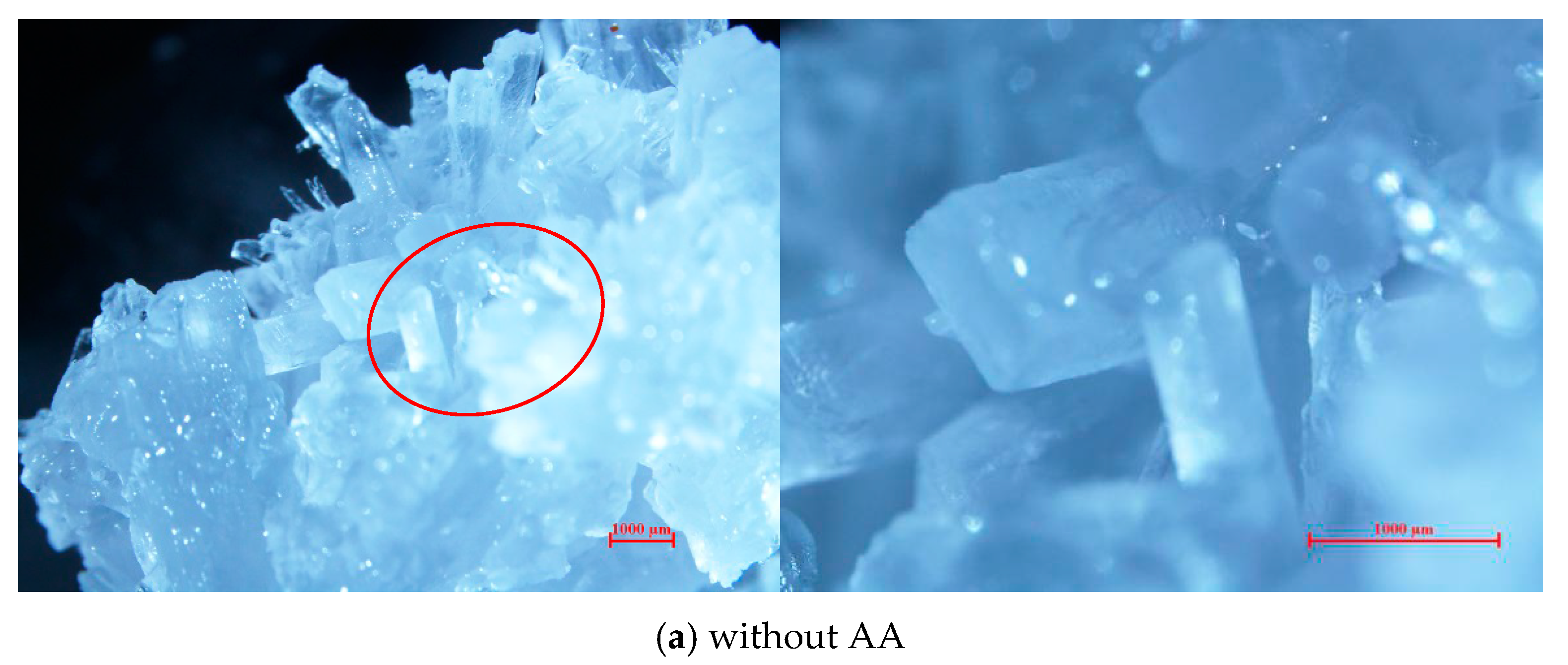
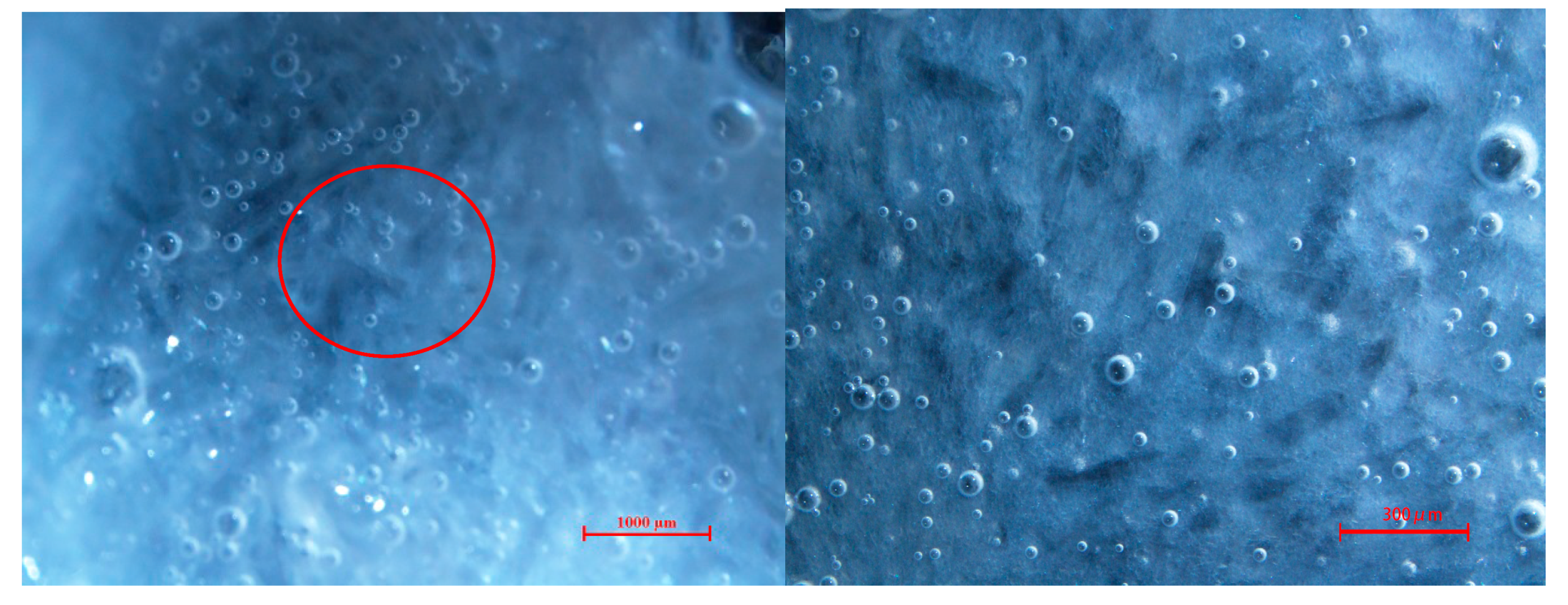
2.4. Micromorphology of TBAB Hydrate Slurry
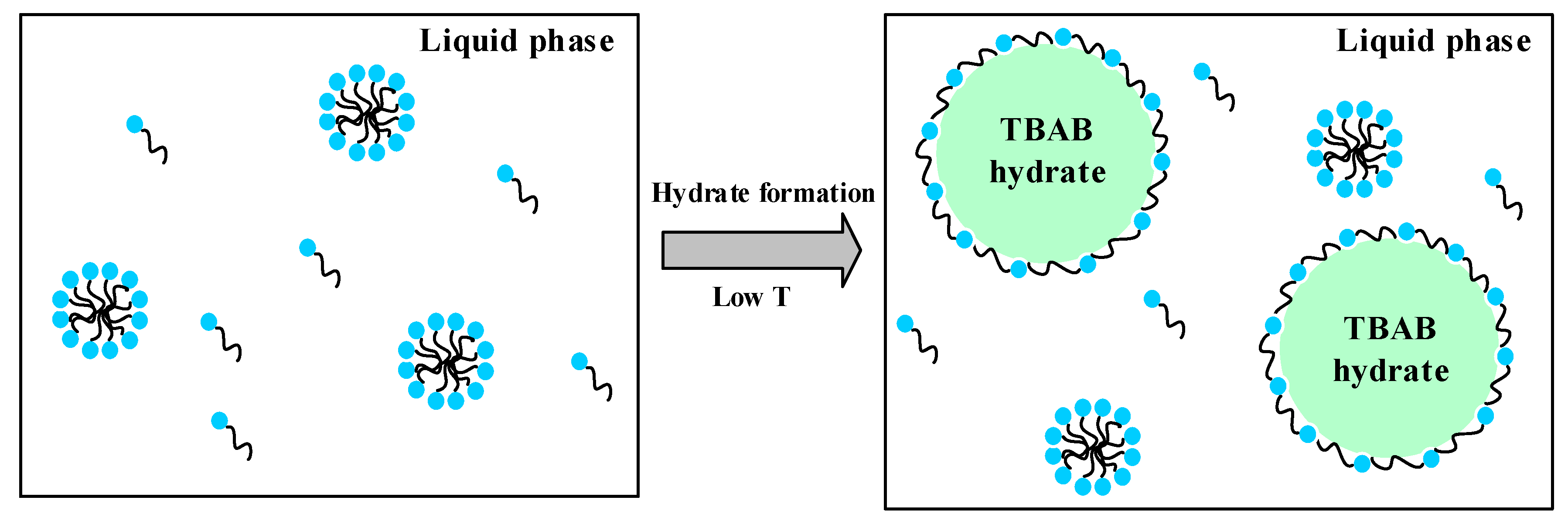
2.5. Analysis of the AA’s Anti-Agglomeration Mechanisms
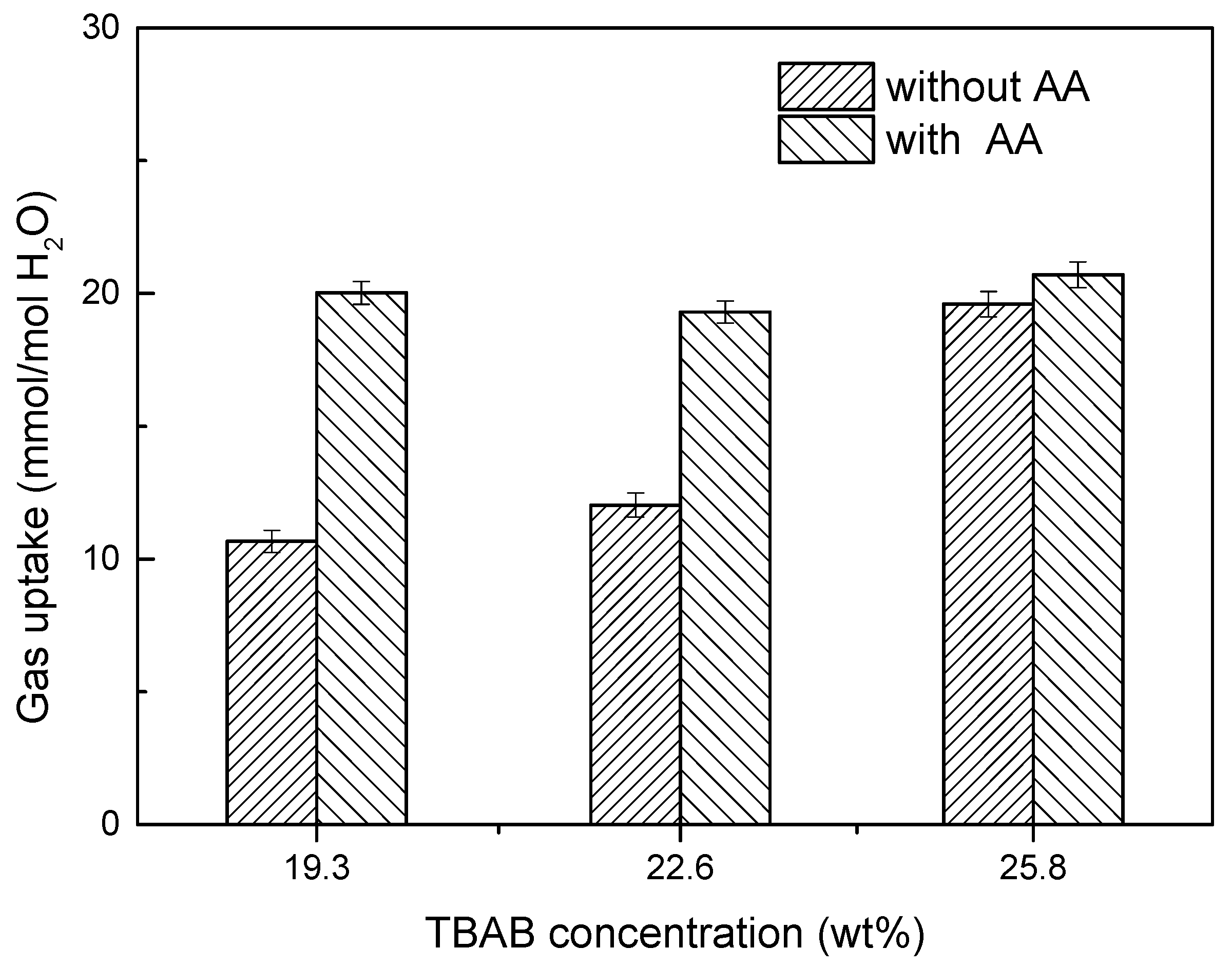
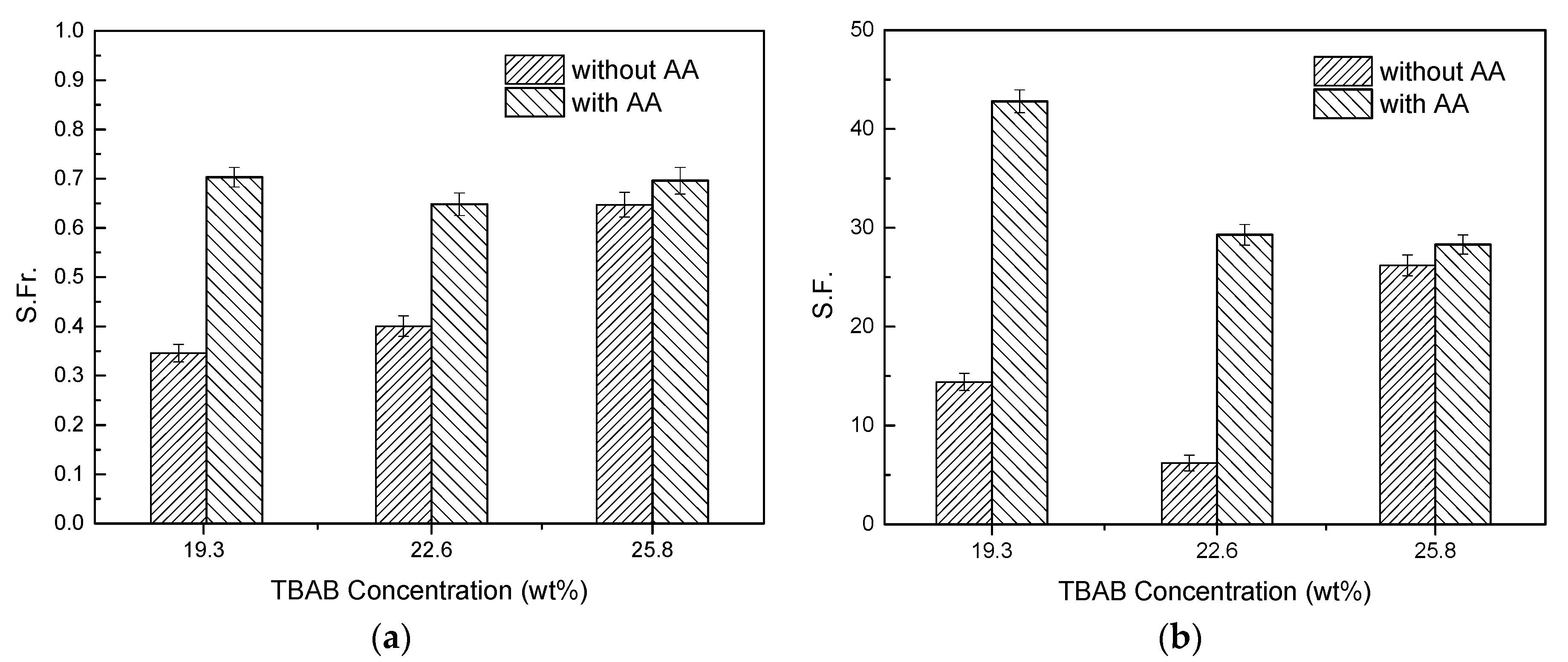
2.6. Effect of AA’s Anti-Agglomeration on CO2 Separation
3. Materials and Methods
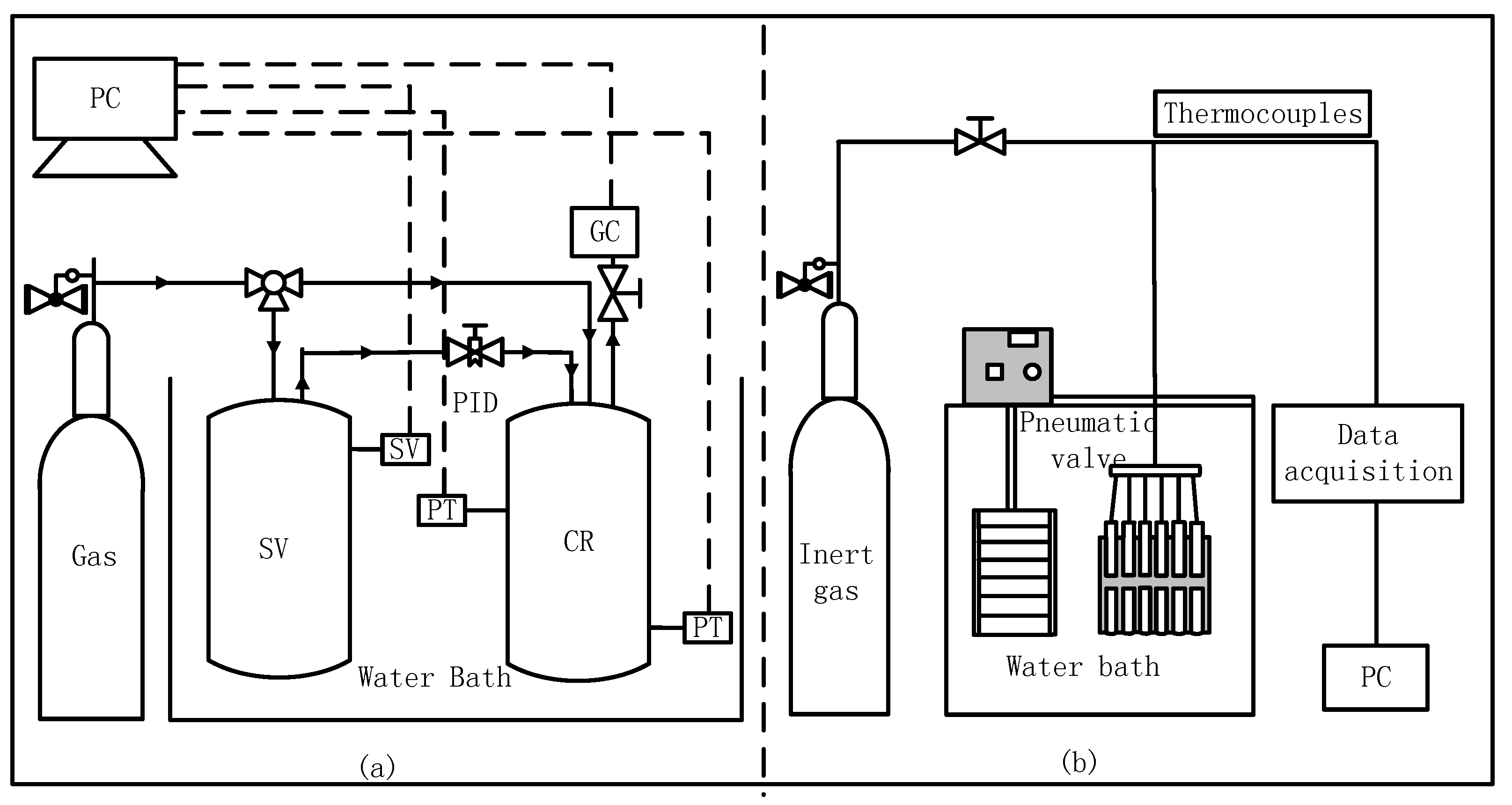
3.1. Apparatus
3.2. Materials
3.3. Methods
3.3.1. Anti-Agglomerant Screening Experiments
3.3.2. Observation of Micromorphology of TBAB Hydrate
3.3.3. CO2 Hydrate Separation Experiments
4. Conclusions
- The minimum AA dosage required increases with TBAB concentration in solution. A 6–8 wt. % AA addition can effectively prevent hydrate particles agglomeration in 19.3–29 wt. % TBAB aqueous solution. The TBAB hydrates slurry maintains stable over 72 h.
- The micromorphology of hydrate particles shows that the addition of AA reduces the particle size of TBAB hydrate markedly because the cocamidopropyl dimethylamine molecules absorbs on the surface of TBAB hydrate crystal nucleus and forms a steric hindrance in the process of hydrate particle growth and agglomeration.
- The IGCC syngas hydrate separating results indicate that AA addition can not only effectively prevent TBAB-CO2 hydrate agglomeration and the blockage of the separating equipment, but also improve the gas transfer in the phase of the hydrate slurry, thus increase the hydrate separation efficiency drastically.
- Compared to the separating results without AA addition, the CO2 concentration in residual gas decreased from 30.62% to 17.33% with AA addition. Gas storage capacity, CO2 split fraction and separation factor increased significantly, and CO2 split fraction and separation factor reached 70.3% and 42.8%, respectively.
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Wigley, T.M.L.; Jain, A.K.; Joos, F.; Nyenzi, B.S.; Shukla, P.R. Implications of Proposed CO2 Emissions Limitations; IPCC Technical Paper 4; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 1997. [Google Scholar]
- Freund, P.; Ormerod, W.G. Progress toward storage of carbon dioxide. Energy Convers. Manag. 1997, 38, S199–S204. [Google Scholar] [CrossRef]
- Hendricks, C.A.; Blok, K.; Turkenburg, W.C. Technology and cost of recovering and storing carbon-dioxide from an integratedgasifier, combined-cycle plant. Energy 1991, 16, 1277–1293. [Google Scholar] [CrossRef]
- Duc, N.H.; Chauvy, F.; Herri, J.M. CO2 capture by hydrate crystallization—A potential solution for gas emission of steelmaking industry. Energy Convers. Manag. 2007, 48, 1313. [Google Scholar] [CrossRef]
- Linga, P.; Kumar, R.N.; Englezos, P. Gas hydrate formation from hydrogen/carbon dioxide and nitrogen/carbon dioxide gas mixtures. Chem. Eng. Sci. 2007, 62, 4268–4276. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.D.; Linga, P.; Englezos, P.; Kim, Y.S.; Lee, M.S.; Kim, Y.D. Gas hydrate formation process for pre-combustion capture of carbon dioxide. Energy 2009, 35, 2729–2733. [Google Scholar] [CrossRef]
- Kumar, R.; Linga, P.; Ripmeester, J.A.; Englezos, P. Two-stage clathrate hydrate/membrane process for precombustion capture of carbon dioxide and hydrogen. J. Environ. Eng. ASCE 2009, 135, 411–417. [Google Scholar] [CrossRef]
- Zhang, J.S.; Yedlapalli, P.; Lee, J.W. Thermodynamic analysis of hydrate-based pre-combustion capture of CO2. Chem. Eng. Sci. 2009, 64, 4732–4736. [Google Scholar] [CrossRef]
- Kang, S.P.; Lee, H. Recovery of CO2 from flue gas using gas hydrate: Thermodynamic verification through phase equilibrium measurements. Environ. Sci. Technol. 2000, 34, 4397–4400. [Google Scholar] [CrossRef]
- Mokhatab, S.; Wilkens, R.J.; Leontaritis, K.J. A review of strategies for solving gas-hydrate problems in subsea pipelines. Energy Source Part A 2007, 29, 39–45. [Google Scholar] [CrossRef]
- Huo, Z.; Freer, E.; Lamar, M.; Sannigrahi, B.; Knauss, D.M.; Sloan, E.D. Hydrate plug prevention by anti-agglomeration. Chem. Eng. Sci. 2001, 56, 4979–4991. [Google Scholar] [CrossRef]
- Huangjing, Z.; Minwei, S.; Abbas, F. Anti-agglomeration of natural gas hydrates in liquid condensate and crude oil at constant pressure conditions. Fuel 2016, 180, 187–193. [Google Scholar]
- Sugier, A.; Bourgmayer, P.; Behar, E.; Freund, E. EP 323307, 5 July 1989.
- Sugier, A.; Bourgmayer, P.; Behar, E.; Freund, E. EP 323774, 12 July 1989.
- Sugier, A.; Bourgmayer, P.; Stern, R. EP 323775, 12 July 1989.
- Zanota, M.L.; Dicharry, C.; Graciaa, A. Hydrate plug prevention by quaternary ammonium salts. Energy Fuels 2005, 19, 584–590. [Google Scholar] [CrossRef]
- Kelland, M.A.; Svartaas, T.M.; Ovsthus, J.; Tomita, T.; Chosa, J. Studies on some zwit-terionic surfactant gas hydrate anti-agglomerants. Chem. Eng. Sci. 2006, 61, 4048–4059. [Google Scholar] [CrossRef]
- Sloan, E.D. A changing hydrate paradigm—From apprehension to avoidance to risk management. Fluid Phase Equilib. 2005, 228, 67–74. [Google Scholar] [CrossRef]
- Mehta, A.P.; Hebert, P.B.; Cadena, E.R.; Weatherman, J.P. Fulfilling the promise of low-dosage hydrate inhibitors: Journey from academic curiosity to successful field implementation. SPE Prod. Facil. 2003, 18, 73–79. [Google Scholar] [CrossRef]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases; CRC: Boca Raton, FL, USA, 2008. [Google Scholar]
- Sun, M.; Firoozabadi, A. New surfactant for hydrate anti-agglomeration in hydrocarbon flowlines and seabed oil capture. J. Colloid. Interface Sci. 2013, 402, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Jebraeel, G.; Antonin, C.; Bahman, T. Separation and capture of carbon dioxide from CO2/H2 syngas mixture using semi-clathrate hydrates. Chem. Eng. Res. Des. 2011, 89, 1747–1751. [Google Scholar]
- Xu, C.; Zhang, S.; Cai, J.; Chen, Z.; Li, X. CO2 (carbon dioxide) separation from CO2-H2 (hydrogen) gas mixtures by gas hydrates in TBAB (tetra-n-butyl ammonium bromide) solution and Raman spectroscopic analysis. Energy 2013, 59, 719–725. [Google Scholar] [CrossRef]
- Soo, M.K.; Ju, D.L.; Hyun, J.L.; Eun, K.L.; Yangdo, K. Gas hydrate formation method to capture the carbon dioxide for pre-combustion process in IGCC plant. Int. J. Hydrogen Energy 2011, 36, 1115–1121. [Google Scholar]
- Taylor, C.J.; Dieker, L.E.; Miller, K.T.; Koh, C.A.; Sloan, E.D., Jr. Micromechanical adhesion force measurements between tetrahyfrofuran hydrate particles. J. Colloid Interface Sci. 2007, 306, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.M.; Van Ness, H.C.; Abbott, M.M. Introduction to Chemical Engineering Thermodynamics; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Praveen, L.; Rajnish, K.; Peter, E. The clathrate hydrate process for post and pre-combustion capture of carbon dioxide. J. Hazard. Mater. 2007, 149, 625–629. [Google Scholar]
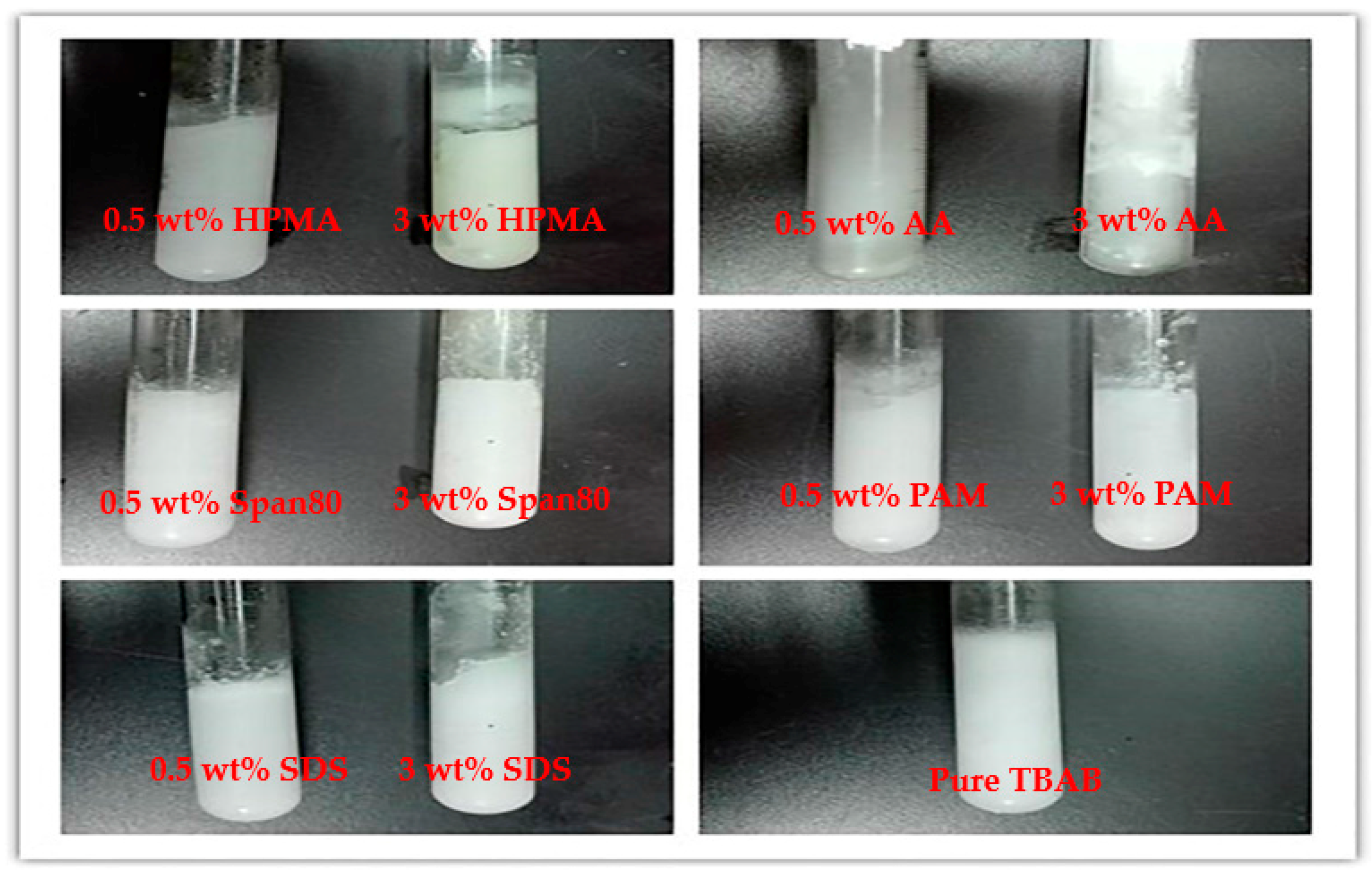
| No. | Anti-Agglomerant | Result f | |||||
|---|---|---|---|---|---|---|---|
| 1 | HPMA g | 0.5 | 16.2 | 30 | 275.15 | 1.5 | No |
| 2 | HPMA | 3.0 | 16.2 | 30 | 275.15 | 1.5 | No |
| 3 | AA h | 0.5 | 16.2 | 30 | 275.15 | 1.5 | Yes |
| 4 | AA | 3.0 | 16.2 | 30 | 275.15 | 1.5 | Yes |
| 5 | Span80 | 0.5 | 16.2 | 30 | 275.15 | 1.5 | No |
| 6 | Span80 | 3.0 | 16.2 | 30 | 275.15 | 1.5 | No |
| 7 | PAM i | 0.5 | 16.2 | 30 | 275.15 | 1.5 | No |
| 8 | PAM | 3.0 | 16.2 | 30 | 275.15 | 1.5 | No |
| 9 | SDS j | 0.5 | 16.2 | 30 | 275.15 | 1.5 | No |
| 10 | SDS | 3.0 | 16.2 | 30 | 275.15 | 1.5 | No |
| 11 | - | 0 | 16.2 | 30 | 275.15 | 1.5 | No |
| Experiment No. | S.Fr.f | S.F.g | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 19.3 | 0 | 277.15 | 4.000 | 125/210 | 30.62 | 0.346 | 14.4 |
| 2 | 19.3 | 6 | 277.15 | 4.000 | 125/210 | 17.33 | 0.703 | 42.8 |
| 3 | 22.6 | 0 | 277.15 | 4.000 | 125/210 | 29.81 | 0.401 | 6.2 |
| 4 | 22.6 | 7 | 277.15 | 4.000 | 125/210 | 19.97 | 0.648 | 29.3 |
| 5 | 25.8 | 0 | 277.15 | 4.000 | 125/210 | 20.25 | 0.647 | 26.2 |
| 6 | 25.8 | 8 | 277.15 | 4.000 | 125/210 | 17.96 | 0.696 | 28.3 |
| CTBAB/wt. % | VTBAB/mL | nTBAB/mol | n1CO2 a/mol | n2CO2 b/mol |
|---|---|---|---|---|
| 19.3 | 125 | 0.075 | 0.225 | 0.155 |
| 22.6 | 125 | 0.088 | 0.264 | 0.155 |
| 25.8 | 125 | 0.100 | 0.300 | 0.155 |
| References | TBAB a | S.Fr. | S.F. | |||
|---|---|---|---|---|---|---|
| Gholinezhad et al. [22] | 5 and 10 wt. % | 273.5–273.9 | 3.8–3.9 | 50/50 | 41–47% | 15.7–28.0 |
| XU et al. [23] | 0.29 mol % | 274.15 | 3.0 | 180/120 | 14.5% | - |
| Kim et al. [24] | 0.5, 1.0 and 3.0 mol % | 284.15–287.15 | 3.0 | - | 10.1–24.1% | 16.1–26.0 |
| This work | 19.3, 22.6 and 25.8 wt. % | 277.15 | 4.0 | 125/210 | 34.6–64.7% | 6.2–26.2 |
| This work | 19.3, 22.6 and 25.8 wt. % with AA | 277.15 | 4.0 | 125/210 | 64.8–70.3% | 28.3–42.8 |
| No. | Name | Purity | Supplier |
|---|---|---|---|
| 1 | TBAB | 99.0% | Aladdin Industrial Corporation (Shanghai agent, China) |
| 2 | Sodium dodecyl sulfate (SDS) | analytical pure | Tianjin Fuchen Chemical Reagent Factory |
| 3 | Span80 | 99.0% | Tianjin Kermel Chemical Reagent Factory Co., Ltd. |
| 4 | Polyacrylamide (PAM) | MW. 5 million | Shanghai Macklin Biochemicals Co., Ltd. |
| 5 | Polymaleic acid | 50 vol % | Shanghai Macklin Biochemicals Co., Ltd. |
| 6 | Cocamidopropyl dimethylamine | Shanghai Yincong New Material Technology Co., Ltd. | |
| 7 | Glycerin | 99.0% | MYM Biological Technology Co., Ltd. |
| 8 | Simulated syngas | 39.98 mol % CO2 + 60.02 mol % H2 | Huate Gas Co., Ltd., China. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Li, X.-S.; Chen, Z.-Y.; Zhang, Y.; Xu, C.-G.; Xia, Z.-M. Anti-Agglomerator of Tetra-n-Butyl Ammonium Bromide Hydrate and Its Effect on Hydrate-Based CO2 Capture. Energies 2018, 11, 399. https://doi.org/10.3390/en11020399
Li R, Li X-S, Chen Z-Y, Zhang Y, Xu C-G, Xia Z-M. Anti-Agglomerator of Tetra-n-Butyl Ammonium Bromide Hydrate and Its Effect on Hydrate-Based CO2 Capture. Energies. 2018; 11(2):399. https://doi.org/10.3390/en11020399
Chicago/Turabian StyleLi, Rong, Xiao-Sen Li, Zhao-Yang Chen, Yu Zhang, Chun-Gang Xu, and Zhi-Ming Xia. 2018. "Anti-Agglomerator of Tetra-n-Butyl Ammonium Bromide Hydrate and Its Effect on Hydrate-Based CO2 Capture" Energies 11, no. 2: 399. https://doi.org/10.3390/en11020399
APA StyleLi, R., Li, X.-S., Chen, Z.-Y., Zhang, Y., Xu, C.-G., & Xia, Z.-M. (2018). Anti-Agglomerator of Tetra-n-Butyl Ammonium Bromide Hydrate and Its Effect on Hydrate-Based CO2 Capture. Energies, 11(2), 399. https://doi.org/10.3390/en11020399