Bioelectrochemical Systems for Removal of Selected Metals and Perchlorate from Groundwater: A Review
Abstract
1. Introduction
2. Groundwater Contamination Issues
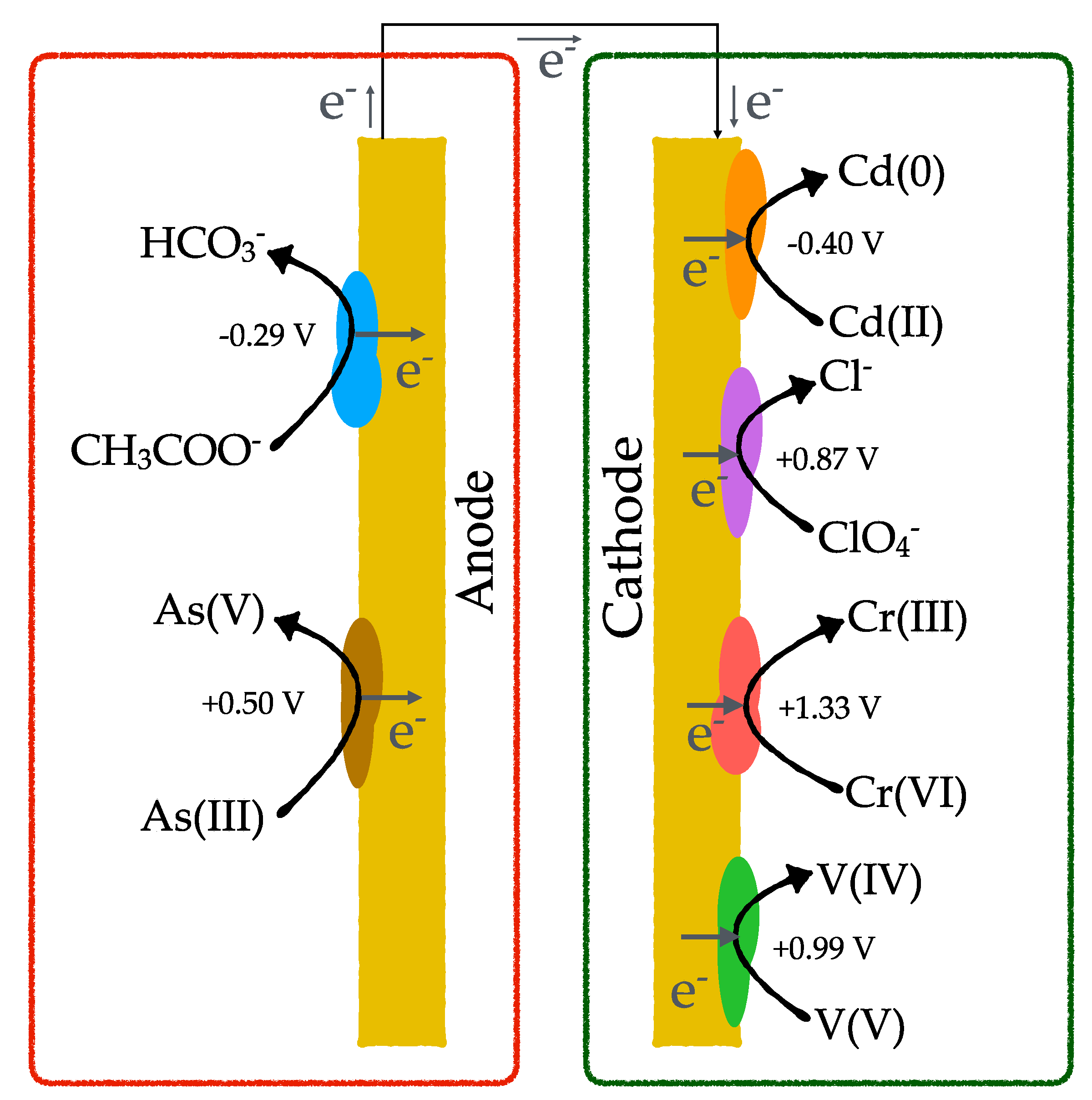
3. Contaminants Removal from Groundwater Using BES
3.1. Arsenic
3.2. Chromium
3.3. Cadmium
3.4. Vanadium
3.5. Perchlorate
4. Perspectives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cecconet, D.; Devecseri, M.; Callegari, A.; Capodaglio, A.G. Effects of process operating conditions on the autotrophic denitrification of nitrate-contaminated groundwater using bioelectrochemical systems. Sci. Total Environ. 2018, 613–614, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Urbansky, E.T.; Schock, M.R. Issues in managing the risks associated with perchlorate in drinking water. J. Environ. Manag. 1999, 56, 79–95. [Google Scholar] [CrossRef]
- Molognoni, D.; Devecseri, M.; Cecconet, D.; Capodaglio, A.G. Cathodic groundwater denitrification with a bioelectrochemical system. J. Water Process Eng. 2017, 19, 67–73. [Google Scholar] [CrossRef]
- Oyem, H.H.; Oyem, I.M.; Usese, A.I. Iron, manganese, cadmium, chromium, zinc and arsenic groundwater contents of Agbor and Owa communities of Nigeria. Springerplus 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, H.; Xiu, W.; Wang, J.; Shen, M. High arsenic groundwater in the Guide basin, northwestern China: Distribution and genesis mechanisms. Sci. Total Environ. 2018, 640–641, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Hausladen, D.M.; Alexander-Ozinskas, A.; McClain, C.; Fendorf, S. Hexavalent Chromium Sources and Distribution in California Groundwater. Environ. Sci. Technol. 2018, 52, 8242–8251. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Caldwell, J.; Sneath, G. Current State and Trend of Cadmium Levels in Soil, Freshwater and Sediments Across the Waikato Region. In Science and Policy: Nutrient Management Challenges for the Next Generation; Fertilizer and Lime Research Centre, Massey University: Palmerston North, New Zealand, 2017; pp. 1–11. [Google Scholar]
- Arena, G.; Copat, C.; Dimartino, A.; Grasso, A.; Fallico, R.; Sciacca, S.; Fiore, M.; Ferrante, M. Determination of total vanadium and vanadium(V) in groundwater from Mt. Etna and estimate of daily intake of vanadium(V) through drinking water. J. Water Health 2015, 13, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.P. Point and nonpoint pollution and restoring groundwater quality in Italy: 30 years of experience. Rend. Lincei 2017, 28, 255–264. [Google Scholar] [CrossRef]
- Hashim, M.A.; Mukhopadhyay, S.; Sahu, J.N.; Sengupta, B. Remediation technologies for heavy metal contaminated groundwater. J. Environ. Manag. 2011, 92, 2355–2388. [Google Scholar] [CrossRef] [PubMed]
- Rozendal, R.A.; Hamelers, H.V.M.; Rabaey, K.; Keller, J.; Buisman, C.J.N. Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol. 2008, 26, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-W.; Choi, S.-J.; Lee, T.-H.; Lee, G.-Y.; Cha, J.-H.; Kim, C.-W. Application of biocathode in microbial fuel cells: Cell performance and microbial community. Appl. Microbiol. Biotechnol. 2008, 79, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Jung, S.H.; Regan, J.M.; Logan, B.E. Electricity generation and microbial community analysis of alcohol powered microbial fuel cells. Bioresour. Technol. 2007, 98, 2568–2577. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cheng, S.A.; Logan, B.E. Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environ. Sci. Technol. 2005, 39, 5488–5493. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Gonçalves, L.; Peixoto, J.M.; Peixoto, L.; Brito, A.G.; Martins, G. Resources recovery in the dairy industry: Bioelectricity production using a continuous microbial fuel cell. J. Clean. Prod. 2017, 140, 971–976. [Google Scholar] [CrossRef]
- Cecconet, D.; Molognoni, D.; Callegari, A.; Capodaglio, A.G. Agro-food industry wastewater treatment with microbial fuel cells: Energetic recovery issues. Int. J. Hydrogen Energy 2018, 43, 500–511. [Google Scholar] [CrossRef]
- Callegari, A.; Cecconet, D.; Molognoni, D.; Capodaglio, A.G. Sustainable processing of dairy wastewater: Long-term pilot application of a bio-electrochemical system. J. Clean. Prod. 2018, 189, 563–569. [Google Scholar] [CrossRef]
- Molognoni, D.; Chiarolla, S.; Cecconet, D.; Callegari, A.; Capodaglio, A.G. Industrial wastewater treatment with a bioelectrochemical process: Assessment of depuration efficiency and energy production. Water Sci. Technol. 2018, 77, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.; Schievano, A.; Trasatti, S.P.; Morrone, R.; D’Antona, N.; Cristiani, P. Signal trends of microbial fuel cells fed with different food-industry residues. Int. J. Hydrogen Energy 2017, 42, 1841–1852. [Google Scholar] [CrossRef]
- Iskander, S.M.; Novak, J.T.; Brazil, B.; He, Z. Simultaneous energy generation and UV quencher removal from landfill leachate using a microbial fuel cell. Environ. Sci. Pollut. Res. 2017, 24, 26040–26048. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, J.M.; Adeloju, S.B.; Ghosh, P.C. Landfill leachate: A promising substrate for microbial fuel cells. Int. J. Hydrogen Energy 2017, 42, 23794–23798. [Google Scholar] [CrossRef]
- Cecconet, D.; Molognoni, D.; Callegari, A.; Capodaglio, A.G. Biological combination processes for efficient removal of pharmaceutically active compounds from wastewater: A review and future perspectives. J. Environ. Chem. Eng. 2017, 5, 3590–3603. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X.; Logan, B.E.; Lee, H. Brewery wastewater treatment using air-cathode microbial fuel cells. Appl. Microbiol. Biotechnol. 2008, 78, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Chen, S.; Babanova, S.; Phadke, S.; Salvacion, M.; Mirhosseini, A.; Chan, S.; Carpenter, K.; Cortese, R.; Bretschger, O. Long-term performance of a 20-L continuous flow microbial fuel cell for treatment of brewery wastewater. J. Power Sources 2017, 356, 274–287. [Google Scholar] [CrossRef]
- Cusick, R.D.; Kiely, P.D.; Logan, B.E. A monetary comparison of energy recovered from microbial fuel cells and microbial electrolysis cells fed winery or domestic wastewaters. Int. J. Hydrogen Energy 2010, 35, 8855–8861. [Google Scholar] [CrossRef]
- Yu, N.; Xing, D.; Li, W.; Yang, Y.; Li, Z.; Li, Y.; Ren, N. Electricity and methane production from soybean edible oil refinery wastewater using microbial electrochemical systems. Int. J. Hydrogen Energy 2017, 42, 96–102. [Google Scholar] [CrossRef]
- Srikanth, S.; Kumar, M.; Singh, D.; Singh, M.P.; Das, B.P. Electro-biocatalytic treatment of petroleum refinery wastewater using microbial fuel cell (MFC) in continuous mode operation. Bioresour. Technol. 2016, 221, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, A.G.; Molognoni, D.; Dallago, E.; Liberale, A.; Cella, R.; Longoni, P.; Pantaleoni, L. Microbial fuel cells for direct electrical energy recovery from urban wastewaters. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Molognoni, D.; Puig, S.; Balaguer, M.D.; Capodaglio, A.G.; Callegari, A.; Colprim, J. Multiparametric control for enhanced biofilm selection in microbial fuel cells. J. Chem. Technol. Biotechnol. 2016, 91, 1720–1727. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Molognoni, D.; Puig, S.; Balaguer, M.D.; Colprim, J. Role of Operating Conditions on Energetic Pathways in a Microbial Fuel Cell. Energy Procedia 2015, 74, 728–735. [Google Scholar] [CrossRef]
- Molognoni, D.; Puig, S.; Balaguer, M.D.; Liberale, A.; Capodaglio, A.G.; Callegari, A.; Colprim, J. Reducing start-up time and minimizing energy losses of Microbial Fuel Cells using Maximum Power Point Tracking strategy. J. Power Sources 2014, 269, 403–411. [Google Scholar] [CrossRef]
- Zou, S.; He, Z. Efficiently “pumping out” value-added resources from wastewater by bioelectrochemical systems: A review from energy perspectives. Water Res. 2018, 131, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; He, Z. Energy production, use and saving in a bioelectrochemical desalination system. RSC Adv. 2012, 2, 10673–10679. [Google Scholar] [CrossRef]
- Saeed, H.M.; Husseini, G.A.; Yousef, S.; Saif, J.; Al-Asheh, S.; Abu Fara, A.; Azzam, S.; Khawaga, R.; Aidan, A. Microbial desalination cell technology: A review and a case study. Desalination 2015, 359, 1–13. [Google Scholar] [CrossRef]
- Kadier, A.; Simayi, Y.; Kalil, M.S.; Abdeshahian, P.; Hamid, A.A. A review of the substrates used in microbial electrolysis cells (MECs) for producing sustainable and clean hydrogen gas. Renew. Energy 2014, 71, 466–472. [Google Scholar] [CrossRef]
- Sleutels, T.H.J.A.; Ter Heijne, A.; Buisman, C.J.N.; Hamelers, H.V.M. Steady-state performance and chemical efficiency of Microbial Electrolysis Cells. Int. J. Hydrogen Energy 2013, 38, 7201–7208. [Google Scholar] [CrossRef]
- Pepè Sciarria, T.P.; Batlle-Vilanova, P.; Colombo, B.; Scaglia, B.; Balaguer, M.D.; Colprim, J.; Puig, S.; Adani, F. Bio-electrorecycling of carbon dioxide into bioplastics. Green Chem. 2018. [Google Scholar] [CrossRef]
- Vassilev, I.; Hernandez, P.A.; Batlle-Vilanova, P.; Freguia, S.; Krömer, J.O.; Keller, J.; Ledezma, P.; Virdis, B. Microbial Electrosynthesis of Isobutyric, Butyric, Caproic Acids, and Corresponding Alcohols from Carbon Dioxide. ACS Sustain. Chem. Eng. 2018, 6, 8485–8493. [Google Scholar] [CrossRef]
- Muñoz-Aguilar, R.; Molognoni, D.; Bosch-Jimenez, P.; Borràs, E.; Della Pirriera, M.; Luna, Á. Design, Operation, Modeling and Grid Integration of Power-to-Gas Bioelectrochemical Systems. Energies 2018, 11, 1947. [Google Scholar] [CrossRef]
- Zeppilli, M.; Mattia, A.; Villano, M.; Majone, M. Three-chamber Bioelectrochemical System for Biogas Upgrading and Nutrient Recovery. Fuel Cells 2017, 17, 593–600. [Google Scholar] [CrossRef]
- Zou, S.; Qin, M.; Moreau, Y.; He, Z. Nutrient-energy-water recovery from synthetic sidestream centrate using a microbial electrolysis cell—Forward osmosis hybrid system. J. Clean. Prod. 2017, 154, 16–25. [Google Scholar] [CrossRef]
- Chouler, J.; Cruz-Izquierdo, Á.; Rengaraj, S.; Scott, J.L.; Di Lorenzo, M. A screen-printed paper microbial fuel cell biosensor for detection of toxic compounds in water. Biosens. Bioelectron. 2018, 102, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Chouler, J.; Di Lorenzo, M. Water quality monitoring in developing countries; Can microbial fuel cells be the answer? Biosensors 2015, 5, 450–470. [Google Scholar] [CrossRef] [PubMed]
- Modin, O.; Aulenta, F. Three promising applications of microbial electrochemistry for the water sector. Environ. Sci. Water Res. Technol. 2017, 3, 391–402. [Google Scholar] [CrossRef]
- Logan, B.E.; Rabaey, K. Conversion of Wastes into Bioelectricity and Chemicals by Using Microbial Electrochemical Technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 2009, 7, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, A.G.; Molognoni, D.; Pons, A.V. A multi-perspective review of microbial fuel-cells for wastewater treatment: Bio-electro-chemical, microbiologic and modeling aspects. AIP Conf. Proc. 2016, 1758. [Google Scholar] [CrossRef]
- Ter Heijne, A.; Liu, F.; van der Weijden, R.; Weijma, J.; Buisman, C.J.N.; Hamelers, H.V.M. Copper Recovery Combined with Electricity Production in a Microbial Fuel Cell. Environ. Sci. Technol. 2010, 44, 4376–4381. [Google Scholar] [CrossRef] [PubMed]
- Pous, N.; Casentini, B.; Rossetti, S.; Fazi, S.; Puig, S.; Aulenta, F. Anaerobic arsenite oxidation with an electrode serving as the sole electron acceptor: A novel approach to the bioremediation of arsenic-polluted groundwater. J. Hazard. Mater. 2015, 283, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.K.; Tran, H.T.; Park, Y.; Yu, J.; Lee, T. Microbial arsenite oxidation with oxygen, nitrate, or an electrode as the sole electron acceptor. J. Ind. Microbiol. Biotechnol. 2017, 44, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Sevda, S.; Sreekishnan, T.R.; Pous, N.; Puig, S.; Pant, D. Bioelectroremediation of perchlorate and nitrate contaminated water: A review. Bioresour. Technol. 2018, 255, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Huang, L.; Zhang, Y. Cathodic reduction of hexavalent chromium [Cr(VI)] coupled with electricity generation in microbial fuel cells. Biotechnol. Lett. 2008, 30, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Feng, C.; Ni, J.; Zhang, J.; Huang, W. Simultaneous reduction of vanadium (V) and chromium (VI) with enhanced energy recovery based on microbial fuel cell technology. J. Power Sources 2012, 204, 34–39. [Google Scholar] [CrossRef]
- Modin, O.; Wang, X.; Wu, X.; Rauch, S.; Fedje, K.K. Bioelectrochemical recovery of Cu, Pb, Cd, and Zn from dilute solutions. J. Hazard. Mater. 2012, 235–236, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Majone, M.; Verdini, R.; Aulenta, F.; Rossetti, S.; Tandoi, V.; Kalogerakis, N.; Agathos, S.; Puig, S.; Zanaroli, G.; Fava, F. In situ groundwater and sediment bioremediation: Barriers and perspectives at European contaminated sites. New Biotechnol. 2015, 32, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Pous, N.; Balaguer, M.D.; Colprim, J.; Puig, S. Opportunities for groundwater microbial electro-remediation. Microb. Biotechnol. 2018, 11, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Cecconet, D.; Zou, S.; Capodaglio, A.G.; He, Z. Evaluation of energy consumption of treating nitrate-contaminated groundwater by bioelectrochemical systems. Sci. Total Environ. 2018, 636, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.J.; Valko, M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011, 31, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Agusa, T.; Trang, P.T.K.; Lan, V.M.; Anh, D.H.; Tanabe, S.; Viet, P.H.; Berg, M. Human exposure to arsenic from drinking water in Vietnam. Sci. Total Environ. 2014, 488–489, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Monrad, M.; Ersbøll, A.K.; Sørensen, M.; Baastrup, R.; Hansen, B.; Gammelmark, A.; Tjønneland, A.; Overvad, K.; Raaschou-Nielsen, O. Low-level arsenic in drinking water and risk of incident myocardial infarction: A cohort study. Environ. Res. 2017, 154, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Rahman, M.M.; Ramanathan, A.; Naidu, R. Arsenic and other elements in drinking water and dietary components from the middle Gangetic plain of Bihar, India: Health risk index. Sci. Total Environ. 2016, 539, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.K.; Ali, I. Arsenic: Occurrence, toxicity and speciation techniques. Water Res. 2000, 34, 4304–4312. [Google Scholar] [CrossRef]
- USEPA. Costs of Arsenic Removal Technologies for Small Water Systems; USEPA: Washington, DC, USA, 2011.
- Liao, L.; Jean, J.S.; Chakraborty, S.; Lee, M.K.; Kar, S.; Yang, H.J.; Li, Z. Hydrogeochemistry of groundwater and arsenic adsorption characteristics of subsurface sediments in an alluvial plain, SW Taiwan. Sustainbility 2016, 8. [Google Scholar] [CrossRef]
- Jadhav, S.V.; Bringas, E.; Yadav, G.D.; Rathod, V.K.; Ortiz, I.; Marathe, K.V. Arsenic and fluoride contaminated groundwaters: A review of current technologies for contaminants removal. J. Environ. Manag. 2015, 162, 306–325. [Google Scholar] [CrossRef] [PubMed]
- Hering, J.G.; Elimelech, M. International perspective on arsenic in groundwater: Problems and treatment strategies. In Proceedings of the American Water Works Association, Annual Conference, Anaheim, CA, USA, 18–22 June 1995. [Google Scholar]
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Khaska, M.; Le Gal La Salle, C.; Verdoux, P.; Boutin, R. Tracking natural and anthropogenic origins of dissolved arsenic during surface and groundwater interaction in a post-closure mining context: Isotopic constraints. J. Contam. Hydrol. 2015, 177–178, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Li, J.; Zhao, J.; Yang, J.; Bai, F.; Dou, Y. Heavy metal in surface sediments of the Liaodong Bay, Bohai Sea: Distribution, contamination, and sources. Environ. Monit. Assess. 2013, 185, 5071–5083. [Google Scholar] [CrossRef] [PubMed]
- Barringer, J.L.; Mumford, A.; Young, L.Y.; Reilly, P.A.; Bonin, J.L.; Rosman, R. Pathways for arsenic from sediments to groundwater to streams: Biogeochemical processes in the Inner Coastal Plain, New Jersey, USA. Water Res. 2010, 44, 5532–5544. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, C.F.; Hamula, C.L.A.; Huang, S.; Gabos, S.; Le, X.C. A review on arsenic concentrations in Canadian drinking water. Environ. Rev. 2010, 18, 291–307. [Google Scholar] [CrossRef]
- Dalla Libera, N.; Fabbri, P.; Mason, L.; Piccinini, L.; Pola, M. Exceedance probability map: A tool helping the definition of arsenic Natural Background Level (NBL) within the Drainage Basin to the Venice Lagoon (NE Italy). In Proceedings of the 19th EGU General Assembly (EGU2017), Vienna, Austria, 23–28 April 2017; Volume 19, p. 6388. [Google Scholar]
- Hoover, J.; Gonzales, M.; Shuey, C.; Barney, Y.; Lewis, J. Elevated Arsenic and Uranium Concentrations in Unregulated Water Sources on the Navajo Nation, USA. Expo. Health 2017, 9, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Sorg, T.J.; Chen, A.S.C.; Wang, L. Arsenic species in drinking water wells in the USA with high arsenic concentrations. Water Res. 2014, 48, 156–169. [Google Scholar] [CrossRef] [PubMed]
- USEPA. 2018 Edition of the Drinking Water Standards and Health Advisories; USEPA: Washington, DC, USA, 2018; pp. 2–6.
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- European Comission; The Council of the European Union. Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption; The Council of the European Union: Brussels, Belgium, 1998; Volume L330, pp. 32–54. [Google Scholar]
- Zhao, X.; Wang, H.; Tang, Z.; Zhao, T.; Qin, N.; Li, H.; Wu, F.; Giesy, J.P. Amendment of water quality standards in China: Viewpoint on strategic considerations. Environ. Sci. Pollut. Res. 2018, 25, 3078–3092. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health in China. GB 5749-2006; Ministry of Health in China: Beijing, China, 2006; pp. 1–18.
- Russo, R.; Sciacca, S.; La Milia, D.I.; Poscia, A.; Moscato, U. Vanadium in drinking water: Toxic or therapeutic?! Systematic literature review and analysis of the population exposure in an Italian volcanic region. Eur. J. Public Health 2014, 24, 2839236. [Google Scholar] [CrossRef]
- Demetriades, A.; Reimann, C.; Birke, M. European Ground Water Geochemistry Using Bottled Water as a Sampling Medium. In Clean Soil and Safe Water; Quercia, F., Vidojevic, D., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 115–139. ISBN 9789400722408. [Google Scholar]
- Hutchison, J.M.; Guest, J.S.; Zilles, J.L. Evaluating the Development of Biocatalytic Technology for the Targeted Removal of Perchlorate from Drinking Water. Environ. Sci. Technol. 2017, 51, 7178–7186. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Nandi, R.; Saha, B. Sources and toxicity of hexavalent chromium. J. Coord. Chem. 2011, 64, 1782–1806. [Google Scholar] [CrossRef]
- Zhitkovich, A. Chromium in drinking water: Sources, metabolism, and cancer risks. Chem. Res. Toxicol. 2011, 24, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Naz, A.; Mishra, B.K.; Gupta, S.K. Human Health Risk Assessment of Chromium in Drinking Water: A Case Study of Sukinda Chromite Mine, Odisha, India. Expo. Health 2016, 8, 253–264. [Google Scholar] [CrossRef]
- Messner, B.; Bernhard, D. Cadmium and cardiovascular diseases: Cell biology, pathophysiology, and epidemiological relevance. BioMetals 2010, 23, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Tellez-Plaza, M.; Guallar, E.; Howard, B.V.; Umans, J.G.; Francesconi, K.A.; Goessler, W.; Silbergeld, E.K.; Devereux, R.B.; Navas-Acien, A. Cadmium Exposure and Incident Cardiovascular Disease. Epidemiology 2013, 24, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.B.; Vahter, M.; Concha, G.; Broberg, K. Low-level environmental cadmium exposure is associated with DNA hypomethylation in Argentinean women. Environ. Health Perspect. 2012, 120, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Engström, A.; Michaëlsson, K.; Suwazono, Y.; Wolk, A.; Vahter, M.; Åkesson, A. Long-term cadmium exposure and the association with bone mineral density and fractures in a population-based study among women. J. Bone Miner. Res. 2011, 26, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Ritter, L.; Solomon, K.; Sibley, P.; Hall, K.; Keen, P.; Mattu, G.; Linton, B. Sources, pathways, and relative risks of contaminants in surface water and groundwater: A perspective prepared for the Walkerton inquiry. J. Toxicol. Environ. Health Part A 2002, 65, 1–142. [Google Scholar] [PubMed]
- Hem, J.D. Chemistry and occurence of cadmium and zinc in surface water and groundwater. Water Resour. Res. 1972, 8, 661–679. [Google Scholar] [CrossRef]
- Wu, C.; Luo, Y.; Deng, S.; Teng, Y.; Song, J. Spatial characteristics of cadmium in topsoils in a typical e-waste recycling area in southeast China and its potential threat to shallow groundwater. Sci. Total Environ. 2014, 472, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.T.; Belitz, K. Factors controlling the regional distribution of vanadium in groundwater. Ground Water 2010, 48, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Cinti, D.; Poncia, P.P.; Brusca, L.; Tassi, F.; Quattrocchi, F.; Vaselli, O. Spatial distribution of arsenic, uranium and vanadium in the volcanic-sedimentary aquifers of the Vicano-Cimino Volcanic District (Central Italy). J. Geochem. Explor. 2015, 152, 123–133. [Google Scholar] [CrossRef]
- Prouty, N.G.; Swarzenski, P.W.; Fackrell, J.K.; Johannesson, K.; Palmore, C.D. Groundwater-derived nutrient and trace element transport to a nearshore Kona coral ecosystem: Experimental mixing model results. J. Hydrol. Reg. Stud. 2017, 11, 166–177. [Google Scholar] [CrossRef]
- Soldi, T.; Riolo, C.; Alberti, G.; Gallorini, M.; Peloso, G.F. Environmental vanadium distribution from an industrial settlement. Sci. Total Environ. 1996, 181, 45–50. [Google Scholar] [CrossRef]
- Mejia, J.A.; Rodriguez, R.; Armienta, A.; Mata, E.; Fiorucci, A. Aquifer vulnerability zoning, an indicator of atmospheric pollutants input? Vanadium in the Salamanca aquifer, Mexico. Water. Air. Soil Pollut. 2007, 185, 95–100. [Google Scholar] [CrossRef]
- Rehder, D. Perspectives for vanadium in health issues. Future Med. Chem. 2016, 8, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Gummow, B.; Botha, C.J.; Noordhuizen, J.P.T.M.; Heesterbeek, J.A.P. The public health implications of farming cattle in areas with high background concentrations of vanadium. Prev. Vet. Med. 2005, 72, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Barth, A.; Schaffer, A.W.; Konnaris, C.; Blauensteiner, R.; Winker, R.; Osterode, W.; Rüdiger, H.W. Neurobehavioral effects of vanadium. J. Toxicol. Environ. Health Part A 2002, 65, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E. Assessing the outlook for perchlorate remediation. Environ. Sci. Technol. 2001, 35, 482A–487A. [Google Scholar] [CrossRef] [PubMed]
- Gullick, R.W.; Lechevallier, M.W.; Barhorst, T.S. Occurrence of perchlorate in drinking water sources. J. Am. Water Works Assoc. 2001, 93, 66–77. [Google Scholar] [CrossRef]
- Rao, B.; Anderson, T.A.; Orris, G.J.; Rainwater, K.A.; Rajagopalan, S.; Sandvig, R.M.; Scanlon, B.R.; Stonestrom, D.A.; Walvoord, M.A.; Jackson, W.A. Widespread natural perchlorate in unsaturated zones of the southwest United States. Environ. Sci. Technol. 2007, 41, 4522–4528. [Google Scholar] [CrossRef] [PubMed]
- Urbansky, E.T.; Brown, S.K.; Magnuson, M.L.; Kelty, C.A. Perchlorate levels in samples of sodium nitrate fertilizer derived from Chilean caliche. Environ. Pollut. 2001, 112, 299–302. [Google Scholar] [CrossRef]
- Crump, K.S.; Gibbs, J.P. Benchmark calculations for perchlorate from three human cohorts. Environ. Health Perspect. 2005, 113, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, F.X.; Byrd, D.; Deyhle, G.M.; Sesser, D.E.; Skeels, M.R.; Lamm, S.H. Neonatal thyroxine level and perchlorate in drinking water. J. Occup. Environ. Med. 2000, 42, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.F.; Wu, J.K. Adsorption of arsenite and arsenate within activated alumina grains: Equilibrium and kinetics. Water Res. 2001, 35, 2049–2057. [Google Scholar] [CrossRef]
- Wickramasinghe, S.R.; Han, B.; Zimbron, J.; Shen, Z.; Karim, M.N. Arsenic removal by coagulation and filtration: Comparison of groundwaters from the United States and Bangladesh. Desalination 2004, 169, 231–244. [Google Scholar] [CrossRef]
- Jiang, J.Q. Removing arsenic from groundwater for the developing world—A review. Water Sci. Technol. 2001, 44, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Callegari, A.; Ferronato, N.; Rada, E.C.; Capodaglio, A.G.; Torretta, V. Assessment of arsenic removal efficiency by an iron oxide-coated sand filter process. Environ. Sci. Pollut. Res. 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.-J.; Li, W.-G.; Zhang, D.-Y.; Fan, W.-B.; Zhang, X.-R. Adsorption of arsenic from micro-polluted water by an innovative coal-based mesoporous activated carbon in the presence of co-existing ions. Int. Biodeterior. Biodegrad. 2015, 102, 256–264. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; Jiang, S.-J. Chitosan-functionalized graphene oxide: A novel adsorbent an efficient adsorption of arsenic from aqueous solution. J. Environ. Chem. Eng. 2016, 4, 1698–1713. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, Y.; Gu, X.; Zhao, Y. Adsorption behavior and mechanism of different arsenic species on mesoporous MnFe2O4 magnetic nanoparticles. Chemosphere 2017, 181, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, B.; Cheng, M.; Li, Y.; Hao, L.; Guo, H. Spontaneous arsenic (III) oxidation with bioelectricity generation in single-chamber microbial fuel cells. J. Hazard. Mater. 2016, 306, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.K.; Park, Y.; Yu, J.; Lee, T. Simultaneous arsenite oxidation and nitrate reduction at the electrodes of bioelectrochemical systems. Environ. Sci. Pollut. Res. 2016, 23, 19978–19988. [Google Scholar] [CrossRef] [PubMed]
- Brastad, K.S.; He, Z. Water softening using microbial desalination cell technology. Desalination 2013, 309, 32–37. [Google Scholar] [CrossRef]
- Bernhardt, P.V.; Santini, J.M. Protein film voltammetry of arsenite oxidase from the chemolithioautotrophic arsenite-oxidizing bacterium NT-26. Biochemistry 2006, 45, 2804–2809. [Google Scholar] [CrossRef] [PubMed]
- Xue, A.; Shen, Z.Z.; Zhao, B.; Zhao, H.Z. Arsenite removal from aqueous solution by a microbial fuel cell-zerovalent iron hybrid process. J. Hazard. Mater. 2013, 261, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, A.G.; Callegari, A.; Molognoni, D. Online monitoring of priority and dangerous pollutants in natural and urban waters. Manag. Environ. Qual. Int. J. 2016, 27, 507–536. [Google Scholar] [CrossRef]
- Su, L.; Jia, W.; Hou, C.; Lei, Y. Microbial biosensors: A review. Biosens. Bioelectron. 2011, 26, 1788–1799. [Google Scholar] [CrossRef] [PubMed]
- Grattieri, M.; Hasan, K.; Minteer, S.D. Bioelectrochemical Systems as a Multipurpose Biosensing Tool: Present Perspective and Future Outlook. ChemElectroChem 2017, 4, 834–842. [Google Scholar] [CrossRef]
- Tan, Y.C.; Kharkwal, S.; Chew, K.K.W.; Alwi, R.; Mak, S.F.W.; Ng, H.Y. Enhancing the robustness of microbial fuel cell sensor for continuous copper(II) detection against organic strength fluctuations by acetate and glucose addition. Bioresour. Technol. 2018, 259, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.; Minteer, S.D. Long-term arsenic monitoring with an Enterobacter cloacae microbial fuel cell. Bioelectrochemistry 2015, 106, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Webster, D.P.; TerAvest, M.A.; Doud, D.F.R.; Chakravorty, A.; Holmes, E.C.; Radens, C.M.; Sureka, S.; Gralnick, J.A.; Angenent, L.T. An arsenic-specific biosensor with genetically engineered Shewanella oneidensis in a bioelectrochemical system. Biosens. Bioelectron. 2014, 62, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Nancharaiah, Y.V.; Venkata Mohan, S.; Lens, P.N.L. Metals removal and recovery in bioelectrochemical systems: A review. Bioresour. Technol. 2015, 195, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Gangadharan, P.; Nambi, I.M. Hexavalent chromium reduction and energy recovery by using dual-chambered microbial fuel cell. Water Sci. Technol. 2015, 71, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, A.; Ding, H.; Jin, S.; Yan, Y.; Wang, C.; Zen, C.; Wang, X. Cr(VI) reduction at rutile-catalyzed cathode in microbial fuel cells. Electrochem. Commun. 2009, 11, 1496–1499. [Google Scholar] [CrossRef]
- Choi, C.; Hu, N.; Lim, B. Cadmium recovery by coupling double microbial fuel cells. Bioresour. Technol. 2014, 170, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, L.; Wu, D.; Huang, L.; Zhou, P.; Quan, X.; Chen, G. Dependency of simultaneous Cr(VI), Cu(II) and Cd(II) reduction on the cathodes of microbial electrolysis cells self-driven by microbial fuel cells. J. Power Sources 2015, 273, 1103–1113. [Google Scholar] [CrossRef]
- Tandukar, M.; Huber, S.J.; Onodera, T.; Pavlostathis, S.G. Biological chromium(VI) reduction in the cathode of a microbial fuel cell. Environ. Sci. Technol. 2009, 43, 8159–8165. [Google Scholar] [CrossRef] [PubMed]
- Xafenias, N.; Zhang, Y.; Banks, C.J. Enhanced performance of hexavalent chromium reducing cathodes in the presence of Shewanella oneidensis MR-1 and lactate. Environ. Sci. Technol. 2013, 47, 4512–4520. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, J.; Quan, X.; Yang, F. Enhancement of hexavalent chromium reduction and electricity production from a biocathode microbial fuel cell. Bioprocess Biosyst. Eng. 2010, 33, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhu, X.; Song, T.; Zhang, L.; Jia, H.; Wei, P. Effect of acclimatization on hexavalent chromium reduction in a biocathode microbial fuel cell. Bioresour. Technol. 2015, 180, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chai, X.; Cheng, S.; Chen, G. Evaluation of carbon-based materials in tubular biocathode microbial fuel cells in terms of hexavalent chromium reduction and electricity generation. Chem. Eng. J. 2011, 166, 652–661. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Q.; Jiang, L.; Zhou, P.; Quan, X.; Logan, B.E. Adaptively Evolving Bacterial Communities for Complete and Selective Reduction of Cr(VI), Cu(II), and Cd(II) in Biocathode Bioelectrochemical Systems. Environ. Sci. Technol. 2015, 49, 9914–9924. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.; Mastorgio, A.; Espinoza, A.; Pedrali, L.; Daghio, M.; Sezenna, E.; Franzetti, A.; Saponaro, S.; Ferrari, G. Microbial assisted hexavalent chromium removal in the cathodic chamber of a Microbial Electrolysis Cell. In Proceedings of the 4th European Meeting of the International Society for Microbial Electrochemistry and Technology (4th EU-ISMET), Newcastle upon Tyne, UK, 12–14 September 2018; p. 152. [Google Scholar]
- Pous, N.; Koch, C.; Colprim, J.; Puig, S.; Harnisch, F. Extracellular electron transfer of biocathodes: Revealing the potentials for nitrate and nitrite reduction of denitrifying microbiomes dominated by Thiobacillus sp. Electrochem. Commun. 2014, 49, 93–97. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, L.; Pan, Y.; Zhou, P.; Quan, X.; Logan, B.E.; Chen, H. Cooperative cathode electrode and in situ deposited copper for subsequent enhanced Cd(II) removal and hydrogen evolution in bioelectrochemical systems. Bioresour. Technol. 2016, 200, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhao, H.; Shi, C.; Zhou, S.; Ni, J. Simultaneous removal of sulfide and organics with vanadium(V) reduction in microbial fuel cells. J. Chem. Technol. Biotechnol. 2009, 84, 1780–1786. [Google Scholar] [CrossRef]
- Qiu, R.; Zhang, B.; Li, J.; Lv, Q.; Wang, S.; Gu, Q. Enhanced vanadium (V) reduction and bioelectricity generation in microbial fuel cells with biocathode. J. Power Sources 2017, 359, 379–383. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, B.; Tian, C.; Liu, Y.; Shi, C.; Cheng, M.; Feng, C. Enhanced microbial reduction of vanadium (V) in groundwater with bioelectricity from microbial fuel cells. J. Power Sources 2015, 287, 43–49. [Google Scholar] [CrossRef]
- Shea, C.; Clauwaert, P.; Verstraete, W.; Nerenberg, R. Adapting a denitrifying biocathode for perchlorate reduction. Water Sci. Technol. 2008, 58, 1941–1946. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.S.; Clauwaert, P.; Green, S.J.; Verstraete, W.; Nerenberg, R. Bioelectrochemical Perchlorate Reduction in a Microbial Fuel Cell. Environ. Sci. Technol. 2010, 44, 4685–4691. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Gao, M.M.; Zhang, G.; Wang, X.H.; Wang, S.G.; Song, C.; Xu, Y.Y. Perchlorate reduction in microbial electrolysis cell with polyaniline modified cathode. Bioresour. Technol. 2015, 177, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Tian, X.; Guo, J.; Guo, Y.; Song, Y.; Yue, L.; Wang, Y.; Liang, X. Effects of resazurin on perchlorate reduction and bioelectricity generation in microbial fuel cells and its catalysing mechanism. Biochem. Eng. J. 2016, 114, 164–172. [Google Scholar] [CrossRef]
- Mieseler, M.; Atiyeh, M.N.; Hernandez, H.H.; Ahmad, F. Direct enrichment of perchlorate-reducing microbial community for efficient electroactive perchlorate reduction in biocathodes. J. Ind. Microbiol. Biotechnol. 2013, 40, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Thrash, J.C.; Van Trump, J.I.; Weber, K.A.; Miller, E.; Achenbach, L.A.; Coates, J.D. Electrochemical stimulation of microbial perchlorate reduction. Environ. Sci. Technol. 2007, 41, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Clauwaert, P.; Rabaey, K.; Aelterman, P.; De Schamphelaire, L.; Pham, T.H.; Boeckx, P.; Boon, N.; Verstraete, W. Biological Denitrification in Microbial Fuel Cells. Environ. Sci. Technol. 2007, 41, 3354–3360. [Google Scholar] [CrossRef] [PubMed]
- Clauwaert, P.; Desloover, J.; Shea, C.; Nerenberg, R.; Boon, N.; Verstraete, W. Enhanced nitrogen removal in bio-electrochemical systems by pH control. Biotechnol. Lett. 2009, 31, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, M.; Zhang, Y.; She, Z.; Ren, Y.; Wang, Z.; Zhao, C. Perchlorate reduction by hydrogen autotrophic bacteria in a bioelectrochemical reactor. J. Environ. Manag. 2014, 142, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Yu, H.; Li, C.; Ren, Y.; Wei, C.; Feng, C. Competitive microbial reduction of perchlorate and nitrate with a cathode directly serving as the electron donor. Electrochim. Acta 2014, 133, 217–223. [Google Scholar] [CrossRef]
- Lian, J.; Tian, X.; Li, Z.; Guo, J.; Guo, Y.; Yue, L.; Ping, J.; Duan, L. The effects of different electron donors and electron acceptors on perchlorate reduction and bioelectricity generation in a microbial fuel cell. Int. J. Hydrogen Energy 2017, 42, 544–552. [Google Scholar] [CrossRef]
- Jiang, C.; Yang, Q.; Wang, D.; Zhong, Y.; Chen, F.; Li, X.; Zeng, G.; Li, X.; Shang, M. Simultaneous perchlorate and nitrate removal coupled with electricity generation in autotrophic denitrifying biocathode microbial fuel cell. Chem. Eng. J. 2017, 308, 783–790. [Google Scholar] [CrossRef]
- Ieropoulos, I.A.; Stinchcombe, A.; Gajda, I.; Forbes, S.; Merino-Jimenez, I.; Pasternak, G.; Sanchez-Herranz, D.; Greenman, J. Pee power urinal-microbial fuel cell technology field trials in the context of sanitation. Environ. Sci. Water Res. Technol. 2016, 2, 336–343. [Google Scholar] [CrossRef]
- Ge, Z.; He, Z. Long-term performance of a 200 liter modularized microbial fuel cell system treating municipal wastewater: Treatment, energy, and cost. Environ. Sci. Water Res. Technol. 2016, 2, 274–281. [Google Scholar] [CrossRef]
- Dong, Y.; Qu, Y.; He, W.; Du, Y.; Liu, J.; Han, X.; Feng, Y. A 90-liter stackable baffled microbial fuel cell for brewery wastewater treatment based on energy self-sufficient mode. Bioresour. Technol. 2015, 195, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Greenman, J.; Ieropoulos, I.A. Allometric scaling of microbial fuel cells and stacks: The lifeform case for scale-up. J. Power Sources 2017, 356, 365–370. [Google Scholar] [CrossRef]
- Xie, X.; Criddle, C.; Cui, Y. Design and fabrication of bioelectrodes for microbial bioelectrochemical systems. Energy Environ. Sci. 2015, 8, 3418–3441. [Google Scholar] [CrossRef]
- Palma, E.; Daghio, M.; Franzetti, A.; Petrangeli Papini, M.; Aulenta, F. The bioelectric well: A novel approach for in situ treatment of hydrocarbon-contaminated groundwater. Microb. Biotechnol. 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.K.; Park, Y.; Yu, J.; Lee, T. Bioelectrochemical denitrification on biocathode buried in simulated aquifer saturated with nitrate-contaminated groundwater. Environ. Sci. Pollut. Res. 2016, 23, 15443–15451. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; He, Z. Current-driven nitrate migration out of groundwater by using a bioelectrochemical system. RSC Adv. 2014, 4, 10290–10294. [Google Scholar] [CrossRef]
- Tong, Y.; He, Z. Nitrate removal from groundwater driven by electricity generation and heterotrophic denitrification in a bioelectrochemical system. J. Hazard. Mater. 2013, 262, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.P.; Srinivasan, B.; Manuel, M.-F.; Tartakovsky, B. A two-population bio-electrochemical model of a microbial fuel cell. Bioresour. Technol. 2010, 101, 5256–5265. [Google Scholar] [CrossRef] [PubMed]
- Gadkari, S.; Gu, S.; Sadhukhan, J. Towards automated design of bioelectrochemical systems: A comprehensive review of mathematical models. Chem. Eng. J. 2018, 343, 303–316. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Cecconet, D.; Molognoni, D. An Integrated Mathematical Model of Microbial Fuel Cell Processes: Bioelectrochemical and Microbiologic Aspects. Processes 2017, 5, 73. [Google Scholar] [CrossRef]
- Pinto, R.P.; Srinivasan, B.; Escapa, A.; Tartakovsky, B. Multi-Population Model of a Microbial Electrolysis Cell. Environ. Sci. Technol. 2011, 45, 5039–5046. [Google Scholar] [CrossRef] [PubMed]
- Ping, Q.; Zhang, C.; Chen, X.; Zhang, B.; Huang, Z.; He, Z. Mathematical Model of Dynamic Behavior of Microbial Desalination Cells for Simultaneous Wastewater Treatment and Water Desalination. Environ. Sci. Technol. 2014, 48, 13010–13019. [Google Scholar] [CrossRef] [PubMed]
- Cecconet, D.; Bolognesi, S.; Daneshgar, S.; Callegari, A.; Capodaglio, A.G. Improved process understanding and optimization by multivariate statistical analysis of Microbial Fuel Cells operation. Int. J. Hydrogen Energy 2018, 43, 16719–16727. [Google Scholar] [CrossRef]
- Luo, S.; Sun, H.; Ping, Q.; Jin, R.; He, Z. A Review of Modeling Bioelectrochemical Systems: Engineering and Statistical Aspects. Energies 2016, 9, 111. [Google Scholar] [CrossRef]
- Srinivasan, V.; Weinrich, J.; Butler, C. Nitrite accumulation in a denitrifying biocathode microbial fuel cell. Environ. Sci. Water Res. Technol. 2016, 2, 344–352. [Google Scholar] [CrossRef]




| Contaminant | Limit/Guideline Value (μg L−1) | Country/Organization |
|---|---|---|
| Arsenic | 10 | USA [75], WHO [76], EU [77], China [78] |
| Chromium | 100 | USA [75] |
| 50 | EU [77], WHO [76], China [78] | |
| Cadmium | 5 | EU [77], USA [75], China [79] |
| 3 | WHO [76] | |
| Vanadium | 140 | Italy [80] |
| 100 | Ukraine [81] | |
| 5 | Bosnia and Herzegovina, Croatia, FYROM [81] | |
| 1 | Serbia [81] | |
| Perchlorate | 70 | WHO [76], China [78] |
| 15 | USA [82] | |
| 6 | California (USA) [82] | |
| 2 | Massachusetts (USA) [82] |
| BES Type | Electrode(s) Type | Applied Potential/Voltage | As (III) Removal Rate | Energy Production | Ref. |
|---|---|---|---|---|---|
| Polarized bioanode | Graphite rod | +0.497 V vs. SHE | 0.42 mg L−1 day−1 | - | [49] |
| MFC | Carbon-fiber felt (anode), plain carbon paper (cathode) | - | n.a. a | 752.6 ± 17 mW m−2 b | [114] |
| MFC | Carbon paper | - | 13.2 mg L−1 day−1 | 1–3 mV c | [115] |
| Polarized bioanode d | Carbon paper | +0.500 V vs. SHE | 29.6 mg L−1 day−1 | - | [115] |
| Polarized bioanode e | Carbon paper | 1 V | 29.6 mg L−1 day−1 | - | [115] |
| MDC | Carbon brush | - | n.a. | 7.67 ± 0.71 mA f | [116] |
| BES Type | Cathode | Electrodes Material | Cr(VI) Removal Rate | Maximum Power Density | Maximum Current Density | Ref. |
|---|---|---|---|---|---|---|
| MFC | Abiotic | Graphite plates | n.a. | 150 mW m−2 | 0.04 mA cm−2 | [52] |
| MFC | Abiotic | Carbon cloth | n.a. | 767.01 mW m−2 | 2.08 mA cm−2 | [126] |
| MFC | Abiotic | Rutile-coated graphite plate (cat.), graphite plate (an.) | n.a. | n.a. | n.a. | [127] |
| MFC | Abiotic | Carbon brush (an.), carbon cloth (cat.) | n.a. | 5367 mW m−2 | n.a. | [128] |
| MFC | Abiotic | Graphite felt (an.), carbon rod (cat.) | 4.28 mg L−1 h−1 | 3.3 W m−3 | 5.1 A m−3 | [129] |
| MFC | Biotic | Graphite plates | 0.46 mg gVSS−1 h−1 | 55.5 mW m−2 | 123.4 mA m−2 | [130] |
| MFC | Biotic | Graphite felt | n.a. | n.a. | 32.5 mA m−2 | [131] |
| MFC | Biotic | Graphite plate (an.), graphite plate and granules (cat.) | 2.4 ± 0.2 mg gVSS−1 h−1 | 2.4 ± 0.1 W m−3 | 6.9 A m−3 | [132] |
| MFC | Biotic | Graphite felt | 0.66 ± 0.01 mg L−1 h−1 | 9.7 ± 0.4 mW m−2 | 69.7 ± 1.4 mA m−2 | [133] |
| MFC | Biotic | Graphite fiber | 20.6 mg gVSS−1 h−1 | 15 W m−3 | 48 A m−3 | [134] |
| MFC | Biotic | Graphite brush (an.), graphite felt (cat.) | 1.24 ± 0.01 mg L−1 h−1 | n.a. a | n.a. b | [135] |
| BES type | Cathode | Electrodes Material | Potential/Voltage Applied | Cd removal Rate | Ref. |
|---|---|---|---|---|---|
| Bioanode + potentiostatically-controlled cathode | Abiotic | Carbon felt (an.), Ti wire (cat.) | −0.66 V vs. SHE | n.a. | [54] |
| Double MFC arrangement | Abiotic | Carbon brush (an.), carbon cloth (cat.) | Energy harvested from upstream MFC | n.a. | [128] |
| MEC | Abiotic | Graphite felt (an.), Ti sheet (cat.) | Energy harvested from 2 stacked MFC | n.a. | [129] |
| MEC | Abiotic | Graphite felt (an.), carbon cloth (cat.) | 0.7 V | 6.59 mg L−1 h−1 | [138] |
| MEC | Biotic | Graphite brush (an.), graphite felt (cat.) | 0.5 V | 0.98 ± 0.01 mg L−1 h−1 | [135] |
| BES Type | Cathode | Electrodes Material | V(V) Removal Rate | Maximum Power Density | Maximum Current Density | Reference |
|---|---|---|---|---|---|---|
| MFC | Abiotic | Carbon fiber felt | n.a. | 572.4 ± 18.2 mW m−2 | 1094.0 ± 50.6 mA m−2 | [139] |
| MFC | Abiotic | Carbon fiber felt | n.a. | 970.2 ± 60.5 mW m−2 | 2462.5 ± 23.1 mA m−2 | [53] |
| MFC | Biotic | Carbon fiber felt | n.a. | 529 ± 12 mW m−2 | n.a. | [140] |
| BES Type | Cathode | Electrodes Material | ClO4− Removal Rate | Potential/Voltage Applied | Power Density | Current Density | Ref. |
|---|---|---|---|---|---|---|---|
| MFC | Biotic | Graphite granules | 12 mg L−1 day−1 | - | n.a. | n.a. a | [142] |
| MFC | Biotic | Graphite granules | 24 mg L−1 day−1 | - | 0.3 mW m−2 | 0.17 mA m−2 | [143] |
| MEC | Biotic | Polyaniline modified graphite (cat.), graphite electrode (an.) | n.a. | −0.4 V vs. SCE | - | - | [144] |
| MFC | Biotic | Carbon cloth (an.), Pt-coated carbon cloth (cat.) | 25.0 mg L−1 h−1 | - | n.a. b | n.a. b | [145] |
| MFC | Biotic | Ti wire–carbon fiber brushes | 2 mg m−2 day−1 | - | n.a. | n.a. | [146] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cecconet, D.; Callegari, A.; Capodaglio, A.G. Bioelectrochemical Systems for Removal of Selected Metals and Perchlorate from Groundwater: A Review. Energies 2018, 11, 2643. https://doi.org/10.3390/en11102643
Cecconet D, Callegari A, Capodaglio AG. Bioelectrochemical Systems for Removal of Selected Metals and Perchlorate from Groundwater: A Review. Energies. 2018; 11(10):2643. https://doi.org/10.3390/en11102643
Chicago/Turabian StyleCecconet, Daniele, Arianna Callegari, and Andrea G. Capodaglio. 2018. "Bioelectrochemical Systems for Removal of Selected Metals and Perchlorate from Groundwater: A Review" Energies 11, no. 10: 2643. https://doi.org/10.3390/en11102643
APA StyleCecconet, D., Callegari, A., & Capodaglio, A. G. (2018). Bioelectrochemical Systems for Removal of Selected Metals and Perchlorate from Groundwater: A Review. Energies, 11(10), 2643. https://doi.org/10.3390/en11102643







