A Roadmap for Achieving Sustainable Energy Conversion and Storage: Graphene-Based Composites Used Both as an Electrocatalyst for Oxygen Reduction Reactions and an Electrode Material for a Supercapacitor
Abstract
:1. Introduction
2. ORR Activity of Graphene-Based Catalysts
2.1. Graphene as Metal Support
2.1.1. Graphene as Pt-Based Electrocatalyst Support
2.1.2. Graphene as Pt-Free Metal Electrocatalyst Support
2.2. Graphene as Standalone ORR Catalyst
2.2.1. Nitrogen-Doped Graphene
2.2.2. Dual-Doped Graphene as ORR Catalyst
2.2.3. Graphene/Carbon Nanostructure Composite
3. Graphene-Based Materials in Supercapacitor Applications
3.1. Graphene-Based EDL Supercapacitor
3.1.1. Anti-Restacking Porous or 3D Architecture
3.1.2. Novel Design and Synthesis Methods
3.1.3. Tailorable and Flexible Device
3.2. Graphene-Based Pseudocapacitive Supercapacitor
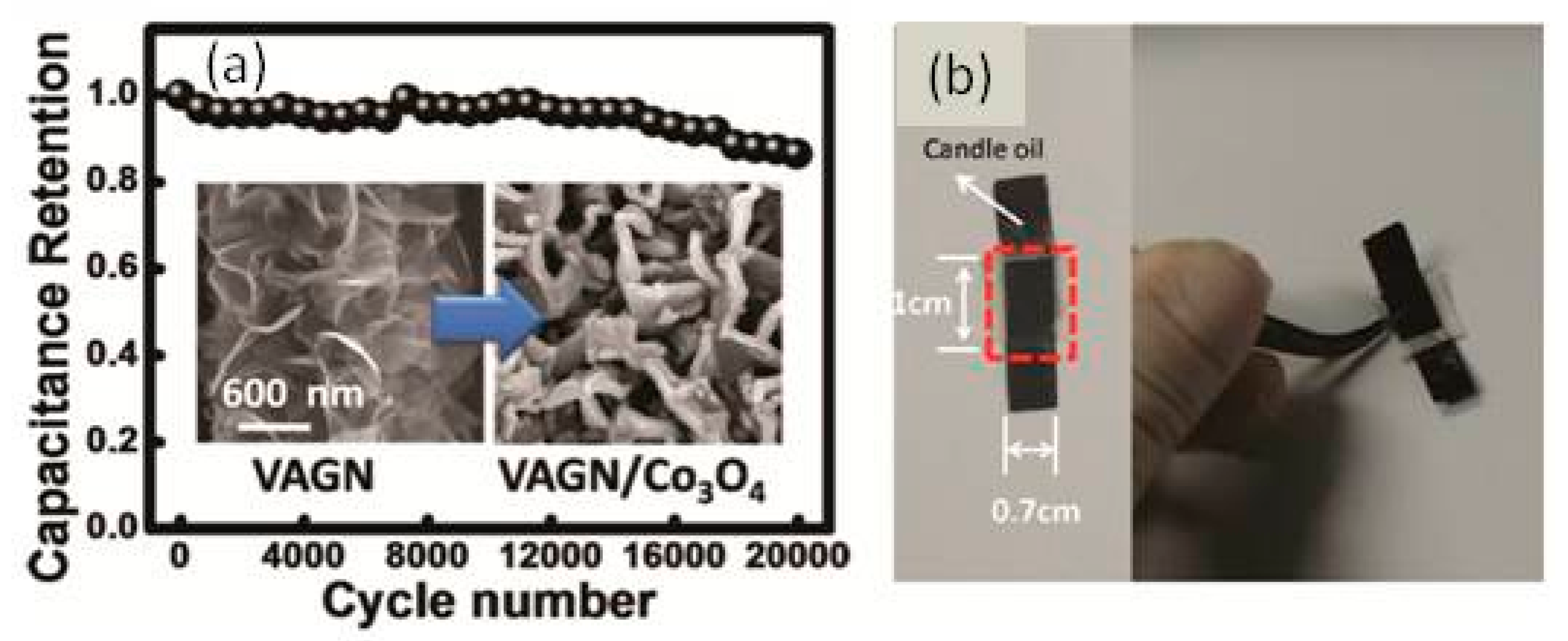
3.2.1. MnO2/Graphene Network
3.2.2. Other Metal Oxide/Graphene Network
3.3. Graphene-Based Hybrid Supercapacitors
3.3.1. As Cathode Materials
3.3.2. As Anode Material
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aricò, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; Schalkwijk, W.V. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2004, 104, 4245–4269. [Google Scholar] [CrossRef] [PubMed]
- Tibaquirá, J.E.; Hristovski, K.D.; Westerhoff, P.; Posner, J.D. Recovery and quality of water produced by commercial fuel cells. Int. J. Hydrogen Energy 2011, 36, 4022–4028. [Google Scholar] [CrossRef]
- Ding, R.; Li, X.D.; Shi, W.; Xu, Q.L.; Liu, E.H. One-pot solvothermal synthesis of ternary Ni-Co-P micro/nano-structured materials for high performance aqueous asymmetric supercapacitors. Chem. Eng. J. 2017, 320, 376–388. [Google Scholar] [CrossRef]
- Thakur, A.K.; Choudhary, R.B.; Majumder, M.; Majhi, M. Fairly improved pseudocapacitance of PTP/PANI/TiO2 nanohybrid composite electrode material for supercapacitor applications. Ionics 2017, 42, 1–12. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.H.; Oh, J.G.; Jang, K.L.; Jeong, B.H.; Hong, B.K.; Kim, T.S. Mechanical behavior of free-standing fuel cell electrodes on water surface. ACS Appl. Mater. Interfaces 2016, 8, 15391–15398. [Google Scholar] [CrossRef] [PubMed]
- Filanovsky, B.; Granot, E.; Dirawi, R.; Presman, I.; Kuras, I.; Patolsky, F. Nanotextured metal copper substrates as powerful and long-lasting fuel cell anodes. Nano Lett. 2011, 11, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Dru, D.; Baranton, S.; Bigarré, J.; Buvat, P.; Coutanceau, C. Fluorine-free Pt nanocomposites for three-phase interfaces in fuel cell electrodes. ACS Catal. 2016, 6, 6993–7001. [Google Scholar] [CrossRef]
- Unni, S.M.; Illathvalappil, R.; Bhange, S.N.; Puthenpediakkal, H.; Kurungot, S. Carbon Nanohorn-derived graphene nanotubes as a platinum-free fuel cell cathode. ACS Appl. Mater. Interfaces 2015, 7, 24256–24264. [Google Scholar] [CrossRef] [PubMed]
- Dombrovskis, J.K.; Jeong, H.Y.; Fossum, K.; Terasaki, O.; Palmqvist, A.E.C. Transition metal ion-chelating ordered mesoporous carbons as noble metal-free fuel cell catalysts. Chem. Mater. 2013, 25, 856–861. [Google Scholar] [CrossRef]
- Singh, D.; Mamtani, K.; Bruening, C.R.; Miller, J.T.; Ozkan, U.S. Use of H2S to probe the active sites in FeNC catalysts for the oxygen reduction reaction (ORR) in acidic media. ACS Catal. 2014, 4, 3454–3462. [Google Scholar] [CrossRef]
- Roche, I.; Chaînet, E.; Chatenet, M.; Vondrák, J. Carbon-supported manganese oxide nanoparticles as electrocatalysts for the oxygen reduction reaction (ORR) in alkaline medium: Physical characterizations and ORR mechanism. J. Phys. Chem. C 2007, 111, 1434–1443. [Google Scholar] [CrossRef]
- Asara, G.G.; Paz-Borbón, L.O.; Baletto, F. “Get in touch and keep in contact”: Interface effect on the oxygen reduction reaction (ORR) activity for supported PtNi nanoparticles. ACS Catal. 2016, 6, 4388–4393. [Google Scholar] [CrossRef]
- Khan, I.A.; Qian, Y.H.; Badshah, A.; Nadeem, M.A.; Zhao, D. Highly porous carbon derived from MOF-5 as a support of ORR electrocatalysts for fuel cells. ACS Appl. Mater. Interfaces 2016, 8, 17268–17275. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.L.; Liu, F.F.; Liu, X.J.; Liao, S.J.; You, C.H.; Tian, X.L.; Nan, H.X.; Luo, F.; Song, H.Y.; Fu, Z.Y.; et al. Effect of transition metals on the structure and performance of the doped carbon catalysts derived from polyaniline and melamine for ORR application. ACS Catal. 2014, 4, 3797–3805. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Y.J.; Chen, H.B.; Yang, D.G.; Li, H.M. Porous N-doped carbon prepared from triazine-based polypyrrole network: A highly efficient metal-free catalyst for oxygen reduction reaction in alkaline electrolytes. ACS Appl. Mater. Interfaces 2016, 8, 28615–28623. [Google Scholar] [CrossRef] [PubMed]
- Bo, X.J.; Han, C.; Zhang, Y.F.; Guo, L.P. Confined nanospace synthesis of less aggregated and porous nitrogen-doped graphene as metal-free electrocatalysts for oxygen reduction reaction in alkaline solution. ACS Appl. Mater. Interfaces 2014, 6, 3023–3030. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yue, Q.L.; Chen, G.F.; Zhai, Y.L.; Wang, L.; Wang, H.S.; Zhao, J.S.; Liu, J.F.; Jia, J.B.; Li, H.B. Effects of acid treatment of Pt-Ni alloy nanoparticles@graphene on the kinetics of the oxygen reduction reaction in acidic and alkaline solutions. J. Phys. Chem. C 2011, 115, 379–389. [Google Scholar] [CrossRef]
- He, Q.G.; Li, Q.; Khene, S.; Ren, X.M.; López-Suárez, F.E.; Lozano-Castelló, D.; Bueno-López, A.; Wu, G. High-loading cobalt oxide coupled with nitrogen-doped graphene for oxygen reduction in anion-exchange-membrane alkaline fuel cells. J. Phys. Chem. C 2013, 117, 8697–8707. [Google Scholar] [CrossRef]
- Kumar, K.; Canaff, C.; Rousseau, J.; Arrii-Clacens, S.; Napporn, T.W.; Habrioux, A.; Kokoh, K.B. Effect of the oxide-carbon heterointerface on the activity of Co3O4/NRGO nanocomposites toward ORR and OER. J. Phys. Chem. C 2016, 120, 7949–7958. [Google Scholar] [CrossRef]
- Deming, C.P.; Mercado, R.; Gadiraju, V.; Sweeney, S.W.; Khan, M.; Chen, S.W. Graphene quantum dots-supported palladium nanoparticles for Efficient electrocatalytic reduction of oxygen in alkaline media. ACS Sustain. Chem. Eng. 2015, 3, 3315–3323. [Google Scholar] [CrossRef]
- Vinoth, R.; Patil, I.M.; Pandikumar, A.; Kakade, B.A.; Huang, N.M.; Dionysios, D.D.; Neppolian, B. Synergistically enhanced electrocatalytic performance of an N-doped graphene quantum dot-decorated 3D MoS2-graphene nanohybrid for oxygen reduction reaction. ACS Omega 2016, 1, 971–980. [Google Scholar] [CrossRef]
- Yin, H.J.; Tang, H.J.; Wang, D.; Gao, Y.; Tang, Z.Y. Facile synthesis of surfactant-free Au cluster/graphene hybrids for high-performance oxygen reduction reaction. ACS Nano 2012, 6, 8288–8297. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Niu, H.T.; Li, Z.Y.; Du, Y.; Cizek, P.; Xie, Z.L.; Xiong, H.G.; Lin, T. High-performance supercapacitor electrode materials from cellulose-derived carbon nanofibers. ACS Appl. Mater. Interfaces 2015, 7, 14946–14953. [Google Scholar] [CrossRef] [PubMed]
- Li, P.X.; Yang, Y.B.; Shi, E.Z.; Shen, Q.C.; Shang, Y.Y.; Wu, S.T.; Wei, J.Q.; Wang, K.L.; Zhu, H.W.; Yuan, Q.; et al. Core-double-shell, carbon nanotube@polypyrrole@MnO2 sponge as freestanding, compressible supercapacitor electrode. ACS Appl. Mater. Interfaces 2014, 6, 5228–5234. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.Y.; Zhang, L.L.; Ji, H.X.; Li, Y.; Zhao, X.; Bai, X.; Fan, X.B.; Zhang, F.B.; Ruoff, R.S. Nanoporous Ni(OH)2 thin film on 3D ultrathin-graphite foam for asymmetric supercapacitor. ACS Nano 2013, 7, 6237–6243. [Google Scholar] [CrossRef] [PubMed]
- Hahm, M.G.; Reddy, A.L.M.; Cole, D.P.; Rivera, M.; Vento, J.A.; Nam, J.; Jung, H.Y.; Kim, Y.L.; Narayanan, N.T.; Hashim, D.P.; et al. Carbon nanotube-nanocup hybrid structures for high power supercapacitor applications. Nano Lett. 2012, 12, 5616–5621. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Nagamuthu, S.; Muralidharan, G. Supercapacitor studies on NiO nanoflakes synthesized through a microwave route. ACS Appl. Mater. Interfaces 2013, 5, 2188–2196. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.P.; Li, L.; Hua, L.; Qian, Q.Q.; Wang, P.F.; Zhou, J.Y.; Sun, G.Z.; Huang, W. Design of amorphous manganese oxide@multiwalled carbon nanotube fiber for robust solid-state supercapacitor. ACS Nano 2017, 11, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Shin, S.S.; Han, H.S.; Oh, L.S.; Kim, D.H.; Kim, J.H.; Hong, K.S.; Kim, J.Y. 1-D structured flexible supercapacitor electrodes with prominent electronic/ionic transport capabilities. ACS Appl. Mater. Interfaces 2014, 6, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.; Li, C.; Chung, H.S.; Moore, J.; Thomas, J.; Jung, Y. High-performance one-body core/shell nanowire supercapacitor enabled by conformal growth of capacitive 2D WS2 layers. ACS Nano 2016, 10, 10726–10735. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.M.; Xu, C.F.; Chen, G.X.; Liu, Z.H.; Ma, M.; Xie, Q.J.; Zheng, N.F.; Yao, S.Z. Synthesis of ultrathin nitrogen-doped graphitic carbon nanocages as advanced electrode materials for supercapacitor. ACS Appl. Mater. Interfaces 2013, 5, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.H.; Hu, S.; Dai, F.; Yi, R.; Gordin, M.L.; Chen, S.R.; Song, J.X.; Wang, D.H. Self-templated synthesis of mesoporous carbon from carbon tetrachloride precursor for supercapacitor electrodes. ACS Appl. Mater. Interfaces 2016, 8, 6779–6783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zou, Q.H.; Hsu, H.S.; Raina, S.; Xu, Y.X.; Kang, J.B.; Chen, J.; Deng, S.Z.; Xu, N.S.; Kang, W.P. Morphology effect of vertical graphene on the high performance of supercapacitor electrode. ACS Appl. Mater. Interfaces 2016, 8, 7363–7369. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Sarkar, D.; Karmakar, K.; Mandal, K.; Khan, G.G. High-performance supercapacitor electrode based on cobalt oxide-manganese dioxide-nickel oxide ternary 1D hybrid nanotubes. ACS Appl. Mater. Interfaces 2016, 8, 20786–20792. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Z.; Li, G.R.; Guo, Y.; Ying, Y.L.; Peng, X.S. Flexible and binder-free hierarchical porous carbon film for supercapacitor electrodes derived from MOFs/CNT. ACS Appl. Mater. Interfaces 2017, 9, 14043–14050. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.J.; Wan, S.G.; Jiang, X.Q.; Wang, Z.; Gao, S.Y. Peanut-shell-like porous carbon from nitrogen-containing poly-N-phenylethanolamine for high-performance supercapacitor. ACS Appl. Mater. Interfaces 2015, 7, 22238–22245. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.H.; Bian, L.J.; Song, Y.; Liu, X.X. Electrochemical codeposition of vanadium oxide and polypyrrole for high-performance supercapacitor with high working voltage. ACS Appl. Mater. Interfaces 2014, 6, 12656–12664. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Tahir, M.N.; Adil, S.F.; Khan, H.U.; Siddiqui, M.R.H.; Al-warthan, A.A.; Tremel, W. Graphene based metal and metal oxide nanocomposites: Synthesis, properties and their applications. J. Mater. Chem. A 2015, 3, 18753–18808. [Google Scholar] [CrossRef]
- Kou, R.; Shao, Y.Y.; Wang, D.H.; Engelhard, M.H.; Kwak, J.H.; Wang, J.; Viswanathan, V.V.; Wang, C.M.; Lin, Y.H.; Wang, Y.; et al. Enhanced activity and stability of Pt catalysts on functionalized graphene sheets for electrocatalytic oxygen reduction. Electrochem. Commun. 2009, 11, 954–957. [Google Scholar] [CrossRef]
- Lu, T.; Zhang, Y.P.; Li, H.B.; Pan, L.K.; Li, Y.L.; Sun, Z. Electrochemical behaviors of graphene-ZnO and graphene-SnO2 composite films for supercapacitors. Electrochi. Acta 2010, 55, 4170–4173. [Google Scholar] [CrossRef]
- Sakthinathan, S.; Kubendhiran, S.; Chen, S.M.; Karuppiah, C.; Chiu, T.W. Novel bifunctional electrocatalyst for ORR activity and methyl parathion detection based on reduced graphene oxide/palladium tetraphenylporphyrin nanocomposite. J. Phys. Chem. C 2017, 121, 14096–14107. [Google Scholar] [CrossRef]
- Guo, S.J.; Sun, S.H. FePt nanoparticles assembled on graphene as enhanced catalyst for oxygen reduction reaction. J. Am. Chem. Soc. 2012, 134, 2492–2495. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.S.; Yang, S.B.; Sun, Y.; Parvez, K.; Feng, X.L.; Müllen, K. 3D nitrogen-doped graphene aerogel-supported Fe3O4 nanoparticles as efficient electrocatalysts for the oxygen reduction reaction. J. Am. Chem. Soc. 2012, 134, 9082–9085. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.Y.; Wang, H.L.; Zhou, J.G.; Li, Y.G.; Wang, J.; Regier, T.; Dai, H.J. Covalent hybrid of spinel manganese-cobalt oxide and graphene as advanced oxygen reduction electrocatalyst. J. Am. Chem. Soc. 2012, 134, 3517–3523. [Google Scholar] [CrossRef] [PubMed]
- Gartia, Y.; Parnell, C.M.; Watanabe, F.; Szwedo, P.; Biris, A.S.; Peddi, N.; Nima, Z.A.; Ghosh, A. Graphene-enhanced oxygen reduction by MN4 type cobalt(III) catalyst. ACS Sustain. Chem. Eng. 2015, 3, 97–102. [Google Scholar] [CrossRef]
- Jahan, M.; Bao, Q.L.; Loh, K.P. Electrocatalytically active graphene-porphyrin MOF composite for oxygen reduction reaction. J. Am. Chem. Soc. 2012, 134, 6707–6713. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.T.; Liu, Y.; Baek, J.B.; Dai, L.M. Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Yu, D.S.; Dai, L.M.; Chang, D.W.; Baek, J.B. Polyelectrolyte-functionalized graphene as metal-free electrocatalysts for oxygen reduction. ACS Nano 2011, 5, 6202–6209. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.L.; Gao, Y.; Zhu, S.M.; Zheng, J.S.; Chen, Z.X.; Yin, C.; Lou, X.H.; Zhang, D. Facile fabrication of N-doped graphene as efficient electrocatalyst for oxygen reduction reaction. ACS Appl. Mater. Interfaces 2015, 7, 19619–19625. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.X.; Wang, J.; Zhang, Y.W.; Xu, W.L.; Xing, W.; Xu, D.; Zhang, Y.F.; Zhang, X.B. ZIF-8 derived graphene-based nitrogen-doped porous carbon sheets as highly efficient and durable oxygen reduction electrocatalysts. Angew. Chem. Int. Ed. 2014, 53, 14235–14239. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Illathvalappil, R.; Kurungot, S.; Nair, B.N.; Mohamed, A.A.P.; Anilkumar, G.M.; Yamaguchi, T.; Hareesh, U.S. Graphene oxide sheathed ZIF-8 microcrystals: Engineered precursors of nitrogen-doped porous carbon for efficient oxygen reduction reaction (ORR) electrocatalysis. ACS Appl. Mater. Interfaces 2016, 8, 29373–29382. [Google Scholar] [CrossRef] [PubMed]
- Stamatin, S.N.; Hussainova, I.; Ivanov, R.; Colavita, P.E. Quantifying graphitic edge exposure in graphene-based materials and its role in oxygen reduction reactions. ACS Catal. 2016, 6, 5215–5221. [Google Scholar] [CrossRef]
- Li, R.; Wei, Z.D.; Gou, X.L. Nitrogen and phosphorus dual-doped graphene/carbon nanosheets as bifunctional electrocatalysts for oxygen reduction and evolution. ACS Catal. 2015, 5, 4133–4142. [Google Scholar] [CrossRef]
- Amiinu, I.S.; Zhang, J.; Kou, Z.K.; Liu, X.B.; Asare, O.K.; Zhou, H.; Cheng, K.; Zhang, H.N.; Mai, L.Q.; Pan, M.; et al. Self-organized 3D porous graphene dual-doped with biomass-sponsored nitrogen and sulfur for oxygen reduction and evolution. ACS Appl. Mater. Interfaces 2016, 8, 29408–29418. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Cheng, H.H.; Hu, Y.; Shi, G.Q.; Dai, L.M.; Qu, L.T. Nitrogen-doped graphene quantum dots with oxygen-rich functional groups. J. Am. Chem. Soc. 2012, 134, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.L.; Ye, R.Q.; Ye, G.L.; Peng, Z.W.; Fan, X.J.; Samuel, E.L.G.; Ajayan, P.M.; Tour, J.M. Boron- and nitrogen-doped graphene quantum dots/graphene hybrid nanoplatelets as efficient electrocatalysts for oxygen reduction. ACS Nano 2014, 8, 10837–10843. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.L.; Huang, H.H.; He, Y.H.; Feng, X.; Wang, S.; Dai, L.M.; Wang, J.C. Graphene quantum dots supported by graphene nanoribbons with ultrahigh electrocatalytic performance for oxygen reduction. J. Am. Chem. Soc. 2015, 137, 7588–7591. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.C.; Hoque, M.A.; Hassan, F.; Choi, J.Y.; Kim, B.J.; Chen, Z.W. Oxygen reduction on graphene-carbon nanotube composites doped sequentially with nitrogen and sulfur. ACS Catal. 2014, 4, 2734–2740. [Google Scholar] [CrossRef]
- Sui, Z.Y.; Meng, Y.N.; Xiao, P.W.; Zhao, Z.Q.; Wei, Z.X.; Han, B.H. Nitrogen-doped graphene aerogels as efficient supercapacitor electrodes and gas adsorbents. ACS Appl. Mater. Interfaces 2015, 7, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.B.; Feng, J.C.; Wu, P.Y. Deposition of three-dimensional graphene aerogel on nickel foam as a binder-free supercapacitor electrode. ACS Appl. Mater. Interfaces 2013, 5, 7122–7129. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.G.; Yu, Z.N.; Neff, D.; Zhamu, A.; Jang, B.Z. Graphene-based supercapacitor with an ultrahigh energy density. Nano Lett. 2010, 10, 4863–4868. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Z.Q.; Huang, Y.; Ma, Y.F.; Wang, C.Y.; Chen, M.M.; Chen, Y.S. Supercapacitor devices based on graphene materials. J. Phys. Chem. C 2009, 113, 13103–13107. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, N.; Kim, B.G.; Jung, D.S.; Im, K.; Hur, J.; Choi, J.W. Restacking-inhibited 3D reduced graphene oxide for high performance supercapacitor electrodes. ACS Nano 2013, 7, 9366–9374. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, J.Z.; Zhao, Y.; Li, Y.; Xian, W.; Amjadipour, M.; MacLeod, J.; Motta, N. Role of graphene oxide liquid crystals in hydrothermal reduction and supercapacitor performance. ACS Appl. Mater. Interfaces 2016, 8, 22316–22323. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.J.; Balakrishnan, K.; Huang, J.S.; Meunier, V.; Sumpter, B.G.; Srivastava, A.; Conway, M.; Reddy, A.L.M.; Yu, J.; Vajtai, R.; et al. Ultrathin planar graphene supercapacitor. Nano Lett. 2011, 11, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, S.I.; Yoon, J.C.; Jang, J.H. Chemical vapor deposition of mesoporous graphene nanoballs for Supercapacitor. ACS Nano 2013, 7, 6047–6055. [Google Scholar] [CrossRef] [PubMed]
- Sahu, V.; Shekhar, S.; Sharma, R.K.; Singh, G. Ultrahigh performance supercapacitor from lacey reduced graphene oxide nanoribbons. ACS Appl. Mater. Interfaces 2015, 7, 3110–3116. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Zhang, L.L.; Lee, S.J.; Oh, J.H.; Lee, K.S.; Potts, J.R.; Ji, J.Y.; Zhao, X.; Ruoff, R.S.; Park, S.J. Generation of B-doped graphene nanoplatelets using a solution process and their supercapacitor applications. ACS Nano 2013, 7, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.G.; Chen, Y.F.; Li, P.J.; He, J.R.; Zhang, W.L.; Guo, Z.; Li, Y.R.; Dong, M.D. Synthesis of silicon-doped reduced graphene oxide and its applications in dye-sensitive solar cells and supercapacitors. RSC Adv. 2016, 6, 15080–15086. [Google Scholar] [CrossRef]
- Xu, Z.W.; Li, Z.; Holt, C.M.B.; Tan, X.H.; Wang, H.L.; Amirkhiz, B.S.; Stephenson, T.; Mitlin, D. Electrochemical supercapacitor electrodes from sponge-like graphene nanoarchitectures with ultrahigh power density. J. Phys. Chem. Lett. 2012, 3, 2928–2933. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.H.; Yang, C.; Zhang, Z.X.; Zou, P.C.; Lin, Z.Y.; Shi, G.Q.; Yang, Q.H.; Kang, F.Y.; Wong, C.P. Shape-tailorable graphene-based ultra-high-rate supercapacitor for wearable electronics. ACS Nano 2015, 9, 5636–5645. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Liu, N.S.; Su, J.; Li, L.Y.; Long, F.; Zou, Z.G.; Jiang, X.L.; Gao, Y.H. Highly stretchable and self-healable supercapacitor with reduced graphene oxide based fiber springs. ACS Nano 2017, 11, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.H.; Hu, L.B.; Liu, N.; Wang, H.L.; Vosgueritchian, M.; Yang, Y.; Cui, Y.; Bao, Z.N. Enhancing the supercapacitor performance of graphene/MnO2 nanostructured electrodes by conductive wrapping. Nano Lett. 2011, 11, 4438–4442. [Google Scholar] [CrossRef] [PubMed]
- Naderi, H.R.; Norouzi, P.; Ganjali, M.R. Electrochemical study of a novel high performance supercapacitor based on MnO2/nitrogen-doped graphene nanocomposite. Appl. Surf. Sci. 2016, 366, 552–560. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, S.X.; Wu, L.M. Porous nickel hydroxide-manganese dioxide-reduced graphene oxide ternary hybrid spheres as excellent supercapacitor electrode materials. ACS Appl. Mater. Interfaces 2014, 6, 8621–8630. [Google Scholar] [CrossRef] [PubMed]
- He, Y.M.; Chen, W.J.; Li, X.D.; Zhang, Z.X.; Fu, J.C.; Zhao, C.H.; Xie, E.Q. Freestanding three-dimensional graphene/MnO2 composite networks as ultralight and flexible supercapacitor electrodes. ACS Nano 2013, 7, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.Y.; Li, N.; Jin, S.X.; Yang, G.W.; Wang, C.X. All-solid-state symmetric supercapacitor based on Co3O4 nanoparticles on vertically aligned graphene. ACS Nano 2015, 9, 5310–5317. [Google Scholar] [CrossRef] [PubMed]
- Heydari, H.; Gholivand, M.B. A novel high-performance supercapacitor based on high-quality CeO2/nitrogen-doped reduced graphene oxide nanocomposite. Appl. Phys. A 2017, 123, 187. [Google Scholar] [CrossRef]
- Quan, H.Y.; Cheng, B.C.; Xiao, Y.H.; Lei, S.J. One-pot synthesis of α-Fe2O3 nanoplates-reduced graphene oxide composite for supercapacitor application. Chem. Eng. J. 2016, 286, 165–173. [Google Scholar] [CrossRef]
- He, W.; Jiang, H.J.; Zhou, Y.; Yang, S.D.; Xue, X.; Zou, Z.Q.; Zhang, X.G.; Akins, D.L.; Yang, H. An efficient reduction route for the production of Pd–Pt nanoparticles anchored on graphene nanosheets for use as durable oxygen reduction electrocatalysts. Carbon 2012, 50, 265–274. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Mun, B.S.; Arenz, M.; Mayrhofer, K.J.J.; Lucas, C.A.; Wang, G.F.; Ross, P.N.; Markovic, N.M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kang, L.T.; Bai, G.L.; Li, P.Y.; Deng, J.C.; Liu, X.G.; Yang, Y.Z.; Gao, F.; Liang, W. Solvothermal synthesis of Fe2O3 loaded activated carbon as electrode materials for high-performance electrochemical capacitors. Electrochim. Acta 2014, 134, 67–75. [Google Scholar] [CrossRef]
- Aravindan, V.; Mhamane, D.; Ling, W.C.; Ogale, S.; Madhavi, S. Nonaqueous lithium-ion capacitors with high energy densities using trigol-reduced graphene oxide nanosheets as cathode-active material. ChemSusChem 2013, 6, 2240–2244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, T.F.; Yang, X.; Zhang, L.; Leng, K.; Huang, Y.; Chen, Y.S. A high-performance supercapacitor-battery hybrid energy storage device based on graphene-enhanced electrode materials with ultrahigh energy density. Energy Environ. Sci. 2013, 6, 1623–1632. [Google Scholar] [CrossRef]
- Wang, H.W.; Guan, C.; Wang, X.F.; Fan, H.J. A high energy and power Li-ion capacitor based on a TiO2 nanobelt array anode and a graphene hydrogel cathode. Small 2015, 11, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, S.L.; Zhang, X.; He, T.B.; Lu, F.X.; Li, H.C.; Ye, J.H. A facile method of preparing LiMnPO4/reduced graphene oxide aerogel as cathodic material for aqueous lithium-ion hybrid Supercapacitors. Appl. Surf. Sci. 2018, 428, 977–985. [Google Scholar] [CrossRef]
- Aswathy, R.; Kesavan, T.; Kumaran, K.T.; Ragupathy, P. Octahedral high voltage LiNi0.5Mn1.5O4 spinel cathode: Enhanced capacity retention of hybrid aqueous capacitors with nitrogen doped grapheme. J. Mater. Chem. A 2015, 3, 12386–12395. [Google Scholar] [CrossRef]
- Qian, Y.; Cai, X.Y.; Zhang, C.Y.; Jiang, H.F.; Zhou, L.J.; Li, B.S.; Lai, L.F. A free-standing Li4Ti5O12/graphene foam composite as anode material for Li-ion hybrid supercapacitor. Electrochim. Acta 2017, 258, 1311–1319. [Google Scholar] [CrossRef]
- Song, H.; Fu, J.J.; Ding, K.; Huang, C.; Wu, K.; Zhang, X.M.; Gao, B.; Huo, K.F.; Peng, X.; Chu, P.K. Flexible Nb2O5 nanowires/graphene film electrode for high-performance hybrid Li-ion supercapacitors. J. Power Sources 2016, 328, 599–606. [Google Scholar] [CrossRef]
- Zhang, F.; Tang, Y.B.; Liu, H.; Ji, H.Y.; Jiang, C.L.; Zhang, J.; Zhang, X.L.; Lee, C.S. Uniform incorporation of flocculent molybdenum disulfide nanostructure into three-dimensional porous graphene as an anode for high-performance lithium ion batteries and hybrid supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 4691–4699. [Google Scholar] [CrossRef] [PubMed]
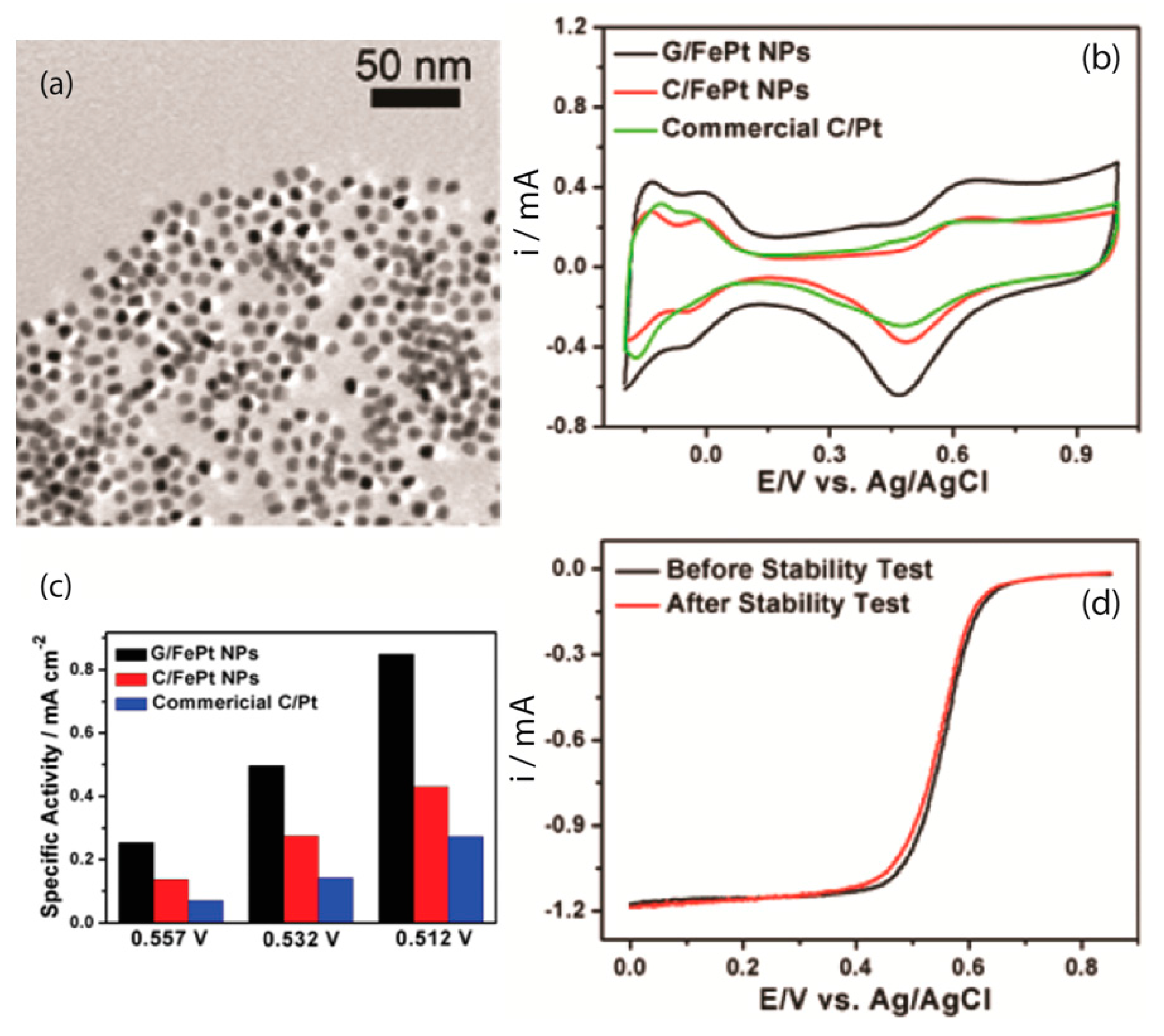
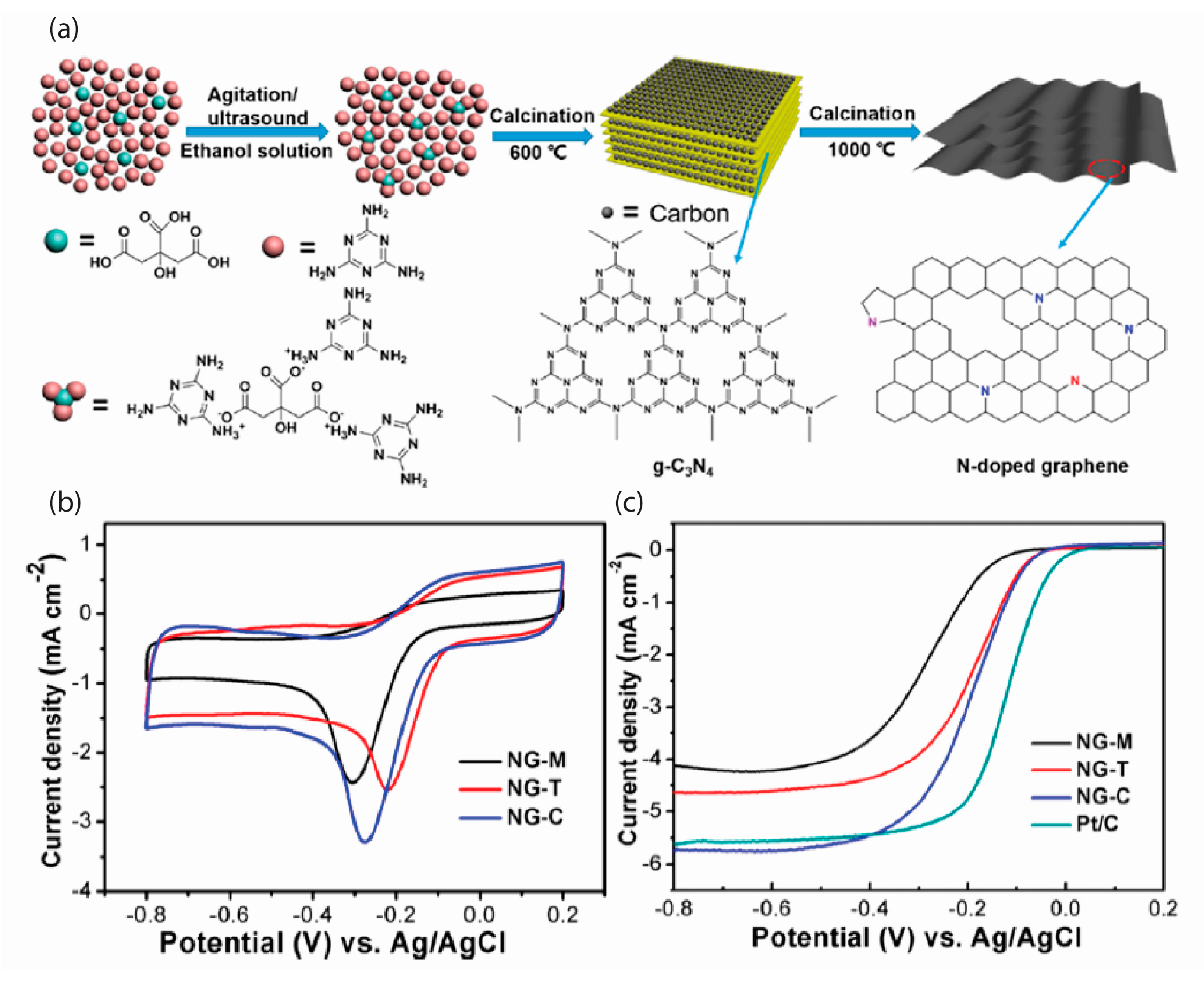
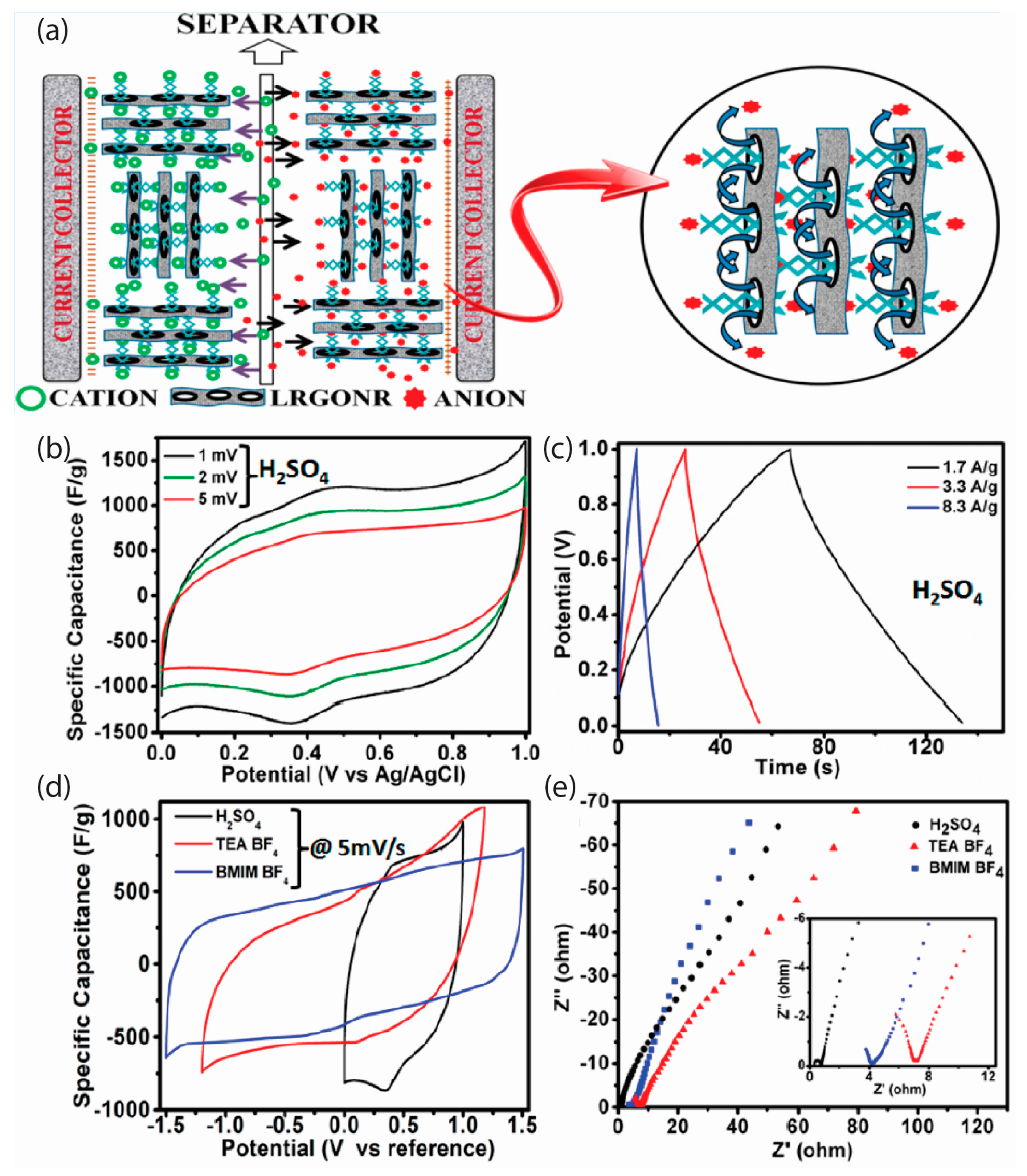
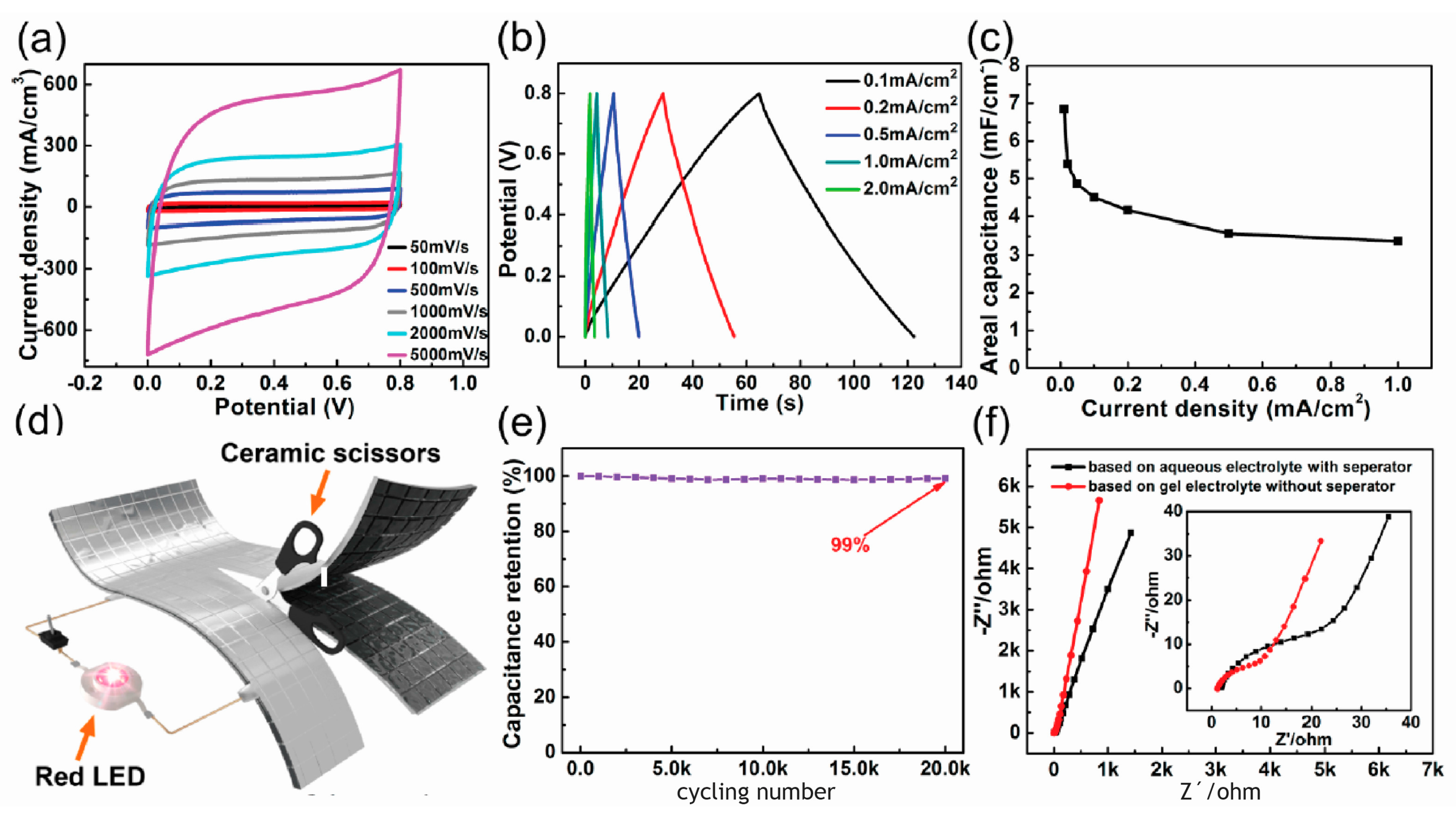
| [Ref.] Catalyst Abbreviation | Mass Activity (mA/mg) | Current Density Specific Density (mA/cm2) | ESCA BET (m2/g) | Onset Potential (V) | Peak Potential(V) | Half-Wave Potential (V) | 4e Selectivity | Electrochemical Stability |
|---|---|---|---|---|---|---|---|---|
| [43] G/FePt | - | 0.85 | - | - | - | 0.557 vs. Ag/AgCl | - | no change after 10,000 cycles in 0.4–0.8 V range |
| [44] Fe3O4/N-GAs | - | −2.56 | - | −0.19 vs. Ag/AgCl | - | - | 3.72–3.95 | 79.3% current retention after 20,000 s |
| [45] MnCo2O4/N-rmGO | - | 151 | - | 0.95 vs. RHE | 0.88 vs. RHE | - | ~3.9 | 3.5% decrease in current density over 20,000 s |
| [46] MN4 type cobalt(III) | - | - | - | - | −0.04 vs. Ag/AgCl | - | 4.04 | - |
| [47] (G-dye-FeP)n MOF | - | 5.9 | 933 | - | −0.23 vs. Ag/AgCl | - | ~4 | - |
| [48] N-Graphene | - | −0.78 | - | - | - | - | 3.6–4 | no obvious current decrease after 200,000 cycles in −1.0–0 V range |
| [49] PDDA-Graphene | - | - | - | −0.15 vs. SCE | −0.35 vs. SCE | - | 3.5–4 | much slower decrease than the Pt/C and leveled off after 17,000 s |
| [50] N-Doped Graphene | - | −2.4(NG-M) −2.6(NG-T) −3.3(NG-C) | - | −0.07(NG-M) 0(NG-T) 0(NG-C) | −0.31(NG-M) −0.22(NG-T) −0.28(NG-C) | - | 3.55 | no significant decrease in current density after 2000 cycles |
| [51] GNPCSs | - | - | 911 | - | - | - | 3.78–3.98 | 94% retention over 28,000 s |
| [52] GZx | - | - | 917 for GZ80 | 0.88 vs. RHE | - | 0.75 | 3.2 | negligible penalty in E1/2 after 5000 cycles |
| [53] G-ANF-1 | 35 | 1.3 | 203(G-ANF-1) | 0.73 vs. RHE | 0.63 | - | 3.2 | - |
| [54] N,P-GCNS | - | 5.56 | 900.2 | 1.01 | 0.85 vs. RHE | - | 3.96 | 4.5% performance attenuation after 16,000 s |
| [55] NSG | - | - | 319.93 | - | −0.22 vs. SCE | −0.23 | 3.52–3.83 | 94.3% current retention after 55,000 s |
| [56] N-GQD | - | - | - | −0.16 | −0.27 | - | 3.6–4.4 | no obvious decrease after 2 days |
| [57] BN-GQD/G30 | - | ~11.1 | - | ~0 vs. Ag/AgCl | - | - | 3.93 | - |
| [58] GQD/GNR | - | - | - | - | −0.19 vs. Ag/AgCl | - | 3.91 | 1% current decrease after 26 h |
| [59] GC-NLs | - | −4.80 | - | 0.85 vs. RHE | - | 0.72 | 3.6–3.8 | - |
| [Ref.] Electrode Material Abbreviation | BET (m2/g) | Electrical Conductiviy (S/m) | Specific Capacitance | Stability/Capacitance Retention | Rate Capability Rate Performance | Energy Density | Power Density | Equivalent Series Resistance |
|---|---|---|---|---|---|---|---|---|
| [60] NGA | 830 | 0.8 | 223 F/g | 8% decrease after 2000 cycles | - | - | - | - |
| [61] GA@NF | 463 | 71.4 | 366 F/g | 60% retention after 2000 cycles | - | - | - | 0.45 Ω |
| [62] curved graphene | - | - | 151.4 F/g@1 A/g | - | Completely recharged in less than 2 min | 90 Wh/kg | - | - |
| [63] graphene material | 320 | 100 | 205 F/g, 64 µF/cm2 | 90% retention after 1200 cycles | - | 28.5 Wh/kg | 10 kW/kg | 3.2 Ω |
| [64] cMR-rGOth | 1040 | - | 210 F/g | 96% retention after 20,000 cycles | - | - | - | - |
| [65] blade-rGO | - | - | 250 F/g | 90% retention over 4000 cycles | - | - | - | 7.5–16 Ω |
| [66] RMGO | - | - | 247.3 F/g | No degradation in performance after 1500 cycles | - | - | - | - |
| [67] p-doped MGB | 346 | 6.5 S/cm | 206 F/g | 96% retention after 10,000 cycles | - | - | - | - |
| [68] LRGONR | 190 | - | 1042 F/g | 97% retention over 3000 GCD cycles | - | 15.06 Wh/kg | - | 0.48 Ω |
| [69] B-doped Graphene | 466 | - | 200 F/g, 43 µF/cm2 | 95% retention after 4500 cycles | - | - | - | 0.8 Ω |
| [70] Si-rGO | - | - | 184.4 F/g | - | - | - | 107 kW/kg | 0.61 Ω |
| [71] SPG | 418 | - | 16.2 µF/cm2 | 87% retention after 10,000 cycles | 2.3 min for one charge-discharge cycle | 21.4 Wh/kg | - | - |
| [72] ErGO-NCA | - | - | 160 F/g, 57.1 mF/cm2 | 94.3% retentioan after 20,000 cycles | - | - | - | - |
| [73] PPy/RGO/MWCNTs | - | - | 25.9 F/cm3 | 9.6% decay over 3000 cycles | - | 0.94 mWh/cm3 | - | 0.7 Ω |
| [74] GMC or GMP | - | - | 380 F/g | 95-96% capacitance retention after 1000 cycles | - | - | - | 41 Ω or 27 Ω |
| [75] MnO2/NRGO | - | - | 522 F/g | 3.7% decrease after applying 4000 CVs | - | - | - | 0.40 Ω |
| [76] Ni(OH)2-MnO2-RGO | 147 | - | 1985 F/g | 75% capacity retention after 2000 cycles | - | 54.0 Wh/kg | 1016 W/kg | - |
| [77] Graphene/MnO2 | 392 | - | 130 F/g, 1.42 F/cm2 | Capacity decrease from 29.8/g–27.8 F/g after 500 cycles | - | 6.8 Wh/kg | - | 2.41 Ω |
| [78] Co3O4/VAGN | - | - | 3480 F/g | - | - | 80 Wh/kg | 20 kW/kg | - |
| [79] CeO2-NRGO | - | - | 230 F/g | 94.1% retention for 4000 cycles tests | - | - | - | 0.43 Ω |
| [80] α-Fe2O3/rGO | 38.04 | - | 930.6 F/g | 70% retention after 1000 cycles | - | - | - | 1.64 Ω |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, P.; Zhao, P.; Wang, Y.; Liu, B.; Yin, G.; Dong, M. A Roadmap for Achieving Sustainable Energy Conversion and Storage: Graphene-Based Composites Used Both as an Electrocatalyst for Oxygen Reduction Reactions and an Electrode Material for a Supercapacitor. Energies 2018, 11, 167. https://doi.org/10.3390/en11010167
Huo P, Zhao P, Wang Y, Liu B, Yin G, Dong M. A Roadmap for Achieving Sustainable Energy Conversion and Storage: Graphene-Based Composites Used Both as an Electrocatalyst for Oxygen Reduction Reactions and an Electrode Material for a Supercapacitor. Energies. 2018; 11(1):167. https://doi.org/10.3390/en11010167
Chicago/Turabian StyleHuo, Peipei, Peng Zhao, Yin Wang, Bo Liu, Guangchao Yin, and Mingdong Dong. 2018. "A Roadmap for Achieving Sustainable Energy Conversion and Storage: Graphene-Based Composites Used Both as an Electrocatalyst for Oxygen Reduction Reactions and an Electrode Material for a Supercapacitor" Energies 11, no. 1: 167. https://doi.org/10.3390/en11010167
APA StyleHuo, P., Zhao, P., Wang, Y., Liu, B., Yin, G., & Dong, M. (2018). A Roadmap for Achieving Sustainable Energy Conversion and Storage: Graphene-Based Composites Used Both as an Electrocatalyst for Oxygen Reduction Reactions and an Electrode Material for a Supercapacitor. Energies, 11(1), 167. https://doi.org/10.3390/en11010167






