Insulation Strength and Decomposition Characteristics of a C6F12O and N2 Gas Mixture
Abstract
1. Introduction
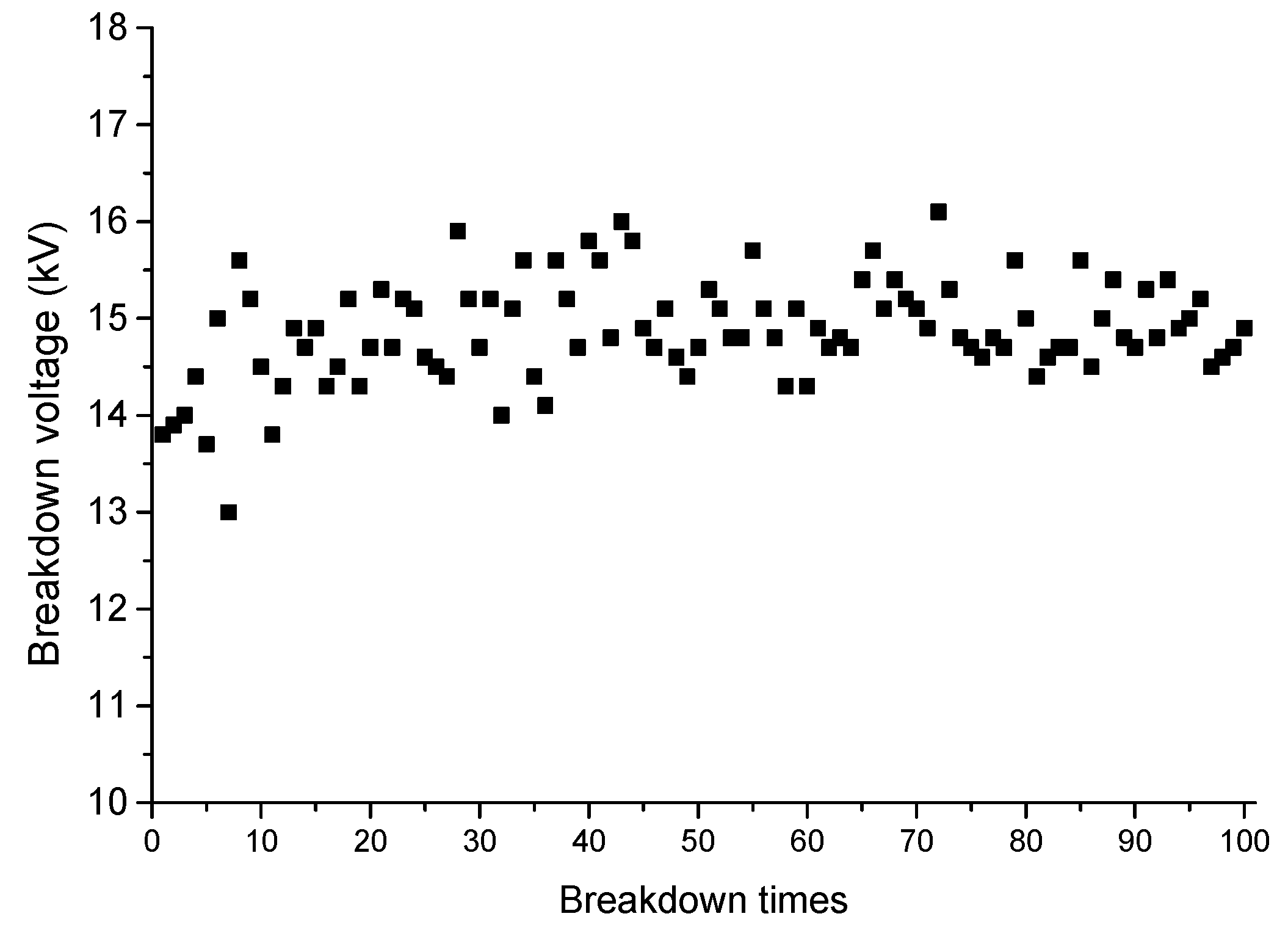
2. Experiment
- T1:
- Induction voltage regulator with an input voltage of 380 V and an output voltage of 0–400 V;
- T2:
- Non-corona test transformer can provide the power frequency test voltage of 100 kV;
- R:
- Protection resistance of R = 10 kΩ, which can protect the transformer from being damaged in breakdown;
- CV:
- Capacitive divider, in which the test voltage with high amplitude can be converted into the lower value which can be measured directly by the voltmeter;
- V:
- Voltmeter measures the voltage through the capacitive divider.
3. Decomposition Process Calculation
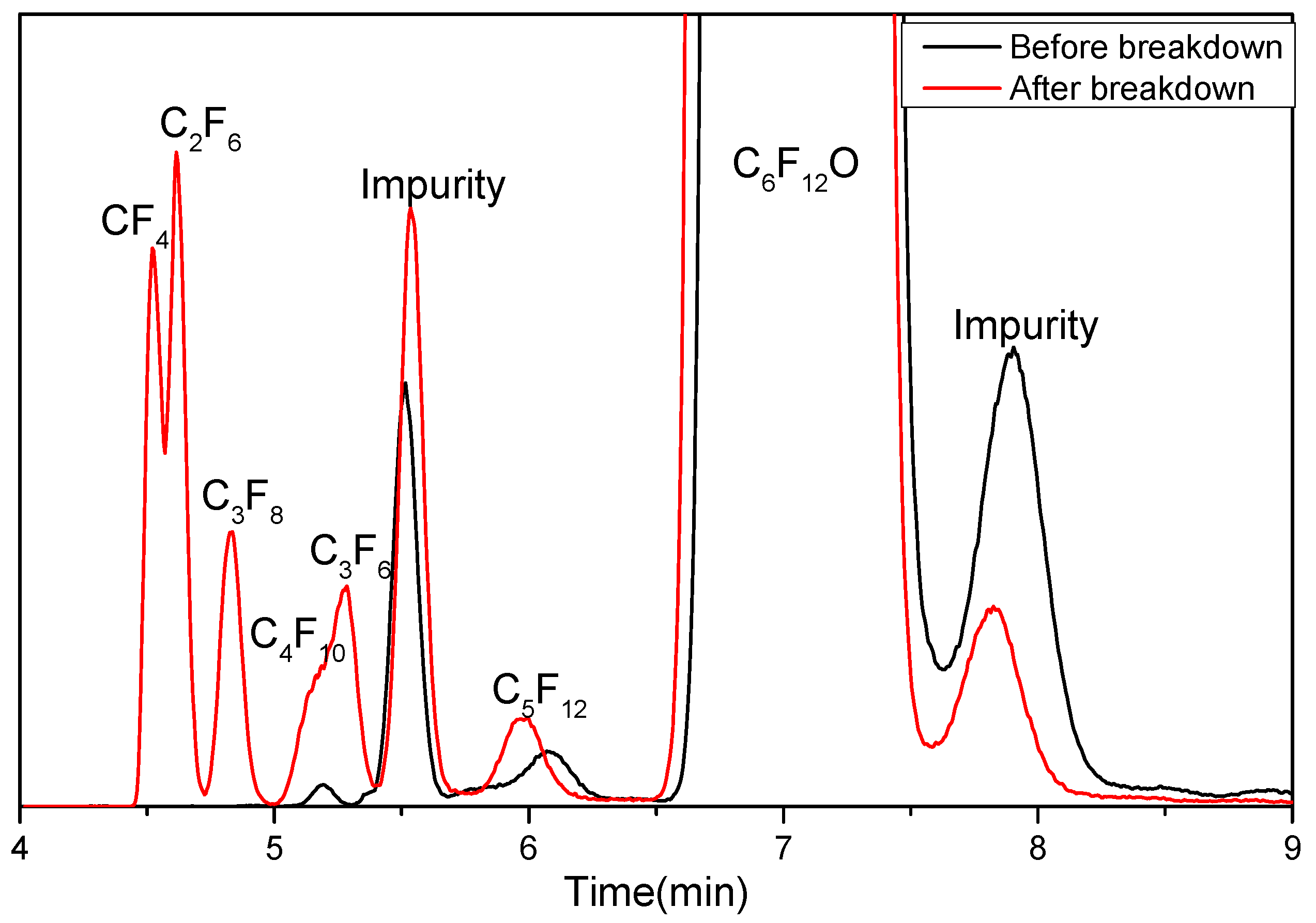
3.1. Formation of Decomposition Products
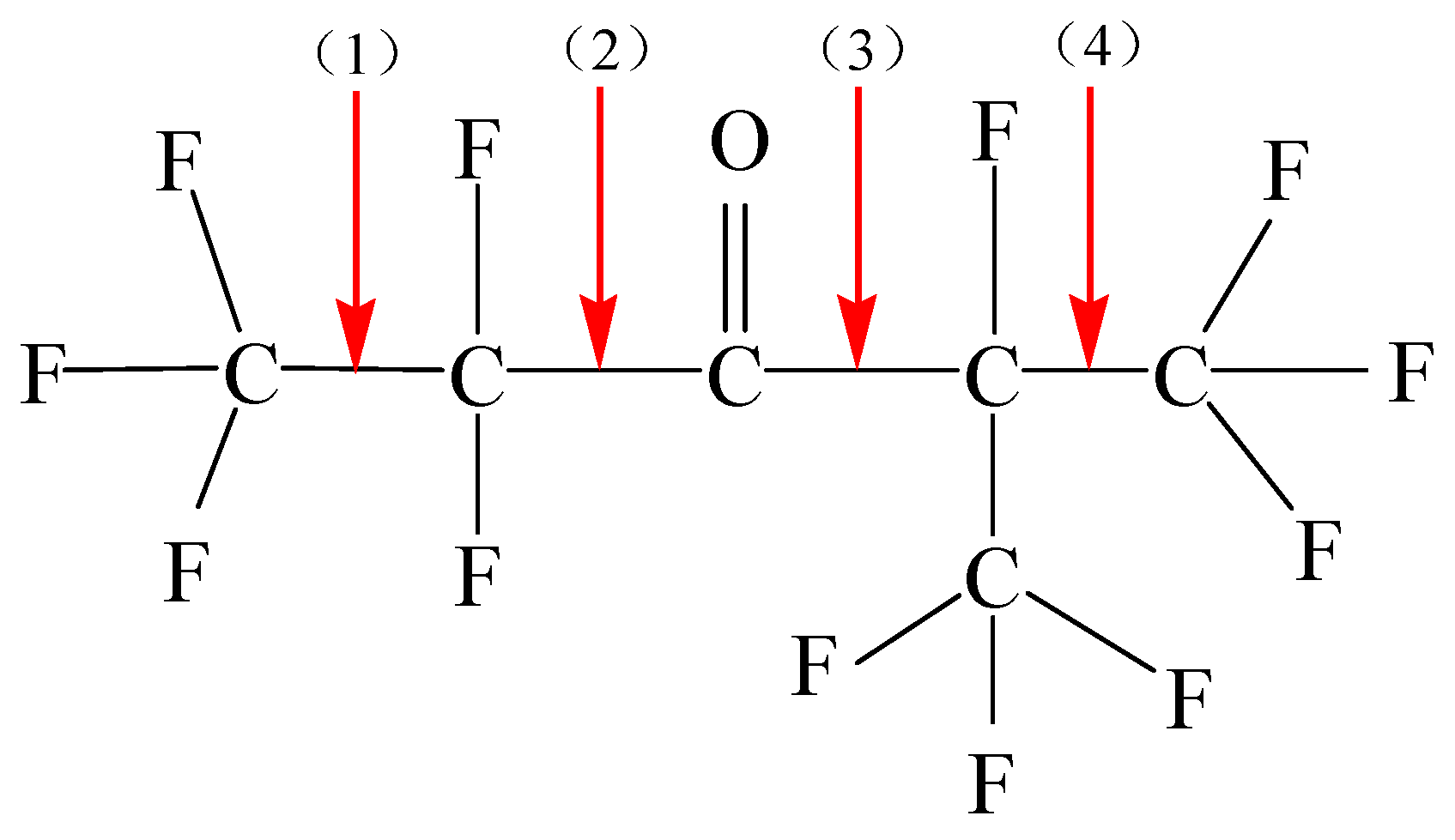
- (1)
- C6F12O → CF3• + C5F9O• ΔH = 86.7857 kcal/mol
- (2)
- C6F12O → C2F5• + C4F7O• ΔH = 85.6439 kcal/mol
- (3)
- C6F12O → C3F5O• + C3F7• ΔH = 83.4267 kcal/mol
- (4)
- C6F12O → C5F9O• + CF3• ΔH = 81.0649 kcal/mol
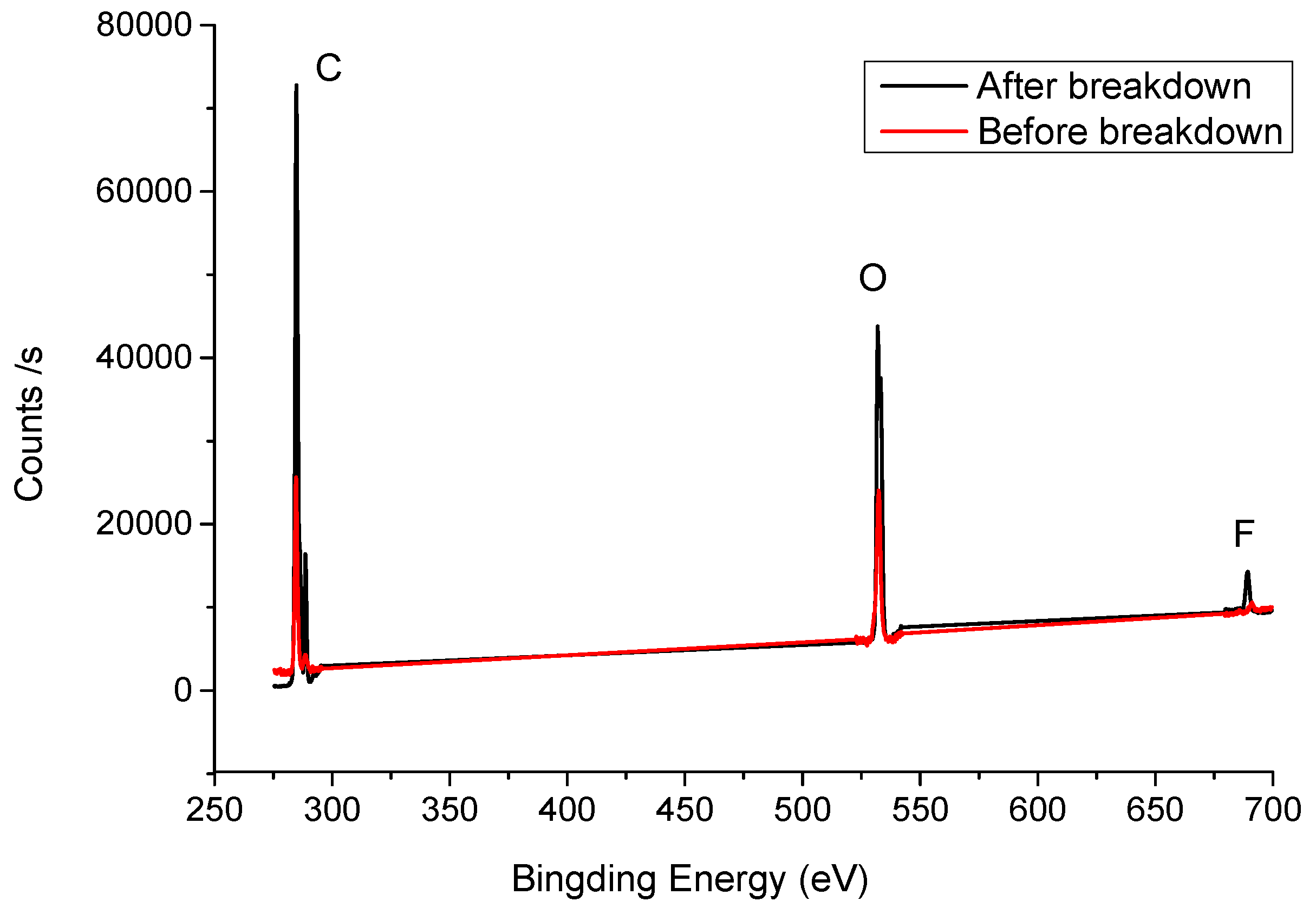
3.2. Basic Properties of C6F12O and Decomposition Products
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Rokunohe, T.; Yagihashi, Y.; Endo, F.; Oomori, T. Fundamental insulation characteristics of air; N2, CO2, N2/O2, and SF6/N2 mixed gases. Electr. Eng. Jpn. 2006, 155, 9–17. [Google Scholar] [CrossRef]
- Nishiwaki, S.; Koshizuka, T.; Uchii, T.; Kawano, H. Discussions on post arc current of a CO2 circuit breaker. In Proceedings of the 17th International Conference on Gas Discharges and Their Applications, Cardiff, UK, 7–12 September 2008. [Google Scholar]
- Uchiii, A.M.T.; Koshizuka, T.; Kawano, H. Thermal interruption capabilities of CO2 gas and CO2-based gas mixtures. In Proceedings of the 18th International Conference on Gas Discharges and Their Applications, Greifswald, Germany, 5–10 September 2010. [Google Scholar]
- Lim, D.Y.; Bae, S. Study on oxygen/nitrogen gas mixtures for the surface insulation performance in gas insulated switchgear. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 1567–1576. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, Y.M.; Seok, B.Y.; Kwon, J.H.; Lim, K.J. A study on the lightning impulse breakdown characteristics of dry air for design of eco-friendly electric power apparatus. In Proceedings of the 18th International Symposium on High Voltage Engineering, Seoul, Korea, 25–30 August 2013. [Google Scholar]
- Koch, H.; Hopkins, M. Overview of gas insulated lines (GIL). In Proceedings of the Power Engineering Society General Meeting, San Francisco, CA, USA, 12–16 June 2005. [Google Scholar]
- Zhang, X.; Xiao, S.; Zhou, J.; Tang, J. Experimental analysis of the feasibility of CF3I/CO2 substituting SF6 as insulation medium using needle-plate electrodes. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1895–1900. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, S.; Han, Y.; Cressault, Y. Experimental studies on power frequency breakdown voltage of CF3I/N2 mixed gas under different electric fields. Appl. Phys. Lett. 2016, 108, 092901-1–092901-4. [Google Scholar] [CrossRef]
- Wu, B.T.; Xiao, D.M.; Liu, Z.S.; Zhang, L.C.; Liu, X.L. Analysis of insulation characteristics of c-C4F8 and N2 gas mixtures by the Monte Carlo method. J. Phys. D Appl. Phys. 2006, 39, 4204–4207. [Google Scholar] [CrossRef]
- Okabe, S.; Wada, J.; Ueta, G. Dielectric properties of gas mixtures with C3F8/C2F6 and N2/CO2. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 2108–2116. [Google Scholar] [CrossRef]
- Linteris, G.T.; Babushok, V.I.; Sunderland, P.B.; Takahashi, F.; Katta, V.R.; Meier, O. Unwanted combustion enhancement by C6F12O fire suppressant. Proc. Combust. Inst. 2013, 34, 2683–2690. [Google Scholar] [CrossRef]
- Tuma, P.E. Fluoroketone C2F5C(O)CF(CF3)2, as a Heat Transfer Fluid for Passive and Pumped 2-Phase Applications. In Proceedings of the Annual IEEE Semiconductor Thermal Measurement and Management Symposium, San Jose, CA, USA, 16–20 March 2008. [Google Scholar]
- Mantilla, J.D.; Gariboldi, N.; Grob, S.; Claessens, M. Investigation of the insulation performance of a new gas mixture with extremely low GWP. In Proceedings of the IEEE Electrical Insulation Conference, Philadelphia, PA, USA, 8–11 June 2014. [Google Scholar]
- Xu, W.; Jiang, Y.; Ren, X. Combustion promotion and extinction of premixed counterflow methane/air flames by C6F12O fire suppressant. J. Fire Sci. 2016, 34, 289–304. [Google Scholar] [CrossRef]
- Deng, Y.; Li, B.; Xiao, D. Analysis of the insulation characteristics of C3F8 gas mixtures with N2 and CO2 using Boltzmann equation method. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 3253–3259. [Google Scholar] [CrossRef]
- Hyrenbach, M.; Hintzen, T.; Muller, P.; Owens, J. Alternative gas insulation in medium-voltage switchgear. In Proceedings of the 23th international conference on electricity distribution, Lyon, France, 15–18 June 2015. [Google Scholar]
- Perdew, J.P.; Wang, Y. Accurate and simple analyticre presentation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Monkhorst, H.; Pack, J. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, S.; Zhang, J.; Li, C.; Dai, Q.; Han, Y. Influence of humidity on the decomposition products and insulating characteristics of CF3I. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 819–828. [Google Scholar] [CrossRef]
- Ai, L.L.; Duan, X.M.; Liu, J.Y. Theoretical studies on the reaction mechanism of CF3CF=CF2 with OH. Comput. Theor. Chem. 2013, 1013, 15–22. [Google Scholar] [CrossRef]
- Swick, D.A.; Karle, I.L. Structure and Internal Motion of C2F6 and Si2Cl6 Vapors. J. Chem. Phys. 1955, 23, 1499–1504. [Google Scholar] [CrossRef]
- Carroll, T.X.; Børve, K.J.; Sæthre, L.J.; Bozek, J.D.; Kukk, E.; Hahne, J.A.; Thomas, T.D. Carbon 1s photoelectron spectroscopy of CF4 and CO: Search for chemical effects on the carbon 1s hole-state lifetime. J. Chem. Phys. 1999, 116, 10221–10228. [Google Scholar] [CrossRef]
- Garg, S.K.; Davidson, D.W.; Ripmeester, J.A. Analysis of NMR lineshapes of rigid-lattice multispin systems II. Bond length and chemical shielding in CF4. J. Magn. Reson. 1979, 36, 325–331. [Google Scholar] [CrossRef]
- Hubers, M.M.; Los, J. Ion pair formation in alkali-SF6 collisions: Dependence on collisional and vibrational energy. Chem. Phys. 1975, 10, 235–259. [Google Scholar] [CrossRef]
- Wada, J.; Ueta, G.; Okabe, S.; Hikita, M. Dielectric properties of gas mixtures with per-fluorocarbon gas and gas with low liquefaction temperature. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 838–847. [Google Scholar] [CrossRef]
- Devins, J.C. Replacement Gases for SF6. IEEE Trans. Dielectr. Electr. Insul. 1980, EI-15, 81–86. [Google Scholar] [CrossRef]
- Deng, Y.; Xiao, D. Analysis of the insulation characteristics of CF3I gas mixtures with Ar, Xe, He, N2, and CO2 using Boltzmann equation method. Jpn. J. Appl. Phys. 2014, 53, 096201. [Google Scholar] [CrossRef]
- Takahashi, K.; Itoh, A.; Nakamura, T.; Tachibana, K. Radical kinetics for polymer film deposition in fluorocarbon (C4F8, C3F6, and C5F8) plasmas. Thin Solid Films 2000, 374, 303–310. [Google Scholar] [CrossRef]
- Ivy, D.J.; Rigby, M.; Baasandorj, M.; Burkholder, J.B.; Prinn, R.G. Global emission estimates and radiative impact of C4F10, C5F12, C6F14, C7F16 and C8F18. Atmos. Chem. Phys. 2012, 12, 7635–7645. [Google Scholar] [CrossRef]
- Ravishankara, A.R.; Solomon, S.; Turnipseed, A.A.; Warren, R.F. Atmospheric lifetimes of long-lived halogenated species. Science 1993, 259, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Surhone, L.M.; Timpledon, M.T.; Marseken, S.F. Novec 1230. Landolt Börnstein Group IV Phys. Chem. 2010, 26, 263–291. [Google Scholar]

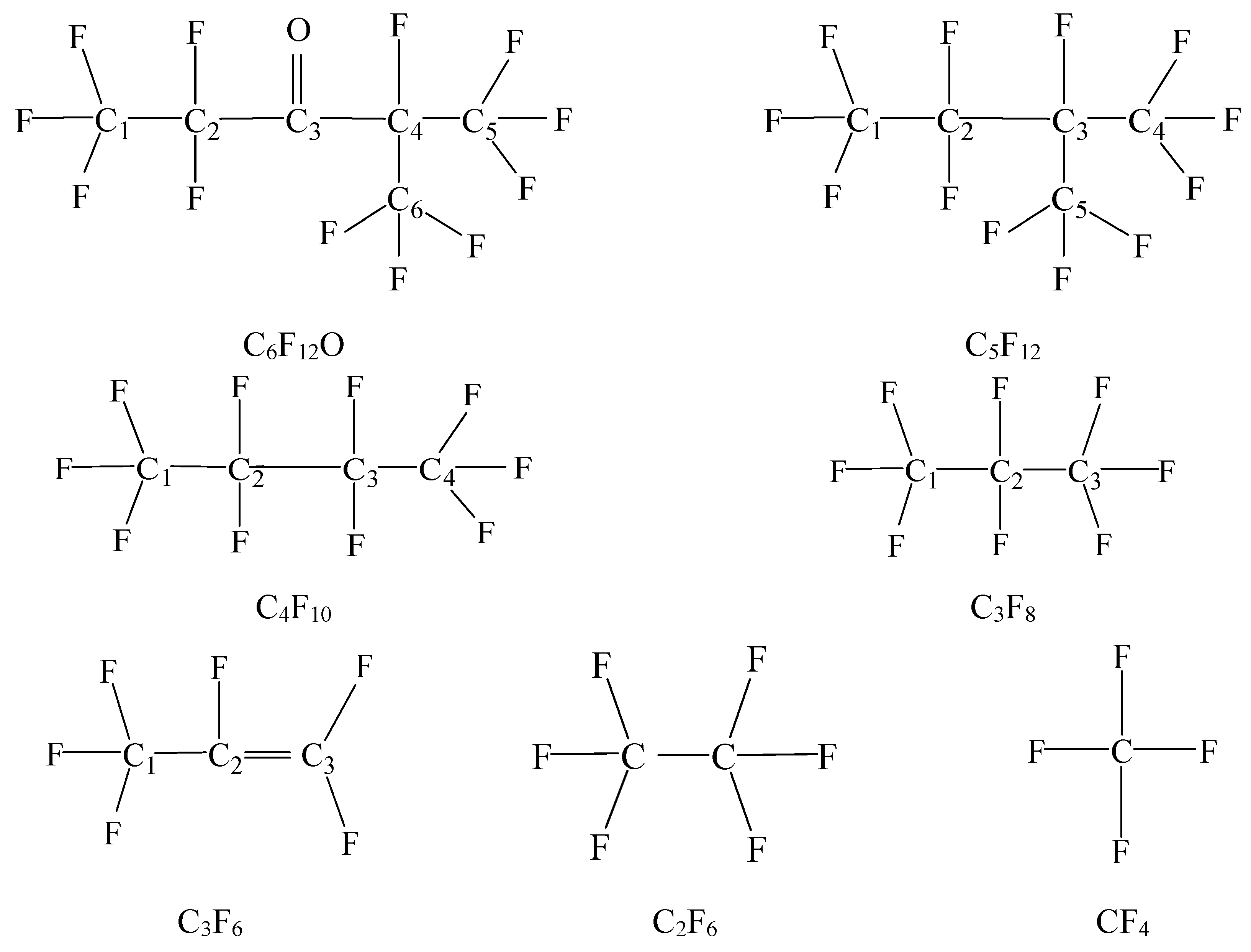

| Gas | Bond Length | Bond Angle | ||||
|---|---|---|---|---|---|---|
| Species | Type | Value(Å) | Reference | Type | Value (°) | Reference |
| C6F12O | F–C1 | 1.402 | - | F–C1–F | 108.511 | - |
| C1–C2 | 1.566 | - | F–C2–F | 108.021 | - | |
| F–C2 | 1.409 | - | C1–C2–C3 | 115.584 | - | |
| C2–C3 | 1.566 | - | C2–C3=O | 117.907 | - | |
| C3=O | 1.277 | - | C2–C3–C4 | 124.115 | - | |
| C3–C4 | 1.594 | - | C3–C4–C5 | 113.574 | - | |
| C4–F | 1.439 | - | F–C4–C6 | 104.340 | - | |
| C4–C5, C4–C6 | 1.565 | - | F–C5–F | 108.519 | - | |
| C5–F, C6–F | 1.401 | - | - | |||
| C5F12 | F–C1 | 1.348 | - | F–C1–F | 108.719 | - |
| C1–C2 | 1.571 | - | F–C1–C2 | 110.792 | - | |
| C2–C3 | 1.579 | - | F–C2–F | 109.044 | - | |
| F–C2 | 1.361 | - | F–C2–C3 | 107.132 | - | |
| F–C3 | 1.376 | - | F–C3–C4 | 106.775 | - | |
| C3–C4, C3–C5 | 1.583 | - | C1–C2–C3 | 118.835 | - | |
| C4–F, C5–F | 1.348 | - | C2–C3–C4 | 114.570 | - | |
| - | C4–C3–C5 | 110.239 | - | |||
| - | F–C4–F | 107.675 | - | |||
| C4F10 | F–C1, F–C4 F–C2, F–C3 C1–C2, C3–C4 C2–C3 | 1.349 1.360 1.568 1.564 | - | F–C1–F F–C2–F, C1–C2–C3 C2–C3–C4 C1–C2–F | 108.692 109.103 115.210 115.185 107.392 | - |
| C3F8 | C1–C2, C2–C3 | 1.563 | 1.54 [19] | F–C1–C2 | 108.783 | 109.1 [19] |
| F–C1, F–C3 | 1.348 | 1.33 [19] | F–C2–F | 108.892 | 108.6 [19] | |
| F–C2 | 1.359 | 1.34 [19] | F–C2–C3 | 109.292 | 109.5 [19] | |
| F–C3–C4 | 107.964 | 108.0 [19] | ||||
| C1–C2–C3 | 115.580 | 115.2 [19] | ||||
| C3F6 | C1–C2 | 1.497 | 1.498 [20] | C2–C3–C4 | 108.200 | 108.40 [20] |
| C2=C3 | 1.341 | 1.329 [20] | C4–C3–C5 | 110.645 | - | |
| F–C1 | 1.362 | 1.341 [20] | F–C4–F | 113.505 | - | |
| F–C2 | 1.350 | 1.337 [20] | F–C2=C3 | 118.849 | - | |
| F–C3 | 1.324 | 1.329 [20] | F–C3–F | 112.210 | 112.04 [20] | |
| C2F6 | C–C | 1.591 | 1.56 ± 0.03 [21] | F–C–F | 108.648 | - |
| C–F | 1.347 | 1.32 ± 0.01 [21] | F–C–C | 110.282 | 109.5 ± 1.5 [21] | |
| CF4 | C–F | 1.341 | 1.3166 [22] 1.330 ± 0.005 [23] | F–C–F | 109.471 | - |
| No. | Reaction | Energy of Reactants (Ha) | Energy of Products (Ha) | Temperature Correction Value (298 K) (kcal/mol) | Energy (kcal/mol) | Barrier (kcal/mol) |
|---|---|---|---|---|---|---|
| ① | CF3• → F• + CF2: | −337.4509077 | −337.3130314 | −3.109 | 83.409 | - |
| ② | CF2: → 2F• + C | −237.5749908 | −237.2185790 | 11.116 | 234.768 | - |
| ③ | CF3• + CF3• → C2F6 | −674.777429 | −674.9387560 | −0.238 | −101.472 | - |
| ④ | CF3• + F• → CF4 | −437.0751517 | −437.2801428 | 6.345 | −122.289 | - |
| ⑤ | C2F5• + F• → C2F6 | −674.7368246 | −674.9387560 | 6.444 | −120.270 | - |
| ⑥ | C2F5• + C2F5• → C4F10 | −1150.1183793 | −1150.2726764 | 3.302 | −93.521 | - |
| ⑦ | C2F5• + CF3• → C3F8 | −912.4463898 | −912.6071230 | 8.819 | −92.043 | - |
| ⑧ | C3F7• + F• → C3F8 | −912.4310489 | −912.6071230 | 6.755 | −103.733 | - |
| ⑨ | C3F7• → C3F6 + F• | −812.7456917 | −812.6434617 | −3.015 | 61.135 | 54.296 |
| ⑩ | C2F5• + C3F7• → C5F12 | −1387.8040065 | −1387.9376407 | 3.565 | −80.292 | - |
| Gas | Eion (eV) | Eaff (eV) | EO (eV) | Insulation Strength Relative to SF6 [25,26,27] | Boiling Point (°C) [27,28] | GWP (100 Years) [27,29] | Lifetime (Years) [27,30,31] |
|---|---|---|---|---|---|---|---|
| SF6 | 15.153 | 0.438 | 8.999 | 1 | −63 | 23900 | 3200 |
| C6F12O | 11.408 | 0.817 | 6.120 | 2.70 | 49 | ~1 | 5 days |
| CF4 | 15.965 | −1.253 | 12.590 | 0.39 | −186.8 | 6300 | 50,000 |
| C2F6 | 14.796 | −1.208 | 11.570 | 0.78–0.79 | −78 | 9200 | 10,000 |
| C3F8 | 14.208 | −0.673 | 10.330 | 0.96–0.97 | −37 | 7000 | 2600 |
| C3F6 | 11.759 | 1.755 | 5.129 | - | −28 | - | <10 |
| C4F10 | 11.674 | −1.841 | 7.973 | 1.25, 1.31 | −2 | 7000 | 2600 |
| C5F12 | 14.657 | −0.906 | 7.680 | - | 28 | 7280 | >2000 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Tian, S.; Xiao, S.; Deng, Z.; Li, Y.; Tang, J. Insulation Strength and Decomposition Characteristics of a C6F12O and N2 Gas Mixture. Energies 2017, 10, 1170. https://doi.org/10.3390/en10081170
Zhang X, Tian S, Xiao S, Deng Z, Li Y, Tang J. Insulation Strength and Decomposition Characteristics of a C6F12O and N2 Gas Mixture. Energies. 2017; 10(8):1170. https://doi.org/10.3390/en10081170
Chicago/Turabian StyleZhang, Xiaoxing, Shuangshuang Tian, Song Xiao, Zaitao Deng, Yi Li, and Ju Tang. 2017. "Insulation Strength and Decomposition Characteristics of a C6F12O and N2 Gas Mixture" Energies 10, no. 8: 1170. https://doi.org/10.3390/en10081170
APA StyleZhang, X., Tian, S., Xiao, S., Deng, Z., Li, Y., & Tang, J. (2017). Insulation Strength and Decomposition Characteristics of a C6F12O and N2 Gas Mixture. Energies, 10(8), 1170. https://doi.org/10.3390/en10081170






