2.1. Co-Digestion of Grass with Cow Dung and Silage with Cow Dung for Methane Production by Self-Fermentation
The experimental set ups for methane production from a co-digestion of grass with cow dung and silage with cow dung by self-fermentation (without anaerobic sludge) and bioaugmentation are tabulated in
Table 1. Methane content, and MY by self-fermentation of co-digestion of grass with cow dung and silage with cow dung at various mixing ratios are shown in
Table 2. Methane content in all experiments ranged from 64–72% and 62–70% from co-digestion of grass with cow dung and silage with cow dung, respectively. Methane production (MP) and lag phase increased with an increase of mixing ratio greater than 1:1 (
Table 2). In contrast, MY decreased when the mixing ratio increased. Similar trends were observed for both grass with cow dung and silage with cow dung. Maximum MY of 176.66 and 184.94 mL CH
4/g-VS
added, from co-digestion of grass with cow dung as well as silage with cow dung, respectively, were obtained at a mixing ratio of 1:1.
An increase of the ratio of grass with cow dung and silage with cow dung greater than 1:1 resulted in an increase in MP. In contrast, MY decreased when the ratio of grass with cow dung and silage to cow dung increased greater than 1:1. This contradicts the previous sentence. An increase of the ratio of grass with cow dung and silage with cow dung ratio greater than 1:1 resulted in a higher C/N ratio (33.09–46.99,
Table 1) than the optimum range of 20–30 [
26]. This results in adverse effects on methane production process [
26]. The low MY obtained might be caused by the imbalance between hydrolytic, fermentative, and acetogenic bacteria, and methanogenic archaea [
27]. The imbalances are caused by an unsuitable substrate ratio, low pH, and accumulation of organic acids, high total ammonia-nitrogen and free ammonia content [
28]. A mixing ratio of 1:1 had the shortest lag phase (
Table 1) suggesting a short hydrolysis step. Hydrolysis is a rate limiting step during anaerobic digestion processes [
10]. The length of the lag phase is important for the efficiency of AD [
10]. However, there was no significantly difference in methane content at different mixing ratios (
Table 3). The results indicated that the indigenous microorganisms present in the co-digestion of grass with cow dung, silage with cow dung and in cow dung are capable of degrading and converting the grass and silage to methane. A mixing ratio of 1:1 is found to be a suitable mixing ratio due to the highest MY obtained and the shortest lag phase (
Table 3). Xie et al. [
29] also reported that the highest MY from co-digestion of pig manure with silage occurred at a mixing ratio of 1:1.
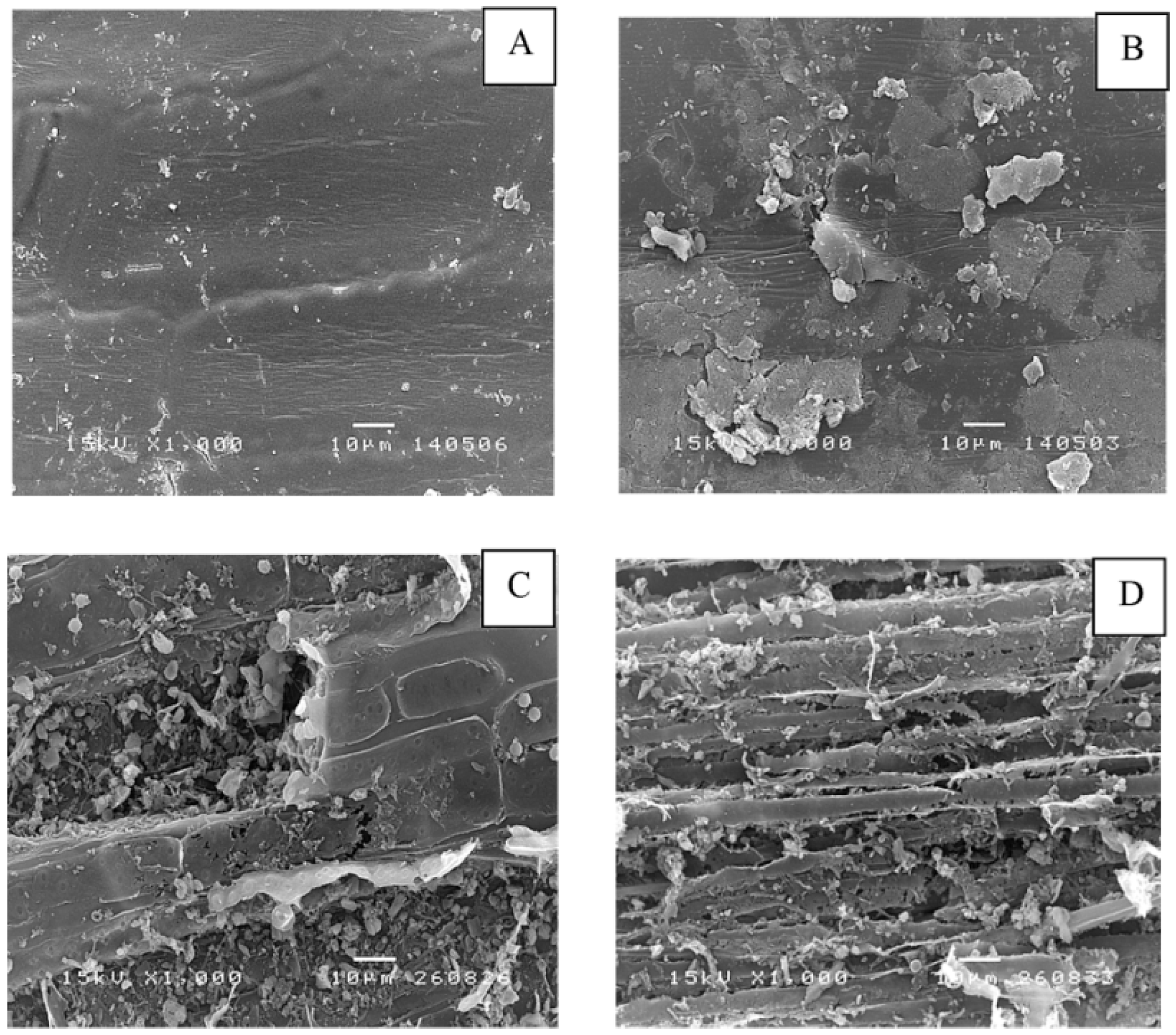
The morphological alteration of grass and silage at the initial and final methane production process was investigated using scanning electron microscope (SEM). Grass shows a smooth surface (
Figure 1A) while a destroyed surface of the native silage was observed. This is possibly due to a degradation of cellulose and hemicellulose by bacteria during an ensiling [
30,
31]. After the end of methane fermentation process, the morphological of grass and silage were much destroyed and disorganized caused by microbial driven decomposition process (
Figure 1C,D).
In order to enhance methane production, the cow dung concentration was increased to 20, 30 and 40 g-VS/L (G/C7–G/C9 and S/C7-S/C9) while keeping a constant grass and silage concentration at 10 g-VS/L. This resulted in a mixing ratio of 1:2, 1:3, and 1:4, respectively (
Table 4). MY from co-digestion of grass with cow dung of 194.73, 158.55, 134.18 mL CH
4/g-VS
added and MY from co-digestion of silage with cow dung of 198.39, 161.35, 130.12 mL CH
4/g-VS
added, respectively, were obtained at respective mixing ratio of 1:2, 1:3 and 1:4 (
Table 3). A maximum Rm of 2.76 and 2.68 mL CH
4/(L·h) from co-digestion of grass with cow dung and silage with cow dung, respectively, were obtained at a mixing ratio of 1:4 (
Table 3).
MY decreased with an increase in the amount of cow dung. A high amount of cow dung resulted in a high nitrogen content in the fermentation system. Therefore, a low MY might be caused by the low carbon source for methanogen and the accumulation of free ammonia concentration from the cow dung. Xie et al. [
29] reported that free NH
3 concentration of 210 mg/L was obtained from co-digestion of pig manure with silage at a mixing ratio of 3:1 while Wu et al. [
32] found that inhibition of methanogens by free NH
3 was reversible when the free NH
3 concentration was as high as 998 mg/L. The varying inhibition concentrations of free NH
3 is attributed to the differences in substrates and inocula, environmental conditions and acclimation periods [
29].
2.2. Methane Production from Co-Digestion of Grass with Cow Dung and Silage with Cow Dung by Bioaugmentation of Anaerobic Sludge
The MP, Rm, MY and methane content were obtained from a grass with cow dung and silage with cow dung at different mixing ratios augmented with anaerobic sludge as showed in
Table 5. A methane content in all experiments ranged from 59–65% and 60–65% from a co-digestion of grass with cow dung and silage with cow dung, respectively. Methane contents from the co-digestion of silage with pig manure were also found in the ranges of 59–65% [
29].
MY increased with an increase in the ratio of grass with cow dung and silage with cow dung from 1:1 to 3:1 (g-VS/g-VS). MY was decreased when the ratio of grass with cow dung and silage with cow dung were greater than 3:1 (g-VS/g-VS) (
Table 5). The grass with cow dung and silage with cow dung ratio of 3:1 gave the maximum MY of 179.59 and 208.11 mL CH
4/g-VS
added, respectively (
Table 5). A high MY observed at the ratio 3:1 implied that at a suitable C/N ratio, the microbial growth and substrate utilization were enhanced [
33]. Thus, a methane production was improved. For a co-digestion of grass with cow dung and silage with cow dung, manure provide buffering capacity and a wide range of nutrients [
34], while the addition of grass and silage containing high carbon content (43–45%) balances the C/N ratio. Co-digestion of silage with cow dung gave a MY higher than a co-digestion of grass with cow dung might be due to the fact that the silage is easier to be degraded than grass. During the ensiling, the grass is degraded by microorganisms resulting in more biodegradability structure of silage than grass [
35].
The final pH in all experiments ranged from 7.01–7.93 for a co-digestion of grass with cow dung and silage with cow dung (
Table 5). pH range of 7.00–8.00 was suitable for obtaining a high biogas production and degradation of VS [
36]. Methanogenic bacteria perform well within a pH range of 6.80–7.20 while drop in pH below 6.60 might inhibit methanogens [
1]. The present study shows that the pH in the range of 7.01–7.55 is suitable for methanogens.
Under the optimum conditions, the MY of 179.59 mL CH4/g-VSadded obtained by bioaugmentation of anaerobic sludge to a co-digestion of grass with cow dung was comparable to the self-fermentation of grass with cow dung (176.66 mL CH4/g-VSadded). However, a maximum MY (208.11 mL CH4/g-VSadded) obtained by bioaugmentation of anaerobic sludge to a co-digestion of silage with cow dung was significantly higher (p < 0.05) than MY of 184.94 mL CH4/g-VSadded obtained from self-fermentation of silage with cow dung. Based on our findings, the bioaugmentation technique effectively enhanced the MY from co-digestion of silage with cow dung only, but could not enhance the MY obtained from co-digestion of grass with cow dung. The discrepancy might be due to the different in the structure of grass and silage. During the ensiling, the compression of the material in tightly closed containers established the anaerobic condition. This promotes the growth of lactic acid bacteria. In consequence, the lactic acid produced by lactic acid bacteria can loosen the structure and allow more biodegradability of the silage by the bioaugmended microbial consortium. In contrast, the structure of grass was more complex comprising of lignin, hemicellulose and crystalline cellulose. Therefore, the accession of the microorganisms augmended into the fermentation system of grass is more difficult than the silage.
2.3. Microbial Community and Methane Fermentation Performance
The bacteria and archaea community detected in a co-digestion of grass with cow dung and silage with cow dung by self-fermentation were analyzed by polymerase chain reaction-denaturing gradient gel electrophoresis (PCR-DGGE) (
Figure 2). Main bacteria found under the optimum conditions i.e., a co-digestion of grass with cow dung (lane A) and silage with cow dung (lane B) ratio of 1:1 by self-fermentation were
Aciditerrimonas sp. (band 1),
Acetoanaerobium sp. (bands 2 and 4),
Ruminococcus sp. (band 3),
Clostridium sp. (band 5) and
Parapedobacter sp. (band 6).
Ruminococcus sp. and
Parapedobacter sp. play substantial roles in degrading polysaccharides of plant biomass and utilize the complex organic substrates in aquatic environments such as cellulose and other biomacromolecules [
37].
Aciditerrimonas sp.,
Acetoanaerobium sp., and
Clostridium sp. can convert the simple sugar to VFAs resulted in hydrogen and carbon dioxide as a by-products. The archaea community found in a methane production by self-fermentation from the co-digestion of grass with cow dung (lane C) and silage with cow dung (lane D) were
Methanomicrobium sp. (bands 2 and 4),
Methanoculleus sp. (band 6) and
Metahnoregula sp. (band 3). These microorganisms are able to utilize VFAs, hydrogen and carbon dioxide as the substrate for a methane production occurred in step 4.
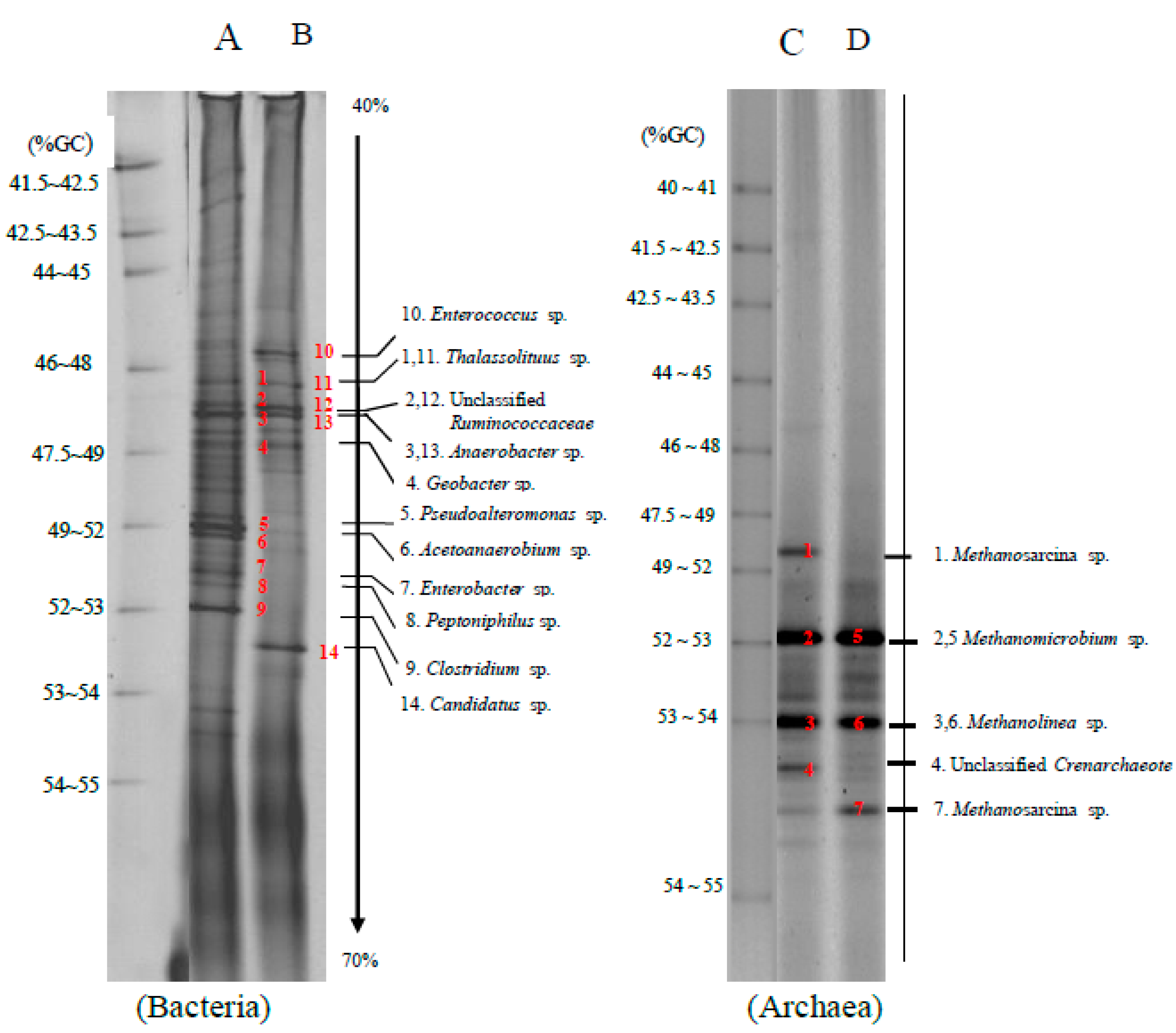
The bacteria and archaea community found in a methane production by bioaugmentation of anaerobic sludge to a co-digestion of grass with cow dung and silage with cow dung was depicted in
Figure 3. The cellulolytic bacteria found in bioaugmentation of anaerobic sludge to a co-digestion of grass with cow dung (lane A) and silage with cow dung (lane B) were
Clostridium sp. (band 9) and Unclassified
Ruminococcaceae (bands 2 and 12). The hydrolytic bacteria found in the bioaugmentation treatments were
Clostridium sp. (band 9),
Enterococcus sp. (band 10),
Thalassolituus sp. (bands 1 and 11), Unclassified
Ruminococcaceae (bands 2 and 12),
Anaerobacter sp. (bands 3 and 13) and
Geobacter sp. (band 4), respectively.
Pseudoalteromonas sp. (band 5) and
Peptoniphilus sp. (band 8) were hydrolytic bacteria found in the bioaugmentation treatments of a co-digestion of grass with cow dung, while
Candidatus sp. (band 14) was found in the bioaugmentation treatments of a co-digestion of silage with cow dung. Acidogenic bacteria found in bioaugmentation treatments of a co-digestion of grass with cow dung and silage with cow dung were
Enterobacter sp. (band 10),
Clostridium sp. (band 9) and Unclassified
Ruminococcaceae (bands 2 and 12). The acetogenic bacteria found in the bioaugmentation treatment of a co-digestion of grass with cow dung was
Acetoanaerobium sp. (band 6) and
Clostridium sp. (band 9).
Clostridium sp. and
Enterococcus sp. are well-known hydrogen producing bacteria capable of converting and hydrolyzing polysaccharides to short chain VFAs, hydrogen and carbon dioxide as the primary fermentation products in the AD process [
38]. A long chain VFAs such as butyrate, propionate can be converted to acetate by
Acetoanaerobium sp. VFAs, hydrogen and carbon dioxide can be further converted to methane by methanogenic bacteria using two paths as follows [
39]:
The archaea community is found in bioaugmentation of anaerobic sludge to a co-digestion of grass with cow dung (lane C) and silage with cow dung (lane D) comprised of
Methanosarcina sp. (bands 1 and 7),
Methanolinea sp. (bands 3 and 6),
Methanomicrobium sp. (bands 2 and 5) and Unclassified
Crenarchaeote. (band 4). Both
Methanomicrobium sp. and
Methanolinea sp. were hydrogenotrophic methanogens that can utilize hydrogen and carbon dioxide as the substrate to produce methane (Equation (1)) [
40].
Methanosarcina sp. is a well-known as methanogenic bacterium capable of converting acetic acid to methane (Equation (2)). Since the main methanogens were
Methanomicrobium sp. and
Methanolinea sp. suggesting that the main methane production process from a co-digestion of grass with cow dung and silage with cow dung is a hydrogenotrophic methanogenic pathway (Equation (1)). The presence of
Methanosarcina sp. suggested that the other methane production process was acetoclastic methanogenic pathway (Equation (2)).
The bacteria community found in self-fermentation and bioaugmentation processes was quite different in terms of species and quantity but the roles were similar. All of bacteria found under the optimum conditions of a self-fermentation (1:1) and bioaugmentation (3:1) process can convert substrate (grass, silage, and cow dung) to VFAs, hydrogen and carbon dioxide. Therefore, the important indications for methane production performance were substrate concentration (VFAs, hydrogen and carbon dioxide), the amount and types of archaea founded in the fermentation system.
Under the optimum conditions of a co-digestion of grass with cow dung and silage with cow dung at a mixing ratio of 1:1 by self-fermentation, the methanogens were
Methanomicrobium sp. and
Methanoculleus sp. At the optimum conditions of a co-digestion of grass with cow dung and silage with cow dung at a mixing ratio of 3:1 by bioaugmentation treatments, the methanogens were
Methanosarcina sp.,
Methanomicrobium sp., and
Methanolinea sp. The comparison of archaea population in both fermentation processes showed that the band of methanogens found in the bioaugmentation treatments were more predominant than self-fermentation treatments of a co-digestion of grass with cow dung and silage with cow dung. These results are correlated with a high MY obtained by bioaugmentation of anaerobic sludge into a co-digestion of silage with cow dung. However, predominant of archaea population in a co-digestion of grass with cow dung in a bioaugmentation treatment did not significantly enhance the MY (
Table 1 and
Table 5). The MY obtained from bioaugmentation treatment was higher than a self-fermentation of silage with cow dung. The discrepancy might be due to the bioaugmentation treatment was predominant with methanogens than a self-fermentation. The bands of the methanogens found in bioaugmentation treatment had a higher intensity than self-fermentation. A predominant of a methanogens found in bioaugmention treatment of a co-digestion of silage with cow dung was correlated with the maximum MY obtained. The results implied that the predominant methanogens efficiently converted the VFAs, hydrogen and carbon dioxide to methane. In addition, the differences of MY at various C/N ratio might be caused by the coordinate matching of antagonistic and symbiotic relationships among different species. Our results demonstrated that the improved MY depended on the C/N ratio of grass with cow dung and silage with cow dung and also the biogumentation of anaerobic sludge. In addition, the changes in the ratio of grass with cow dung and silage with cow dung caused the changes in indigenous bacteria and archaea community structure. Therefore, we speculated that a normal flora resided in silage and cow dung may serve as the source of inoculum to produce methane.
2.4. Methane Production from Solid Residue Left over after Methane Production Process
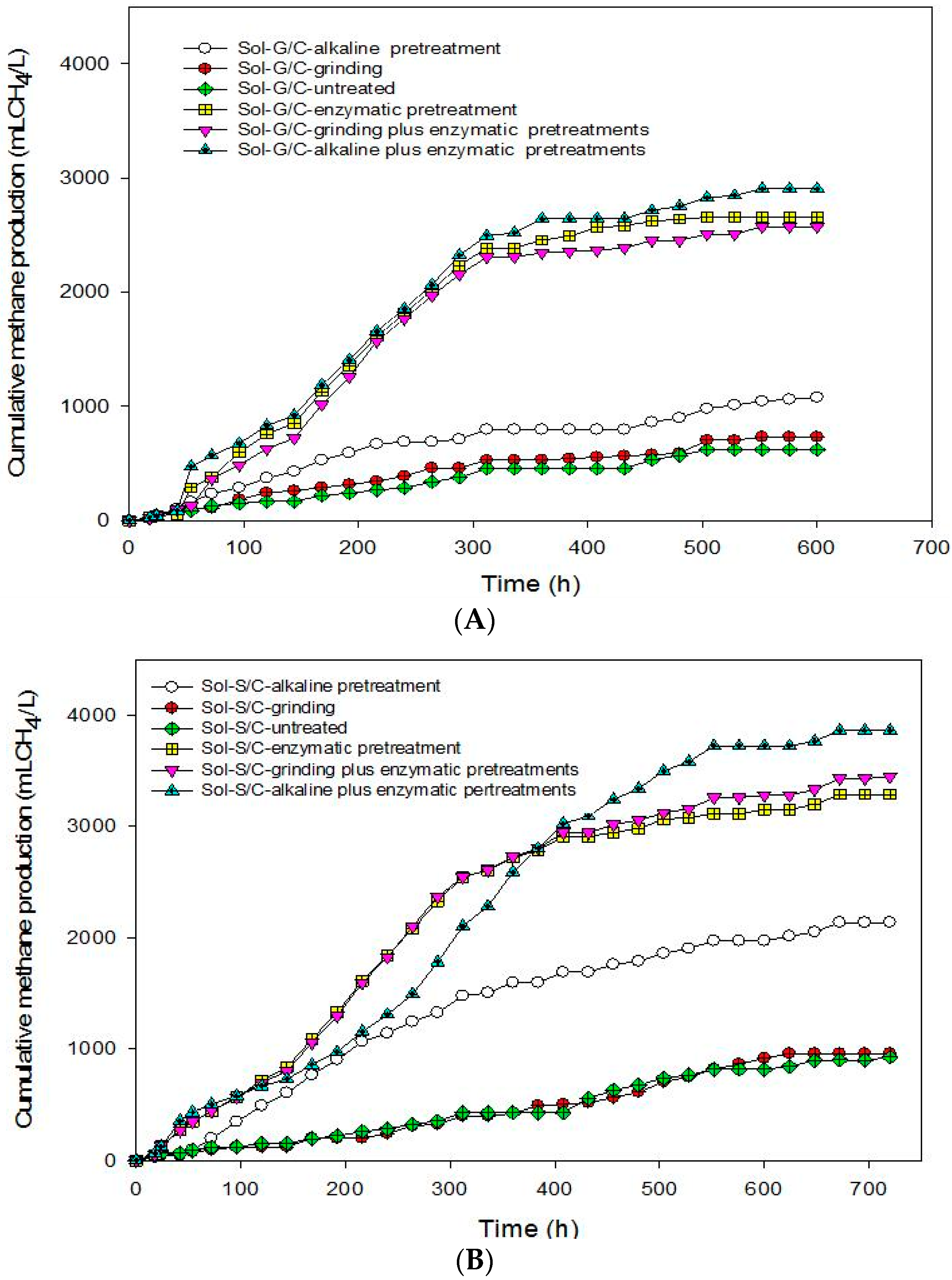
This experiment was conducted in order to recover total energy from grass and silage by producing methane from the solid residues left over after the methane production process at the optimum ratio of grass with cow dung and silage with cow dung of 3:1 by a bioaugmentation treatment. The solid residue was subjected to different pretreatment methods including grinding, alkaline, enzyme, grinding plus enzyme, and alkaline plus enzyme. The effects of different pretreatment methods on cumulative MP are shown in
Figure 4. The pretreated solid residues showed a significant effect on cumulative MP (
Figure 4). The results indicate that the enzyme pretreatment method was suitable to degrade cellulose and hemicellulose in grass, silage and some macromolecules in cow dung resulted in a higher cumulative MP (
Figure 4) and MY (
Table 6).
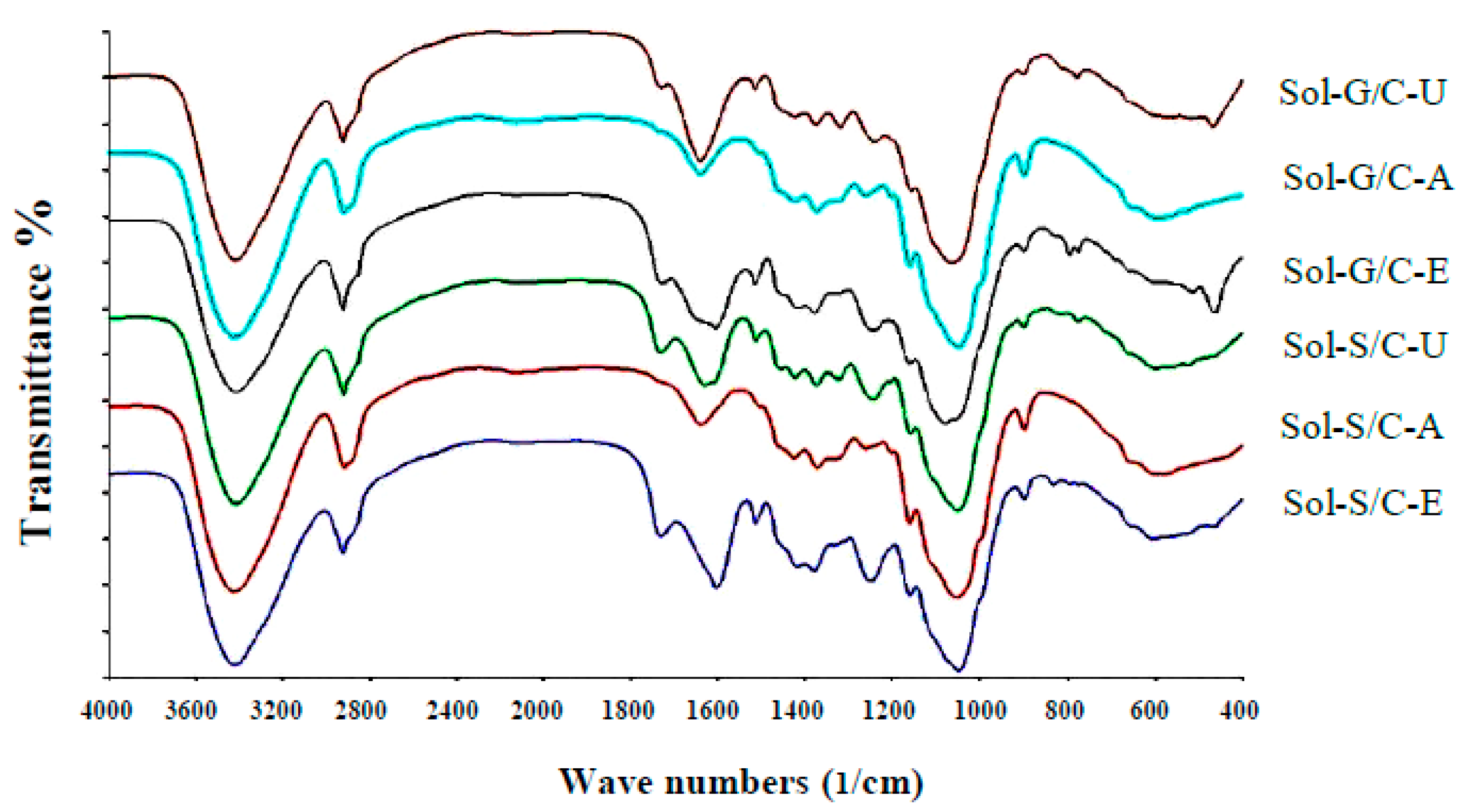
Fourier transform infrared spectrometer (FTIR) spectra showed the structure differences among untreated, alkaline pretreatment and enzyme pretreatment (
Figure 5). Application of pretreatment caused the changes in peak intensity at the wave number ranges from 1063–1700/cm in comparison to untreated materials (control). The changes in peak intensity at these ranges is correlated with the changes in lignin structure (1400–1700/cm) [
41] and crystallinity of cellulose (1100–1200/cm) [
42]. The results implied that the alkaline and enzyme pretreatment can partially destroy the lignin and crystallinity of cellulose inside grass and silage. Solid residues from co-digestion of grass with cow dung and silage with cow dung pretreated with enzyme and alkaline showed a different spectrum in comparison to untreated solid residues from a co-digestion of grass with cow dung and silage with cow dung (
Figure 5).
The intensity of the peak, approximately 1600/cm, was lower after pretreatment than untreated solid residues of a co-digestion of grass with cow dung and silage with cow dung, indicating that the structure of lignin was changed after the pretreatments. The previous observations on spectra showed that the aromatic skeletal/C–O stretching ratio, R = I (1157/cm)/I(1063/cm), represent the crystallinity of cellulose. As the aromatic skeletal/C–O stretching ratio increase, the crystallinity decrease [
42]. The aromatic skeletal/C–O stretching ratio of solid residue of a co-digestion of grass with cow dung pretreated with an alkaline and enzyme were found to increase from 0.70 to 0.17 and 0.07 to 0.13, respectively, and for the solid residue of co-digestion of silage with cow dung were found to increase from 0.12 to 0.20 and 0.12 to 0.14, respectively. A high aromatic skeletal/C–O stretching ratio indicated that the pretreatment effectively destroys the lignin structure and disrupt the crystalline cellulose to reduce crystallinity. The highest aromatic skeletal/C–O stretching ratio was found with the alkali pretreatment method indicating that the alkali method has the highest efficiency to degrade lignin compound and disrupt the crystalline cellulose. Alkali pretreatment leads to an increase in porosity and internal surface area, structural swelling, a decrease in the degree of polymerization and crystallinity and a breakdown of links between lignin and other polymers [
43]. These would allow a better accessibility of cellulose and hemicellulose by enzymes and [
17,
24]. More accessible cellulose is the key success of the methane production process due to it can be easily degraded and converted to methane by microorganisms [
44]. In this study, solid residues from co-digestion of grass with cow dung and silage with cow dung pretreated with alkali gave 10.49 and 13.42-fold increases in MY in comparison to the untreated solid residues of co-digestion of grass with cow dung and silage with cow dung. However, the MP obtained from solid residue of grass with cow dung pretreated by alkali was 1.76 times less than MP obtained from solid residue of grass with cow dung pretreated by enzyme pretreatment. Thus, the alkaline pretreatment can remove lignin and yield accessible cellulose as a substrate for methanogens, while the enzyme pretreatment can specifically degrade the cellulose to sugars resulting in a higher obtained MP.
Based on these results, in order to enhance the methane production from solid residue obtained from a co-digestion of grass with cow dung and silage with cow dung the combination of chemical and physical with enzyme pretreatments method were conducted. Grinding plus enzyme and alkali plus enzyme pretreatment methods were used. The results showed that the combined pretreatment enhanced the MY from solid residue obtained from a co-digestion of grass with cow dung and silage with cow dung (
Table 6). Alkali plus enzyme pretreatment gave the maximum cumulative MP from the solid residues from a co-digestion of grass with cow dung and silage with cow dung. The MP from the solid residue of silage with cow dung was higher than the solid residue of grass with cow dung in all treatments (
Table 6). MP from pretreated solid residues was in the order of alkali plus enzyme pretreatment > enzyme pretreatment > grinding plus enzyme pretreatment > alkaline pretreatment > grinding > untreated for solid residues from a co-digestion of grass with cow dung while alkali plus enzyme pretreatment > alkali pretreatment > grinding plus enzyme pretreatment > enzyme pretreatment > grinding > untreated from silage with cow dung. MP from the combined pretreatment was higher than a sole pretreatment. A sole pretreatment does not provide efficient results due to its limited specific effect, e.g., NaOH mainly targets lignin, but not hemicellulose [
45]. Maximum MP of 3,547 mL CH
4/L was obtained from the solid residues of a co-digestion of silage with cow dung pretreated by an alkali plus enzyme treatment. Moreover, alkali pretreatment can break the lignin barrier and disrupt the crystallinity of cellulose, thus increased the susceptibility of cellulose to enzyme [
41,
46,
47].
Results indicated that Rm was greatly improved after the pretreatment of solid residues obtained from a co-digestion of grass with cow dung and silage with cow dung. The untreated solid residues of grass with cow dung and silage with cow dung yielded a low Rm of 1.32 and 1.58 mL CH
4/(L·h), respectively. The highest Rm of 10.33 and 10.02 mL CH
4/(L·h) were obtained from the solid residues of grass with cow dung and silage with cow dung, respectively, pretreated by an enzyme pretreatment which are six times higher than that of the untreated sample (1.32 and 1.58 mL CH
4/(L·h), respectively). In contrast, the effects of grinding and alkali pretreatment on Rm were less obvious. Our results indicated that the enzymatic hydrolysis is much more efficient than the grinding and alkali pretreatment. Similarly, a maximum MY of 333.63 and 301.38 mL CH
4/g-VS
added, from the solid residues of grass with cow dung and silage with cow dung, respectively, were obtained by an alkali plus enzyme pretreatment. Alkali pretreatment is necessary for effective biogas generation from lignocellulosic biomass due to its high efficiency in delignification which can increase the accessibility of cellulose for the enzymatic reaction [
48]. NaOH induces the saponification of the uronic bonds between hemicelluloses and lignin, swells the biomass and increases pore size, and also facilitates the diffusion of the hydrolytic enzymes [
49]. Michalska and Ledakowicz [
50] also reported that a combination of alkali treatment with enzymatic hydrolysis of
Sorghum moench resulted in 30% and 50% higher MY than alkali and enzymatic treatment alone, respectively. The results from the combined alkali plus enzyme pretreatment improve MY about 50% greater than the enzymatic hydrolysis alone which was coincided with the result of [
48].
2.5. Energy Production from a Co-Digestion of Grass with Cow Dung and Silage with Cow Dung by Self-Fermentation, Bioaugmentation and Pretreated Solid Residues
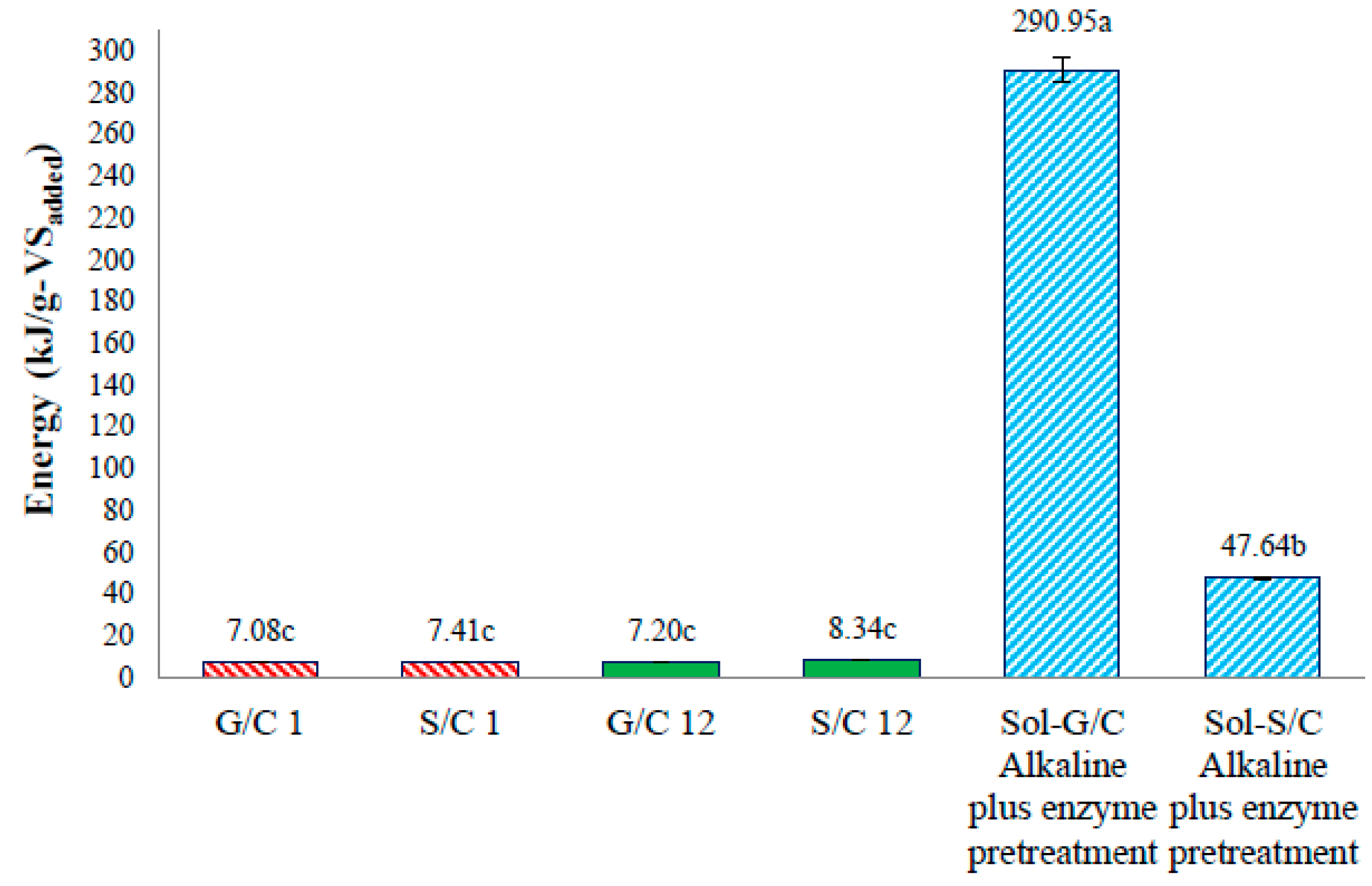
The energy production from a co-digestion of grass with cow dung and silage with cow dung by self-fermentation, bioaugmentation and from pretreated solid residue were depicted in
Figure 6. The energy production of a co-digestion of grass with cow dung and silage with cow dung by self-fermentation were 7.08 and 7.41 kJ/g-VS
added while the energy production from a co-digestion of grass with cow dung and silage with cow dung by bioaugmentation treatment were 7.20 and 8.34 kJ/g-VS
added, respectively (
Figure 6). The energy production from a co-digestion of silage with cow dung by a bioaugmentation treatment was 1.18 times greater than self-fermentation. Therefore, the bioaugmentation of anaerobic sludge into a co-digestion of silage with cow dung showed a positive effect on biogas production and energy production.
The maximum energy production of solid residue from a co-digestion of grass with cow dung and silage with cow dung pretreated by an alkaline plus enzyme were 290.95 and 47.64 kJ/g-VS
added, respectively (
Figure 6). The energy production of solid residue from co-digestion of grass with cow dung and silage with cow dung pretreated by alkaline plus enzyme were 29.16 and 5.71 times, respectively, higher than a co-digestion of grass and silage with cow dung bioaugmended with the anaerobic sludge, respectively. These results revealed that the pretreatment of solid residue obtained from a co-digestion of grass with cow dung gave a better benefit in terms of increasing the energy production (
Figure 7). The pretreatment method can destroy the crystalline cellulose inside grass and silage and gained more glucose which caused an increase in MP and MY. Therefore, an application of pretreatment methods on the solid residue left over after co-digestion of grass with cow dung was a good approach for totally recover the methane from lignocellulosic materials.
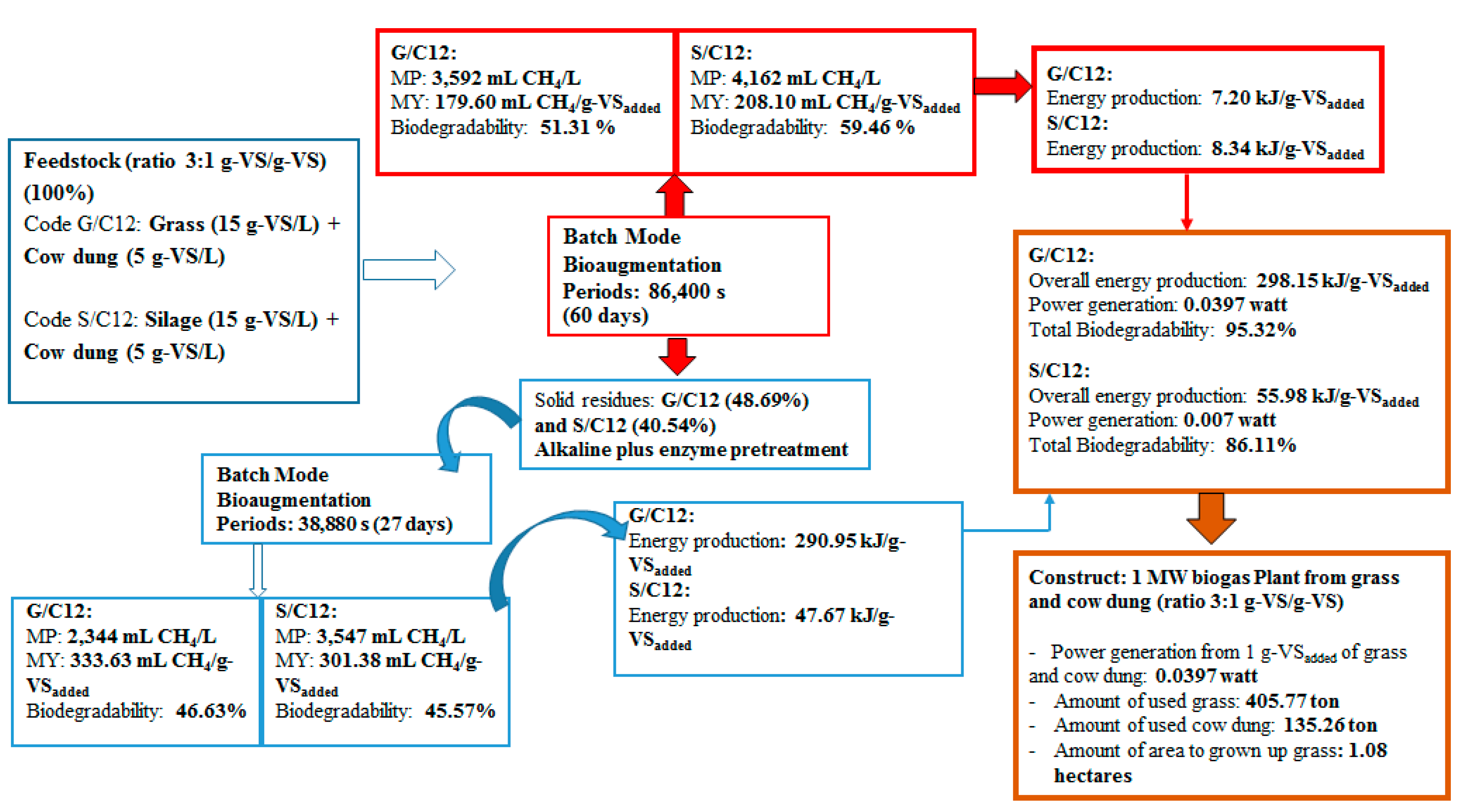
The overall energy production from co-digestion of grass with cow dung and silage with cow dung was depicted in
Figure 7. The overall energy production from a co-digestion of grass with cow dung and silage with cow dung were 298.15 and 55.98 kJ/g-VS
added respectively. This implies that the use of 1 g-VS of grass and cow dung at a ratio of 3:1 (0.75 g-VS
grass and 0.25 g-VS
cow dung) as the substrate to produce biogas via the bioaugmentation technique gave a maximum energy production of 298.15 kJ, whereas the use of 1 g-VS of silage and cow dung gave a maximum energy production of 55.98 kJ. The power generation (watt) was then calculated by dividing overall energy production (J) by overall fermentation time (s). In this study, an overall fermentation time for biogas production from a co-digestion of grass with cow dung and silage with cow dung were approximately 87 days. Therefore, the power generation from co-digestion of grass with cow dung and silage with cow dung were 0.0397 and 0.007 watts, respectively. The amount of grass and cow dung were further estimated for establishing 1 MW biomass power plant. In case of grass, 18.89 × 10
3 kg-VS, equivalent to 75.56 × 10
3 kg-dw, was needed for a power generation of 1 MW (1 × 10
6 watt). Moisture content of grass was 78.14%, therefore the wet weight (ww) of grass required were 96.72 × 10
3 kg. Results implied that in 87 days of fermentation time, 96.72 tons of grass or 405.77 ton of grass/year were sufficient to establish 1 MW biomass power plant. The amount of cow dung was estimated in the same manner and 135.26 ton of cow dung was required to co-digest with 405.77 ton of grass to establish a 1 MW biomass power plant. Since the production rate of grass in Thailand were average 375 tons/hectares/year (Department of Alternative Energy Development and Efficiency, Ministry of Energy, Thailand), therefore, the total area to grow grass for 1 MW biomass power plant would be 1.08 hectares/year. However, it is worth noting that the amount of energy is depending on the concentration and quality of the gas and how the energy in methane is used.