Chemical Characteristics and NaCl Component Behavior of Biochar Derived from the Salty Food Waste by Water Flushing
Abstract
1. Introduction
2. Experimental
2.1. Raw Material
2.2. Experimental Methods
2.3. Analytical Methods
2.3.1. Characterization
2.3.2. X-ray Diffraction Spectroscopy
2.3.3. FT-IR
2.3.4. NMR
3. Results and Discussion
3.1. Chemical Composition of Salty Food-Waste-Derived Biochar
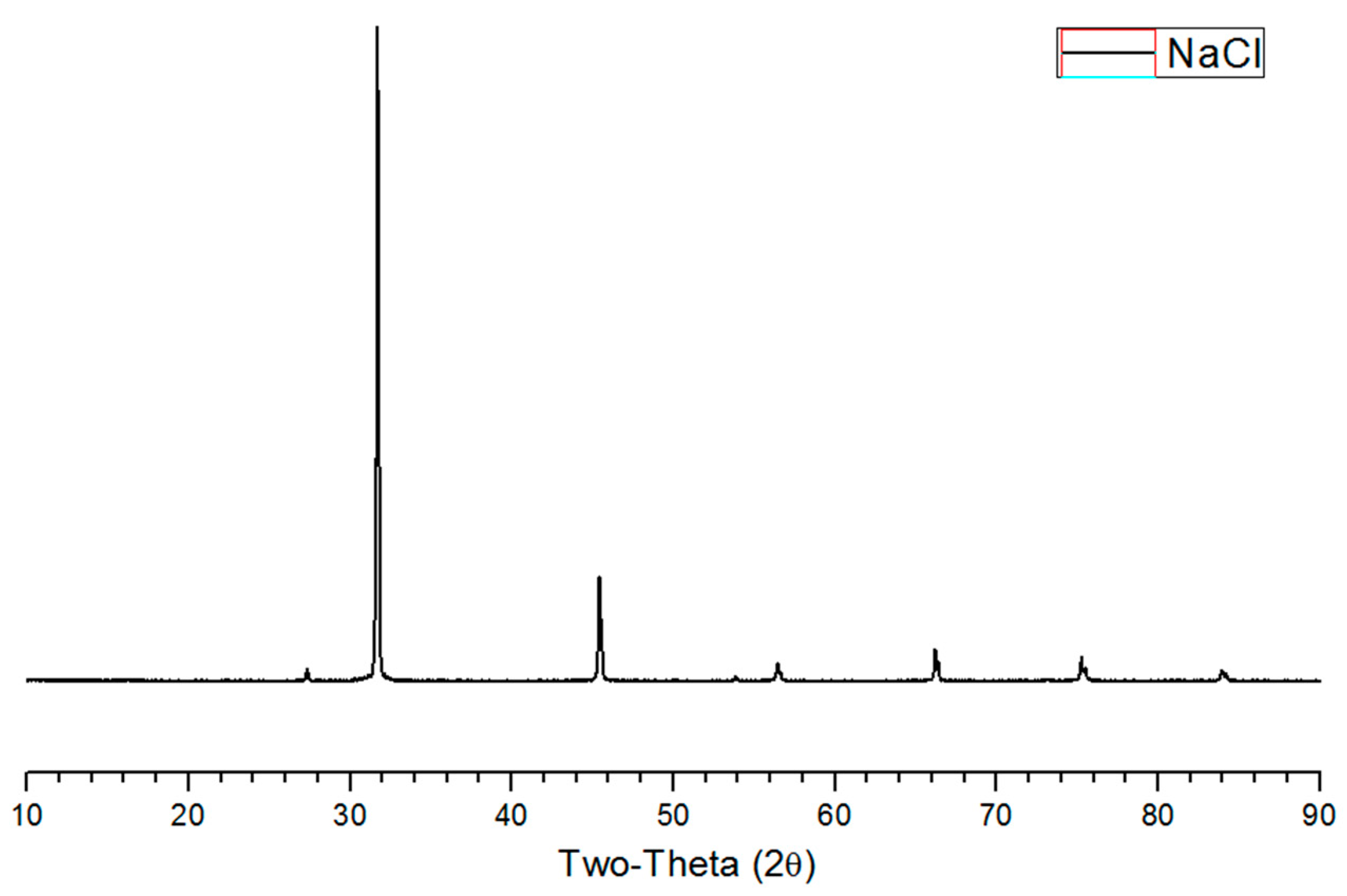
3.2. XRD Analysis of Salty Food-Waste-Derived Biochar
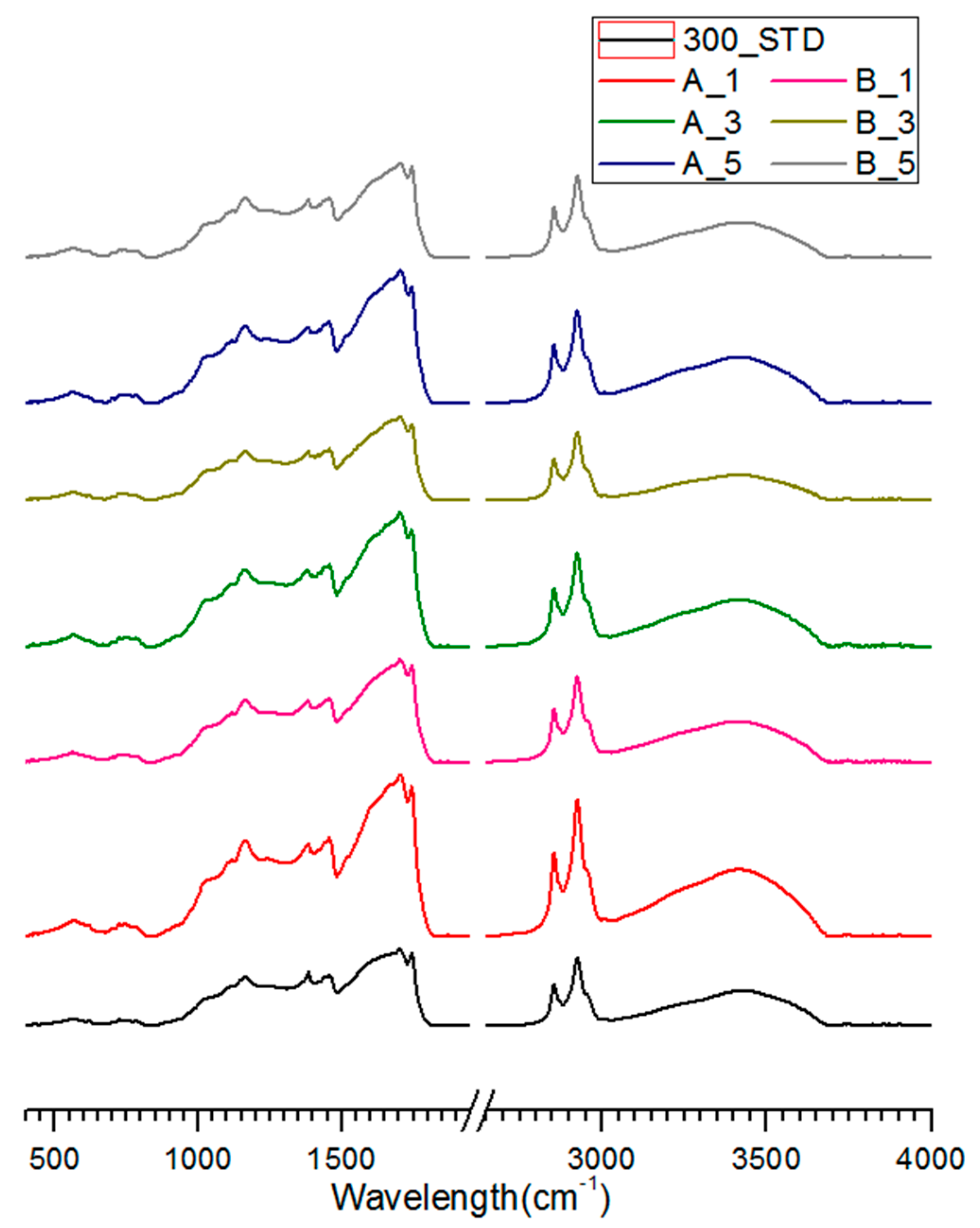
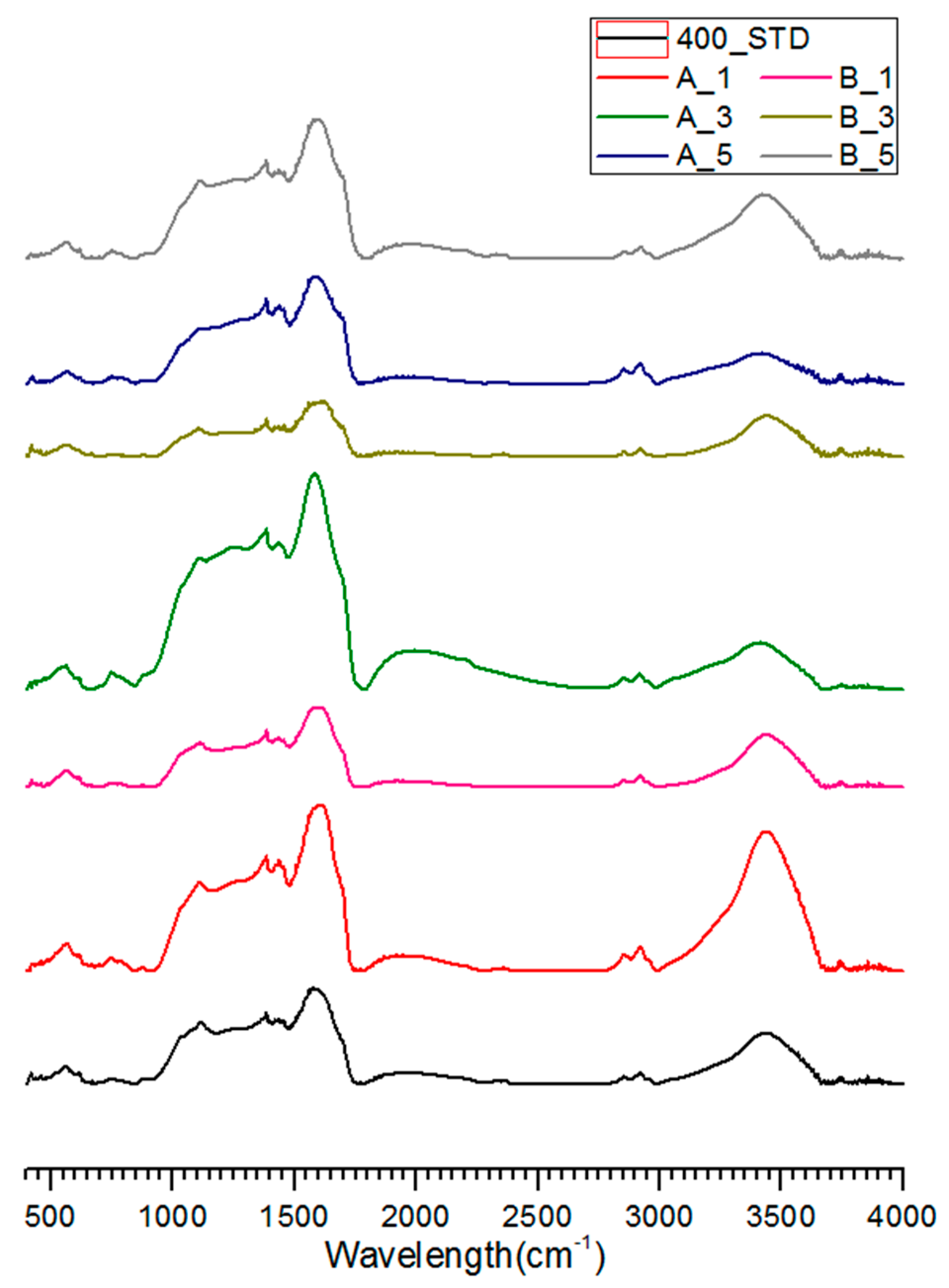
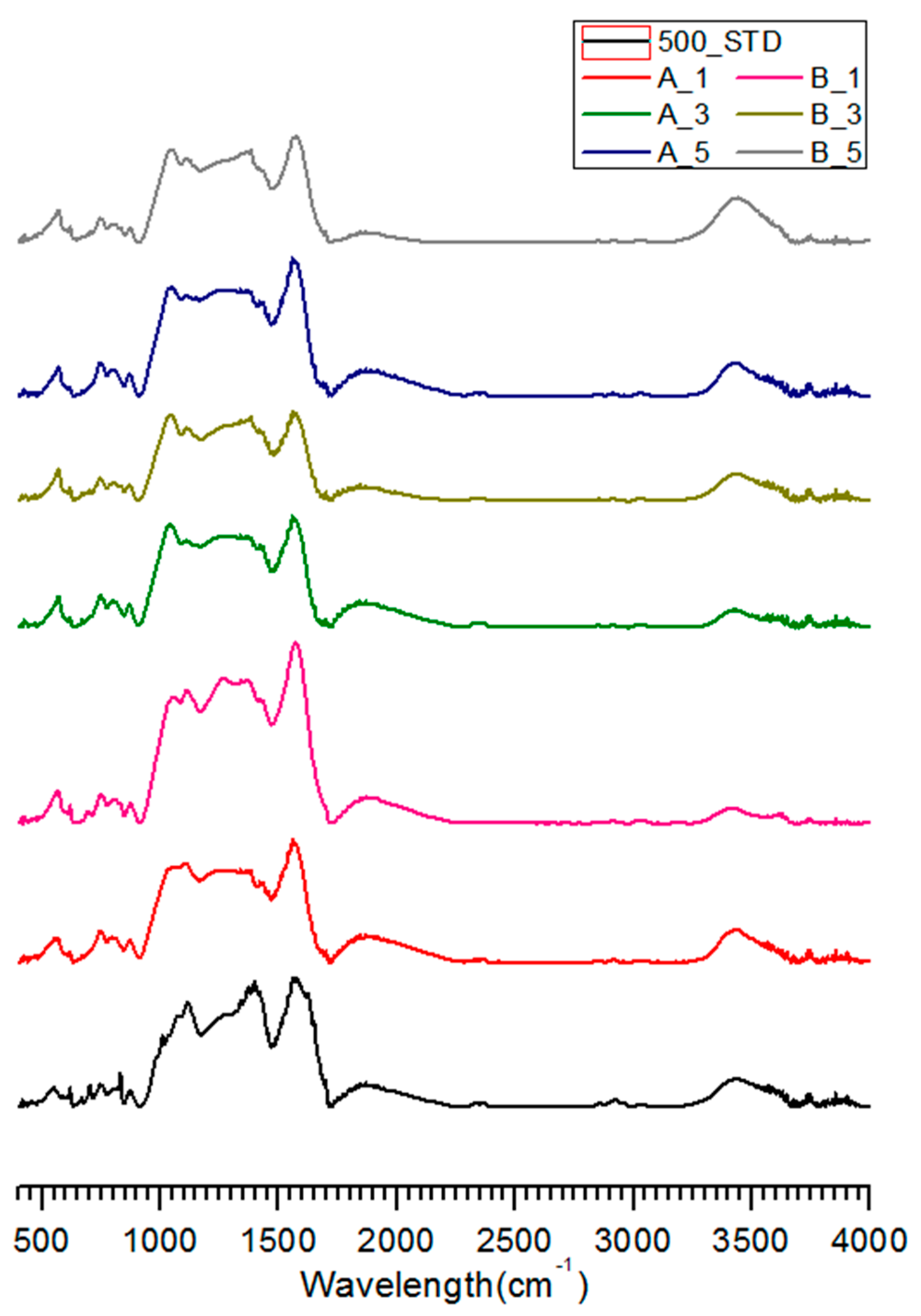
3.3. FT-IR Analysis of Salty Food-Waste-Derived Biochar
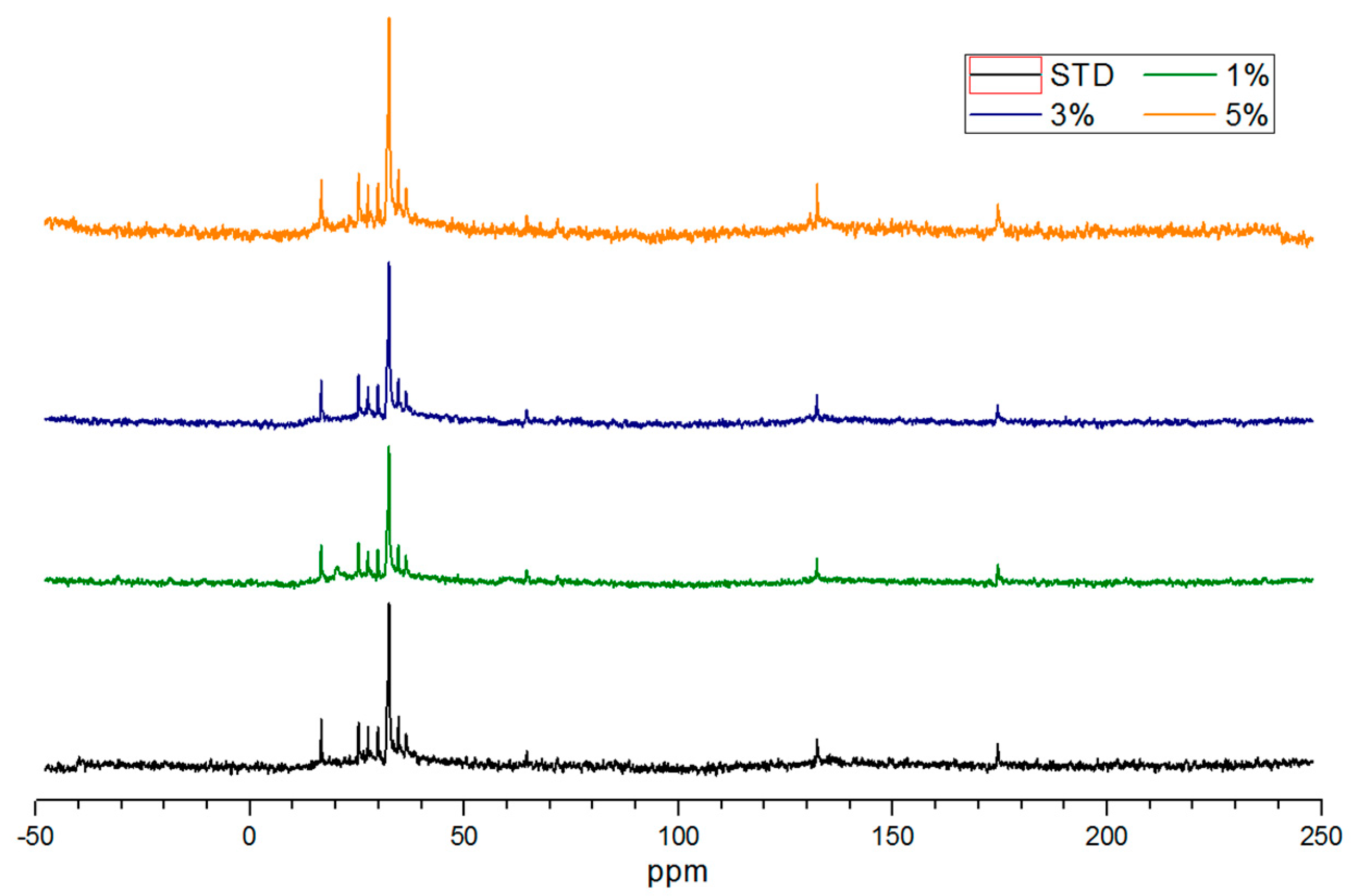
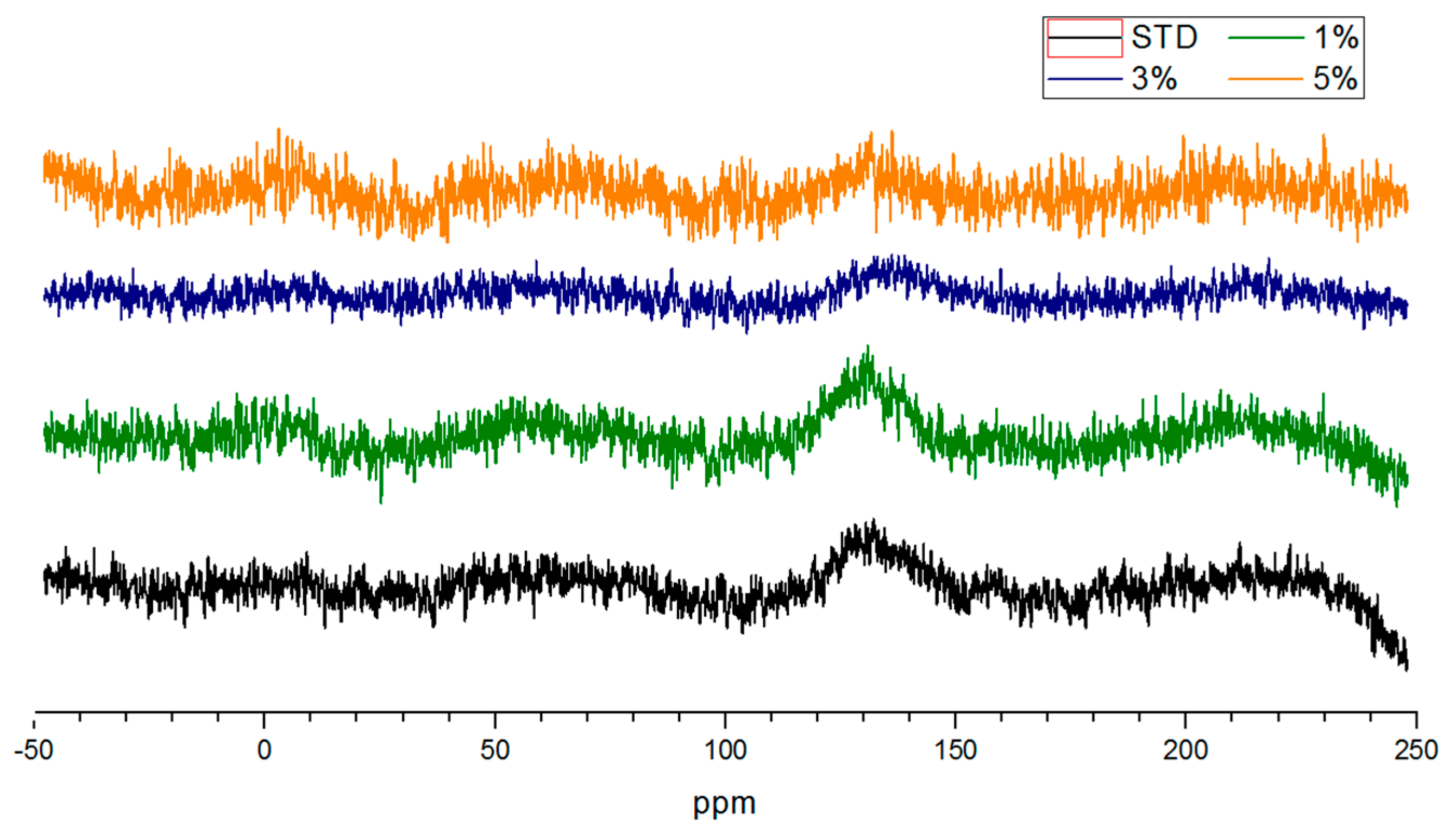
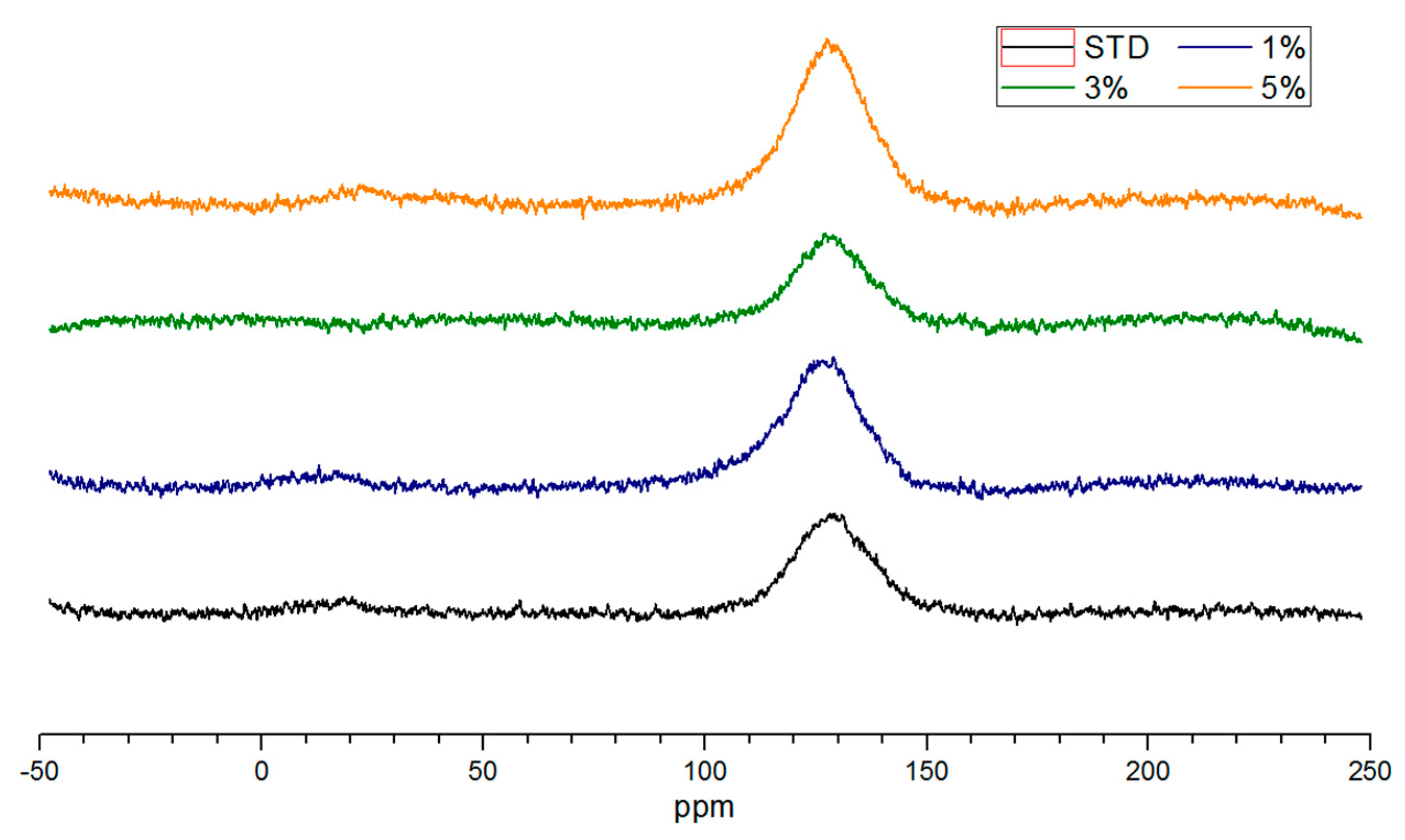
3.4. NMR Analysis of Salty Food Waste Derived Biochar
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hall, K.D.; Guo, J.; Dore, M.; Chow, C.C. The progressive increase of food waste in America and its environmental impact. PLoS ONE 2009, 4, e7940. [Google Scholar] [CrossRef] [PubMed]
- Venkat, K. The climate change and economic impacts of food waste in the United States. Int. J. Food Syst. Dyn. 2011, 2, 431–446. [Google Scholar]
- Lehmann, J.; Joseph, S. Biochar for environmental management: An introduction. In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009. [Google Scholar]
- Ioannidou, O.; Zabaniotou, A. Agricultural residues as precursors for activated carbon production—A review. Renew. Sustain. Energy Rev. 2007, 11, 1966–2005. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Brassard, P.; Godbout, S.; Raghavan, V.G.S.; Palacios, J.H.; Grenier, M.; Zegan, D. The production of engineered biochars in a vertical auger pyrolysis reactor for carbon sequestration. Energies 2017, 10, 288. [Google Scholar] [CrossRef]
- Taghizadeh-Toosi, A.; Clough, T.J.; Condron, L.M.; Sherlock, R.R.; Anderson, C.R.; Craigie, R.A. Biochar incorporation into pasture soil suppresses in situ nitrous oxide emissions from ruminant urine patches. J. Environ. Qual. 2011, 40, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.J.; Dalley, T.S.L.; Helleur, R.J. Preliminary laboratory production and characterization of biochars from lignocellulosic municipal waste. J. Anal. Appl. Pyrolysis 2013, 99, 71–78. [Google Scholar] [CrossRef]
- Prakongkep, N.; Gilkes, R.J.; Wiriyakitnateekul, W. Forms and solubility of plant nutrient elements in tropical plant waste biochars. J. Plant Nutr. Soil Sci. 2015, 178, 732–740. [Google Scholar] [CrossRef]
- Kwapinski, W.; Byrne, C.M.P.; Kryachko, E.; Wolfram, P.; Adley, C.; Leahy, J.J.; Novotny, E.H.; Hayes, M.H.B. Biochar from biomass and waste. Waste Biomass Valoriz. 2010, 1, 177–189. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Lehmann, J.; Hockaday, W.C.; Joseph, S.; Masiello, C.A. Temperature sensitivity of black carbon decomposition and oxidation. Environ. Sci. Technol. 2010, 44, 3324–3331. [Google Scholar] [CrossRef] [PubMed]
- Downie, A.; Alan, C.; Paul, M. Physical properties of biochar. In Biochar for Environmental Management: Science and Technology; Earthscan: London, UK, 2009. [Google Scholar]
- Jin, H. Characterization of Microbial Life Colonizing Biochar and Biochar-Amended Soils. Ph.D. Thesis, Cornell University, Ithaca, NY, USA, 2010. [Google Scholar]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowleye, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Deenik, J.L.; McClellan, T.; Uehara, G.; Antal, M.J.; Campbell, S. Charcoal volatile matter content influences plant growth and soil nitrogen transformations. Soil Sci. Soc. Am. J. 2010, 74, 1259–1270. [Google Scholar] [CrossRef]
- Guizani, C.; Jeguirim, M.; Valin, S.; Limousy, L.; Salvador, S. Biomass Chars: The Effects of Pyrolysis Conditions on Their Morphology, Structure, Chemical Properties and Reactivity. Energies 2017, 10, 796. [Google Scholar] [CrossRef]
- Hossain, M.K.; Strezov, V.; Chan, K.Y.; Ziolkowski, A.; Nelsona, P.F. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J. Environ. Manag. 2011, 92, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Selvam, A.; Chan, M.; Wong, J.W.C. Nitrogen conservation and acidity control during food wastes composting through struvite formation. Bioresour. Technol. 2013, 147, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.T.; Selvam, A.; Wong, J.W.C. Reducing nitrogen loss and salinity during ‘struvite’ food waste composting by zeolite amendment. Bioresour. Technol. 2016, 200, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Environment (MOE). A Study on FoodWaste Reduction Equipment Guidelines and Quality Standard P; Ministry of Environment: Sejong City, Korea, 2009.
- Yang, X.; Wang, H.; Strong, P.J.; Xu, S.; Liu, S.; Lu, K.; Sheng, K.; Guo, J.; Che, L.; He, L.; et al. Thermal Properties of Biochars Derived from Waste Biomass Generated by Agricultural and Forestry Sectors. Energies 2017, 10, 469. [Google Scholar] [CrossRef]
- Quyn, D.M.; Wu, H.; Li, C.-Z. Volatilisation and catalytic effects of alkali and alkaline earth metallic species during the pyrolysis and gasification of Victorian brown coal. Part I. Volatilisation of Na and Cl from a set of NaCl-loaded samples. Fuel 2002, 81, 143–149. [Google Scholar] [CrossRef]
- Korea Rural Development Administration, National Academy of Agricultural Science. A Method of Inspection of Physical and Chemical Fertilizers; Rural Development Administration: Jeonju-si, Korea, 2016.
- Korea Rural Development Administration, National Academy of Agricultural Science. Methods of Soil Chemical Analysis; Rural Development Administration: Jeonju-si, Korea, 2010.
- Zambon, I.; Colosimo, F.; Monarca, D.; Cecchini, M.; Gallucci, F.; Proto, A.R.; Lord, R.; Colantoni, A. An innovative agro-forestry supply chain for residual biomass: Physicochemical characterisation of biochar from olive and hazelnut pellets. Energies 2016, 9, 526. [Google Scholar] [CrossRef]
- Spokas, K.A. Review of the stability of biochar in soils: Predictability of O:C molar ratios. Carbon Manag. 2010, 1, 289–303. [Google Scholar] [CrossRef]
- Mukome, F.N.D.; Zhang, X.; Silva, L.C.R.; Six, J.; Parikh, S.J. Use of chemical and physical characteristics to investigate trends in biochar feedstocks. J. Agric. Food Chem. 2013, 61, 2196–2204. [Google Scholar] [CrossRef] [PubMed]
- Mukome, F.N.D.; Parikh, S.J. Chemical, Physical, and Surface characterization of Biochar. In Biochar: Production, Characterization, and Applications; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Ok, Y.S.; Uchimiya, S.M.; Chang, S.X.; Bolan, N. (Eds.) Biochar: Production, Characterization, and Applications; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Yuan, J.-H.; Xu, R.-K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yang, M.; Feng, Q.; McGrouther, K.; Wang, H.; Lu, H.; Chen, Y. Chemical characterization of rice straw-derived biochar for soil amendment. Biomass Bioenergy 2012, 47, 268–276. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar]
- Zhao, S.-X.; Ta, N.; Wang, X.-D. Effect of Temperature on the Structural and Physicochemical Properties of Biochar with Apple Tree Branches as Feedstock Material. Energies 2017, 10, 1293. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Ketterings, Q.; Reid, S.; Rao, R. Cornell University Agronomy Fact Sheet #22: Cation Exchange Capacity (CEC); Cornell University: Ithaca, NY, USA, 2007. [Google Scholar]
- Bourke, J.; Manley-Harris, M.; Fushimi, C.; Dowaki, K.; Nunoura, T.; Antal, M.J., Jr. Do all carbonized charcoals have the same chemical structure? 2. A model of the chemical structure of carbonized charcoal. Ind. Eng. Chem. Res. 2007, 46, 5954–5967. [Google Scholar] [CrossRef]
- Guerrero, M.; Ruiz, M.P.; Millera, Á.; Alzueta, M.U.; Bilbao, R. Characterization of biomass chars formed under different devolatilization conditions: Differences between rice husk and eucalyptus. Energy Fuels 2008, 22, 1275–1284. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Herbert, L.; Hosek, I.; Kripalani, R. The Characterization and Comparison of Biochar Produced from a Decentralized Reactor Using Forced Air and Natural Draft Pyrolysis; California Polytechnic State University: San Luis Obispo, CA, USA, 2012. [Google Scholar]
- Cheng, C.-H.; Lehmann, J.; Thies, J.E.; Burton, S.D.; Engelhardb, M.H. Oxidation of black carbon by biotic and abiotic processes. Org. Geochem. 2006, 37, 1477–1488. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Bravo, D.; Gosselink, R.J.A.; van Dam, J.E.G. Characterisation of structure-dependent functional properties of lignin with infrared spectroscopy. Ind. Crop. Prod. 2004, 20, 205–218. [Google Scholar] [CrossRef]
- Kačuráková, M.; Wilson, R.H. Developments in mid-infrared FT-IR spectroscopy of selected carbohydrates. Carbohydr. Polym. 2001, 44, 291–303. [Google Scholar] [CrossRef]
- Lammers, K.; Arbuckle-Keil, G.; Dighton, J. FT-IR study of the changes in carbohydrate chemistry of three New Jersey pine barrens leaf litters during simulated control burning. Soil Biol. Biochem. 2009, 41, 340–347. [Google Scholar] [CrossRef]
- Kubo, S.; Kadla, J.F. Hydrogen bonding in lignin: A Fourier transform infrared model compound study. Biomacromolecules 2005, 6, 2815–2821. [Google Scholar] [CrossRef] [PubMed]
- Parimala, K.; Balachandran, V. Structural study, NCA, FT-IR, FT-Raman spectral investigations, NBO analysis and thermodynamic properties of 2′, 4′-difluoroacetophenone by HF and DFT calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 110, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Porchelvi, E.E.; Muthu, S. The spectroscopic (FT-IR, FT-Raman and NMR), NCA, Fukui function analysis first order hyperpolarizability, TGA of 6-chloro-3, 4dihydro-2H-1, 2, 4-benzothiazine-7-sulphonamide1, 1-dioxide by ab initio HF and Density Functional method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 123, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Mcbeath, A.V.; Smernik, R.J.; Krull, E.S.; Lehmann, J. The influence of feedstock and production temperature on biochar carbon chemistry: A solid-state 13 C NMR study. Biomass Bioenergy 2014, 60, 121–129. [Google Scholar] [CrossRef]
- Knicker, H.; Hilscher, A.; González-Vila, F.J.; Almendros, G. A new conceptual model for the structural properties of char produced during vegetation fires. Org. Geochem. 2008, 39, 935–939. [Google Scholar] [CrossRef]
- Brewer, C.E.; Unger, R.; Schmidt-Rohr, K.; Brown, R.C. Criteria to select biochars for field studies based on biochar chemical properties. BioEnergy Res. 2011, 4, 312–323. [Google Scholar] [CrossRef]
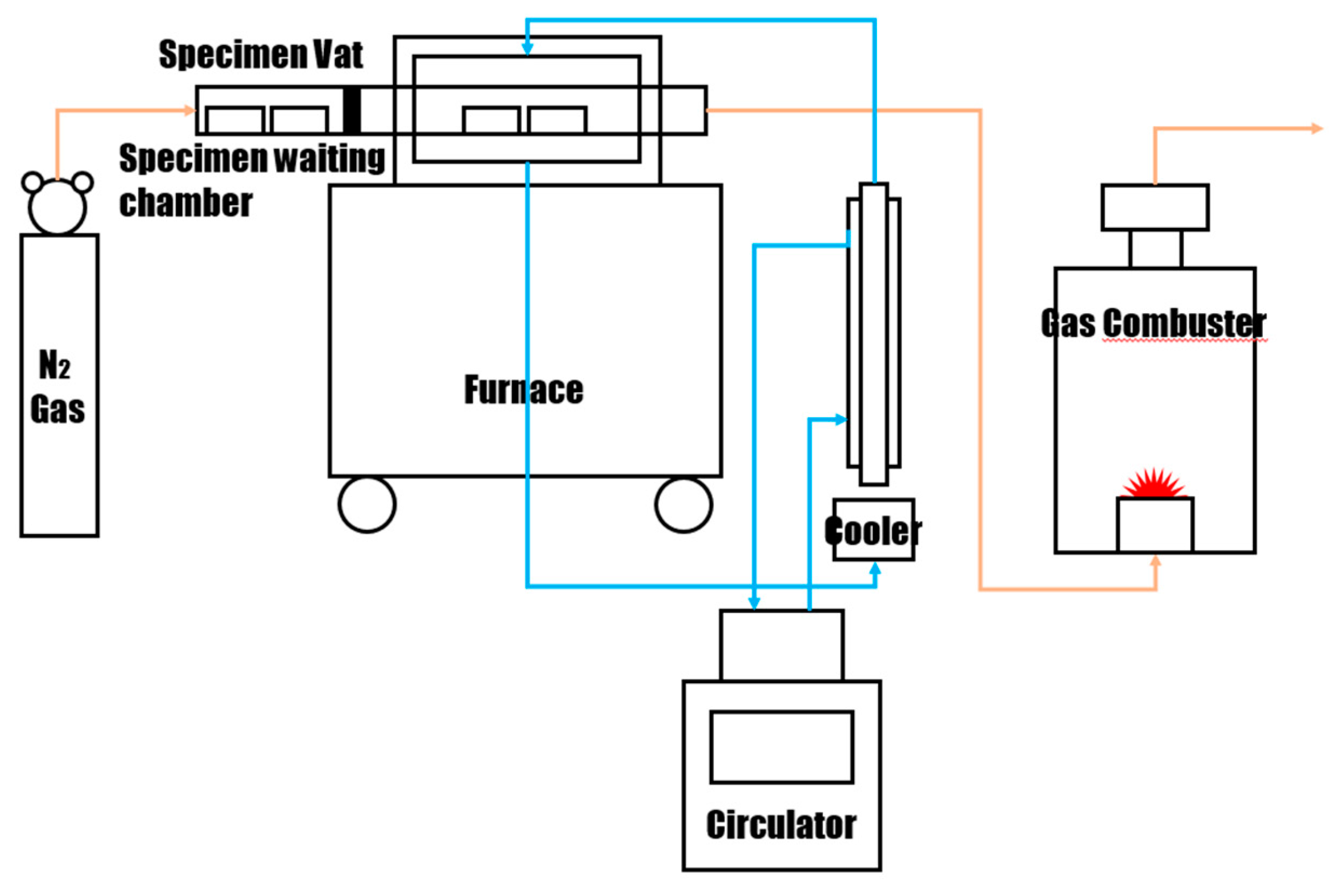
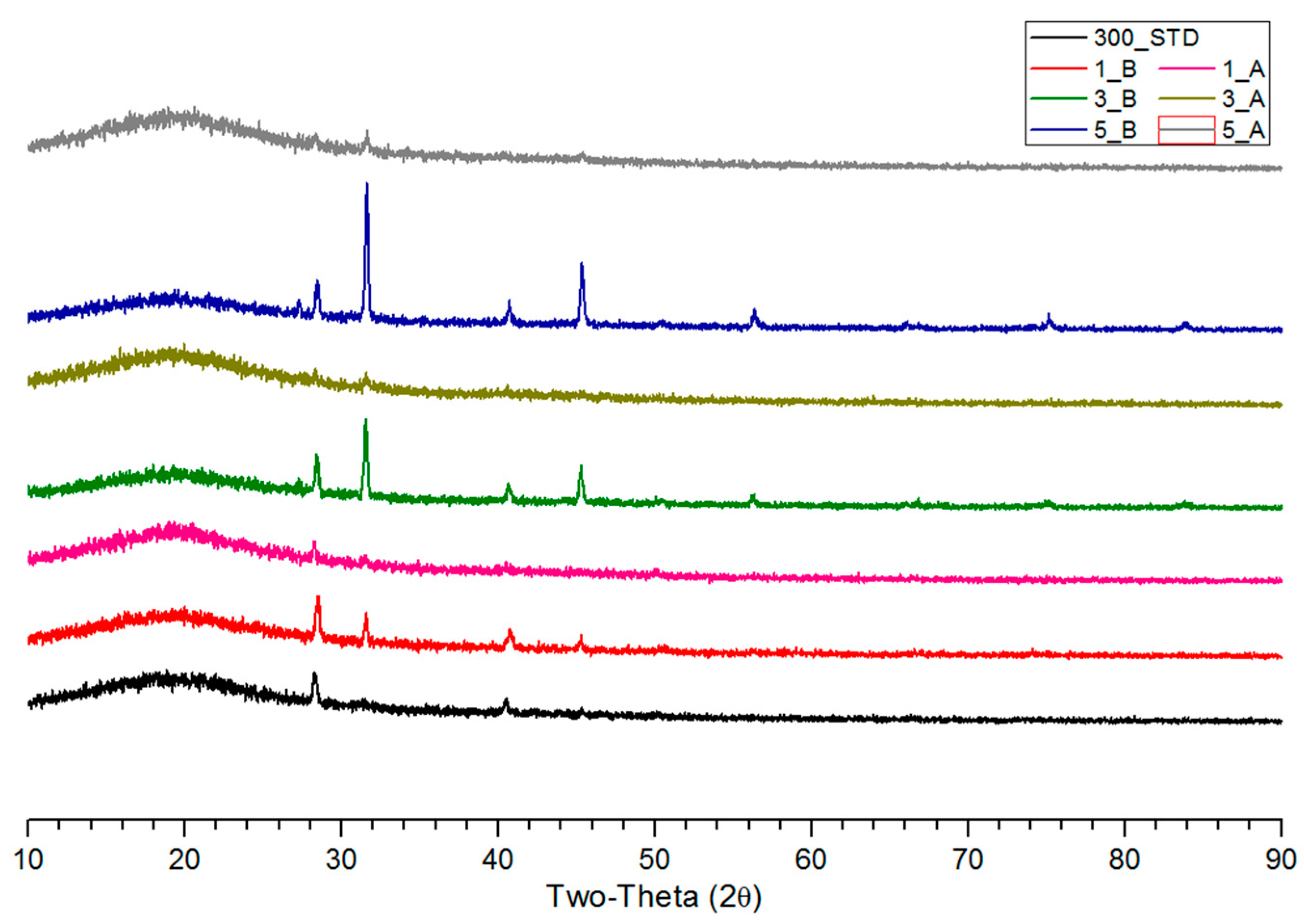
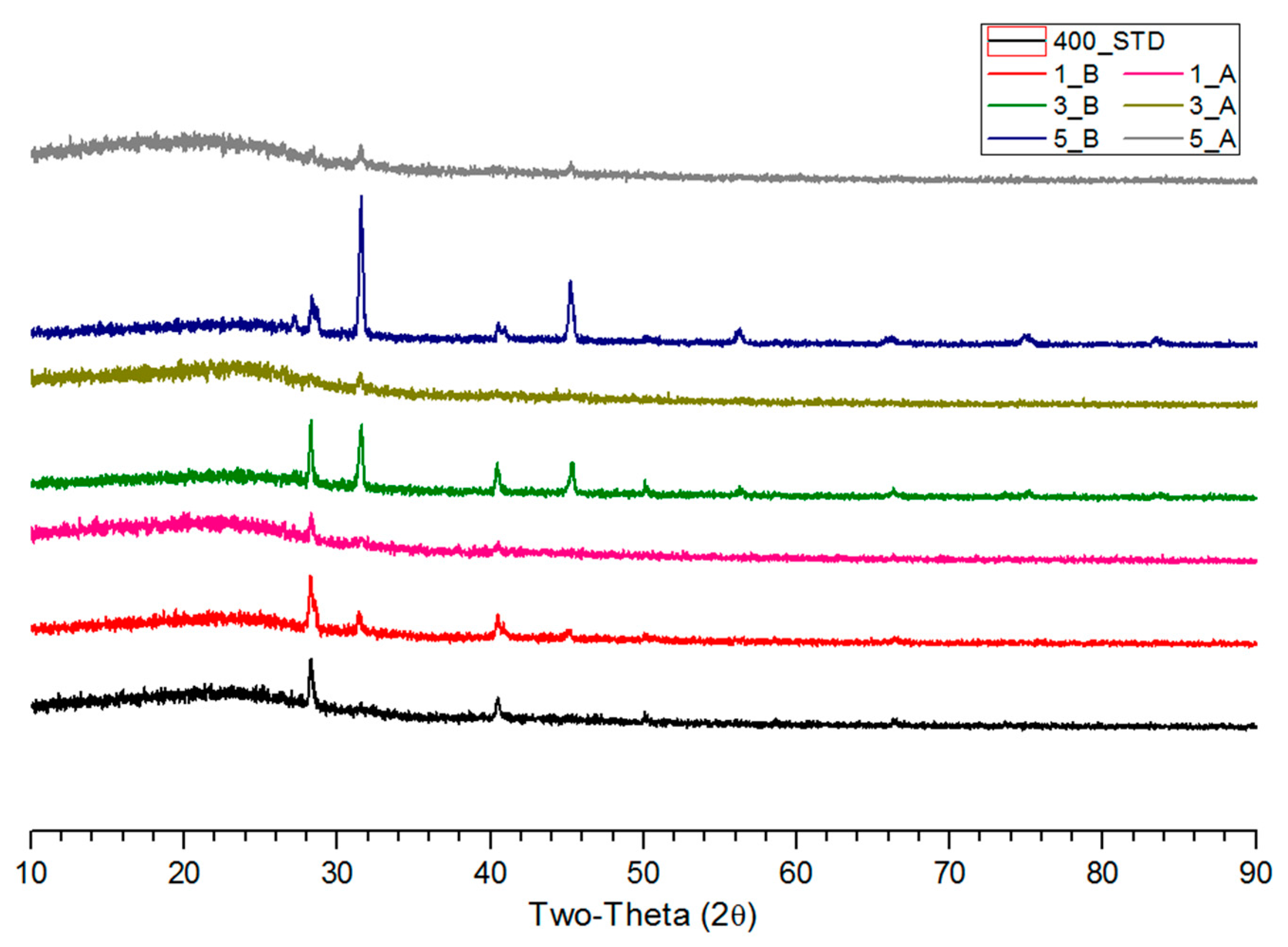
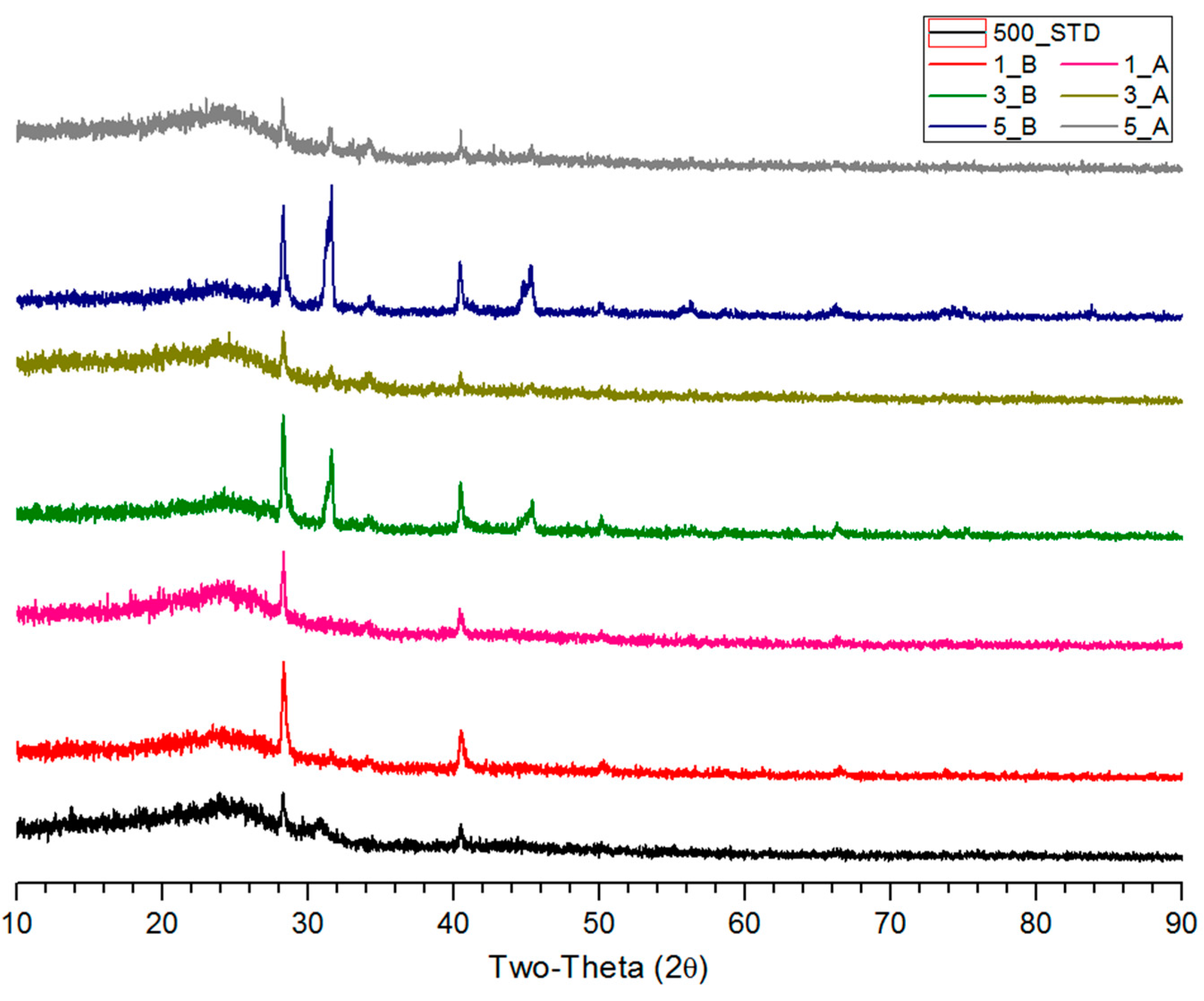
| Classification | Composition Ratio (wt %) | Methods of Food Ingredient Processing | |
|---|---|---|---|
| Food Ingredients | Processing Method | ||
| Grains | 16 | Rice (16) | |
| Vegetables | 51 | Napa cabbage (9) | Cutting width less than 100 mm. |
| Potato (20) | Chop into 5 mm size pieces. | ||
| Onion (20) | |||
| Daikon (2) | |||
| Fruits | 14 | Apple (7) | Split into 8 pieces in lengthwise. |
| Mandarin/Orange (7) | |||
| Meat and Fish | 19 | Meat (4) | Cutting width around 3 cm. |
| Fish (12) | Split into 4 pieces. | ||
| Egg shell (3) | |||
| Total | 100 | 100 | |
| Sample ID | Component, wt % | Atomic Ratio | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O | Na | Cl | Ash | C/N | H/C | O/C | |
| Dry | 51.46 | 13.22 | 3.14 | 32.18 | - | - | - | 16.38 | 0.257 | 0.63 |
| 300 °C_STD | 60.97 | 5.37 | 5.28 | 23.66 | 1.21 | 1.56 | 4.72 | 11.55 | 0.088 | 0.39 |
| 300 °C_1%_B | 60.18 | 5.32 | 5.29 | 25.88 | 3.08 | 3.17 | 3.33 | 11.38 | 0.088 | 0.43 |
| 300 °C_1%_A | 61.70 | 5.59 | 5.24 | 25.19 | 2.02 | 1.51 | 2.28 | 11.77 | 0.091 | 0.41 |
| 300 °C_3%_B | 58.96 | 5.17 | 5.09 | 22.53 | 6.63 | 6.26 | 8.25 | 11.58 | 0.088 | 0.38 |
| 300 °C_3%_A | 63.21 | 5.46 | 5.16 | 24.34 | 2.67 | 1.50 | 1.83 | 12.25 | 0.086 | 0.39 |
| 300°C_5%_B | 57.29 | 5.04 | 4.98 | 20.83 | 9.30 | 8.74 | 11.86 | 11.5 | 0.088 | 0.36 |
| 300 °C_5%_A | 62.08 | 5.81 | 5.42 | 23.03 | 2.62 | 1.50 | 3.66 | 11.45 | 0.094 | 0.37 |
| 400 °C_STD | 64.69 | 3.64 | 5.09 | 17.82 | 1.92 | 1.90 | 8.76 | 12.71 | 0.056 | 0.28 |
| 400 °C_1%_B | 63.27 | 3.60 | 5.38 | 17.98 | 4.74 | 4.18 | 9.77 | 11.76 | 0.057 | 0.28 |
| 400 °C_1%_A | 68.71 | 4.28 | 5.04 | 17.60 | 4.01 | 1.20 | 4.37 | 13.63 | 0.062 | 0.26 |
| 400 °C_3%_B | 57.85 | 3.24 | 4.16 | 21.08 | 9.22 | 8.25 | 13.67 | 13.91 | 0.056 | 0.36 |
| 400 °C_3%_A | 65.43 | 3.70 | 5.26 | 20.94 | 3.43 | 1.19 | 4.67 | 12.44 | 0.057 | 0.32 |
| 400 °C_5%_B | 56.15 | 3.20 | 3.99 | 18.74 | 16.53 | 12.66 | 17.92 | 14.07 | 0.057 | 0.33 |
| 400 °C_5%_A | 68.43 | 4.34 | 4.51 | 19.47 | 4.64 | 1.36 | 3.25 | 15.17 | 0.063 | 0.28 |
| 500 °C_STD | 70.11 | 2.81 | 5.23 | 8.83 | 0.51 | 0.90 | 13.02 | 13.41 | 0.04 | 0.13 |
| 500 °C_1%_B | 68.82 | 2.71 | 5.22 | 10.45 | 3.98 | 3.74 | 12.80 | 13.18 | 0.039 | 0.15 |
| 500 °C_1%_A | 72.07 | 2.93 | 5.13 | 9.43 | 3.43 | 1.03 | 10.44 | 14.05 | 0.041 | 0.13 |
| 500 °C_3%_B | 65.72 | 2.69 | 4.63 | 10.33 | 9.65 | 9.42 | 16.63 | 14.19 | 0.041 | 0.16 |
| 500 °C_3%_A | 72.10 | 2.98 | 5.13 | 11.32 | 5.14 | 1.49 | 8.47 | 14.05 | 0.041 | 0.16 |
| 500 °C_5%_B | 61.78 | 2.43 | 4.44 | 10.50 | 15.56 | 13.80 | 20.85 | 13.91 | 0.039 | 0.17 |
| 500 °C_5%_A | 73.28 | 2.92 | 4.92 | 11.80 | 5.35 | 1.45 | 7.08 | 14.89 | 0.04 | 0.16 |
| Sample ID | CEC (cmol/kg) | Extractable Cations (cmolc/kg) | |||
|---|---|---|---|---|---|
| Ca | K | Mg | Na | ||
| 300 °C_STD | 25.57 | 0.39 | 12.68 | 0.34 | 5.56 |
| 300 °C_1%_B | 22.20 | 0.29 | 7.77 | 0.12 | 6.99 |
| 300 °C_1%_A | 18.14 | 0.45 | 4.95 | 0.15 | 4.67 |
| 300 °C_3%_B | 22.42 | 0.17 | 4.06 | 0.06 | 11.08 |
| 300 °C_3%_A | 20.35 | 0.47 | 3.99 | 0.16 | 8.04 |
| 300 °C_5%_B | 110.71 | 0.35 | 16.59 | 0.24 | 86.26 |
| 300 °C_5%_A | 22.67 | 0.58 | 3.89 | 0.22 | 10.07 |
| 400 °C_STD | 62.94 | 1.10 | 44.07 | 1.37 | 14.21 |
| 400 °C_1%_B | 120.39 | 1.19 | 62.59 | 1.15 | 49.98 |
| 400 °C_1%_A | 36.65 | 0.90 | 18.00 | 0.64 | 12.71 |
| 400 °C_3%_B | 163.48 | 0.96 | 52.17 | 1.23 | 108.91 |
| 400 °C_3%_A | 52.60 | 1.30 | 16.17 | 1.00 | 24.68 |
| 400 °C_5%_B | 250.72 | 0.65 | 53.19 | 1.29 | 185.03 |
| 400 °C_5%_A | 39.46 | 1.06 | 9.90 | 0.86 | 22.80 |
| 500 °C_STD | 54.91 | 0.89 | 60.38 | 1.58 | 1.07 |
| 500 °C_1%_B | 75.22 | 0.83 | 62.37 | 1.10 | 14.88 |
| 500 °C_1%_A | 24.79 | 1.07 | 18.23 | 0.68 | 7.67 |
| 500 °C_3%_B | 156.81 | 0.76 | 67.59 | 1.22 | 92.30 |
| 500 °C_3%_A | 18.90 | 1.29 | 10.63 | 1.00 | 9.27 |
| 500 °C_5%_B | 262.08 | 0.63 | 73.55 | 1.31 | 189.90 |
| 500 °C_5%_A | 24.86 | 1.34 | 10.29 | 1.07 | 14.58 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-E.; Jo, J.-H.; Kim, I.-T.; Yoo, Y.-S. Chemical Characteristics and NaCl Component Behavior of Biochar Derived from the Salty Food Waste by Water Flushing. Energies 2017, 10, 1555. https://doi.org/10.3390/en10101555
Lee Y-E, Jo J-H, Kim I-T, Yoo Y-S. Chemical Characteristics and NaCl Component Behavior of Biochar Derived from the Salty Food Waste by Water Flushing. Energies. 2017; 10(10):1555. https://doi.org/10.3390/en10101555
Chicago/Turabian StyleLee, Ye-Eun, Jun-Ho Jo, I-Tae Kim, and Yeong-Seok Yoo. 2017. "Chemical Characteristics and NaCl Component Behavior of Biochar Derived from the Salty Food Waste by Water Flushing" Energies 10, no. 10: 1555. https://doi.org/10.3390/en10101555
APA StyleLee, Y.-E., Jo, J.-H., Kim, I.-T., & Yoo, Y.-S. (2017). Chemical Characteristics and NaCl Component Behavior of Biochar Derived from the Salty Food Waste by Water Flushing. Energies, 10(10), 1555. https://doi.org/10.3390/en10101555




