Abstract
It has long been known that the path (trajectory) taken by the eye to land on a target is rarely straight (Yarbus, 1967). Furthermore, the magnitude and direction of this natural tendency for curvature can be modulated by the presence of a competing distractor stimulus presented along with the saccade target. The distractor-related modulation of saccade trajectories provides a subtle measure of the underlying competitive processes involved in saccade target selection. Here we review some of our own studies into the effects distractors have on saccade trajectories, which can be regarded as a way of probing the competitive balance between target and distractor salience.
Introduction
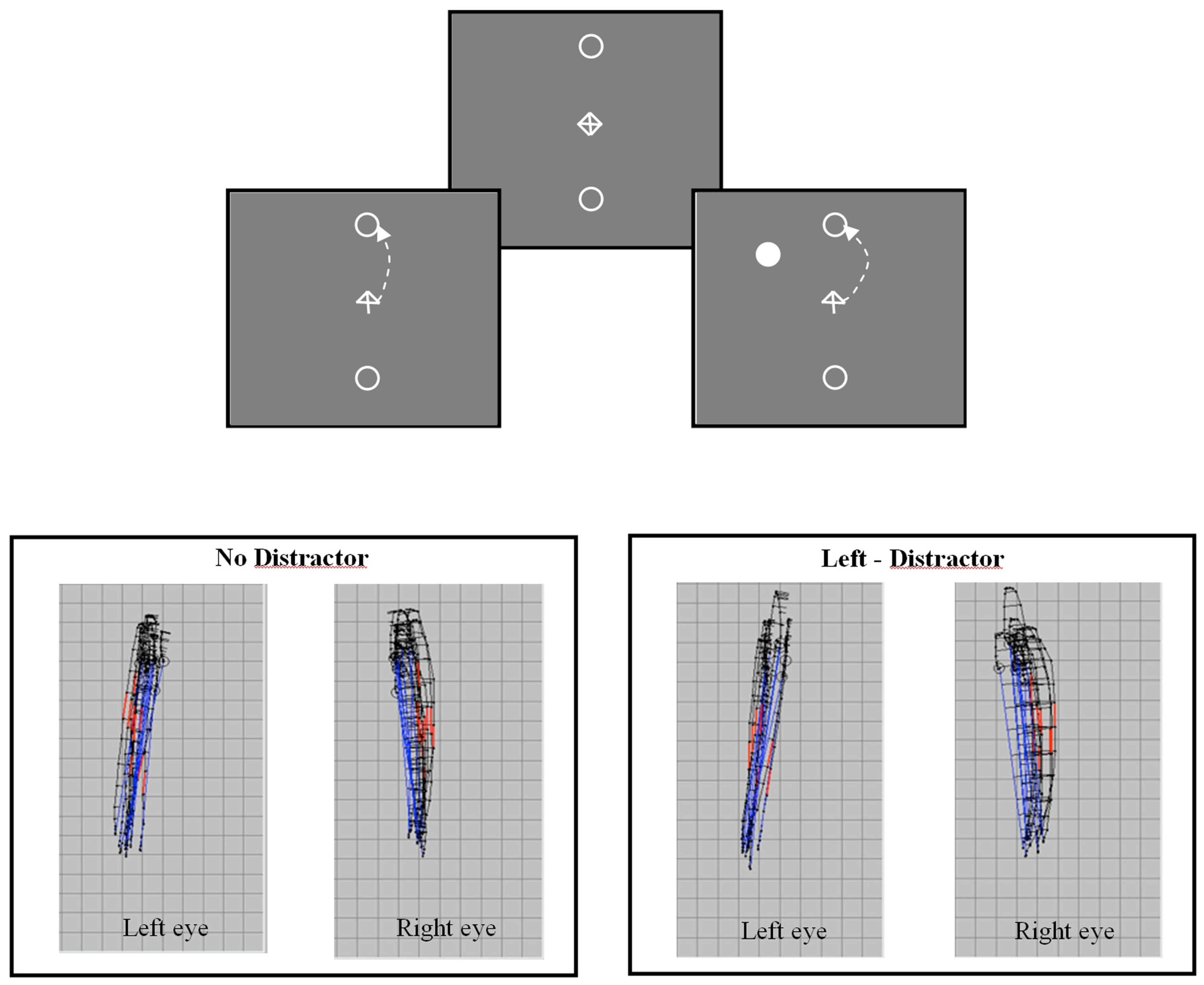
The trajectories of saccadic eye movements are often found to be curved (Collewijin, Erkelens, & Steinman, 1988; Viviani, Berthoz, & Tracey, 1977; Yarbus, 1967). This natural tendency for curvature can be seen in the trajectories of vertical saccades shown in Figure 1 (see also: Kapoula et al. this volume). Figure 1a shows the stimulus display used to elicit voluntary saccades to a target ‘goal’ located above or below fixation. An arrow at central fixation indicates the direction of the saccade on each trial. Binocular recordings of ten upwardly directed saccades, from a single observer, are shown in Figure 1b. The trajectories of upwardly directed saccades for both eyes can be seen to diverge in the temporal direction (Collewijin, Erkelens, & Steinman, 1988). The mean deviation of trajectory (peak deviation divided by amplitude in pixels) without a distractor is: left eye = 0.049, right eye = 0.102. This natural tendency for curva ture can be modulated by the presence of a competing distractor. Figure 1c shows binocular recordings of ten saccade trajectories made by the same observer when a distractor appeared in the upper left visual field. The overall effect of the distractor is to produce a deviation of trajectory in the direction away from the distractor location. Thus, the effects of a distractor in the left visual field are different for the two eyes. The trajectories of the left eye become straighter, while those of the right eye become more curved. The mean deviation of the saccades made in the presence of a distractor as shown in Figure 1c is: left eye = 0.035, right eye = 0.12. The overall modulation of saccade trajectory is, however, broadly similar for the two eyes (left eye change curvature = 0.014, right eye change curvature = 0.018). The effect of distractors on saccade trajectories for the left and right eyes would therefore be highly correlated (Ludwig & Gilchrist, 2003).
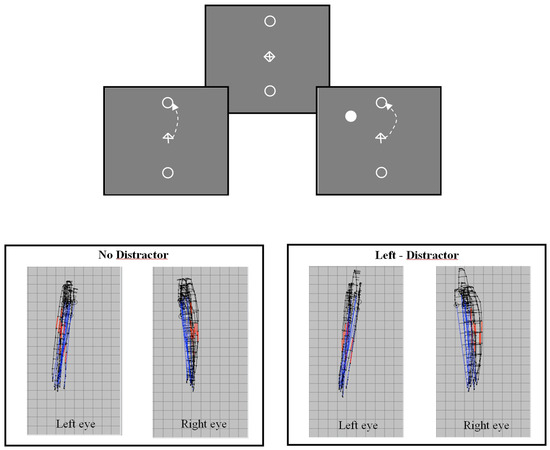
Figure 1.
Figure 1a (Upper panel) Schematic diagram of the stimulus display used to elicit voluntary vertical saccades to target goals located 8 deg in the upper and lower visual field. An arrow cue indicates saccade direction. Saccades can be made with or without a competing task-irrelevant distractor. Figure1b (Lower left) Examples of binocular saccade trajectories (n=10) from a single observer (RW) observed without a distractor showing the natural tendency for saccade trajectories to be curved. Figure 1c (Lower right) shows the modulation of trajectory deviation observed when a distractor appeared in the upper left visual field (45deg from vertical axis).
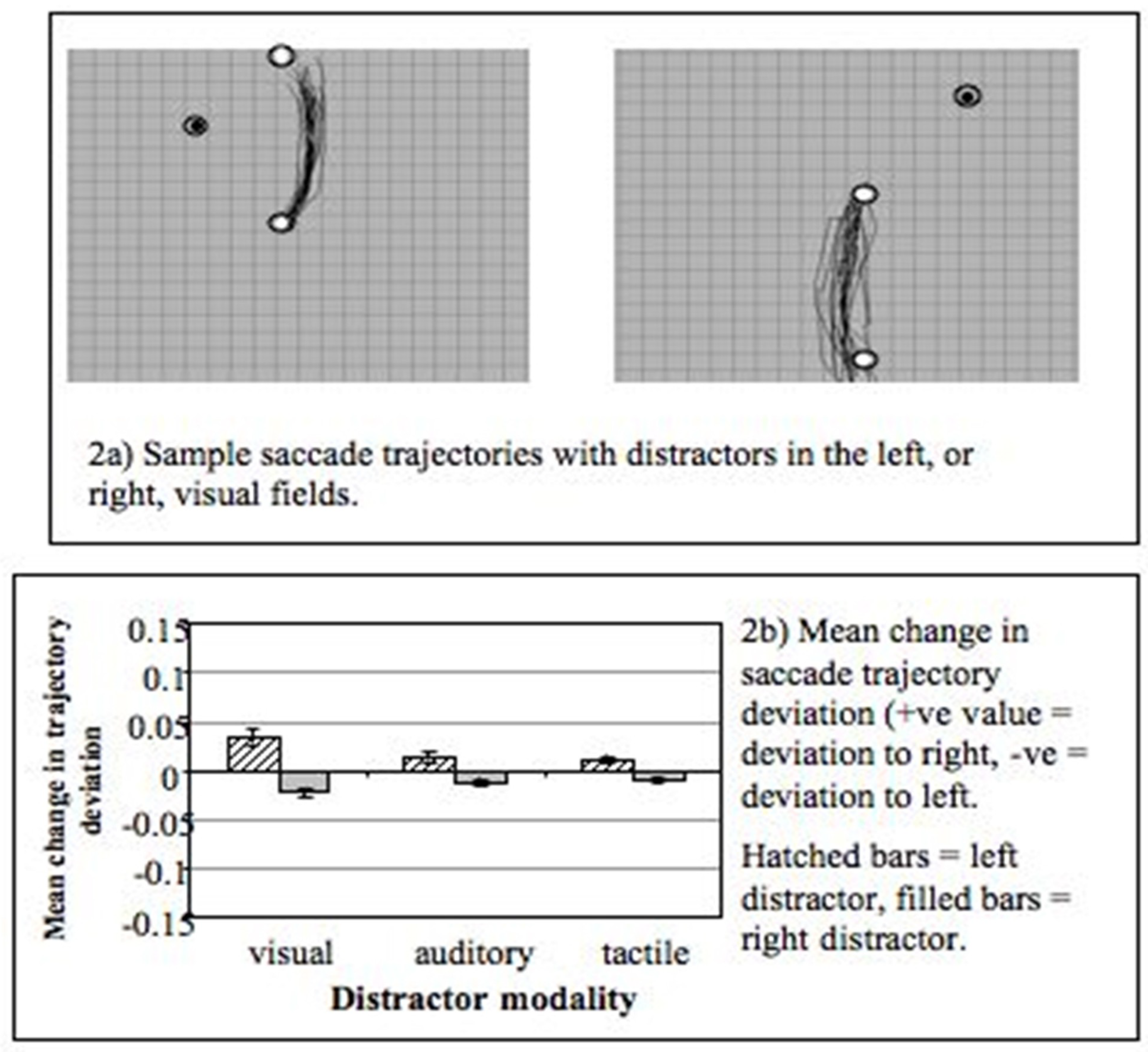
The modulation of saccade trajectories by a competing distractor has become the focus of a number of behavioural and neurophysiological investigations over the past decade (Godijn & Theeuwes, 2004; Ludwig & Gilchrist, 2003; Ludwig & Gilchrist, 2002; McPeek, 2006; McPeek, Han, & Keller, 2003; McPeek & Keller, 2001; Nummenmaa & Hietanen, 2006; Quaia, Aizawa, Optican, & Wurtz, 1998; Van der Stigchel, Meeter, & Theeuwes, 2006, 2007b; Van der Stigchel & Theeuwes, 2005, 2006a, 2006b; van Zoest, Van der Stigchel, & Barton, 2007). Studies by Sheliga, Rizzollati and colleagues (Rizzolatti, Riggio, & Sheliga, 1994; Sheliga, Riggio, Craighero, & Rizzolatti, 1995; Sheliga, Riggio, & Rizzolatti, 1994, 1995) showed that saccade trajectories can deviate away from a location to which covert attention had previously been oriented (to discriminate the direction of a symbolic cue indicating saccade direction). Sheliga and colleagues interpreted this finding in terms of the pre-motor model of attention (Sheliga, Riggio, Craighero, & Rizzolatti, 1995) see also: (Tipper, Howard, & Paul, 2001). In terms of this theory covertly attending to a location involves programming a saccade to that location (which is not executed). The suppression of this saccade programme causes the actual vertical saccade response to deviate away from the location where attention had previously been directed. My own interest (RW) in this topic came from a need for a behavioural paradigm that could be used to investigate crossmodal interaction effects in saccade generation (Doyle & Walker, 2002). Our early studies (Doyle & Walker, 2001) showed that saccades also deviate away from task-irrelevant distractors that have to be ignored (see: Figure 2). The magnitude of the observed trajectory deviation was similar for both stimulus-elicited and voluntary saccades made on the basis of an arrow cue at fixation. Furthermore, the deviation of the trajectory, in the direction away from distractors, was observed with distractors in both the same and opposite hemifield to the saccade target (or goal). These early findings show that the deviation of saccade trajectories is a robust effect, which does not depend on the voluntary orienting of covert attention and that manipulating the spatial relationship between the distractor and saccade goal has rather small effects (Doyle & Walker, 2001). We then developed the basic paradigm to include visual, auditory and tactile distractors (motivated by a study from (Groh & Sparks, 1996a, 1996b) and found that saccades deviated away from distractors presented in all three modalities - although the greatest deviation was observed with visual distractors (Figure 2b).
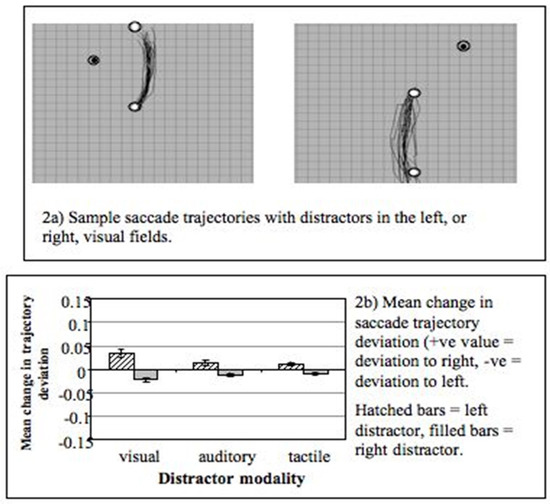
Figure 2.
Figure 2 (upper) Examples of vertical saccade trajectories (data from one observer) made to targets in the upper (left panel), or lower (right panel), visual field. The trajectories deviated away from task-irrelevant visual distractors (adapted from: (Doyle & Walker, 2001)). Figure 2 (lower panel) Mean change in curvature of saccade trajectories (n = 6) with visual, auditory and tactile distractors (from: (Doyle & Walker, 2002).
Explanation of distractor effects
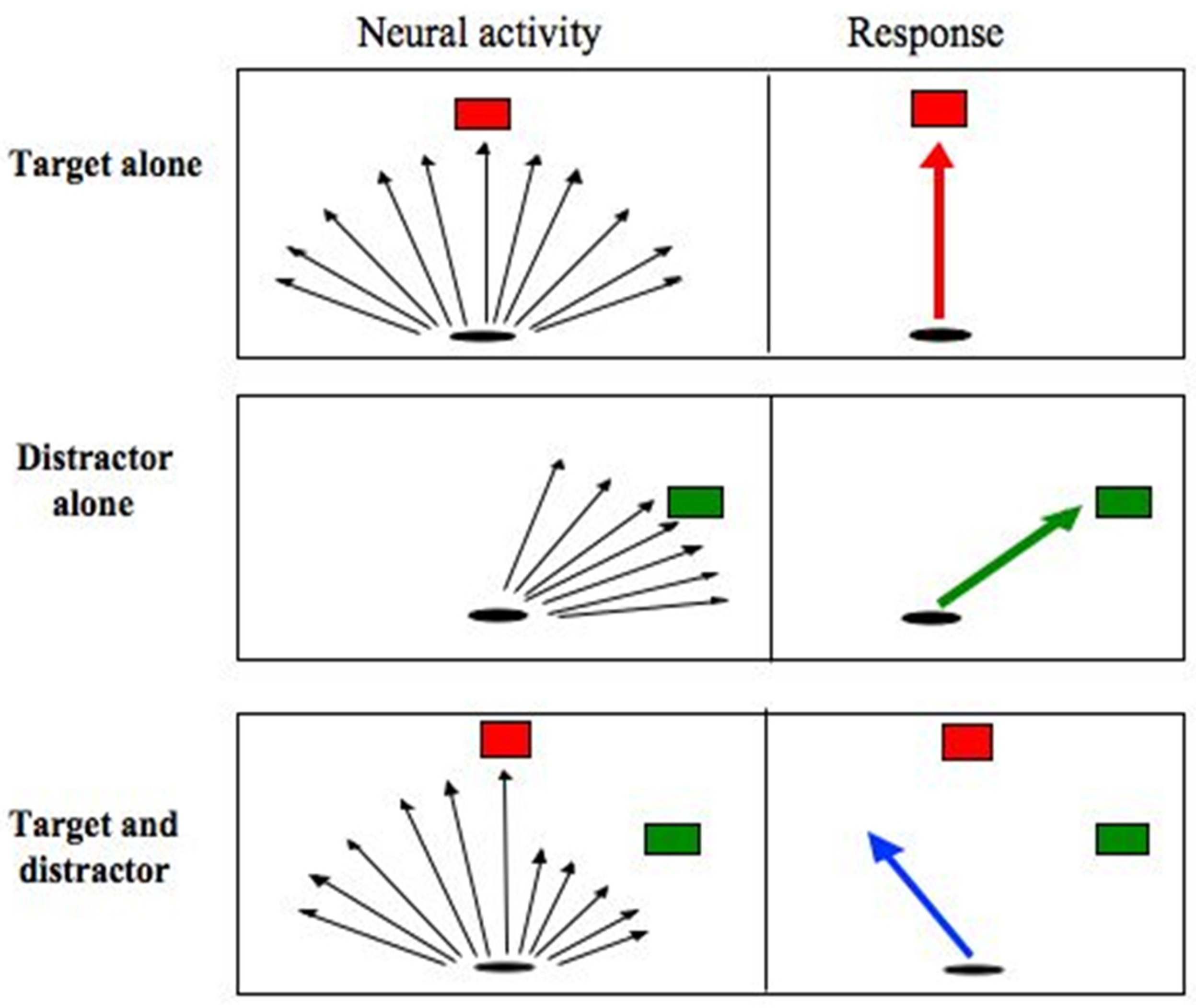
The counterintuitive observation that saccades deviate away from a competing distractor, has been confirmed by numerous other studies (Doyle & Walker, 2001, 2002; Godijn & Theeuwes, 2004; Ludwig & Gilchrist, 2003; McSorley, Haggard, & Walker, 2004, 2005, 2006; Sheliga, Riggio, Craighero, & Rizzolatti, 1995; Sheliga, Riggio, & Rizzolatti, 1994, 1995; Tipper, Howard, & Paul, 2001; Van der Stigchel, Meeter, & Theeuwes, 2006, 2007b; Van der Stigchel & Theeuwes, 2006a; Walker, McSorley, & Haggard, 2006). Explanations of why saccades deviate away from (and not towards as might be expected) distractors have invoked ‘spatial maps’ in which saccade direction is encoded by populations of neurons with broad and overlapping receptive fields. In such models neural activity associated with the distractor is thought to be averaged along with activity associated with the target (or ‘goal’). As the onset of a visual distractor would be expected to produce a separate population of neural activity the natural prediction would be that saccades should deviate towards the distractor location, rather than away from it as is typically observed. The theory, therefore, requires an additional assumption to account for the deviation of saccades away from distractors. The assumption is that neurons encoding the distractor are inhibited, below a baseline level, so they make a negative contribution to the saccade direction vector. This situation is displayed in Figure 3.
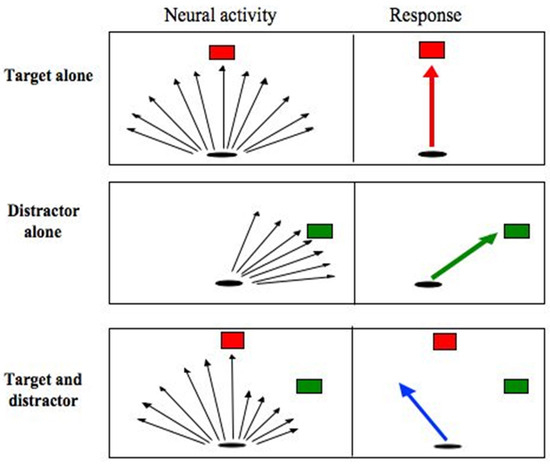
Figure 3.
Schematic representation of populations of neural activity in response to different stimulus configurations (left hand column) and the corresponding saccadic response (right hand column). Black arrows represent population vectors that encode the potential saccade goal. When the target or distractor is shown alone the population vectors centre around their direction. When a target and distractor are presented together activity at the distractor site must be inhibited so it makes a negative contribution to the computation of initial saccade direction.
The intermediate layers of the superior colliculus (SC) form a plausible neurophysiological substrate for the model of saccade deviation described above (Sparks & Hartwich-Young, 1989). The intermediate layers of the SC contain a large population of neurons with large overlapping receptive fields that encode desired saccade direction (Lee, Rohrer, & Sparks, 1988; McIlwain, 1991) which can be regarded as forming a ‘motor map’ (Wurtz, 2000). When the population of neurons encoding the potential target overlaps with a second population encoding the distractor, an error in the computation of initial saccade direction occurs. The direction of initial saccade deviation either towards, or away from, a competing distractor is thought to reflect the level of neural activity at the distractor site at the time the saccade is initiated (McPeek, Han, & Keller, 2003). The involvement of the SC in the modulation of saccade trajectories has been demonstrated by neurophysiological studies, involving single-cell recording (McPeek, Han, & Keller, 2003; Port & Wurtz, 2003) and reversible deactivation (Aizawa & Wurtz, 1998; Quaia, Aizawa, Optican, & Wurtz, 1998). Higher-level influences from cortical regions, such as the frontal eye fields (FEFs), are thought to be involved in the process of selecting a stimulus as the desired target and in inhibiting competing alternatives (Schlag-Rey, Schlag, & Dassonville, 1992). The involvement of the FEFs in saccade curvature has been revealed by McPeek (2006) who showed that the level of distractor-related activity in the frontal eye fields (FEFs) can be related to the direction of initial saccade direction (McPeek, 2006). Thus, it is plausible that the inhibition of distractor locations in the motor map in the SC relies on inhibitory projections from the frontal eye fields.
Neural interactions between target-related activity and distractor-related inhibition, in a two-dimensional motor map such as that formed by neurons in the intermediate layers of the superior colliculus, can account for the initial deviation of saccade trajectory away from the distractor location. Explanations of how saccades may then curve back towards the target, have introduced separate extracollicular feedback processes to control saccade trajectory ‘on-line’ enabling small corrections to be made. Quaia et al. (1999) outlined a detailed model in which the feedback process is attributed to the cerebellum, which provides a separate directional drive signal to the brainstem saccade generator, enabling the on-line correction of a saccade that deviates away from the desired saccade target. The processes involved in the control of trajectory have been discussed elsewhere (McSorley et al., 2004; Quaia et al., 1999) and are not further considered here.
Spatial-effects
A prediction arising from the model outlined above is that manipulating the target-to-distractor spatial separation would be expected to modulate the magnitude of the distractor effects on saccade trajectories. Consistent with this prediction studies have shown that saccades deviate away from distractors, and the magnitude of trajectory deviation may be greater when distractors appear in the same-hemifield to the target (Doyle & Walker, 2001; Tipper, Howard, & Paul, 2001). McSorley, Haggard and Walker (McSorley, Haggard, & Walker, 2004) systematically examined the effect of manipulating target-to-distractor distance in more detail and also the influence of bilateral distractors presented in both visual fields. Manipulating the spatial relationship between target and distractor had little effect on the magnitude of trajectory deviation, although there was a tendency for distractors in the same hemifield as the target to produce greater deviation than those in the opposite hemifield. When two distractors were presented bilaterally at mirror-symmetric locations trajectories tended to be straightened. Manipulating the horizontal distance between the bilateral distractors did not modulate this straightening effect. Thus, it appears that the effect of distractors on saccade trajectories is coarsely coded. This finding is consistent with neurophysiological evidence that a large number of collicular neurons, with broad receptive fields are active for any particular saccade (Munoz & Wurtz, 1995) and that topographically organised projections from the frontal eye fields inhibit broad regions of the collicular map to suppress non-target activity (Schlag-Rey, Schlag, & Dassonville, 1992). Thus, saccade trajectories appear sensitive to the presence of competing distractors, although this does not appear to be a highly spatially specific effect. Recently, however, Van der Stigchel, Meeter & Theeuwes, (2007) (Van der Stigchel, Meeter, & Theeuwes, 2007a) have shown that manipulating the vertical distance of distractors from central fixation can modulate the trajectory effect – with an increase in deviation observed for distractors closer to fixation (although this may only be the case for vertical saccades as these were the only ones investigated). Van der Stigchel et al. speculate that distractors closer to fixation may have greater salience than those further away. This greater distractor salience, produces more potent competition, that in turn requires greater inhibition. Some support for this interpretation comes from another report showing that distractors which share visual similarities with the saccade target, which are assumed to be more salient, produce greater trajectory deviation than dissimilar distractors (Ludwig & Gilchrist, 2003). Differences in the spatial location of targets and distractors may account for the apparent discrepancy between our results and those of Van der Stigchel and colleagues.
What mediates deviation towards or away from a location?
As noted above a puzzle about the effects of distractors on trajectories is that in some situations saccades deviate towards competing locations, while in other situations trajectories deviate away from distractors. The factors involved in the direction of deviation towards, or away from, competing locations have been the subject of some of our investigations. In visual search paradigms, using monkeys, incorrect saccades directed towards distractors have been shown to deviate towards the subsequent saccade goal (McPeek, Han, & Keller, 2003; McPeek & Keller, 2001). By contrast, in situations with more predictable target locations (for example on the vertical axis above or below fixation), with human participants saccades deviate away from distractors. We predicted that the use of predictable target locations could give inhibitory processes involved in suppressing distractor-related activity an early advantage. This was examined in a study in which a pre-cue was used to indicate the target location in order to explore the deviation of horizontal, vertical and oblique saccade trajectories (Walker, McSorley, & Haggard, 2006).
Predictable target locations
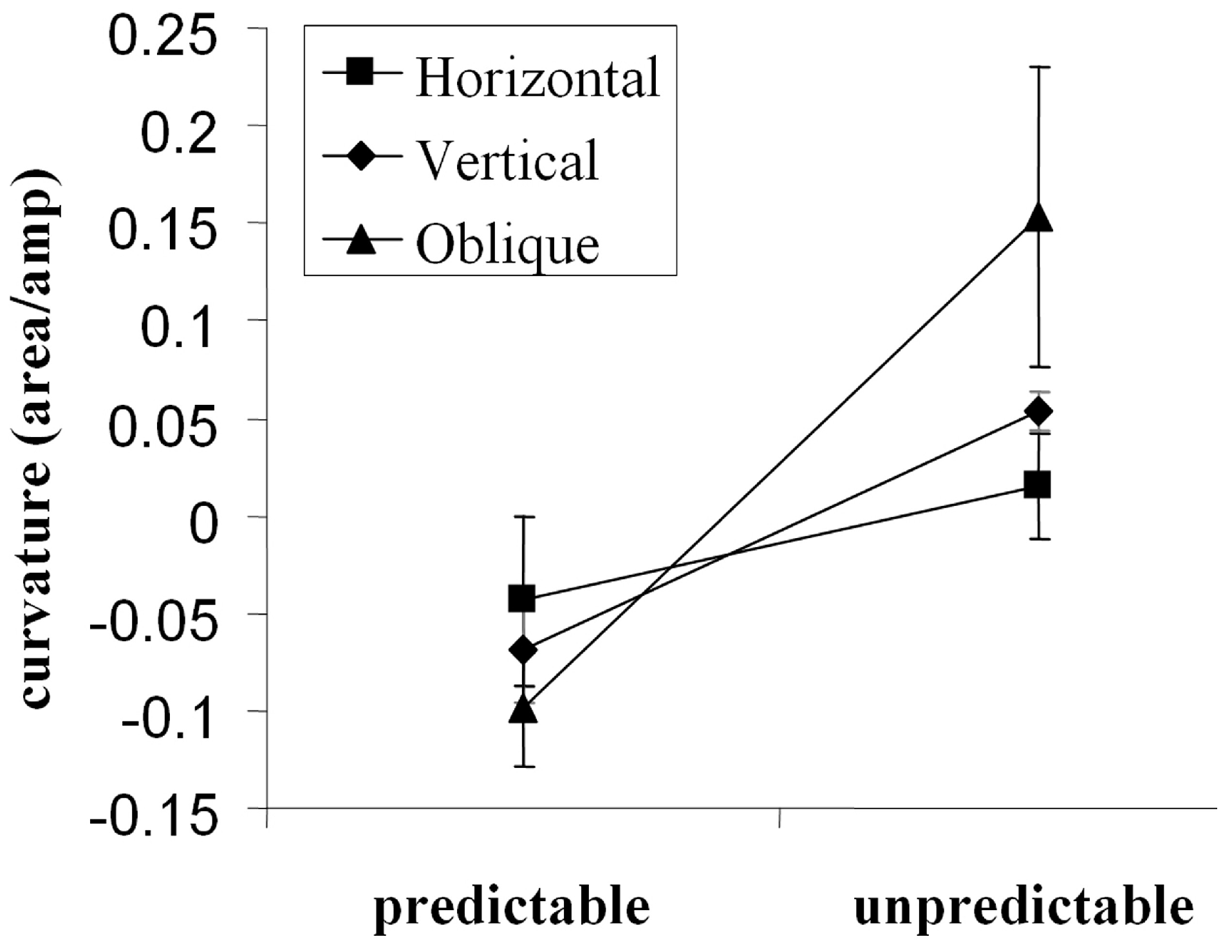
Walker at al. (Walker, McSorley, & Haggard, 2006) investigated the importance of predictable target locations with a wide range of saccade directions. We found that saccades deviated away from distractors when a pre-cue indicated target location. However, when target and distractor location were unpredictable (as is the case in visual search) trajectories deviated towards distractors. This effect was found for all saccade directions (horizontal, vertical and oblique) although the greatest deviation was found for the oblique direction (see Figure 4). As theories of saccade deviation assume that inhibition develops over time, a post-hoc analysis of the relationship between saccade deviation and saccade latency was performed. This analysis showed that the tendency for saccades to deviate away from a distractor increased at longer latencies. A limitation of this analysis, however, was the natural tendency for a positive skew in the underlying latency distribution, resulting in relatively few short latency saccades.
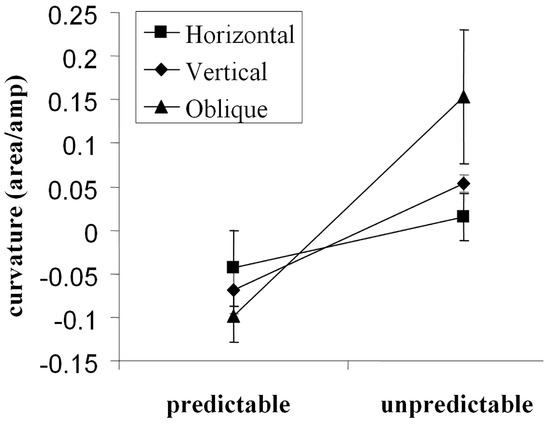
Figure 4.
Saccade trajectory deviation (here shown as normalized area under the curve formed by the saccade path and the most direct route to the saccade target) as a function of cue condition (target location pre-cued, or uncued) and target axis. Saccades to oblique targets deviated most strongly followed by those made to vertical then horizontal targets (McSorley, Haggard, & Walker, 2006). Positive values on the ordinate indicate deviation towards, and negative values deviation away from, a competing distractor.
Relationship between trajectory deviation and saccade latency
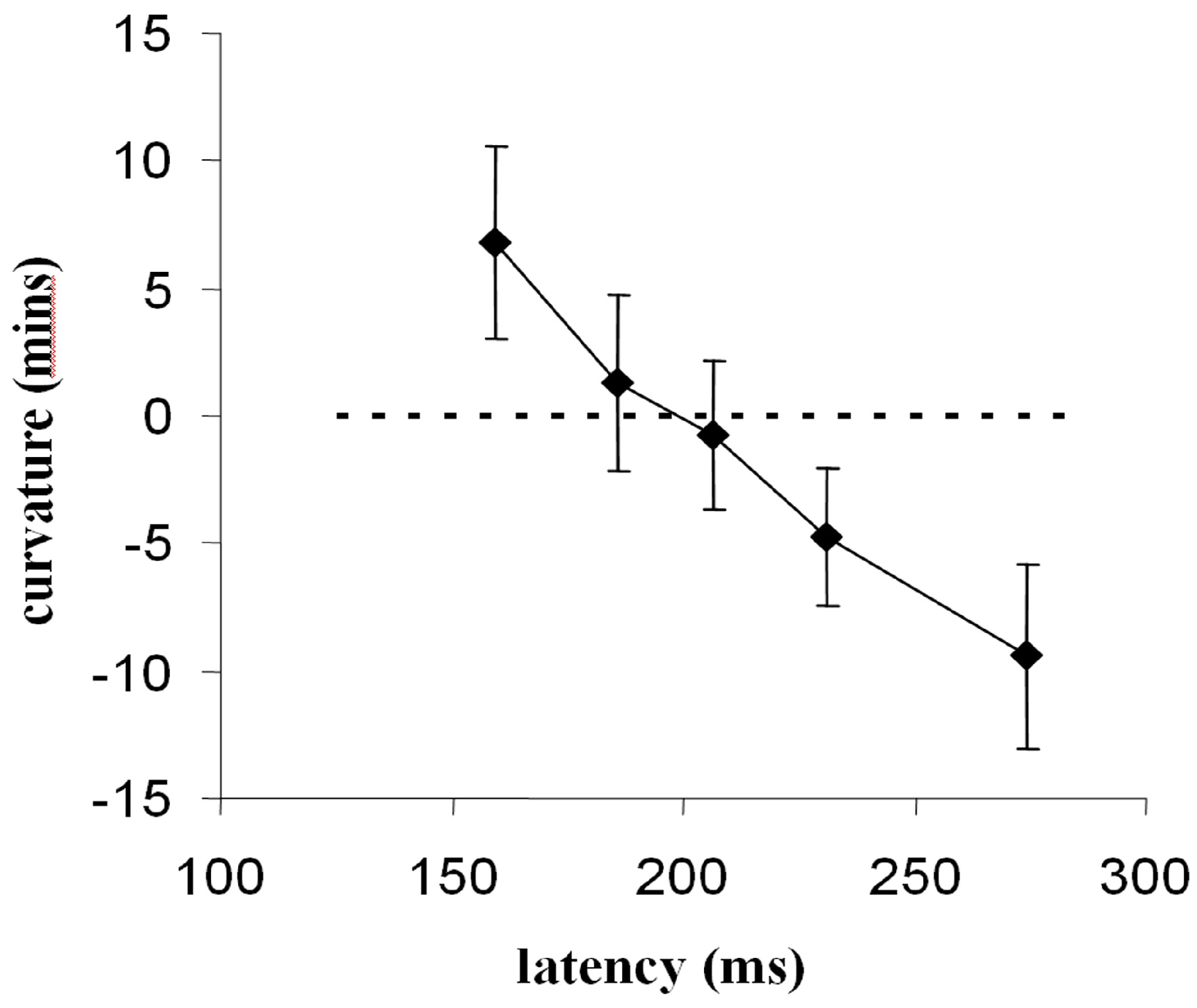
As discussed our analysis showed a trend for longerlatency saccades to deviate away from distractors more strongly. However, the paucity of saccades with latency less than 200-250ms restricts the evidence for this conclusion. In order to establish the relationship between saccade latency and direction of trajectory deviation more clearly we used a simple fixation gap-paradigm (Saslow, 1967) to increase the frequency of short latency saccades (McSorley, Haggard, & Walker, 2006). The use of a fixation-offset manipulation should modulate saccade latency independently of any effects distractors have on latency (Walker, Kentridge, & Findlay, 1995). In our gap-overlap study of trajectory effects saccades were made to a range of unpredictable target locations (horizontal, vertical and oblique) with fixation removed at intervals from 200ms before, to 200ms after, target onset. A clear relation between latency and direction of saccade deviation was observed. Saccades with latency less than 200ms deviated towards distractors while those with latency greater than 200ms deviated away. Similar effects were observed for all saccade directions.
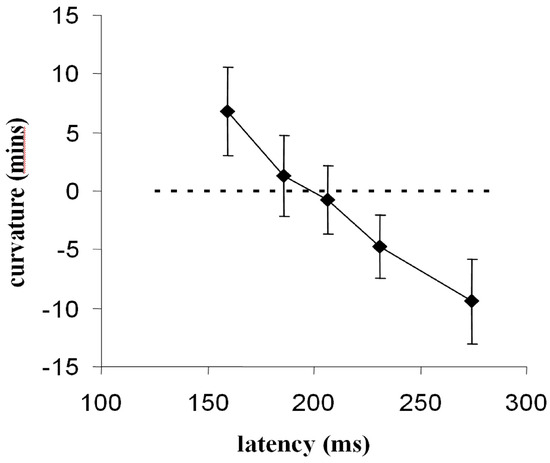
Figure 5.
The relation between saccade latency and saccade deviation (+ve values indicate deviation towards, and -ve values deviation away from, a competing distractor). Data are split into five temporal intervals across all gap-overlap conditions (from: McSorley, Haggard, & Walker, 2006).
Figure 5.
The relation between saccade latency and saccade deviation (+ve values indicate deviation towards, and -ve values deviation away from, a competing distractor). Data are split into five temporal intervals across all gap-overlap conditions (from: McSorley, Haggard, & Walker, 2006).
To summarise, we have shown that the distractorrelated deviation of saccade trajectories is observed for visual, auditory and tactile distractors. The trajectory modulation shows a weak spatial effect and is greater when the distractor appears in the same hemifield as the target. The direction of deviation, towards, or away from, the distractor depends on the predictability of the target location, and on saccade latency. These two factors may be related. When target location is predictable, inhibition may be applied broadly across all non-target locations. This gives an advantage to the process of suppressing distractor-related activity following the onset of the stimuli when latency is long. In effect, the use of predictable target locations may give the inhibitory processes involved in saccade-target selection an advantage as they may be applied prior to stimulus onset. The relationship between direction of deviation and latency is consistent with the time-course of the inhibitory process. When latency is short the distractor-related activity has not been suppressed below baseline. At longer latencies inhibitory processes have suppressed distractor activity below baseline resulting in a negative contribution to the computation of initial saccade direction. Like others we suggest that the modulation of saccade trajectories provides a signature of the underlying state of the saccadic system at the time the saccade is initiated (McPeek, 2006; McPeek, Han, & Keller, 2003).
Stimulation of the frontal eye fields using Transcranial magnetic stimulation
Current explanations of distractor-related modulation of saccade trajectory have been based on the idea that inhibition suppresses the neural activity associated with the distractor, thus enabling a single saccade goal to be selected. The frontal eye fields (FEFs) are an obvious candidate for such top-down modulation of target and distractor related activity (or salience). A recent study (with Patrick Haggard and Puncharat Techawachirakul at University College London- submitted) enabled us to investigate the role of the FEFs in saccade curvature using transcranial magnetic stimulation (TMS). TMS is a technique for stimulating the cortical regions of the brain that has been described as a ‘virtual lesion’ (O’Shea & Walsh, 2007). If the FEFs are involved in inhibiting the distractor we reasoned that applying TMS to the FEFs should interfere with this inhibitory process resulting in a decrease in the distractor effect.
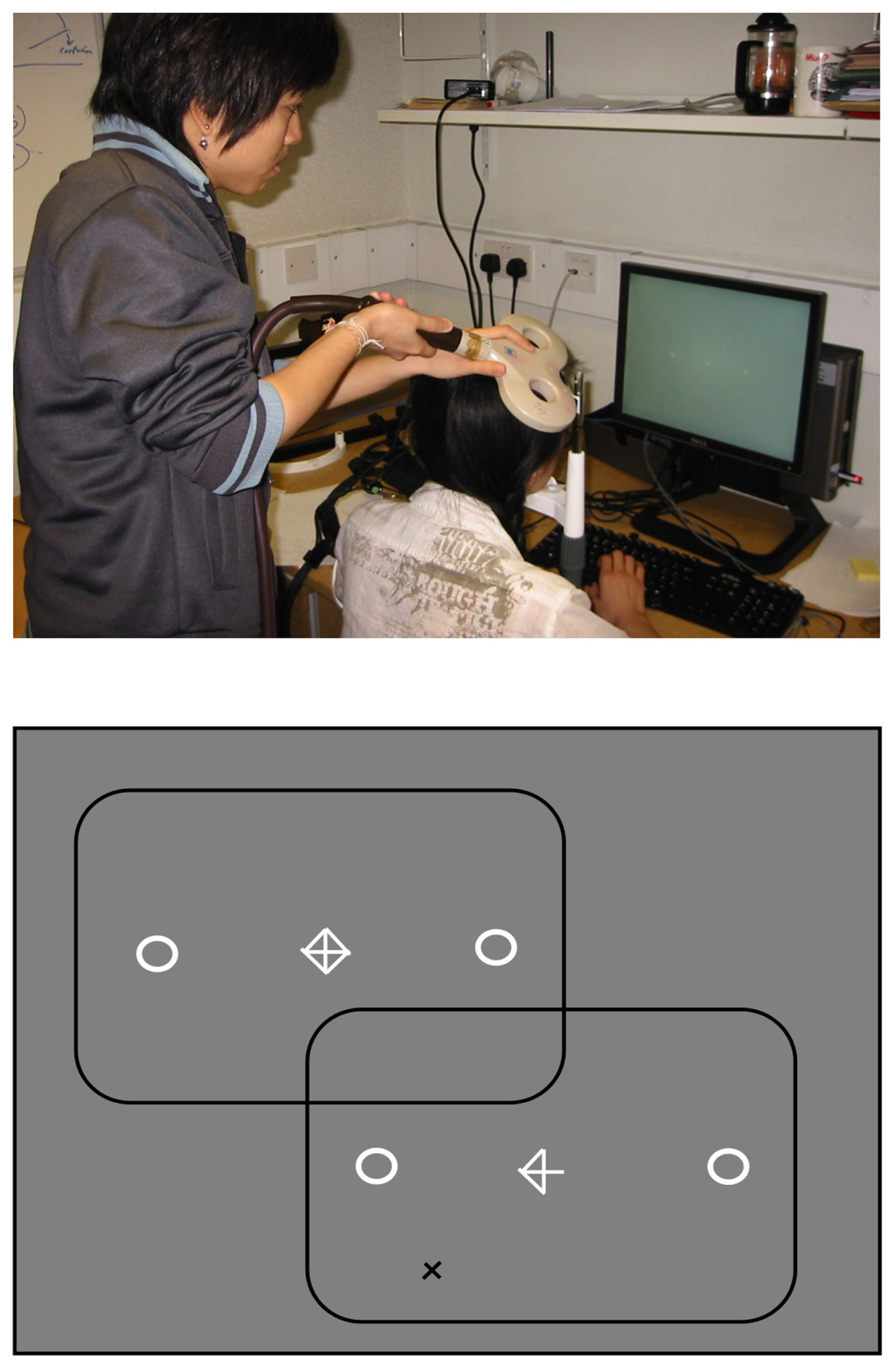
Single-pulse TMS was applied using a MagStim Rapid2 generator using the set-up shown in Figure 6. The frontal eye field region was localised using previously published methods (Muri, Hess, & Meienberg, 1991; Ro, Cheifet, Ingle, Shoup, & Rafal, 1999; Ro, Farne, & Chang, 2002). In brief, the right hemisphere motor cortex hand area was localised in each participant by exploring laterally from the vertex until the optimal scalp position to induce twitches in the left thumb and index finger was found. This location was marked on the scalp and used as a landmark. The coil was then moved 2.5 cm anterior to this landmark to locate the FEF region (Leff, Scott, Rothwell, & Wise, 2001; Muri, Hess, & Meienberg, 1991; O’Shea, Muggleton, Cowey, & Walsh, 2006; Ro, Cheifet, Ingle, Shoup, & Rafal, 1999; Ro, Farne, & Chang, 2002). The level of TMS stimulation was set above the participants’ motor threshold (which varied from 35-60% between participants). In order to control for non-specific effects of TMS, such as the audible ‘click’ and scalp sensation, stimulation was also applied to a control location (vertex) in separate blocks of trials.
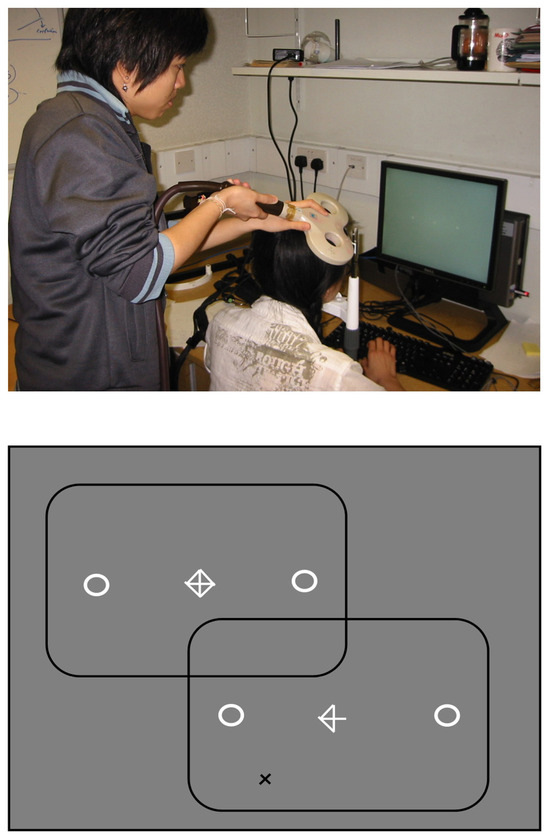
Figure 6.
Eye-movements were recorded using the Eyelink II camera mounted onto a head/chin rest (shown on the left) while participants made horizontal saccades to visual marker boxes, in the direction indicated by a central arrow-cue (shown on the right). On two-thirds of trials distractors appeared at 45 deg from horizontal, in either the upper or lower field, on the remaining trials saccades were made without a distractor. TMS stimulation was applied 150 ms or 250 ms following the onset of the arrow-cue, used to signal saccade direction, to the right frontal eye field region, or vertex, in separate blocks of trials.
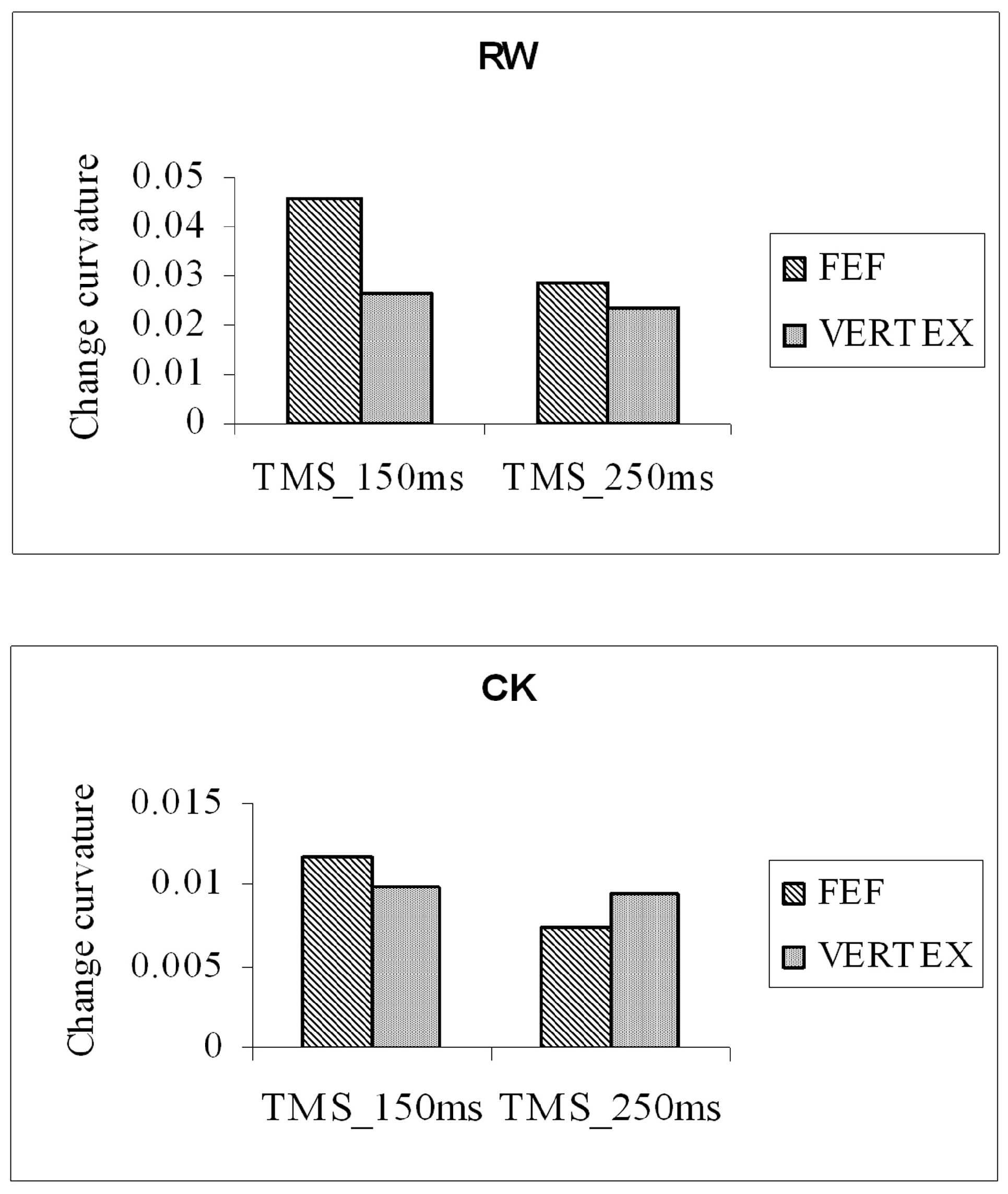
The main measure of interest was the magnitude of the distractor-induced deviation of saccade trajectory observed in the two TMS conditions (FEF and vertex). Figure 7 shows the mean change in saccade deviation (distractor mean deviation – no-distractor mean deviation) for two participants. Positive values on the ordinate indicate the direction of trajectory deviation away from the distractor. Saccade trajectories can be seen to have deviated away from distractors in all conditions. The magnitude of the distractor-related deviation was increased when TMS stimulation was applied to the right-FEF 150 ms after the onset of the gosignal, but not when applied 250ms after cue onset. Findings from a group of eleven participants confirmed this finding. The distractor-induced deviation of saccade trajectory was significantly greater when stimulation was applied to the right FEF 150 ms after distractor and arrow-cue onset than was observed with stimulation of the vertex control location (t(10)=2.35, p<0.05). Stimulation of the FEF 250ms after distractor/cue onset did not enhance the deviation of trajectory (t(10)=0.27, p>0.05). TMS over the right FEF increased the deviation of saccade trajectory away from a distractor, and similar effects were observed for both leftward and rightward saccades.
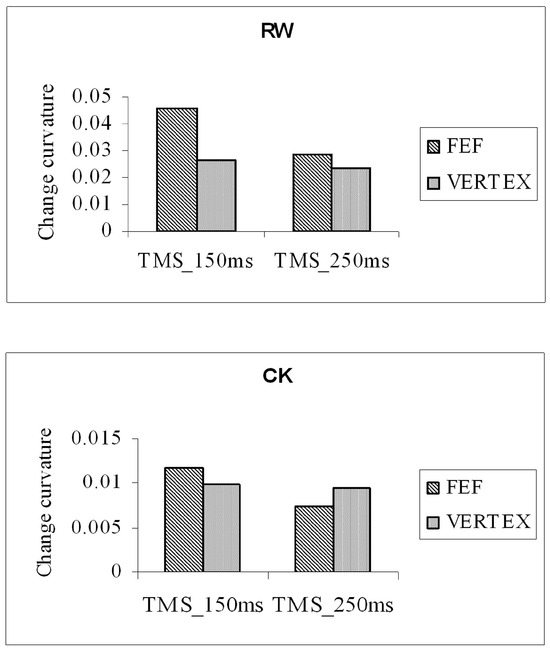
Figure 7.
Mean change in trajectory deviation (distractor mean – no distractor mean) for two participants (RW and CK) collapsed across saccade direction and distractor location. TMS stimulation was applied 150 ms or 250 ms after the onset of the central arrow-cue.
Figure 7 shows that stimulation of the right FEF, 150ms after arrow-cue onset, increased the magnitude of trajectory deviation away from the distractor location. The significant increase in trajectory deviation was consistently observed across participants and could not be explained by an underlying effect of TMS on saccade latency. Our initial prediction was that TMS stimulation applied to the right frontal eye field would disrupt the inhibitory influenced of the FEFs on distractor-related activity in the SC, resulting in a decrease in the magnitude of trajectory deviation. Unexpected consequences of TMS stimulation are not, however, without precedence. For example, Grosbras and Paus (2002, 2003) showed that TMS stimulation of the FEFs facilitated attentional and perceptual processing (Grosbras & Paus, 2002, 2003).
One possible explanation of the increase in trajectory deviation observed following FEF stimulation is that it interferes with the balance between target-related activity and distractor-related inhibition (salience). Stimulation of the FEFs (150 ms after cue onset) may decrease the salience of the target representation, without reducing the distractor inhibition, with the consequence that the population of inhibition makes a greater negative contribution to the computation of initial saccade direction. Some support for this interpretation comes from a recent report that memory-guided saccades (made in the absence of a visual target) show a greater trajectory deviation than saccades made in the presence of a visual target (van Zoest, Van der Stigchel, & Barton, 2007). VanZoest and colleagues suggest that there is a rapid decay of target-related activity in the memory-guided situation that interferes with the overall balance between target and distractor activity. Our results are open to a similar interpretation. Alternatively, FEF stimulation could increase the salience of the distractor with the consequence that greater inhibitory processes are required to suppress the more potent distractor (Tipper, Howard, & Houghton, 2000; Tipper, Howard, & Paul, 2001). The increase in inhibition would increase the deviation of trajectory away from the distractor. Indirect support for a role of distractor salience in trajectory deviation comes from Ludwig & Gilchrist’s (2003) finding that deviation away from a distractor increases when the distractor shares visual properties with the saccade target (Ludwig & Gilchrist, 2003). Although these explanations are speculative these findings provide further support for a role of the FEFs in the distractor-related deviation of saccade trajectory (McPeek, 2006).
Summary
This selective review has described a number of experimental studies of the effects of distractors on the trajectories of saccades. Together this work has developed our understanding of the processes underlying saccade generation. The distractor-related modulation of saccade trajectories has been taken as a proxy measure of the state of the saccadic system at the time the saccade is initiated (McSorley, Haggard, & Walker, 2006). Studies of activity of single neurons in the superior colliculus and frontal eye fields have revealed the neurophysiological substrate of such effects (McPeek, 2006; McPeek, Han, & Keller, 2003). In common with others studying trajectory effects our studies have largely been concerned with the initial deviation of saccade direction, rather than the processes involved with curving the saccade back on target, which are thought to involve the cerebellum rather than colliculus (Quaia, Lefévre, & Optican, 1999). It remains for future studies to investigate the nature of these two processes and to incorporate them into models of saccade generation.
Acknowledgments
Much of the work described in this paper has been performed in collaboration with Patrick Haggard at UCL. The TMS experiments were performed as part of a sabbatical spent at the Institute of Cognitive Neuroscience (UCL) and I am grateful to Vincent Walsh and Neil Muggleton for their help in combining TMS with eye movement recording. The TMS experiment was performed by a graduate student - Puncharat Techawachirakul and we would like to thank her for her assistance.
References
- Aizawa, H., and R. H. Wurtz. 1998. Reversible inactivation of monkey superior colliculus. I. Curvature of saccadic trajectory. Journal of Neurophysiology 79: 2082–2096. [Google Scholar]
- Collewijin, H., C. J. Erkelens, and R. M. Steinman. 1988. Binocular co-ordination of human vertical saccadic eye movements. Journal of Physiology 404: 183–197. [Google Scholar]
- Doyle, M., and R. Walker. 2001. Curved saccade trajectories: Voluntary and reflexive saccades curve away from irrelevant distractors. Experimental Brain Research 139: 333–344. [Google Scholar]
- Doyle, M., and R. Walker. 2002. Multisensory interactions in saccade target selection: curved saccade trajectories. Experimental Brain Research 142: 116–130. [Google Scholar]
- Godijn, R., and J. Theeuwes. 2004. The relationship between inhibition of return and saccade trajectory deviations. Journal of Experimental Psychology: Human Perception and Performance 30: 538–554. [Google Scholar]
- Groh, J. M., and D. L. Sparks. 1996a. Saccades to somatosensory targets I. Behavioral Characteristics. Journal of Neurophysiology 75, 1: 412–427. [Google Scholar] [PubMed]
- Groh, J. M., and D. L. Sparks. 1996b. Saccades to somatosensory targets II. Motor convergence in primate superior colliculus. Journal of Neurophysiology 75, 1: 428–438. [Google Scholar] [PubMed]
- Grosbras, M. H., and T. Paus. 2002. Transcranial magnetic stimulation of the human frontal eye field: Effects on visual perception and attention. Journal of Cognitive Neuroscience 14, 7: 1109–1120. [Google Scholar]
- Grosbras, M. H., and T. Paus. 2003. Transcranial magnetic stimulation of the human frontal eye field facilitates visual awareness. European Journal of Neuroscience 18, 11: 3121–3126. [Google Scholar]
- Kapoula, Z., M. Vernet, Q. Yang, and M. P. Bucci. 2008. Binocular coordination of saccades: development, aging and cerebral substrates. Journal of Eye Movement Research 2, 3: 1–19. [Google Scholar]
- Lee, C., W. H. Rohrer, and D. L. Sparks. 1988. Population coding of saccadic eye movements by neurons in the superior colliculus. Nature 332, March: 357–360. [Google Scholar] [PubMed]
- Leff, A. P., S. K. Scott, J. C. Rothwell, and R. J. S. Wise. 2001. The planning and guiding of reading saccades: a repetitive transcranial magnetic stimulation study. Cerebral Cortex 11, 10: 918–923. [Google Scholar] [PubMed]
- Ludwig, C. J. H., and I. D. Gilchrist. 2003. Target similarity affects saccade curvature away from irrelevant onsets. Experimental Brain Research 152: 60–69. [Google Scholar]
- Ludwig, C. J. H., and I. G. Gilchrist. 2002. Measuring saccade curvature: A curve fitting approach. Bevavior Research Methods, Instruments and Computers 34, 4: 618–624. [Google Scholar]
- McIlwain, J. T. 1991. Distributed spatial coding in the superior colliculus: a review. Visual Neuroscience 6: 3–13. [Google Scholar] [CrossRef]
- McPeek, R. M. 2006. Incomplete suppression of distractor-related activity in the frontal eye field results in curved saccades. Journal of Neurophysiology 96, 5: 2699–2711. [Google Scholar]
- McPeek, R. M., J. H. Han, and E. L. Keller. 2003. Competition between saccade goals in the superior colliculus produces saccade curvature. Journal of Neurophysiology 89, 5: 2577–2590. [Google Scholar]
- McPeek, R. M., and E. L. Keller. 2001. Short-term priming, concurrent processing, and saccade curvature during a target selection task in the monkey. Vision Research 41: 785–800. [Google Scholar]
- McSorley, E., P. Haggard, and R. Walker. 2004. Distractor modulation of saccade trajectories: spatial separation and symmetry effects. Experimental Brain Research 155: 320–333. [Google Scholar]
- McSorley, E., P. Haggard, and R. Walker. 2005. Spatial and temporal aspects of oculomotor inhibition as revealed by saccade trajectories. Vision Research 48: 2492–2499. [Google Scholar]
- McSorley, E., P. Haggard, and R. Walker. 2006. Timecourse of oculomotor inhibition revealed by saccade trajectory modulation. Journal of Neurophysiology 96, 3: 1420–1424. [Google Scholar] [PubMed]
- Munoz, D. P., and R. H. Wurtz. 1995. Saccade-related activity in monkey superior colliculus 2. Spread of activity during saccades. Journal of Neurophysiology 73, 6: 2334–2348. [Google Scholar] [PubMed]
- Muri, R. M., C. W. Hess, and O. Meienberg. 1991. Transcranial Stimulation of the Human Frontal Eye Field by Magnetic Pulses. Experimental Brain Research 86, 1: 219–223. [Google Scholar]
- Nummenmaa, L., and J. K. Hietanen. 2006. Gaze distractors influence saccadic curvature: Evidence for the role of the oculomotor system in gaze-cued orienting. Vision Research 46, 21: 3674–3680. [Google Scholar]
- O’Shea, J., N. G. Muggleton, A. Cowey, and V. Walsh. 2006. On the roles of the human frontal eye fields and parietal cortex in visual search. Visual Cognition 14: 934–957. [Google Scholar] [CrossRef]
- O’Shea, J., and V. Walsh. 2007. Transcranial magnetic stimulation. Current Biology 17, 6: R196–R199. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Port, N. L., and R. H. Wurtz. 2003. Sequential activity of simultaneously recorded neurons in the superior colliculus during curved saccades. Journal of Neurophysiology 90: 1887–1903. [Google Scholar]
- Quaia, C., H. Aizawa, L. M. Optican, and R. H. Wurtz. 1998. Reversible inactivation of monkey superior colliculus. II. maps of saccadic deficits. Journal of Neurophysiology 79, 4: 2097–2110. [Google Scholar]
- Quaia, C., P. Lefévre, and L. M. Optican. 1999. Model of the control of saccades by superior colliculus and cerebellum. Journal of Neurophysiology 82: 999–1018. [Google Scholar] [CrossRef]
- Rizzolatti, G., L. Riggio, and B. M. Sheliga. 1994. Edited by C. Umiltà and M. Moscovitch. Space and selective attention. In Attention and performance XV. MIT press: pp. 231–265. [Google Scholar]
- Ro, T., S. Cheifet, H. Ingle, R. Shoup, and R. Rafal. 1999. Localization of the human frontal eye fields and motor hand area with transcranial magnetic stimulation and magnetic resonance imaging. Neuropsychologia 37, 2: 225–231. [Google Scholar]
- Ro, T., A. Farne, and E. Chang. 2002. Locating the human frontal eye fields with transcranial magnetic stimulation. Journal of Clinical and Experimental Neuropsychology 24, 7: 930–940. [Google Scholar] [PubMed]
- Saslow, M. G. 1967. Effects of components of displacement-step stimuli upon latency for saccadic eye movement. Journal of the Optical Society of America 57, 8: 1024–1029. [Google Scholar]
- Schlag-Rey, M., J. Schlag, and P. Dassonville. 1992. How the frontal eye field can impose a saccade goal on superior colliculus neurons. Journal of Neurophysiology 67, 4: 1003–1005. [Google Scholar]
- Sheliga, B. M., L. Riggio, L. Craighero, and G. Rizzolatti. 1995. Spatial attention-determined modifications in saccade trajectories. NeuroReport 6: 585–588. [Google Scholar]
- Sheliga, B. M., L. Riggio, and G. Rizzolatti. 1994. Orienting of attention and eye movements. Experimental Brain Research 98: 507–522. [Google Scholar] [PubMed]
- Sheliga, B. M., L. Riggio, and G. Rizzolatti. 1995. Spatial attention and eye movements. Experimental Brain Research 105: 261–275. [Google Scholar] [PubMed]
- Sparks, D. L., and R. Hartwich-Young. 1989. Edited by R. H. Wurtz and M. E. Goldberg. The deep layers of the superior colliculus. In The Neurobiology of Saccadic Eye Movements. Elsevier Science Publishers B.V.: pp. 213–255. [Google Scholar]
- Tipper, S. P., L. A. Howard, and G. Houghton. 2000. Edited by S. Monsell and J. Driver. Behavioural consequences of selection from neural population codes. In Control of cognitive processes: Attention and Performance XVIII. MIT press. [Google Scholar]
- Tipper, S. P., L. A. Howard, and M. A. Paul. 2001. Reaching affects saccade trajectories. Experimental Brain Research 136: 241–249. [Google Scholar]
- Van der Stigchel, S., M. Meeter, and J. Theeuwes. 2006. Eye movement trajectories and what they tell us. Neuroscience & Biobehavioral Reviews 30, 5: 666–679. [Google Scholar]
- Van der Stigchel, S., M. Meeter, and J. Theeuwes. 2007a. The spatial coding of the inhibition evoked by distractors. Vision Research 47, 2: 210–218. [Google Scholar]
- Van der Stigchel, S., M. Meeter, and J. Theeuwes. 2007b. Top-down influences make saccades deviate away: The case of endogenous cues. Acta Psychologica 125, 3: 279–290. [Google Scholar]
- Van der Stigchel, S., and J. Theeuwes. 2005. Relation between saccade trajectories and spatial distractor locations. Cognitive Brain Research 25, 2: 579–582. [Google Scholar] [PubMed]
- Van der Stigchel, S., and J. Theeuwes. 2006a. Our eyes deviate away from a location where a distractor is expected to appear. Experimental Brain Research 169: 338–349. [Google Scholar] [PubMed]
- Van der Stigchel, S., and J. Theeuwes. 2006b. Relation between saccade trajectories and spatial distractor locations. Cognitive Brain Research 25: 579–582. [Google Scholar]
- van Zoest, W., S. Van der Stigchel, and J. J. S. Barton. 2008. Distractor effects on saccade trajectories: a comparison of prosaccades, antisaccades, and memory-guided saccades. Experimental Brain Research 186: 431–442. [Google Scholar]
- Viviani, P., A. Berthoz, and D. Tracey. 1977. The curvature of oblique saccades. Vision Research 17: 661–664. [Google Scholar]
- Walker, R., R. W. Kentridge, and J. M. Findlay. 1995. Independent contributions of the orienting of attention, fixation offset and bilateral stimulation on human saccadic latency. Experimental Brain Research 103, 2: 294–310. [Google Scholar]
- Walker, R., E. McSorley, and P. Haggard. 2006. The control of saccade trajectories: direction of curvature depends on advanced knowledge of target location. Perception and Psychophysics 68: 129–138. [Google Scholar]
- Walker, R., P. Techawachirakul, and P. Haggard. submtted. Frontal eye field stimulation modulates the balance of salience between target and distractors. Brain Research. [Google Scholar]
- Wurtz, R. H. 2000. Edited by S. Gazzaniga M. Vision for the control of movement. In Cognitive Neuroscience: A reader. Blackwell Pub. Inc.: pp. 341–365. [Google Scholar]
- Yarbus, A. 1967. Eye movements and vision. Plenium Press. [Google Scholar]
Copyright © 2008. This article is licensed under a Creative Commons Attribution 4.0 International License.