Pupillary Response to Moving Stimuli of Different Speeds
Abstract
Introduction
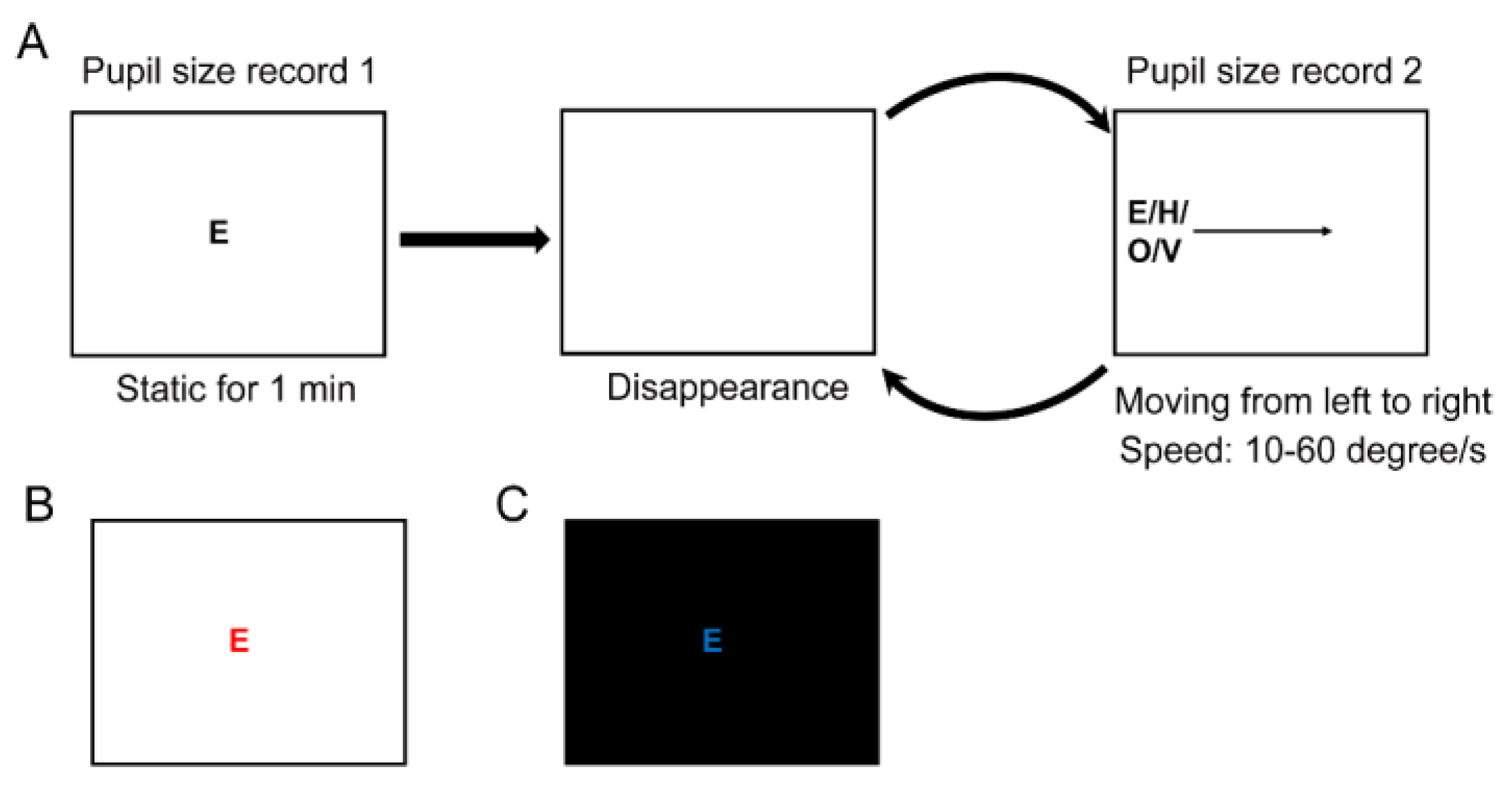
Methods
Experiment 1
Participants
Stimuli Design
Procedure
Experiment 2
Stimuli Design
Procedure
Statistical Analysis
Results
Experiment 1
Experiment 2
Discussion
Ethics and Conflict of Interest
Acknowledgments
References
- Ahnelt, P. K., and H. Kolb. 2000. The mammalian photoreceptor mosaic-adaptive design. Prog Retin Eye Res 19, 6: 711–777. [Google Scholar]
- Alahyane, N., D. C. Brien, B. C. Coe, P. W. Stroman, and D. P. Munoz. 2014. Developmental improvements in voluntary control of behavior: effect of preparation in the fronto-parietal network? Neuroimage 98: 103–117. [Google Scholar] [CrossRef] [PubMed]
- Aston-Jones, G., and J. D. Cohen. 2005. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28: 403–450. [Google Scholar] [CrossRef] [PubMed]
- Barbur, J. L., A. J. Harlow, and A. Sahraie. 1992. Pupillary responses to stimulus structure, colour and movement. Ophthalmic Physiol Opt 12, 2: 137–141. [Google Scholar] [CrossRef]
- Beatty, J., and B. L. Wagoner. 1978. Pupillometric signs of brain activation vary with level of cognitive processing. Science 199, 4334: 1216–1218. [Google Scholar] [CrossRef]
- Braunstein, M. L. 1966. Sensitivity of the observer to transformations of the visual field. J Exp Psychol 72, 5: 683–689. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R. J., H. Zhang, and P. D. Gamlin. 2003. Primate pupillary light reflex: receptive field characteristics of pretectal luminance neurons. J Neurophysiol 89, 6: 3168–3178. [Google Scholar] [CrossRef]
- Connolly, J. D., M. A. Goodale, R. S. Menon, and D. P. Munoz. 2002. Human fMRI evidence for the neural correlates of preparatory set. Nat Neurosci 5, 12: 1345–1352. [Google Scholar] [CrossRef]
- Conway, C. A., B. C. Jones, L. M. DeBruine, A. C. Little, and A. Sahraie. 2008. Transient pupil constrictions to faces are sensitive to orientation and species. J Vis 8, 3: 17.11–11. [Google Scholar] [CrossRef][Green Version]
- Dacey, D. M. 1994. Physiology, morphology and spatial densities of identified ganglion cell types in primate retina. Ciba Found Symp 184: 12–28; discussion 28–34. [Google Scholar]
- Dacey, D. M., B. B. Peterson, F. R. Robinson, and P. D. Gamlin. 2003. Fireworks in the primate retina: in vitro photodynamics reveals diverse LGN-projecting ganglion cell types. Neuron 37, 1: 15–27. [Google Scholar] [CrossRef]
- Dalmaso, M., L. Castelli, and G. Galfano. 2020. Microsaccadic rate and pupil size dynamics in pro/anti-saccade preparation: the impact of intermixed vs. blocked trial administration. Psychol Res 84, 5: 1320–1332. [Google Scholar] [CrossRef]
- de Gee, J. W., T. Knapen, and T. H. Donner. 2014. Decision-related pupil dilation reflects upcoming choice and individual bias. Proc Natl Acad Sci U S A 111, 5: E618–625. [Google Scholar] [CrossRef] [PubMed]
- Dorris, M. C., M. Pare, and D. P. Munoz. 1997. Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J Neurosci 17, 21: 8566–8579. [Google Scholar] [CrossRef] [PubMed]
- Einhauser, W., J. Stout, C. Koch, and O. Carter. 2008. Pupil dilation reflects perceptual selection and predicts subsequent stability in perceptual rivalry. Proc Natl Acad Sci U S A 105, 5: 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Everling, S., and D. P. Munoz. 2000. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J Neurosci 20, 1: 387–400. [Google Scholar] [CrossRef]
- Fink, L. K., B. K. Hurley, J. J. Geng, and P. Janata. 2018. A linear oscillator model predicts dynamic temporal attention and pupillary entrainment to rhythmic patterns. Journal of Eye Movement Research 11, 2. [Google Scholar] [CrossRef]
- Fink, L. K., E. B. Lange, and R. Groner. 2019. The application of eye-tracking in music research. Journal of Eye Movement Research 11, 2. [Google Scholar] [CrossRef]
- Gamlin, P. D. 2006. The pretectum: connections and oculomotor-related roles. Prog Brain Res 151: 379–405. [Google Scholar] [CrossRef]
- Gegenfurtner, K. R., H. M. Mayser, and L. T. Sharpe. 2000. Motion perception at scotopic light levels. J Opt Soc Am A Opt Image Sci Vis 17, 9: 1505–1515. [Google Scholar] [CrossRef]
- Groner, R., M.T. Groner, and K. Koga. 2000. Human motion perception, eye movements, and orientation in visual space: Editorial. Swiss Journal of Psychology 59: 85–88. [Google Scholar] [CrossRef]
- Groner, R., and E. Schollerer. 2005. Perceived velocity of point-light walkers under complex viewing and background conditions. Japanese Psychological Research 47: 204–215. [Google Scholar] [CrossRef]
- Hadjikhani, N., and R. B. Tootell. 2000. Projection of rods and cones within human visual cortex. Hum Brain Mapp 9, 1: 55–63. [Google Scholar] [CrossRef]
- Hasegawa, T., M. Yamashita, T. Suzuki, Y. Hisa, and Y. Wada. 2009. Active linear head motion improves dynamic visual acuity in pursuing a high-speed moving object. Exp Brain Res 194, 4: 505–516. [Google Scholar] [CrossRef]
- Heinrich, S. P. 2007. A primer on motion visual evoked potentials. Doc Ophthalmol 114, 2: 83–105. [Google Scholar] [CrossRef] [PubMed]
- Hupe, J. M., C. Lamirel, and J. Lorenceau. 2009. Pupil dynamics during bistable motion perception. J Vis 9, 7: 10. [Google Scholar] [CrossRef] [PubMed]
- Jainta, S., M. Vernet, Q. Yang, and Z. Kapoula. 2011. The Pupil Reflects Motor Preparation for Saccades Even before the Eye Starts to Move. Front Hum Neurosci 5: 97. [Google Scholar] [CrossRef]
- Jaschinski, W. 2016. Pupil size affects measures of eye position in video eye tracking: implications for recording vergence accuracy. Journal of Eye Movement Research 9, 4. [Google Scholar] [CrossRef]
- Krejtz, K., J. Żurawska, A. Duchowski, and S. Wichary. 2020. Pupillary and microsaccadic responses to cognitive effort and emotional arousal during complex decision making. Journal of Eye Movement Research 13, 5. [Google Scholar] [CrossRef]
- Kübler, T. C., E. Kasneci, and F. Vintila. 2017. Pupil response as an indicator of hazard perception during simulator driving. Journal of Eye Movement Research 10, 4. [Google Scholar] [CrossRef]
- Lappi, O. 2015. Eye Tracking in the Wild: the Good, the Bad and the Ugly. Journal of Eye Movement Research 8, 5. [Google Scholar] [CrossRef]
- Lee, B. B., J. Pokorny, V. C. Smith, P. R. Martin, and A. Valberg. 1990. Luminance and chromatic modulation sensitivity of macaque ganglion cells and human observers. J Opt Soc Am A 7, 12: 2223–2236. [Google Scholar] [CrossRef] [PubMed]
- Lee, B. B., V. C. Smith, J. Pokorny, and J. Kremers. 1997. Rod inputs to macaque ganglion cells. Vision Res 37, 20: 2813–2828. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-I., Y. Makoto, M. Kashino, and S. Furukawa. 2018. Pupillary dilation response reflects surprising moments in music. Journal of Eye Movement Research 11, 2. [Google Scholar] [CrossRef]
- Marc, R. E., B. W. Jones, C. B. Watt, and E. Strettoi. 2003. Neural remodeling in retinal degeneration. Prog Retin Eye Res 22, 5: 607–655. [Google Scholar] [CrossRef]
- Mathot, S., J. B. Melmi, and E. Castet. 2015. Intrasaccadic perception triggers pupillary constriction. Peerj 3: e1150. [Google Scholar] [CrossRef] [PubMed]
- Maunsell, J. H., and D. C. van Essen. 1983. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci 3, 12: 2563–2586. [Google Scholar] [CrossRef]
- Merritt, S. L., H. C. Schnyders, M. Patel, R. C. Basner, and W. O’Neill. 2004. Pupil staging and EEG measurement of sleepiness. Int J Psychophysiol 52, 1: 97–112. [Google Scholar] [CrossRef]
- Munoz, D. P., and S. Everling. 2004. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci 5, 3: 218–228. [Google Scholar] [CrossRef]
- Netser, S., S. Ohayon, and Y. Gutfreund. 2010. Multiple manifestations of microstimulation in the optic tectum: eye movements, pupil dilations, and sensory priming. J Neurophysiol 104, 1: 108–118. [Google Scholar] [CrossRef]
- Purpura, K., E. Kaplan, and R. M. Shapley. 1988. Background light and the contrast gain of primate P and M retinal ganglion cells. Proc Natl Acad Sci U S A 85, 12: 4534–4537. [Google Scholar] [CrossRef] [PubMed]
- Rokszin, A., Z. Markus, G. Braunitzer, A. Berenyi, G. Benedek, and A. Nagy. 2010. Visual pathways serving motion detection in the mammalian brain. Sensors (Basel) 10, 4: 3218–3242. [Google Scholar] [CrossRef] [PubMed]
- Sahraie, A., and J. L. Barbur. 1997. Pupil response triggered by the onset of coherent motion. Graefes Arch Clin Exp Ophthalmol 235, 8: 494–500. [Google Scholar] [CrossRef] [PubMed]
- Schiller, P. H., S. D. True, and J. L. Conway. 1980. Deficits in eye movements following frontal eye-field and superior colliculus ablations. J Neurophysiol 44, 6: 1175–1189. [Google Scholar] [CrossRef]
- Schollerer, E., and R. Groner. 2004. The effect of observer perspective on the perceived velocity of human walkers. Swiss Journal of Psychology 63: 191–199. [Google Scholar] [CrossRef]
- Skottun, B. C. 2016. A few words on differentiating magnoand parvocellular contributions to vision on the basis of temporal frequency. Neurosci Biobehav Rev 71: 756–760. [Google Scholar] [CrossRef]
- Snowden, R. J., R. F. Hess, and S. J. Waugh. 1995. The processing of temporal modulation at different levels of retinal illuminance. Vision Res 35, 6: 775–789. [Google Scholar] [CrossRef]
- Takeuchi, T., and K. K. De Valois. 2000. Velocity discrimination in scotopic vision. Vision Res 40, 15: 2011–2024. [Google Scholar] [CrossRef]
- Ueda, T., Y. Nawa, M. Okamoto, and Y. Hara. 2007. Effect of pupil size on dynamic visual acuity. Percept Mot Skills 104, 1: 267–272. [Google Scholar] [CrossRef]
- Wang, C. A., D. C. Brien, and D. P. Munoz. 2015. Pupil size reveals preparatory processes in the generation of pro-saccades and anti-saccades. Eur J Neurosci 41, 8: 1102–1110. [Google Scholar] [CrossRef]
- Wierda, S. M., H. van Rijn, N. A. Taatgen, and S. Martens. 2012. Pupil dilation deconvolution reveals the dynamics of attention at high temporal resolution. Proc Natl Acad Sci U S A 109, 22: 8456–8460. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, B. J., H. Wilhelm, S. Moro, and J. L. Barbur. 2002. Pupil response components: studies in patients with Parinaud’s syndrome. Brain 125, Pt 10: 2296–2307. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, S., K. Okajima, and T. Takeuchi. 2016. Motion perception under mesopic vision. J Vis 16, 1: 16. [Google Scholar] [CrossRef] [PubMed]

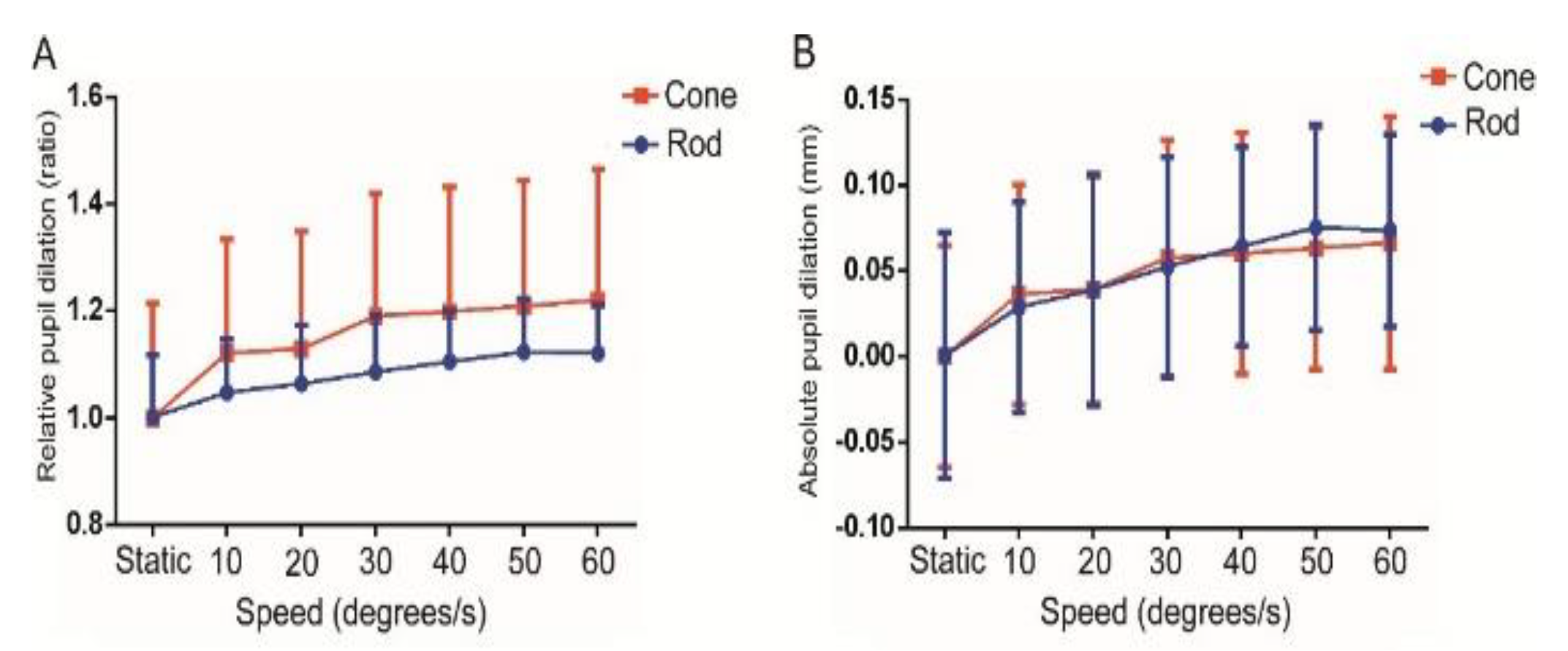
| Speed (deree/s) | PD (mm) Mean (SD) | APD (mm) Mean (SD) | RPD (ratio) Mean (SD) |
|---|---|---|---|
| Static | 3.88 (0.65) | −0.005 (0.65) | 1.0 (0.167) |
| 10 | 4.04 (0.63) | 0.16 (0.63) | 1.04 (0.161) |
| 20 | 4.09 (0.61) | 0.21 (0.61) | 1.05 (0.158) |
| 30 | 4.17 (0.58) | 0.29 (0.58) | 1.08 (0.149) |
| 40 | 4.19 (0.5) | 0.31 (0.5) | 1.08 (0.129) |
| 50 | 4.34 (0.6) | 0.46 (0.6) | 1.12 (0.155) |
| 60 | 4.32 (0.58) | 0.44 (0.58) | 1.11 (0.149) |
| Relative pupil dilation | |||||||
| 10 | 20 | 30 | 40 | 50 | 60 | Speed | (degree/s) |
| 0.010 | 0.046 | <0.001 | 0.010 | <0.001 | <0.001 | 0 | |
| 1.000 | 0.087 | 0.813 | 0.021 | 0.029 | 10 | ||
| 1.000 | 1.000 | 0.240 | 0.326 | 20 | |||
| 1.000 | 0.334 | 1.000 | 30 | ||||
| 1.000 | 1.000 | 40 | |||||
| 1.000 | 50 | ||||||
| Absolute pupil dilation | |||||||
| 10 | 20 | 30 | 40 | 50 | 60 | Speed | (degree/s) |
| 0.010 | 0.047 | <0.001 | 0.010 | <0.001 | <0.001 | 0 | |
| 1.000 | 0.081 | 0.783 | 0.021 | 0.027 | 10 | ||
| 1.000 | 1.000 | 0.236 | 0.314 | 20 | |||
| 1.000 | 0.349 | 1.000 | 30 | ||||
| 1.000 | 1.000 | 40 | |||||
| 1.000 | 50 | ||||||
| Rod paradigm | |||
| Speed (degree/s) | PD (mm) mean (SD) | APD (mm) mean (SD) | RPD (ratio) mean (SD) |
| Static | 6.12 (0.72) | 0.005 (0.72) | 1.0 (0.117) |
| 10 | 6.4 (0.61) | 0.29 (0.61) | 1.05 (0.1) |
| 20 | 6.5 (0.68) | 0.39 (0.68) | 1.063 (0.11) |
| 30 | 6.63 (0.64) | 0.52 (0.64) | 1.086 (0.105) |
| 40 | 6.75 (0.58) | 0.64 (0.58) | 1.105 (0.095) |
| 50 | 6.86 (0.6) | 0.75 (0.6) | 1.123 (0.099) |
| 60 | 6.85 (0.56) | 0.74 (0.56) | 1.12 (0.092) |
| Cone paradigm | |||
| Speed (degree/s) | PD (mm) mean (SD) | APD (mm) mean (SD) | RPD (ratio) mean (SD) |
| Static | 3.02 (0.65) | 0.00 (0.65) | 1.0 (0.214) |
| 10 | 3.39 (0.64) | 0.37 (0.64) | 1.121 (0.212) |
| 20 | 3.41 (0.66) | 0.39 (0.66) | 1.129 (0.219) |
| 30 | 3.6 (0.69) | 0.58 (0.69) | 1.19 (0.228) |
| 40 | 3.62 (0.7) | 0.6 (0.7) | 1.198 (0.233) |
| 50 | 3.65 (0.71) | 0.63 (0.71) | 1.209 (0.234) |
| 60 | 3.68 (0.74) | 0.66 (0.74) | 1.22 (0.245) |
| Relative pupil dilation in rod paradigm (ratio) | |||||||
| 10 | 20 | 30 | 40 | 50 | 60 | Speed | (degree/s) |
| 0.003 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0 | |
| 1.000 | 0.003 | <0.001 | <0.001 | <0.001 | 10 | ||
| 0.037 | <0.001 | <0.001 | 0.003 | 20 | |||
| 0.006 | 0.002 | 0.072 | 30 | ||||
| 1.000 | 1.000 | 40 | |||||
| 1.000 | 50 | ||||||
| Relative pupil dilation in cone paradigm (ratio) | |||||||
| 10 | 20 | 30 | 40 | 50 | 60 | Speed | (degree/s) |
| 0.003 | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 | 0 | |
| 1.000 | 0.120 | 0.075 | 0.028 | 0.016 | 10 | ||
| 0.033 | 0.007 | 0.002 | 0.001 | 20 | |||
| 1.000 | 1.000 | 1.000 | 30 | ||||
| 1.000 | 1.000 | 40 | |||||
| 1.000 | 50 | ||||||
| Absolute pupil dilation in rod paradigm | |||||||
| 10 | 20 | 30 | 40 | 50 | 60 | Speed | (degree/s) |
| 0.003 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0 | |
| 1.000 | 0.003 | <0.001 | <0.001 | <0.001 | 10 | ||
| 0.034 | <0.001 | <0.001 | 0.003 | 20 | |||
| 0.006 | 0.002 | 0.073 | 30 | ||||
| 1.000 | 1.000 | 40 | |||||
| 1.000 | 50 | ||||||
| Absolute pupil dilation in cone paradigm | |||||||
| 10 | 20 | 30 | 40 | 50 | 60 | Speed | (degree/s) |
| 0.003 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0 | |
| 1.000 | 0.003 | <0.001 | <0.001 | <0.001 | 10 | ||
| 0.034 | <0.001 | <0.001 | 0.003 | 20 | |||
| 0.006 | 0.002 | 0.073 | 30 | ||||
| 1.000 | 1.000 | 40 | |||||
| 1.000 | 50 | ||||||
Copyright © 2021. This article is licensed under a Creative Commons Attribution 4.0 International License.
Share and Cite
Wang, Y.; Guo, Y.; Wang, J.; Liu, Z.; Li, X. Pupillary Response to Moving Stimuli of Different Speeds. J. Eye Mov. Res. 2021, 14, 1-12. https://doi.org/10.16910/jemr.14.1.3
Wang Y, Guo Y, Wang J, Liu Z, Li X. Pupillary Response to Moving Stimuli of Different Speeds. Journal of Eye Movement Research. 2021; 14(1):1-12. https://doi.org/10.16910/jemr.14.1.3
Chicago/Turabian StyleWang, Yuexin, Yining Guo, Jiajia Wang, Ziyuan Liu, and Xuemin Li. 2021. "Pupillary Response to Moving Stimuli of Different Speeds" Journal of Eye Movement Research 14, no. 1: 1-12. https://doi.org/10.16910/jemr.14.1.3
APA StyleWang, Y., Guo, Y., Wang, J., Liu, Z., & Li, X. (2021). Pupillary Response to Moving Stimuli of Different Speeds. Journal of Eye Movement Research, 14(1), 1-12. https://doi.org/10.16910/jemr.14.1.3



