Oculomotor Fatigue and Neuropsychological Assessments Mirror Multiple Sclerosis Fatigue
Abstract
Introduction
Methods
Fatigue scoring
Clinical measures
Eye movement recordings
Infrared - Oculogram (IR-OG) - Instruments.
Experimental protocol, stimuli, stress test
- (1)
- calibration,
- (2)
- for 10 sec each sinusoidal pursuit eye movement of 0.7 Hz, predictive square wave jerks of ± 10 deg, and random square wave jerks between 2deg and 30deg.
- (3)
- To induce oculomotor fatigue in our 3 groups, participants had to complete four optokinetic (30°/sec) stress tests of 4 minutes each: alternating between right and left direction.
- (4)
- for 10 sec each sinusoidal pursuit EM of 0.7 Hz, predictive square wave jerks of ± 10 deg, and random square wave jerks between 2deg and 30deg. Total test duration was about 20 minutes.
Phase plane analysis of saccades
TAP – alertness
Statistical analysis
Test-abbriviation
Results
Demographic and clinical characteristics
| MS Fatigue | MS non-Fatigue | P* | |
| (n=28) | (n=21) | ||
| Age | 41.7 +-1.4 | 40.05+-2.16 | n.s. |
| Gender (F/M) | 26 / 2 | 15 / 6 | 0.06 |
| Disease duration | 6.00+-1.0 | 5.62+-1.1 | n.s. |
| EDSS | 3.0+-0.1 | 2.3+-0.3 | 0.06 |
| SDMT | 56.4+-1.6 | 60.1+-3.1 | n.s. |
| 9-HPT | 18.2+-0.5 | 18.2+-0.7 | n.s. |
| pV/Ampl | N | Pre stress test | Post stress test |
| P* | P* | ||
| NOR | 15 | 0.42 | |
| NOR/ MS-Non-Fatigue | 0.29 | 0.05 | |
| MS Non-Fatigue | 21 | 0.1 | |
| MS Non-Fatigue/ MS Fatigue | 0.83 | 0.01 | |
| MS Fatigue | 28 | 0.0005 | |
| NOR/ MS Fatigue | 0.24 | 0.0001 |
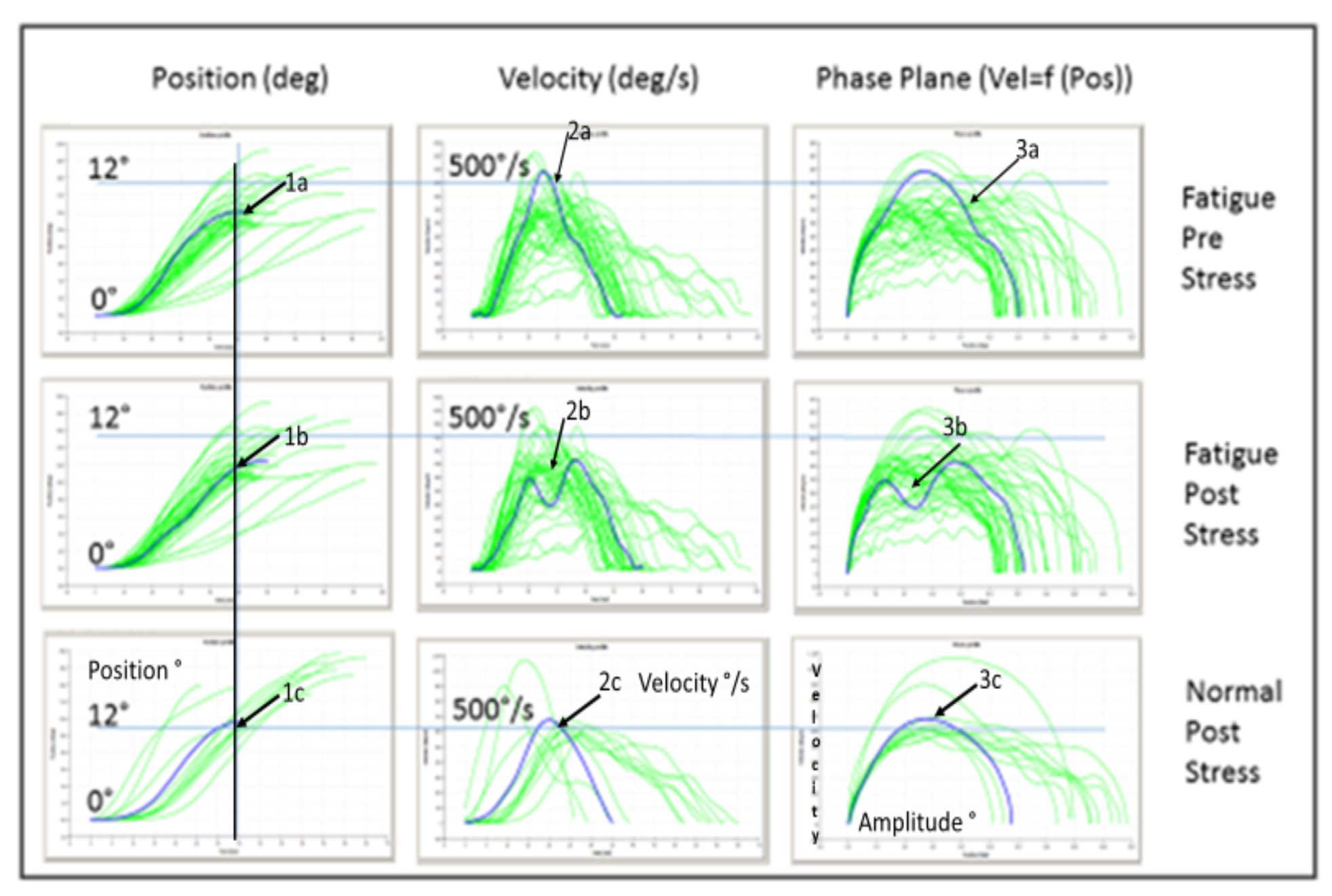

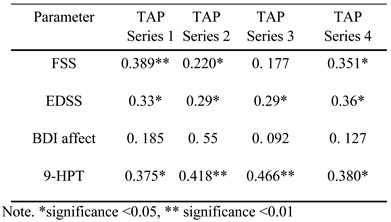
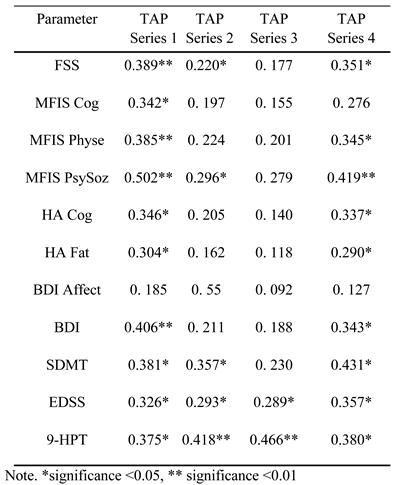
Influence of disability and affective state
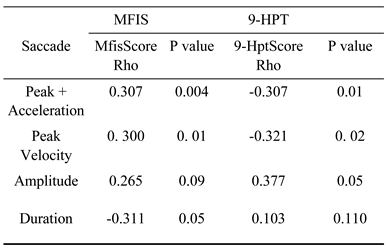
 |
 |
Discussion
Decrease of peak saccadic velocity
Increase of saccadic peak velocity
Pupillary oscillations
Level of fatigue symptoms
Variability of peak velocity
Fatigue and deficits of alertness in sustained attention
Chronotype influence
Fatigue and Depression
Conclusion
Ethics and Conflict of Interest
Appendix A
Inside IR-OG data
The standard deviations of our data were small
Appendix B
References
- Aschoff, J. C. 1968. Veränderungen rascher Blickbewegungen (Saccaden) beim Menschen unter Diazepam (Valium®). Archiv für Psychiatrie und Nervenkrankheiten 211, 4: 325–332. [Google Scholar] [CrossRef] [PubMed]
- Bahill, A. T., M. R. Clark, and L. Stark. 1975a. The main sequence, a tool for studying human eye movements. Mathematical biosciences 24, 3-4: 191–204. [Google Scholar] [CrossRef]
- Bahill, A. T., and L. Stark. 1975b. Overlapping saccades and glissades are produced by fatigue in the saccadic eye movement system. Experimental neurology 48, 1: 95–106. [Google Scholar] [CrossRef] [PubMed]
- Bahill, A. T., A. Brockenbrough, and B. T. Troost. 1981. Variability and development of a normative data base for saccadic eye movements. Investigative ophthalmology & visual science 21, 1: 116–125. [Google Scholar]
- Bakshi, R., Z. A. Shaikh, R. S. Miletich, D. Czarnecki, J. Dmochowski, K. Henschel, and P. R. Kinkel. 2000. Fatigue in multiple sclerosis and its relationship to depression and neurologic disability. Multiple Sclerosis Journal 6, 3: 181–185. [Google Scholar] [CrossRef]
- Bakshi, R. 2003. Fatigue associated with multiple sclerosis: diagnosis, impact and management. Multiple Sclerosis Journal 9, 3: 219–227. [Google Scholar] [CrossRef]
- Bares, M., O. Lungu, T. Liu, T. Waechter, C. M. Gomez, and J. Ashe. 2007. Impaired predictive motor timing in patients with cerebellar disorders. Experimental brain research 180, 2: 355–365. [Google Scholar] [CrossRef]
- Barker, L. M., and M. A. Nussbaum. 2011. The effects of fatigue on performance in simulated nursing work. Ergonomics 54, 9: 815–829. [Google Scholar] [CrossRef]
- Barnes, C.M., and Linn Van Dyne. 2009. I'm tired': Differential effects of physical and emotional fatigue on workload management strategies. Human Relations 62, 1: 59–92. [Google Scholar] [CrossRef]
- Bartlett, F. C. 1943. Ferrier Lecture-Fatigue following highly skilled work. Proceedings of the Royal Society of London. Series B-Biological Sciences 131, 864: 247–257. [Google Scholar] [CrossRef]
- Beck, A., C. Ward, M. Mendelsohn, J. Mock, and J. Erbaugh. 1961. An inventory for measuring depression. Archives of General Psychiatry 4: 561–571. [Google Scholar] [CrossRef]
- Benko, W., M. Ries, E. A. Wiggs, R. O. Brady, R. Schiffmann, and E. J. FitzGibbon. 2011. The saccadic and neurological deficits in type 3 Gaucher disease. PloS one 6, 7: e22410. [Google Scholar] [CrossRef] [PubMed]
- Boksem, M. A., and M. Tops. 2008. Mental fatigue: costs and benefits. Brain Research Reviews 59, 1: 125–139. [Google Scholar] [CrossRef] [PubMed]
- Bol, Y., A. A. Duits, R. M. Hupperts, J. W. Vlaeyen, and F. R. Verhey. 2009. The psychology of fatigue in patients with multiple sclerosis: a review. Journal of Psychosomatic Research 66, 1: 3–11. [Google Scholar] [CrossRef] [PubMed]
- Bower, J. E., P. A. Ganz, and N. Aziz. 2005. Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosomatic Medicine 67, 2: 277–80. [Google Scholar] [CrossRef]
- Buhmann, C., and et al. 2015. Visual attention and saccadic oculomotor control in parkinson's disease. European Neurology 73, 5-6: 283–293. [Google Scholar] [CrossRef]
- Calabrese, M., F. Rinaldi, P. Grossi, I. Mattisi, V. Bernardi, A. Favaretto, and P. Gallo. 2010. Basal ganglia and frontal/parietal cortical atrophy is associated with fatigue in relapsing—remitting multiple sclerosis. Multiple Sclerosis Journal 16, 10: 1220–1228. [Google Scholar] [CrossRef]
- Cazzoli, D., C. A. Antoniades, C. Kennard, T. Nyffeler, C. L. Bassetti, and R. M. Müri. 2014. Eye movements discriminate fatigue due to chronotypical factors and time spent on task–a double dissociation. PloS one 9, 1: e87146. [Google Scholar] [CrossRef]
- Chalder, T., G. Berelowitz, T. Pawlikowska, L. Watts, S. Wessely, D. Wright, and E. P. Wallace. 1993. Development of a fatigue scale. Journal of psychosomatic research 37, 2: 147–153. [Google Scholar] [CrossRef]
- Chaudhuri, A., and P.O Behan. 2000. Fatigue and basalganglia. Journal of the Neurological Sciences 179: 34–42. [Google Scholar] [CrossRef]
- Clark, M. R., and L. Stark. 1974. Control of human eye movements: III. Dynamic characteristics of the eye tracking mechanism. Mathematical Biosciences 20, 3-4: 239–265. [Google Scholar] [CrossRef]
- Cook, G., L. Stark, and B. L. Zuber. 1966. Horizontal eye movements studied with the online computer. Archives of Ophthalmology 76, 4: 589–595. [Google Scholar] [CrossRef]
- Cook, D. B., P. J. O’Connor, G. Lange, and J. Steffener. 2007. Functional neuroimaging correlates of mental fatigue induced by cognition among chronic fatigue syndrome patients and controls. Neuroimage 36, 1: 108–122. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R. A., and M. Fisher. 1989. Amantadine treatment of fatigue associated with multiple sclerosis. Archives of neurology 46, 6: 676–680. [Google Scholar] [CrossRef]
- Colnaghi, S., G. Beltrami, A. Cortese, W. H. Zangemeister, V. Cosi, and M. Versino. 2008. Multiple memory-guided saccades: movement memory improves the accuracy of memory-guided saccades. In Progress in Brain Research. Elsevier: Vol. 171, pp. 425–427. [Google Scholar] [CrossRef]
- Cutter, G. R., M. L. Baier, R. A. Rudick, D. L. Cookfair, J. S. Fischer, J. Petkau, and G. W. Ellison. 1999. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain 122, 5: 871–882. [Google Scholar] [CrossRef] [PubMed]
- Diefendorf, A. R., and R. Dodge. 1908. An experimental study of the ocular reactions of the insane from photographic records. Brain 31, 3: 451–489. [Google Scholar] [CrossRef]
- Di Stasi, L. L., R. Renner, A. Catena, J. J. Cañas, B. M. Velichkovsky, and S. Pannasch. 2012. Towards a driver fatigue test based on the saccadic main sequence: A partial validation by subjective report data. Transportation research part C: emerging technologies 21, 1: 122–133. [Google Scholar] [CrossRef]
- Di Stasi, L. L., R. Renner, P. Staehr, J. R. Helmert, B. M. Velichkovsky, J. J. Cañas, and S. Pannasch. 2010. Saccadic peak velocity sensitivity to variations in mental workload. Aviation, space, and environmental medicine 81, 4: 413–417. [Google Scholar] [CrossRef]
- Di Stasi, L. L., M. B. McCamy, A. Catena, S. L. Macknik, J. J. Canas, and S. Martinez-Conde. 2013a. Microsaccade and drift dynamics reflect mental fatigue. European Journal of Neuroscience 38, 3: 2389–2398. [Google Scholar] [CrossRef]
- Di Stasi, L. L., A. Catena, J. J. Canas, S. L. Macknik, and S. Martinez-Conde. 2013b. Saccadic velocity as an arousal index in naturalistic tasks. Neuroscience & Biobehavioral Reviews 37, 5: 968–975. [Google Scholar] [CrossRef]
- Dodge, R. 1917. The laws of relative fatigue. Psychological Review 24, 2: 89. [Google Scholar] [CrossRef][Green Version]
- Dodge, R., and T. S. Cline. 1901. The angle velocity of eye movements. Psychological Review 8, 2: 145. [Google Scholar] [CrossRef]
- Edelman, J. A., and M. E. Goldberg. 2002. Effect of short-term saccadic adaptation on saccades evoked by electrical stimulation in the primate superior colliculus. Journal of Neurophysiology 87, 4: 1915–1923. [Google Scholar] [CrossRef]
- Egg, R., B. Högl, S. Glatzl, R. Beer, and T. Berger. 2002. Autonomic instability, as measured by pupillary unrest, is not associated with multiple sclerosis fatigue severity. Multiple Sclerosis Journal 8, 3: 256–260. [Google Scholar] [CrossRef] [PubMed]
- Erbaugh, J. 1961. An Inventory for Measuring Depression. Archives of General Psychiatry (4): 561–71. [Google Scholar]
- Guidelines, M. S. C. P. 1998. Fatigue and multiple sclerosis: evidence-based management strategies for fatigue in multiple sclerosis. Paralyzed Veterans of America. [Google Scholar]
- Ferreira, M., P. A. Pereira, M. Parreira, I. Sousa, J. Figueiredo, J. J. Cerqueira, and A. F. Macedo. 2017. Using endogenous saccades to characterize fatigue in multiple sclerosis. Multiple sclerosis and related disorders 14: 16–22. [Google Scholar] [CrossRef][Green Version]
- Fielding, J., T. Kilpatrick, L. Millist, and O. White. 2009. Control of visually guided saccades in multiple sclerosis: disruption to higherorder processes. Neuropsychologia 47, 7: 1647–1653. [Google Scholar] [CrossRef]
- Filippi, M., and M. A. Rocca. 2007. Toward a definition of structural and functional MRI substrates of fatigue in multiple sclerosis. Journal of the Neurological Sciences 263, 1-2: 1–2. [Google Scholar] [CrossRef]
- Finke, C., L. M. Pech, C. Sömmer, J. Schlichting, S. Stricker, M. Endres, and F. Paul. 2012. Dynamics of saccade parameters in multiple sclerosis patients with fatigue. Journal of neurology 259, 12: 2656–2663. [Google Scholar] [CrossRef]
- Fischer, B., and H. Weber. 1993. Express saccades and visual attention. Behavioral and Brain Sciences 16, 3: 553–567. [Google Scholar] [CrossRef]
- Flachenecker, P., and H. Meissner. 2008. Fatigue in multiple sclerosis presenting as acute relapse: subjective and objective assessment. Multiple Sclerosis Journal 14, 2: 274–277. [Google Scholar] [CrossRef]
- Galley, N. 1989. Saccadic eye movement velocity as an indicator of (de)activation: a review and some speculations. Journal of Psychophysiology (3): 229–244. [Google Scholar]
- Gijsman, H. J., J. M. van Gerven, R. J. Verkes, R. C. Schoemaker, M. S. Pieters, E. J. Pennings, and A. F. Cohen. 2002. Saccadic peak velocity and EEG as end-points for a serotonergic challenge test. Human Psychopharmacology: Clinical and Experimental 17, 2: 83–89. [Google Scholar] [CrossRef]
- Gold, S. M., C. Heesen, H. Schulz, U. Guder, A. Mönch, J. Gbadamosi, and K. H. Schulz. 2001. Disease specific quality of life instruments in multiple sclerosis: validation of the Hamburg Quality of Life Questionnaire in Multiple Sclerosis (HAQUAMS). Multiple Sclerosis Journal 7, 2: 119–130. [Google Scholar] [CrossRef] [PubMed]
- Gooding, D. C., and M. A. Basso. 2008. The tell-tale tasks: a review of saccadic research in psychiatric patient populations. Brain and cognition 68, 3: 371–390. [Google Scholar] [CrossRef] [PubMed]
- Grace, P. M., T. Stanford, M. Gentgall, and P. E. Rolan. 2010. Utility of saccadic eye movement analysis as an objective biomarker to detect the sedative interaction between opioids and sleep deprivation in opioidnaive and opioidtolerant populations. Journal of Psychopharmacology 24, 11: 1631–1640. [Google Scholar] [CrossRef]
- Guidelines, M. S. C. P. 1998. Fatigue and multiple sclerosis: evidence-based management strategies for fatigue in multiple sclerosis. Paralyzed Veterans of America. [Google Scholar]
- Harrison, A. M., R. das Nair, and R. Moss-Morris. 2017. Operationalising cognitive fatigability in multiple sclerosis: a Gordian knot that can be cut? Multiple Sclerosis Journal 23, 13: 1682–1696. [Google Scholar] [CrossRef]
- Heesen, C., L. Nawrath, C. Reich, N. Bauer, K. H. Schulz, and S. M. Gold. 2006. Fatigue in multiple sclerosis: an example of cytokine mediated sickness behaviour? Journal of Neurology, Neurosurgery & Psychiatry 77, 1: 34–39. [Google Scholar] [CrossRef]
- Hein, O., and W. H. Zangemeister. 2017. Topology for gaze analyses-Raw data segmentation. Journal of Eye Movement Research 10, 1: 1–25. [Google Scholar] [CrossRef]
- Hirvonen, K., S. Puttonen, K. Gould, J. Korpela, V. F. Koefoed, and K. Müller. 2010. Improving the saccade peak velocity measurement for detecting fatigue. Journal of neuroscience methods 187, 2: 199–206. [Google Scholar] [CrossRef] [PubMed]
- Julian, L. J., L. Vella, T. Vollmer, O. Hadjimichael, and D. C. Mohr. 2008. Employment in multiple sclerosis. Journal of neurology 255, 9: 1354–1360. [Google Scholar] [CrossRef]
- Johansson, S., C. Ytterberg, J. Hillert, L. W. Holmqvist, and L. Von Koch. 2008. A longitudinal study of variations in and predictors of fatigue in multiple sclerosis. Journal of Neurology, Neurosurgery & Psychiatry 79, 4: 454–457. [Google Scholar] [CrossRef]
- Kley, F. 2010. Evaluierung der Testbatterie zur Aufmerksamkeitsprüfung (TAP) als kognitiver Endophänotyp der Schizophrenie. Doctoral dissertation. [Google Scholar] [CrossRef]
- Kluger, B. M., L. B. Krupp, and R. M. Enoka. 2013. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology 80, 4: 409–416. [Google Scholar] [CrossRef] [PubMed]
- Kos, D., E. Kerckhofs, G. Nagels, M. B. D'hooghe, and S. Ilsbroukx. 2008. Origin of fatigue in multiple sclerosis: review of the literature. Neurorehabilitation and neural repair 22, 1: 91–100. [Google Scholar] [CrossRef]
- Kuppuswamy, A. 2017. The fatigue conundrum. Brain 140, 8: 2240–2245. [Google Scholar] [CrossRef]
- Krupp, L. B., D. J. Serafin, and C. Christodoulou. 2010. Multiple sclerosis-associated fatigue. Expert review of neurotherapeutics 10, 9: 1437–1447. [Google Scholar] [CrossRef]
- Krupp, L. B., N. G. LaRocca, J. Muir-Nash, and A. D. Steinberg. 1989. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of neurology 46, 10: 1121–1123. [Google Scholar] [CrossRef]
- Kurtzke, J. F. 1983. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale. Neurology 33, 11: 1444–1444. [Google Scholar] [CrossRef]
- Leigh, J.R., and D.S. Zee. 1999. The neurology of eye movements. F.A. Davis Company. [Google Scholar]
- Liepert, J., D. Mingers, C. Heesen, T. Bäumer, and C. Weiller. 2005. Motor cortex excitability and fatigue in multiple sclerosis: a transcranial magnetic stimulation study. Multiple Sclerosis Journal 11, 3: 316–321. [Google Scholar] [CrossRef]
- Lorist, M. M., M. A. Boksem, and K. R. Ridderinkhof. 2005. Impaired cognitive control and reduced cingulate activity during mental fatigue. Cognitive Brain Research 24, 2: 199–205. [Google Scholar] [CrossRef] [PubMed]
- Maclachlan, L., S. Watson, P. Gallagher, A. Finkelmeyer, L. A. Jason, M. Sunnquist, and J. L. Newton. 2017. Are current chronic fatigue syndrome criteria diagnosing different disease phenotypes? PloS one 12, 10: e0186885. [Google Scholar] [CrossRef]
- Matta, M., R. J. Leigh, M. Pugliatti, I. Aiello, and Serra. 2009. Using fast eye movements to study fatigue in multiple sclerosis. Neurology 73, 10: 798–804. [Google Scholar] [CrossRef]
- McDonald, W. I., A. Compston, G. Edan, D. Goodkin, H. P. Hartung, F. D. Lublin, and M. Sandberg-Wollheim. 2001. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society 50, 1: 121–127. [Google Scholar] [CrossRef]
- Miles, W. R. 1929. Horizontal eye movements at the onset of sleep. Psychological Review 36, 2: 122. [Google Scholar] [CrossRef]
- Morrow, S. A., B. Weinstock-Guttman, F. E. Munschauer, D. Hojnacki, and R. H. B. Benedict. 2009. Subjective fatigue is not associated with cognitive impairment in multiple sclerosis: cross-sectional and longitudinal analysis. Multiple Sclerosis Journal 15, 8: 998–1005. [Google Scholar] [CrossRef]
- Munoz, D. P., and S. Everling. 2004. Look away: the anti-saccade task and the voluntary control of eye movement. Nature Reviews Neuroscience 5, 3: 218–228. [Google Scholar] [CrossRef] [PubMed]
- Nagel, M., H. Behrmann, and W. H. Zangemeister. 2008. Disturbance of predictive response initiation of eye and head movements in cerebellar patients. European Neurology 60, 4: 179–185. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, T., T. M. Nelson, and D. Carlson. 1997. Development of fatigue symptoms during simulated driving. Accident Analysis & Prevention 29, 4: 479–488. [Google Scholar] [CrossRef]
- Peng, G. C., D. S. Zee, and L. B. Minor. 2004. Phase-plane analysis of gaze stabilization to high acceleration head thrusts: a continuum across normal subjects and patients with loss of vestibular function. Journal of Neurophysiology 91, 4: 1763–1781. [Google Scholar] [CrossRef]
- Penner, I. K., N. Bechtel, C. Raselli, M. Stöcklin, K. Opwis, L. Kappos, and P. Calabrese. 2007. Fatigue in multiple sclerosis: relation to depression, physical impairment, personality and action control. Multiple Sclerosis Journal 13, 9: 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Posner, M. I., and S. E. Petersen. 1990. The attention system of the human brain. Annual Review of Neuroscience 13, 1: 25–42. [Google Scholar] [CrossRef]
- Prsa, M., P. W. Dicke, and P. Thier. 2010. The absence of eye muscle fatigue indicates that the nervous system compensates for non-motor disturbances of oculomotor function. Journal of Neuroscience 30, 47: 15834–15842. [Google Scholar] [CrossRef]
- Röhr, D. 2015. Okulomotorik-und Aufmerksamkeitsprüfung als objektive Diagnoseverfahren für Fatigue bei Multipler Sklerose. [Doctoral dissertation, University of Hamburg], Staats- und Universitätsbibliothek Hamburg Archive. Available online: https://ediss.sub.uni-hamburg.de/volltexte/2015/7539/pdf/Dissertation.pdf.
- Schleicher, R., N. Galley, S. Briest, and L. Galley. 2008. Blinks and saccades as indicators of fatigue in sleepiness warnings: looking tired? Ergonomics 51, 7: 982–1010. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D., L. A. Abel, L. F. DellOsso, and R. Daroff. 1979. Saccadic velocity characteristics-Intrinsic variability and fatigue. Aviation, Space, and Environmental Medicine 50, 4: 393–395. [Google Scholar]
- Sepulcre, J., J. C. Masdeu, J. Goni, G. Arrondo, N. Vélez de Mendizábal, B. Bejarano, and P. Villoslada. 2009. Fatigue in multiple sclerosis is associated with the disruption of frontal and parietal pathways. Multiple Sclerosis Journal 15, 3: 337–344. [Google Scholar] [CrossRef]
- Serra, A., C. G. Chisari, and M. Matta. 2018. Eye movement abnormalities in multiple sclerosis: pathogenesis, modeling, and treatment. Frontiers in Neurology 9: 31. [Google Scholar] [CrossRef]
- Shen, K. Q., X. P. Li, C. J. Ong, S. Y. Shao, and E. P. Wilder-Smith. 2008. EEG-based mental fatigue measurement using multiclass support vector machines with confidence estimate. Clinical neurophysiology 119, 7: 1524–1533. [Google Scholar] [CrossRef]
- Siegert, R. J., and D. A. Abernethy. 2005. Depression in multiple sclerosis: a review. Journal of Neurology, Neurosurgery & Psychiatry 76, 4: 469–475. [Google Scholar] [CrossRef]
- Smith, A. 1968. The symbol-digit modalities test: a neuropsychological test of learning and other cerebral disorders. Learning Disorders, 83–91. [Google Scholar]
- Sparks, D. L., and L. E. Mays. 1990. Signal transformations required for the generation of saccadic eye movements. Annual Review of Neuroscience 13, 1: 309–336. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D. L. 2002. The brainstem control of saccadic eye movements. Nature Reviews Neuroscience 3, 12: 952–964. [Google Scholar] [CrossRef]
- Spiteri, S., T. Hassa, D. Claros-Salinas, C. Dettmers, and M. A. Schoenfeld. 2019. Neural correlates of effort-dependent and effort-independent cognitive fatigue components in patients with multiple sclerosis. Multiple sclerosis journal 25, 2: 256–266. [Google Scholar] [CrossRef]
- Straube, A., F. R. Robinson, and A. F. Fuchs. 1997. Decrease in saccadic performance after many visually guided saccadic eye movements in monkeys. Investigative ophthalmology & visual science 38, 13: 2810–2816. [Google Scholar]
- Takagi, M., D. S. Zee, and R. J. Tamargo. 1996. Effect of dorsal cerebellar lesions on saccades and pursuit in monkeys. In Society of Neuroscience Abstracts 22: 1458. [Google Scholar]
- Takikawa, Y., R. Kawagoe, H. Itoh, H. Nakahara, and O. Hikosaka. 2002. Modulation of saccadic eye movements by predicted reward outcome. Experimental Brain Research 142, 2: 284–291. [Google Scholar] [CrossRef]
- Tedeschi, G., D. Dinacci, L. Lavorgna, A. Prinster, G. Savettieri, A. Quattrone, and V. Bresciamorra. 2007. Correlation between fatigue and brain atrophy and lesion load in multiple sclerosis patients independent of disability. Journal of the Neurological Sciences 263, 1-2: 15–19. [Google Scholar] [CrossRef]
- Thompson, A. J., C. H. Polman, D. H. Miller, W. I. McDonald, B. Brochet, X. Filippi M Montalban, and J. De Sa. 1997. Primary progressive multiple sclerosis. Brain: a Journal of Neurology 120, 6: 1085–1096. [Google Scholar] [CrossRef]
- Thorndike, E. 1900. Mental fatigue. Psychological Review 7: 466–482. [Google Scholar] [CrossRef]
- Walker, L. A., J. A. Berard, L. I. Berrigan, L. M. Rees, and M. S. Freedman. 2012. Detecting cognitive fatigue in multiple sclerosis: method matters. Journal of the Neurological Sciences 316, 1-2: 86–92. [Google Scholar] [CrossRef]
- Weinges-Evers, N., A. U. Brandt, M. Bock, C. F. Pfueller, J. Dörr, J. Bellmann-Strobl, and F. Zipp. 2010. Correlation of self-assessed fatigue and alertness in multiple sclerosis. Multiple Sclerosis Journal 16, 9: 1134–1140. [Google Scholar] [CrossRef]
- Wilhelm, B., H. Wilhelm, H. Lüdtke, M. Adler, and P. Streicher. 1996. Pupillography for objective vigilance assessment. Methodological problems and possible solutions. Der Ophthalmologe: Zeitschrift der Deutschen Ophthalmologischen Gesellschaft 93, 4: 446. [Google Scholar] [PubMed]
- Williamson, A., D. A. Lombardi, S. Folkard, J. Stutts, T. K. Courtney, and J. L. Connor. 2011. The link between fatigue and safety. Accident Analysis & Prevention 43, 2: 498–515. [Google Scholar] [CrossRef]
- Winograd-Gurvich, C., N. Georgiou-Karistianis, P. B. Fitzgerald, L. Millist, and O. B. White. 2006. Selfpaced and reprogrammed saccades: differences between melancholic and non-melancholic depression. Neuroscience Research 56, 3: 253–260. [Google Scholar] [CrossRef]
- Yaldizli, Ö., S. Glassl, D. Sturm, A. Papadopoulou, A. Gass, B. Tettenborn, and N. Putzki. 2011. Fatigue and progression of corpus callosum atrophy in multiple sclerosis. Journal of neurology 258, 12: 2199–2205. [Google Scholar] [CrossRef] [PubMed]
- Zangemeister, W. H., and L. Stark. 1982. Gaze latency: variable interactions of head and eye latency. Experimental Neurology 75, 2: 389–406. [Google Scholar] [CrossRef] [PubMed]
- Zangemeister, W. H., U. Oechsner, and C. Freksa. 1995. Short-term adaptation of eye movements in patients with visual hemifield defects indicates high level control of human scanpath. Optometry and vision science: official publication of the American Academy of Optometry 72, 7: 467. [Google Scholar] [CrossRef]
- Zangemeister, W. H., C. Buhmann, L. Maintz, and J Hierling. 2009. Influence of STN-stimulation on Parkinson patient's simulator driving. NCM-Neural Control of Movement 14, 42–43. [Google Scholar]
- Zangemeister, W. H., H. S. Stiehl, and C. Freksa, eds. 1996. Visual attention and cognition. In Advances in psychology. Elsevier. [Google Scholar]
- Zifko, U. A. 2004. Management of fatigue in patients with multiple sclerosis. Drugs 64, 12: 1295–1304. [Google Scholar] [CrossRef]
- Zils, E., A. Sprenger, W. Heide, J. Born, and S. Gais. 2005. Differential effects of sleep deprivation on saccadic eye movements. Sleep 28, 9: 1109–1115. [Google Scholar] [CrossRef]
- Zimmermann, P., and B. Fimm. 1994. Testbatterie zur Aufmerksamkeitsprüfung (Version 1.02c). Psytest. [Google Scholar]
| MS Fatigue | MS non-Fatigue | P* | |
| (n=28) | (n=21) | ||
| FSS | 5.60+-0.15 | 1.73 +-0.24 | p< .001 |
| MFIS phys | 23.25+-1.18 | 6.00+-1.28 | p< .001 |
| MFIS cog | 25.68+-1.30 | 6.95+-1.68 | p< .001 |
| MFIS psych | 4.39+-0.35 | 0.86+-0.27 | p< .001 |
| BDI | 14.17+-1.25 | 4.86+-0.96 | p< .001 |
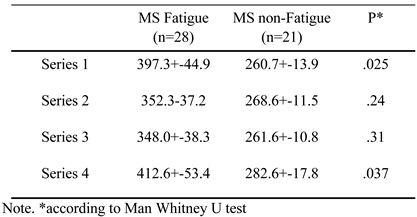 |
© 2020 by the authors. This article is licensed under a Creative Commons Attribution 4.0 International license.
Share and Cite
Zangemeister, W.H.; Heesen, C.; Röhr, D.; Gold, S.M. Oculomotor Fatigue and Neuropsychological Assessments Mirror Multiple Sclerosis Fatigue. J. Eye Mov. Res. 2020, 13, 1-19. https://doi.org/10.16910/jemr.13.4.6
Zangemeister WH, Heesen C, Röhr D, Gold SM. Oculomotor Fatigue and Neuropsychological Assessments Mirror Multiple Sclerosis Fatigue. Journal of Eye Movement Research. 2020; 13(4):1-19. https://doi.org/10.16910/jemr.13.4.6
Chicago/Turabian StyleZangemeister, Wolfgang H., Christof Heesen, Dorit Röhr, and Stefan M. Gold. 2020. "Oculomotor Fatigue and Neuropsychological Assessments Mirror Multiple Sclerosis Fatigue" Journal of Eye Movement Research 13, no. 4: 1-19. https://doi.org/10.16910/jemr.13.4.6
APA StyleZangemeister, W. H., Heesen, C., Röhr, D., & Gold, S. M. (2020). Oculomotor Fatigue and Neuropsychological Assessments Mirror Multiple Sclerosis Fatigue. Journal of Eye Movement Research, 13(4), 1-19. https://doi.org/10.16910/jemr.13.4.6



