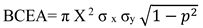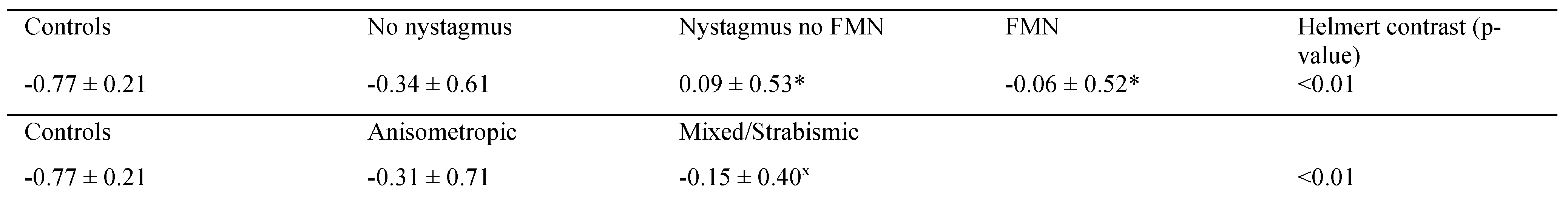Introduction
The postnatal experience is critical to the continuing development of the visual system. If correlated activity between the two eyes is disrupted during critical developmental periods, amblyopia can result, in which one eye has worse visual acuity that cannot be attributed to any structural abnormality and cannot be corrected by corrective lenses alone (
Kiorpes & Daw, 2018; Kiorpes, Kiper, O’Keefe, Cavanaugh, & Movshon, 1998;
Tychsen et al., 2010). Amblyopia is the most common cause of blindness in children (
Abrahamsson & Sjostrand, 1988;
Ciuffreda & Fisher, 1987;
Thomas, 1978). Amblyopia can arise due to a difference in refractive error between the two eyes (anisometropia), eye misalignment (strabismus), or a mixed mechanism (presence of both anisometropia and strabismus). Amblyopia causes changes in organization and function at the level of the primary visual cortex (V1), with under-representation of the ocular dominance columns corresponding to the amblyopic eye and loss of binocular horizontal cells between ocular dominance columns of opposite ocularity in area V1 (Hubel, Wiesel, & LeVay, 1977;
Kiorpes et al., 1998;
Wiesel, 1982;
Wiesel & Hubel, 1963). It is also known that amblyopic patients who have experienced a disruption in binocularity in the first six months of life develop fusion maldevelopment nystagmus (FMN), formerly called latent nystagmus, and nasotemporal (NT) pursuit asymmetry (
Calcutt & Murray, 1998; Ciuffreda, Kenyon, & Stark, 1979;
Gresty et al., 1992;
Kommerell, 1996;
Lang, 2000; Tychsen, Leibole, & Drake, 1996;
Tychsen et al., 2010). Sophisticated psychophysical and visual exploration studies in amblyopia patients have shown that amblyopia impacts the function of both the amblyopic and fellow eyes and is now increasingly being recognized as a disorder of binocular vision, in which both eyes are affected, not just the amblyopic eye (Chen, Otero-Millan, Kumar, Shaikh, & Ghasia, 2018; Kelly, Cheng-Patel, Jost, Wang, & Birch, 2018; Zhou, Liu, Feng, Zhou, & Hess, 2016). Obtaining reliable measurements to quantify the dysfunction of the fellow and amblyopic eye using complex psychophysical tasks can be daunting in children. The advent of remote video-based eye trackers has allowed for reliable and accurate eye movement assessment in children. The purpose of this paper is to systematically assess the fixation eye movement traces in amblyopia patients and categorize amblyopia patients based on the eye movement waveform characteristics obtained under monocular and binocular viewing conditions. Our overarching goal is to identify oculomotor disease biomarkers that are reflective of the severity and type of amblyopia as well as the binocular function impairment seen in amblyopic patients.
During fixation, when the highest level of visual acuity is achieved, the eyes are not motionless but instead show miniature involuntary fixation movements such as micro-saccades or fixational saccades, inter-saccadic drift, and tremor. Microsaccades induce neuronal modulation in cortical area V1 and in the extra-striate cortex. Microsaccades have been shown to play an important role in visual perception by thwarting neural adaptation and preventing visual fading (S. Martinez-Conde, Macknik, & Hubel, 2004; Susana Martinez-Conde, Macknik, Troncoso, & Dyar, 2006;
McCamy et al., 2012; Troncoso, Macknik, & Martinez-Conde, 2008). Thus, the study of fixational eye movements in amblyopia represents a unique opportunity in understanding their role in abnormal visual processing states. A few studies have evaluated fixational saccades in amblyopia patients and have shown that microsaccades are less frequent and have greater amplitude in the amblyopic eye (Shaikh, Otero-Millan, Kumar, & Ghasia, 2016;
Shi et al., 2012). Also, the increase in amplitude of fixational saccades correlates with the severity of amblyopia (
Shaikh et al., 2016). Thus, although small physiological microsaccades are known to reduce the effects of Troxler fading, the increased fixational saccade amplitude seen in amblyopia can have unfavorable effects on monocular visual function. In addition, amblyopic patients without nystagmus have increase in the inter-saccadic drift during visual fixation in both the fellow and amblyopic eye (Chung, Kumar, Li, & Levi, 2015;
Ciuffreda et al., 1979;
Shaikh et al., 2016). Instability of fixation has been reported in amblyopia patients (Gonzalez, Wong, Niechwiej-Szwedo, Tarita-Nistor, & Steinbach, 2012;
Shaikh et al., 2016; Subramanian, Jost, & Birch, 2013). The increased amplitude of fixational saccade, increase inter-saccadic drift and nystagmus can all contribute to increased monocular fixation instability in patients with amblyopia.
Fixational instability has been quantified in most studies to date using the bivariate contour ellipse (BCEA), which is a metric that measures the area over which eye position is dispersed during fixation (
Kelly et al., 2018). BCEA as a measure has several limitations including assumption of normality of the underlying position distributions; thus, the values can be affected by the presence of outliers (
Castet & Crossland, 2012). Because BCEA is a measure of dispersion of eye position, it takes into account both the fast and slow eye movements and does not identify the presence of FMN (
Upadhyaya et al., 2017). FMN is a characteristic oculomotor deficit suggestive of disruption of binocularity in the first six months of life. Thus, FMN serves as a marker that the amblyogenic risk factors were likely present in early infancy. To identify FMN, eye movements traces have to be systematically analyzed for the direction of fast and slow phase under monocular and binocular viewing conditions (
Tychsen et al., 1996;
Tychsen et al., 2010).
The presence of FMN can have implications in terms of monocular and binocular visual function deficits and on treatment effectiveness (
Simonsz & Kommerell, 1989). Thus, to analyze fixation eye movements in amblyopia patients, it is important to evaluate the waveforms for the presence of nystagmus. Since BCEA is a dispersion measure of eye position, it depicts the spread of eye position around the fixation point but does not reflect how fast the eyes are moving. In other words, it does not take into account the eye velocity during fixation, which is equally important in determining the impact of fixation instability on visual functions (
Chen et al., 2018;
Ghasia & Gallagher, 2018; McKee, Levi, & Movshon, 2003). We specifically hypothesize that eye movement parameters, namely fixational saccade amplitude and inter-saccadic drift in patients without nystagmus, and quick and slow phase in patients with nystagmus, will be better reflective of the type and severity of amblyopia and binocular function impairment rather than measures of BCEA alone.
Increased fixation instability is also seen in amblyopic patients during binocular viewing. This increased instability during binocular viewing is thought to reflect abnormalities in the vergence pathways (Economides, Adams, & Horton, 2016; Ghasia, Otero-Millan, & Shaikh, 2017;
Pirdankar & Das, 2016; Richards, Wong, Foeller, Bradley, & Tychsen, 2008;
Subramanian et al., 2013). Vergence BCEA is similar to BCEA in that it involves calculating the standard deviation of eye movements, but uses the difference in eye position between the two eyes at a given time. Thus, similar to monocular BCEA, we hypothesize that vergence BCEA does not provide information about presence of nystagmus and difference of eye velocities between the two eyes, measures that would significantly predict the extent of binocular function impairment in amblyopia. In the presence of normal binocular function, the fixation disparity (the difference between the right and left eye alignment) arising due to the motion of the eyes during fixation stays below a critical threshold thus facilitating binocular fusion (Otero-Millan, Macknik, & Martinez-Conde, 2014). In other words, in healthy subjects the microsaccades occur in the two eyes at the same time with similar amplitudes and direction and have a very small degree of disconjugacy (difference in amplitude between the two eyes) that does not impede binocular fusion (
Shaikh & Ghasia, 2017). We have found that fixational saccade amplitude of the amblyopic eye was greater in patients with absent stereopsis as compared to patients with gross stereopsis, suggesting that patients with amblyopia have impaired binocular coordination (
Shaikh et al., 2016). We have also found that the strabismic patients without amblyopia have increased disconjugacy of fixational saccades (
Ghasia et al., 2017). Less is known about the effects of presence of microstrabismus, binocular functions, and severity of amblyopia on the disconjugacy of fixational saccades in amblyopic patients without nystagmus and differences in the quick phase amplitude between the two eyes in patients with nystagmus.
We will first characterize monocular and binocular fixational instability by analyzing eye movement waveforms of fellow and amblyopic eye and compare the BCEA values across eye movement waveforms. We will then quantify several eye movement parameters, namely fixational saccade amplitude and inter-saccadic drift in amblyopia patients without nystagmus and slow phase velocities in patients with nystagmus. We will also compute the disconjugacy between fixational saccades in patients without nystagmus and disconjugacy of quick phases in patients with nystagmus. We hypothesize that the eye position and eye velocity information obtained from eye movement waveform analysis will be better reflective of fellow eye and amblyopic eye instability rather than using the global measure of BCEA alone. We will also examine the effects of amblyopia type, severity and presence of stereopsis on eye movement dynamics across eye movement waveforms. We hypothesize that the fixation instability will systematically increase per the eye movement waveforms irrespective of the severity of amblyopia. We also hypothesize that eye movement waveforms and dynamic eye movement properties would be a better predictor of amblyopia type, severity and stereopsis function than BCEA alone.
Methods
We recruited 64 subjects (20 controls and 44 amblyopes). The subjects were also grouped based on type of amblyopia (anisometropic = 19, mixed = 18, strabismic = 7) and severity of amblyopia (mild = 21, moderate = 11, severe = 12). There was no significant difference in age between amblyopes and controls (p=0.23, t-test), or between subjects based on presence/absence of nystagmus (p=0.61, one-way ANOVA), severity (p=0.07, one-way ANOVA), or type of amblyopia (p=0.18, one-way ANOVA). Clinical characteristics of each subject are described in supplemental table 1. The subjects did not have any other structural abnormality of the eye or any neurologic disorders. The experimental protocol was approved by the Cleveland Clinic institutional review board and written informed consent was obtained from each participant or parent/legal guardian in accordance with the Declaration of Helsinki.
Eye Movement Recording
Eye movements were measured using the EyeLink 1000 (SR Research, Ontario, Canada), a noninvasive, high-resolution video-oculography tracker that has a spatial resolution of 0.01° and a temporal resolution of 500 Hz. Subjects wore their corrective lenses if applicable for the experiments to achieve their best-corrected vision. Subjects were seated comfortably in a dark room and the subject’s head was stabilized on a chin rest and forehead support, 55cm away from an LCD computer monitor where the visual stimuli were displayed. The resolution of the monitor was 1024x768 and the monitor was 35cm by 27cm. Eye movements were recorded under monocular viewing conditions, both fellow eye viewing (FEV) and amblyopic eye viewing (AEV) and then binocular viewing conditions. In healthy controls, the right eye was labeled as the fellow eye and the left eye was labeled as the amblyopic eye for the purpose of running eye movement analyses. For monocular viewing conditions, an infrared filter was used to cover the eye, which allows infrared rays to measure eye position but blocks the subject from seeing visible light. Monocular calibration and validation of the right and left eye were done under monocular viewing conditions per the manufacturer’s guidelines. Binocular eye positions were measured during both eye viewing and monocular (fellow and amblyopic eye viewing) conditions.
Subjects were instructed to fixate their gaze on a red circular visual target on the screen with a white background (luminance 144 cd/m2) for 45 seconds. The target diameter subtended a 0.5° visual angle.
Data Analysis
The eye position data was then subject to further analysis. All analyses were performed in Matlab (Mathworks, Natick, MA, USA), GraphPad Prism 7 (La Jolla, CA, USA), and SPSS (IBM, Armonk, NY, USA). Blinks and partial blinks were identified and removed. Blinks were defined as portions of raw data where the pupil information was missing, and partial blinks were defined as portions of data where there was a sudden change in pupil size >50 units/sample. In addition, 100 units (200 milliseconds) of data before and after each blink and partial blink was removed to account for periods when the pupil may have been partially occluded by the eyelid. The characteristics of fixation eye movements were analyzed for all study participants.
The fixation stability was quantified by calculating the bivariate contour ellipse (BCEA) using the following equation:
where 2.291 is the Χ
2 value (2 degrees of freedom) corresponding to a probability of 0.68, σ
x and σ
y are the horizontal and vertical standard deviations of eye position respectively, and
p is the product moment correlation of two position components. The resulting BCEA value has been used in previous studies (
Gonzalez et al., 2012; Steinman, Cushman, & Martins, 1982;
Subramanian et al., 2013) as a measure of fixation stability, where a lower BCEA indicates more stable fixation. A log10 transformation was used to normalize the BCEA values. Vergence BCEA values were also calculated for subjects with binocular viewing data. In vergence BCEA, the standard deviation is taken of the disconjugacy, which is the absolute difference between the viewing and non-viewing eye position in the horizontal and vertical direction.
Fixational saccades and quick phases of nystagmus were identified using the unsupervised clustering method described by Otero-Millan et al. (Otero-Millan, Castro, Macknik, & Martinez-Conde, 2014). This method uses clustering to automatically distinguish fixational saccades/quick phases from noise, rather than relying on an arbitrary cutoff. It also produces an index of reliability, which provides a measure of the signal-to-noise ratio in the data. The index of reliability is a number between 0 and 1 and a value greater than 0.75 indicates that error rates are below 0.3 errors per second. Subjects with an index of reliability <0.75 were excluded. Saccade amplitude was defined as the absolute difference between the eye positions at the start and end of a fixational saccade in patients without nystagmus, or quick phase in patients with nystagmus. The horizontal and vertical eye amplitude of the viewing and non-viewing eye were measured and used to calculate a composite amplitude for each eye using the following equation:
The disconjugacy (difference) of composite amplitude between the viewing and non-viewing eye were computed during fellow, amblyopic and binocular viewing conditions.
Drift analysis was also performed using custom-prepared scripts in Matlab. Drifts were defined as epochs between fixational saccades and blinks in patients without nystagmus, or slow phase velocity in patients with nystagmus. We removed 20 milliseconds of data from the start and end of each of these epochs to exclude periods of acceleration and deceleration of the eye during fixational saccades and blinks. Using horizontal and vertical eye velocity data, the composite mean velocity and composite variability of eye position was computed for the viewing eye and non-viewing eye using the equation given above. The composite mean velocity was compared between controls and groups of amblyopes using one-way ANOVA and planned contrasts. Correlation was also calculated between composite mean velocity and the amplitude of the subsequent microsaccade of the corresponding eye to see if there was a relationship between drift velocity and amplitude of the subsequent microsaccade.
A series of ANOVA analyses were carried out to compare BCEA and eye movement parameters among controls and amblyopic subjects, and between amblyopic subjects with different type and nystagmus waveform characteristics. Visual acuity and stereopsis were included as covariates in all these analyses. We first did a mixed ANOVA to compare BCEA values between controls and amblyopic subjects under fellow and amblyopic eye viewing conditions. Next, a two-way between-subjects ANCOVA was done to compare BCEA values between controls and different types of amblyopia and eye movement waveforms of amblyopic patients. BCEA was the dependent variable and type and waveform were independent variables. Controls were included as a control level of waveform and type. Planned comparisons (Helmert contrasts) were used to compare controls versus amblyopic patients grouped per fixation eye movement waveforms and per the type of amblyopia.
Next, we analyzed fixational saccade amplitude, disconjugacy of amplitude of fixational saccade/quick phase of nystagmus, eye position variance and drift velocity. We performed a series of two-way between-subjects ANCOVA with each of these parameters as the dependent variable in turn, and type and waveform as the independent variables separately for the fellow eye and amblyopic eye. We also included control subjects in this analysis as a control level of waveform and type. We used stereopsis and acuity of the corresponding fellow eye and amblyopic eye as covariates. We used Helmert contrasts to compare controls versus amblyopic patients grouped per fixation eye movement waveforms and per the type of amblyopia.
To analyze binocular eye movements, we first performed a linear correlation between vergence BCEA and stereopsis. Next, a two-way between-subjects ANCOVA was performed with vergence BCEA as the dependent variable and type and waveform as the independent variables. Finally, a two-way between-subjects ANCOVA was performed with disconjugacy under binocular viewing conditions as the dependent variable, and type and waveform as the independent variables. Visual acuity and stereopsis were included as covariates in both analyses.
Results
Amblyopic patients are known to have fixation instability, particularly of the amblyopic eye. The presence of nystagmus, the amplitude of fixational saccades and inter-saccadic drifts affect the fixation stability. We have previously shown that amblyopic patients without nystagmus have increased amplitude of fixational saccade, which correlates with the severity of amblyopia (
Shaikh et al., 2016). The fixation instability of the fellow and amblyopic eye would be dependent on various factors including eye movement waveforms, the type and severity of amblyopia and the stereopsis function. We will first characterize the fixation instability in amblyopia patients based on their eye movement waveform.
Characteristics of fixational eye movements in amblyopia patients
We characterized fixational eye movements in amblyopic patients based on their waveform characteristics as those without nystagmus and those with nystagmus. Patients with nystagmus were further evaluated for presence of fusion maldevelopment nystagmus (FMN), defined as having a nasally directed slow phase under monocular viewing with classic reversal in the direction of quick phase towards the uncovered eye. We categorized patients with nystagmus who did not meet the criteria of the signature FMN deficits as having nystagmus without FMN. Patients with nystagmus but no FMN did not have the dissociated vertical deviation frequently seen in FMN patients.
Figure 1 contains representative raw data traces of fixational eye movements obtained in a healthy control (A) and amblyopic subjects with no nystagmus (B), nystagmus no FMN (C), and FMN (D) during both eyes viewing, fellow and amblyopic eye viewing conditions. In controls (A) and patients without nystagmus (B) the microsaccades (black arrows) are separated by periods of inter-saccadic drift (red arrows). In the normal subject (N14) the microsaccades are binocular, conjugate movements with small amplitude (< 1°). In patients without nystagmus (S23), there is an increase in the amplitude of the fixational saccades of the amblyopic eye during amblyopic eye viewing condition (2.2°) compared to controls (right eye viewing: 0.74°). The fixation saccade amplitude of the fellow eye is similar to controls (0.92°). Besides the amplitude, there is an increase in the disconjugacy of fixational saccade, which is worse under amblyopic eye viewing compared to fellow eye viewing condition (1.13° vs. 0.20°). Interestingly, the disconjugacy was also increased under both eyes viewing (0.52°). On the other hand, there is minimal disconjugacy in microsaccades during both eyes, right eye and left eye viewing conditions (0.06°, 0.04°, 0.02° respectively). There is also an increase in the inter-saccadic drift velocity and eye position variance of the amblyopic eye (red trace) during both eyes (1.14°/sec, 0.53°) and amblyopic eye viewing (0.4°/sec, 0.08°) viewing conditions compared to controls (both eyes viewing: 0.06°/sec, 0.01°; right eye viewing: 0.3°/sec, 0.01°; left eye viewing: 0.3°/sec, 0.01°). In patients with nystagmus without FMN (S30), the horizontal and vertical eye velocities of the amblyopic eye are greater during amblyopic eye viewing (0.7°/sec and 0.15°) followed by fellow eye viewing (0.6°/sec and 0.01°) conditions. There is no reversal in the direction of the quick phase of the nystagmus between the fellow and amblyopic eye viewing condition. There is an increase in the disconjugacy of the quick phase, which is worst under amblyopic eye viewing (0.41°), but is still increased under fellow eye (0.14°) and both eyes viewing (0.35°) conditions compared to controls. In patients with FMN (S44), the nystagmus is present under all viewing conditions, but the composite eye velocities and eye position variance during slow phases are greater in the amblyopic eye during AEV (19.05°/sec and 3.25°) compared to the fellow eye during FEV condition (4.3°/sec and 0.06°). During both eye viewing condition, the velocity and eye position variance of the amblyopic eye is greater (1.5°/sec and 0.02°) than the fellow eye viewing (0.85°/sec and 0.02°). The slow phases are directed nasally – with rightward movement during the amblyopic left eye viewing condition and the leftward movement during the fellow right eye viewing condition. There is a reversal in the direction of the quick phase of the nystagmus between the fellow and amblyopic eye viewing condition. There is an increase in the disconjugacy during amblyopic eye viewing (0.25°) and fellow eye viewing (0.31°). The disconjugacy is least during both eyes viewing (0.10°) conditions.
In the subsequent sections, we investigated the effects of type, severity of amblyopia, and binocular function on fixational stability across the eye movement waveforms. We carried out a set of analyses for eye movements obtained under monocular and binocular viewing conditions. We first compared the values obtained using bivariate contour ellipse area (BCEA) analysis between healthy controls and amblyopic subjects. We then examined the correlation of BCEA with amblyopia type and eye movement waveform within amblyopic subjects, while controlling for visual acuity and stereopsis. Next, we repeated this analysis but instead of BCEA we used the composite eye velocity and eye position variance of inter-saccadic drift of controls and patients without nystagmus and composite eye velocity and eye position variance during the slow phases in patients with nystagmus. We also analyzed the composite fixational saccade amplitude and fixational saccade disconjugacy in controls and amblyopic patients without nystagmus. We did a similar analysis of amplitude of quick phase and disconjugacy of amplitude between the two eyes in patients with nystagmus. Lastly, we examined vergence BCEA and disconjugacy as it correlated with amblyopia type and nystagmus waveform in subjects under binocular eye viewing data, while controlling for visual acuity and stereopsis. We used planned contrast analysis to do the following comparisons for waveform characteristics 1) controls versus patients without nystagmus, 2) controls versus patients with nystagmus no FMN, 3) controls versus nystagmus patients with FMN, 4) patients without nystagmus versus patients with nystagmus (with and without FMN), and 5) nystagmus patients without FMN versus nystagmus patients with FMN. Similarly for type of amblyopia, we used planned contrasts for following comparisons 1) controls versus anisometropic amblyopes, 2) controls versus mixed/strabismic, 3) anisometropic versus mixed/strabismic.
Bivariate Contour Ellipse Area (BCEA)
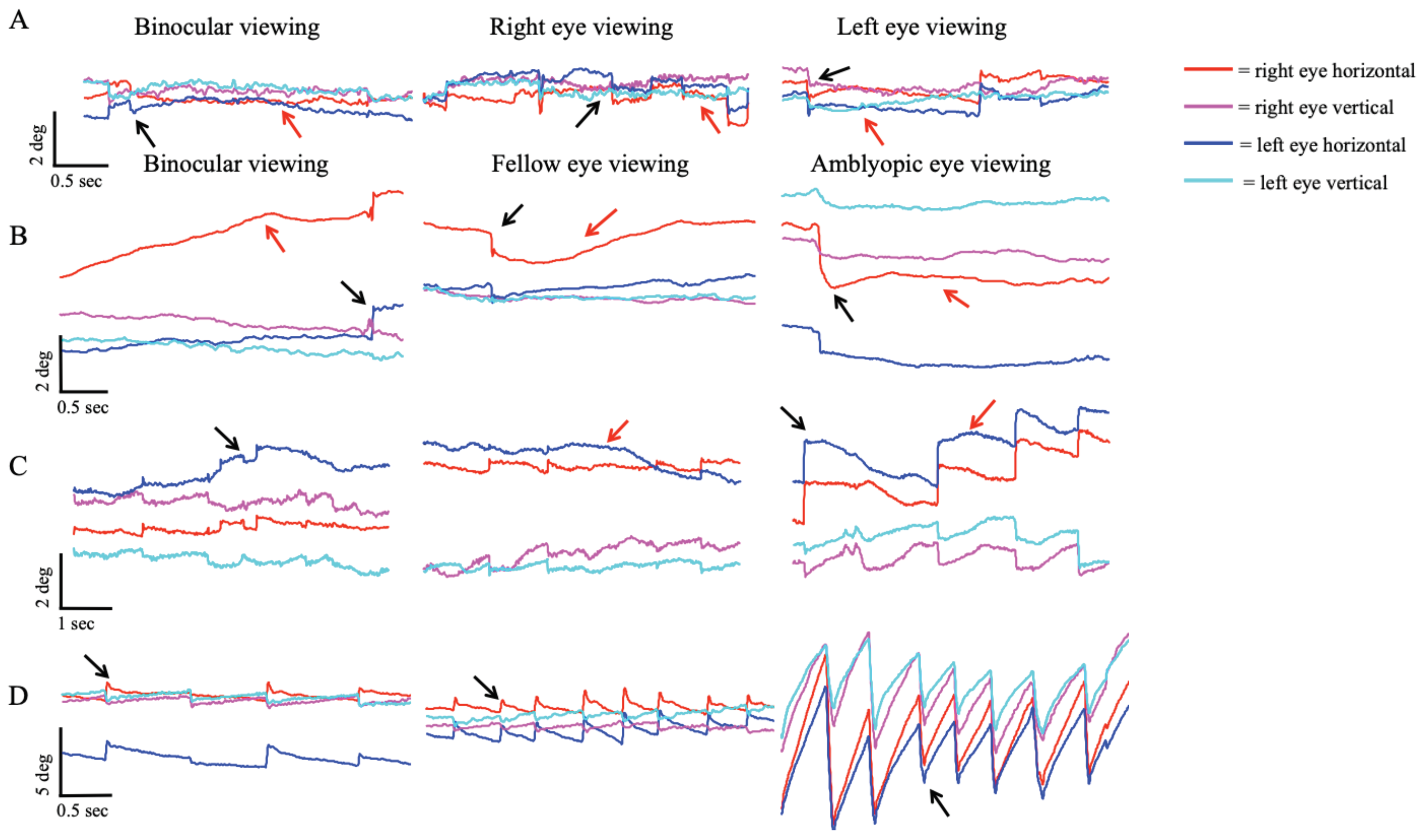
Figure 2 plots the horizontal and vertical eye position of controls and subjects with no nystagmus (severe amblyopia), nystagmus no FMN (moderate amblyopia), and FMN (moderate amblyopia) obtained during a 45-second visual fixation trial in primary position during both eyes viewing, fellow eye viewing, and amblyopic eye viewing conditions. These subjects are the same subjects whose eye movement tracings were depicted in
Figure 1. The log10BCEA values for both eyes are included next to the scatter plots as a quantitative measure of fixation scatter. As demonstrated in these examples, subjects with amblyopia have greater BCEA values with increased scatter of eye positions particularly during amblyopic eye viewing conditions. We first performed a mixed ANOVA comparing BCEA values in healthy controls and all amblyopic subjects (between-subjects factor) under fellow eye viewing (FEV) and amblyopic eye viewing (AEV) conditions (within-subjects factor). As expected, amblyopic patients had significantly greater BCEA than controls under FEV (controls: -0.41 ± 0.39, amblyopes: 0.32 ± 0.59; F=20.7, p<0.01) and AEV conditions (controls: -0.41 ± 0.38, amblyopes: -0.17 ± 0.38; F=20.7, p<0.01). The difference was more pronounced under AEV as indicated by a significant effect between viewing condition and subject group (F=12.9, p<0.01). These findings are in agreement with previous studies that have shown that amblyopic patients have increased BCEA of the amblyopic eye (
Gonzalez et al., 2012;
Shaikh et al., 2016;
Subramanian et al., 2013). We also found increased BCEA values of the amblyopic eye during binocular viewing conditions (controls right eye: -0.55 ± 0.23, fellow eye: -0.20 ± 0.55, amblyopic eye: 0.10 ± 0.60; F=8.6, p<0.01).
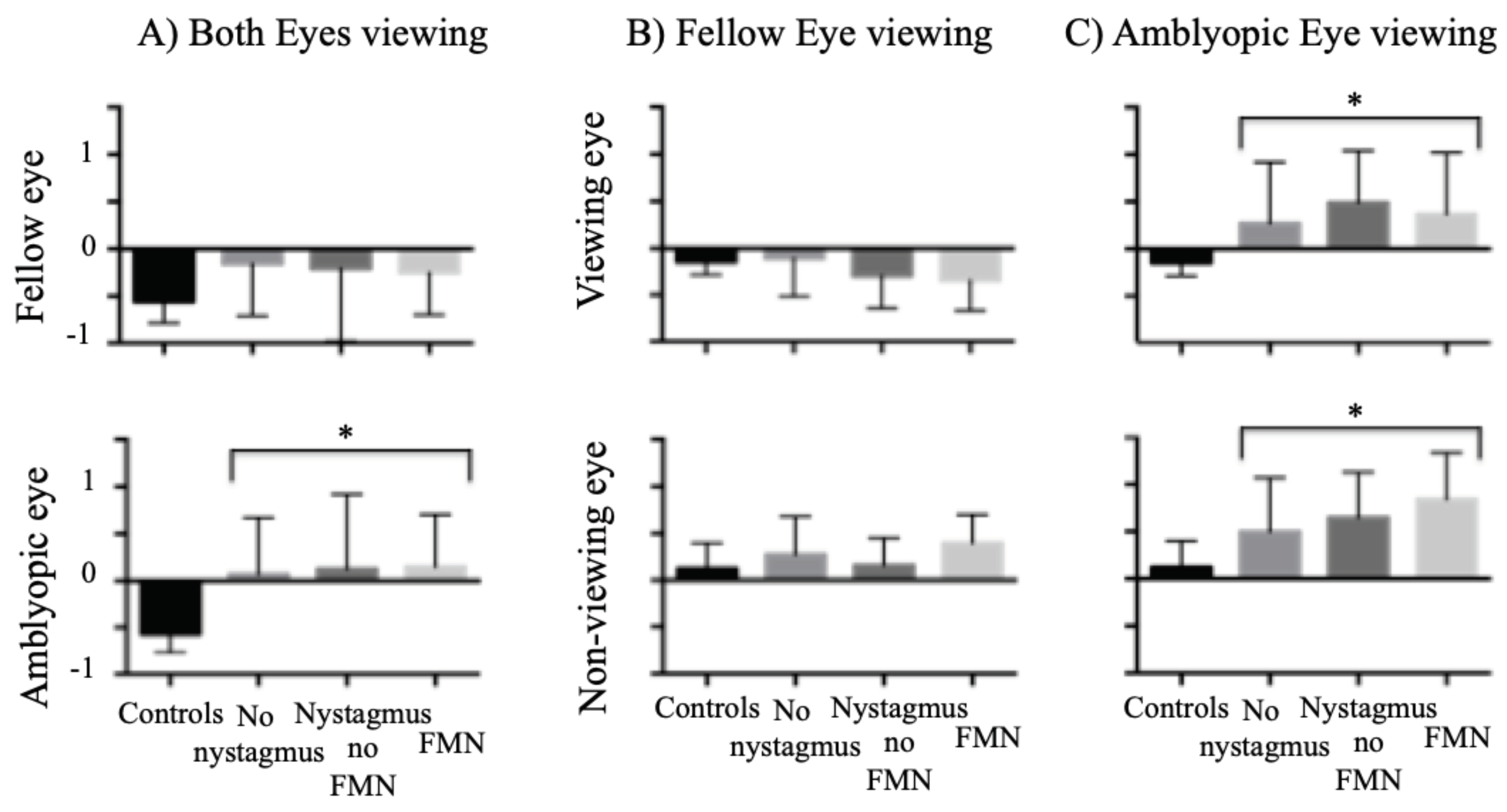
Next we wanted to discern the effects of waveform and type of amblyopia on BCEA. We looked at BCEA values of controls and amblyopes grouped by eye movement waveform during both eyes viewing (BEV), fellow eye viewing (FEV) and amblyopic eye viewing (AEV) conditions (
Figure 3). Under BEV (
Figure 3A), the BCEA values were significantly higher in the amblyopic eye compared to controls (controls: -0.57 ± 0.19, no nystagmus: 0.06 ± 0.60, nystagmus no FMN: 0.12 ±0.79, FMN: 0.15 ± 0.55; F=6.6, p<0.01) with no significant difference in the fellow eye (controls: -0.55 ± 0.23, no nystagmus: -0.15 ± 0.55, nystagmus no FMN: -0.20 ± 0.76, FMN: -0.25 ± 0.44; F=2.1, p=0.10). When comparing the BCEA values between groups under FEV (
Figure 3B), there was no significant difference between controls and amblyopic patients (controls: -0.15 ± 0.23, no nystagmus: -0.11 ± 0.40, nystagmus no FMN: -0.29 ± 0.35, FMN: -0.34 ± 0.32; F=1.6, p=0.18). In patients with nystagmus, the rhythmic to-and-fro nature of the nystagmus results in densely packed eye positions thus producing less scatter. Thus, during FEV conditions, despite having nystagmus, the BCEA values were actually better for patients with FMN than controls. During AEV (
Figure 3C), the BCEA values were higher in amblyopic patients compared to controls (controls: -0.41 ± 0.39, no nystagmus: 0.19 ± 0.59, nystagmus no FMN: 0.46 ± 0.55, FMN: 0.33 ± 0.61; F=9.1; p<0.01). A planned contrast analysis identified differences between controls versus patients without nystagmus, controls versus patients with nystagmus without FMN, controls versus patients with FMN (all Helmert contrasts were significant at p<0.01). No differences were observed between patients without nystagmus versus those with nystagmus and patients without nystagmus without FMN and patients with FMN (p>0.05). Thus, under amblyopic eye viewing similar to fellow eye viewing data, BCEA measures do not reflect the fixation instability occurring due to nystagmus. For the non-viewing eye, significant differences were noted between controls and amblyopia patients across eye movement waveforms under AEV (controls: 0.12 ± 0.27, no nystagmus: 0.49 ± 0.57, nystagmus no FMN: 0.64 ± 0.48, FMN: 0.84 ±0.49; F=5.5, p<0.01). No such differences were seen in the non-viewing eye under FEV (controls: 0.12 ± 0.27, no nystagmus: 0.27 ± 0.40, nystagmus no FMN: 0.15 ± 0.29, FMN: 0.40 ± 0.30; F=1.8, p=0.14).
We also evaluated BCEA values as a function of type of amblyopia during BEV, FEV and AEV conditions.
Table 1 summarizes the BCEA values as a function of type of amblyopia. The amblyopic eye had greater BCEA values compared to controls under BEV irrespective of the type of amblyopia. During BEV, the BCEA values of the fellow eye and amblyopic eye in amblyopic patients were worse than controls irrespective of the type of amblyopia. A planned contrast analysis identified differences between controls and fellow eye of anisometropic amblyopia (p=0.04) and controls and patients with mixed/strabismic amblyopia (p=0.01). Similarly, differences were seen between controls and the amblyopic eye of amblyopes during BEV. No differences were observed between anisometropic versus mixed/strabismic amblyopia in the fellow eye (p=0.78) and amblyopic eye (p=0.46).
During FEV, the BCEA values of the fellow eye were similar in amblyopic patients compared to controls irrespective of the type of amblyopia. The amblyopic eye had greater BCEA values compared to controls under AEV irrespective of the type of amblyopia. A planned contrast analysis identified differences between controls and amblyopic eye of anisometropic amblyopia (p<0.01), as well as controls and amblyopic eye of patients with mixed/strabismic amblyopia (p<0.01). No differences were observed between anisometropic versus mixed/strabismic amblyopia (p=0.11).
Next, we used a two-way between-subjects AN-COVA to compare BCEA values of the amblyopic eye obtained under amblyopic eye viewing condition across different types of amblyopia (anisometropic and mixed/ strabismic) and nystagmus waveforms (no nystagmus, nystagmus no FMN, and FMN). We also included control subjects in this analysis as a control level of waveform and type. Previous studies have shown that BCEA values are affected by visual acuity and stereopsis (
Gonzalez et al., 2012;
Shaikh et al., 2016;
Subramanian et al., 2013). Thus, we included visual acuity and stereopsis as covariates to control for these factors. There was no significant effect of waveform and type on BCEA when we controlled for acuity of amblyopic eye and stereopsis (type: F=2.9, p=0.09; waveform: F=2.0, p=0.14). There was a significant effect of the covariate stereopsis (F=12.8, p=0.01) but not the visual acuity (F=0.8, p=0.77).
Fixation instability has been reported in amblyopic patients under both eyes viewing condition (
Kelly et al., 2018). We further calculated a vergence BCEA (right eye position minus left eye position) that assesses fixational stability between the two eyes obtained under both eyes viewing (BEV) conditions. We examined vergence BCEA across fixation waveforms and amblyopia types (
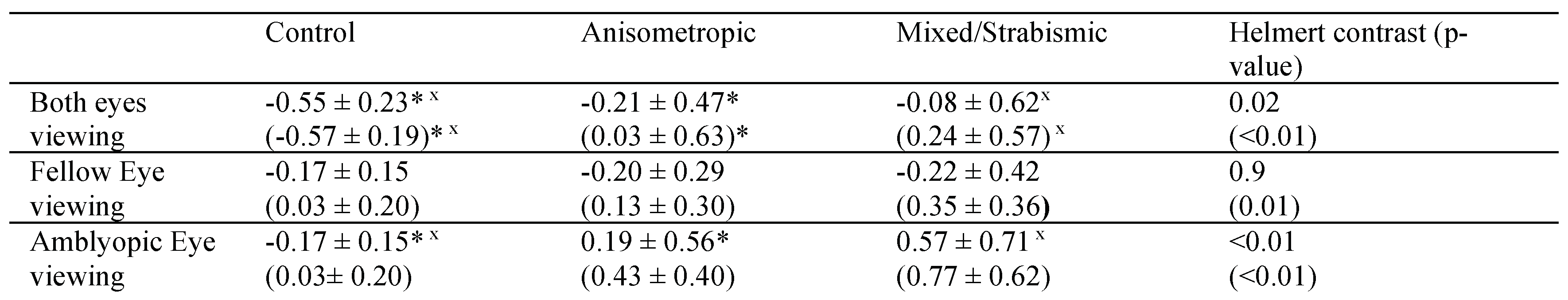
Table 2). BCEA values were greater in amblyopic patients compared to controls. A planned contrast analysis identified differences between controls versus patients without nystagmus, controls versus patients with nystagmus no FMN, and controls versus patients with FMN (all Helmert contrasts were significant at p<0.01). No differences were observed between patients without nystagmus versus those with nystagmus and patients without nystagmus no FMN and patients with FMN. A planned contrast analysis identified differences between controls and anisometropic amblyopia patients (p=0.02), as well as controls and mixed/strabismic amblyopia patients (p<0.01). No differences were observed between anisometropic versus mixed/strabismic amblyopia (p=0.27).
We also investigated the relationship between fixational eye movements by using vergence BCEA and stereopsis. A positive correlation was noted between smaller vergence BCEA suggestive of stable fixation and stereopsis function (Spearman’s rho correlation coefficient=0.64, p<0.01).
We used a two-way between-subjects ANCOVA to compare vergence BCEA values between amblyopic subjects, comparing between types of amblyopia (anisometropic, mixed, and strabismic) and nystagmus waveforms (no nystagmus, nystagmus no FMN, and FMN). We also included control subjects in this analysis as a control level of waveform and type. We included visual acuity and stereopsis as covariates to control for these factors. There was no significant effect of waveform and type on BCEA when we controlled for acuity of amblyopic eye and stereopsis (type: F=0.19, p=0.66; waveform: F=0.44, p=0.65).
Thus, BCEA and vergence BCEA are able to distinguish amblyopic patients from controls. However, the BCEA measures are not able to identify differences between amblyopic patients based on clinical type or fixation eye movement waveforms. This highlights the importance of incorporating dynamic eye movement parameters such as eye velocity, eye position variance, fixational saccade amplitude, and disconjugacy that would better reflect the fixation instability during monocular and binocular viewing conditions.
Both eyes viewing condition
Composite Amplitude
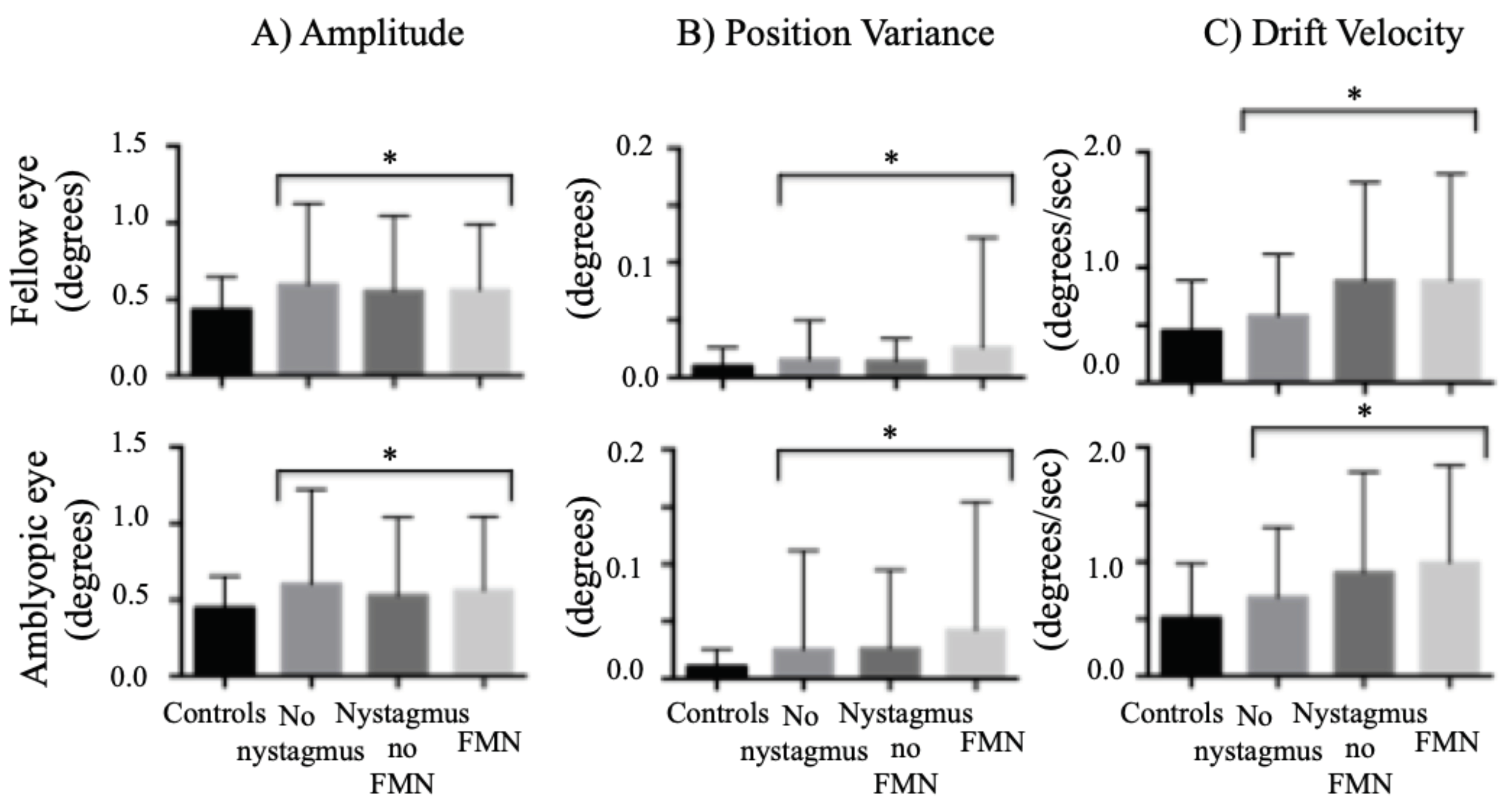
We found an increase in the amplitude of the fixational saccades of both the fellow and amblyopic eye during both eye viewing conditions. We found a similar increase in the amplitude of the quick phase of patients with nystagmus (
Figure 4A). The differences were statistically significant across fixation eye movement waveforms (fellow eye: controls: 0.43° ± 0.21°, no nystagmus: 0.59° ± 0.52°, nystagmus no FMN: 0.55° ± 0.48°, FMN: 0.56° ± 0.42°; F=10.9, p<0.01; amblyopic eye: controls: 0.44° ± 0.20°, no nystagmus: 0.61° ± 0.61°, nystagmus no FMN: 0.52° ± 0.51°, FMN: 0.56° ± 0.48°; F=7.1, p<0.01). A planned contrast analysis identified differences between controls and patients without nystagmus, controls and patients with nystagmus no FMN and control and patients with FMN for fellow and amblyopic eye (Helmert contrasts were significant at p<0.05). No differences were seen between patients without nystagmus and patients with nystagmus, and patients with nystagmus with and without FMN for fellow and amblyopic eye (p>0.05).
We also evaluated composite amplitude as a function of type of amblyopia during both eyes viewing condition. The fellow (controls: 0.43° ± 0.21°, anisometropia: 0.57° ± 0.50°, mixed/strabismic: 0.57° ± 0.47°; p<0.01) and amblyopic eye (controls: 0.44° ± 0.29°, anisometropia: 0.56° ± 0.61°, mixed/strabismic: 0.57° ± 0.52°, p<0.01) had greater amplitude compared to controls irrespective of the type of amblyopia. A planned contrast analysis identified differences between controls and fellow eye of patients with anisometropia (p<0.01) and controls and patients with mixed/strabismic amblyopia (p<0.01). Similarly, differences were seen between controls and amblyopic eye of anisometropic (p<0.01) and mixed/strabismic amblyopia (p<0.01) patients during BEV. No differences were observed between anisometropic versus mixed/strabismic amblyopia in the fellow eye (p=0.95) and amblyopic eye (p=0.43).
A two-way between-subjects ANCOVA was carried out to examine the effects of type and waveform on the amplitude of the fellow and amblyopic eye obtained under both eye viewing. There was a statistically significant main effect of type (F=9.5, p<0.01) and waveform (F=6.3, p<0.01), on amplitude of the fellow eye whilst controlling for visual acuity and stereopsis. In addition, the covariates visual acuity (F=8.4, p<0.01) and stereopsis (F=8.1, p <0.01) did have significant effects on the amplitude of the fellow eye. Similarly, there was a statistically significant main effect of type (F=6.2, p=0.01) and waveform (F=6.6, p<0.01), on amplitude of the amblyopic eye whilst controlling for visual acuity and stereopsis. In addition, the covariates acuity (F=5.9, p=0.01) and stereopsis (F=9.4, p<0.01) did have main effects on the amplitude of the amblyopic eye.
Eye position variance during inter-saccadic drifts and slow phase velocity
We first compared position variance across eye movement waveforms. We found an increase in the eye position variance of the fellow eye of amblyopic patients under both eyes viewing conditions (controls: 0.01° ± 0.01°, no nystagmus: 0.01° ± 0.03°, nystagmus no FMN: 0.01° ± 0.01°, FMN: 0.02° ± 0.09°; F=6.3, p<0.01). A similar increase was seen in the amblyopic eye across all eye movement waveforms (controls: 0.01° ± 0.01°, no nystagmus: 0.02° ± 0.08°, nystagmus no FMN: 0.02° ± 0.06°, FMN: 0.04° ± 0.11°; F=13.8, p<0.01) (
Figure 4B). A planned contrast analysis showed a significant increase in variance in patients without nystagmus compared to controls, patients with nystagmus without FMN compared to controls, patients with FMN compared to controls and FMN patients compared to patients with nystagmus without FMN for both the fellow and amblyopic eye (all Helmert contrasts were significant at p<0.01).
We also evaluated eye position variance as a function of type of amblyopia during both eyes viewing conditions. The fellow eye (controls: 0.01° ± 0.01°, anisometropia: 0.02° ± 0.03°, mixed/strabismic: 0.02° ± 0.07°; F=11.3, p<0.01) and amblyopic eye (controls: 0.01° ± 0.01°, anisometropia: 0.03° ± 0.11°, mixed/strabismic: 0.03° ± 0.08°; F=20.76, p<0.01) had greater eye position variance compared to controls irrespective of the type of amblyopia. A planned contrast analysis identified differences between controls and fellow eye of patients with anisometropia (p<0.01) and controls and patients with mixed/strabismic amblyopia (p<0.01). Similarly, differences were seen between controls and amblyopic eye of anisometropic (p<0.01) and mixed/strabismic amblyopia (p<0.01) patients during BEV. No differences were observed between anisometropic versus mixed/strabismic amblyopia in the fellow eye (p=0.72) and amblyopic eye (p=0.75).
A two-way between-subjects ANCOVA was carried out to examine the effects of type and waveform on the amplitude of the fellow and amblyopic eye obtained under both eye viewing condition while controlling for visual acuity and stereopsis. There was no statistically significant main effect of the type (F=0.27, p=0.6) and waveform (F=0.94, p=0.39) on eye position variance of the fellow eye. On the other hand, for the amblyopic eye there was a statistically significant main effect of the type (F=10.1, p<0.01) and waveform (F=9.5, p<0.01), whilst controlling for visual acuity and stereopsis. The covariate acuity of the amblyopic eye showed a significant effect on eye position variance of the amblyopic eye under both eyes viewing condition (F=8.9, p<0.01).
Eye velocity during inter-saccadic drifts and slow phase velocity
We also found an increase in the inter-saccadic drift and slow phase velocity of both the fellow and amblyopic eye during both eye viewing conditions across eye movement waveforms (
Figure 4C). The differences were statistically significant for both the fellow eye (controls: 0.45°/s ± 0.43°/s, no nystagmus: 0.57°/s ± 0.53°/s, nystagmus no FMN: 0.88°/s ± 0.85°/s, FMN: 0.88°/s ± 0.92°/s; F=30.1, p<0.01) and amblyopic eye (controls: 0.51°/s ± 0.47°/s, no nystagmus: 0.68°/s ± 0.61°/s, nystagmus no FMN: 0.90°/s ± 0.87°/s, FMN: 0.99°/s ± 0.84°/s; F=34.3, p<0.01). A planned contrast analysis identified differences between controls and patients without nystagmus, controls and patients with nystagmus no FMN and control and patients with FMN for fellow and amblyopic eye (Helmert contrasts were significant at p<0.01). The eye velocities were greater in patients with nystagmus compared to patients without nystagmus for fellow and amblyopic eye (p<0.01).
We also evaluated eye velocity as a function of type of amblyopia during both eyes viewing conditions. The fellow (controls: 0.45°/s ± 0.43°/s, anisometropia: 0.80°/s ± 0.84°/s, mixed/strabismic: 0.75°/s ± 0.77°/s; F=39.4, p<0.01) and amblyopic eye (controls: 0.51°/s ± 0.47°/s, anisometropia: 0.80°/s ± 0.85°/s, mixed/strabismic: 0.87°/s ± 0.75°/s; F=46.3, p<0.01) had greater eye velocities compared to controls irrespective of the type of amblyopia. A planned contrast analysis identified differences between controls and fellow eye of patients with anisometropia and controls and patients with mixed/strabismic amblyopia. Similarly, differences were seen between controls and amblyopic eye of anisometropic and mixed/strabismic amblyopia patients during BEV. All Helmert contrasts were significant at p<0.01. No differences were observed between anisometropic versus mixed/strabismic amblyopia in the fellow eye (p=0.43) and amblyopic eye (p=0.37).
A two-way between-subjects ANCOVA was carried out to examine the effects of type and waveform on the eye velocity of the fellow and amblyopic eye obtained under both eye viewing condition while controlling for visual acuity and stereopsis. There was a statistically significant main effect of type (F=13.9, p<0.01) and waveform (F=21.7, p<0.01) on the velocity of the fellow eye. A similar statistically significant main effect of type (F=8.1, p<0.01) and waveform (F=11.0, p<0.01) was seen on the velocity of the amblyopic eye while controlling for visual acuity and stereopsis. In addition, there was a significant effect of covariate stereopsis (F=6.4, p=0.01) but not the visual acuity (F=1.2, p=0.26) on the eye velocity of the amblyopic eye.
Fellow and amblyopic eye viewing conditions
Composite Amplitude
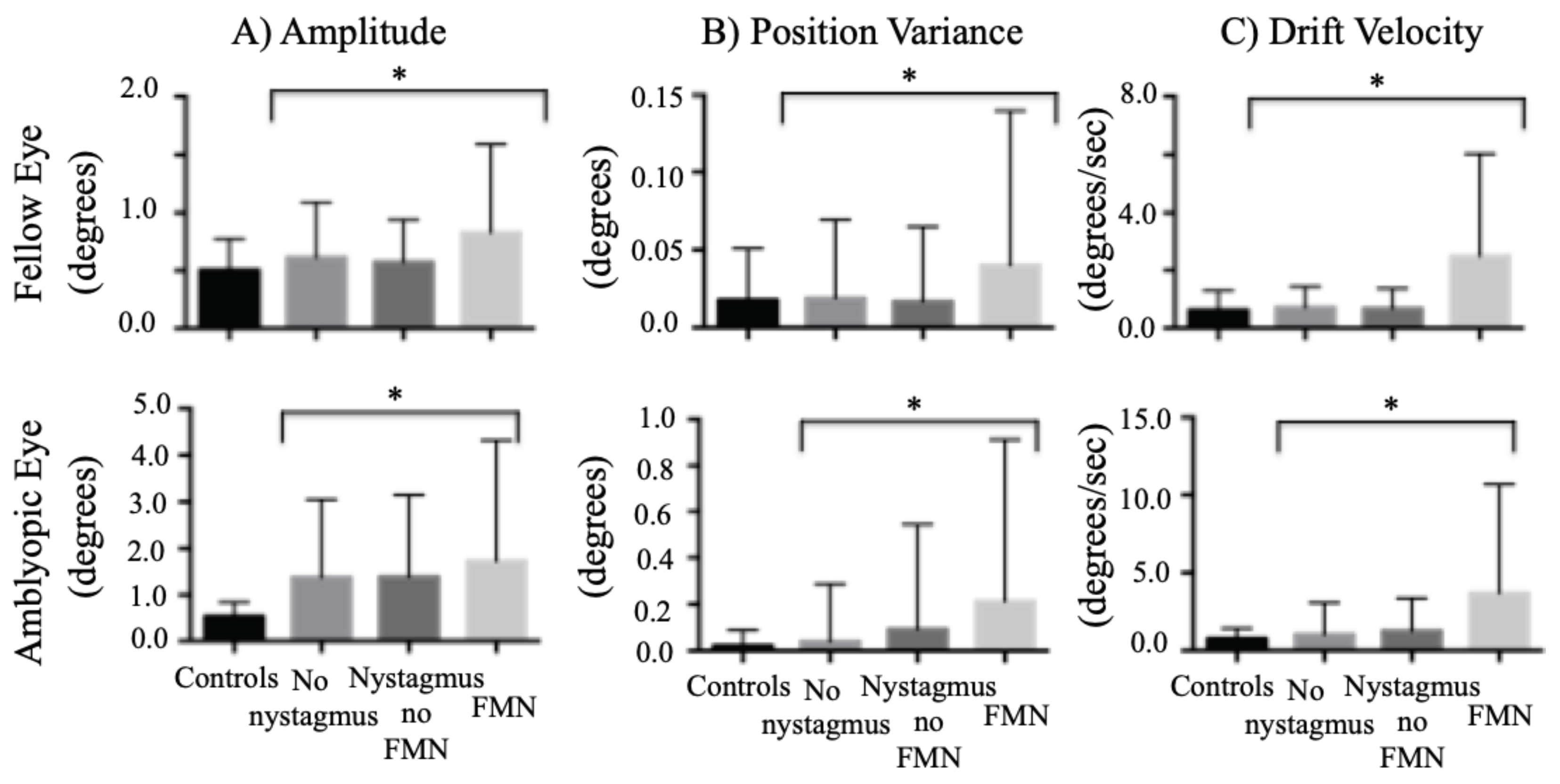
When comparing the composite amplitude between groups of nystagmus waveforms, the amplitude was greater in amblyopic patients during both fellow eye viewing (controls: 0.51° ± 0.27°, no nystagmus: 0.61° ± 0.47°, nystagmus no FMN: 0.57° ± 0.36°, FMN: 0.83° ± 0.75°; F=58.9, p<0.01) and amblyopic eye viewing conditions (controls: 0.54° ± 0.30°, no nystagmus: 1.30° ± 1.60°, nystagmus no FMN: 1.20° ± 1.20°, FMN: 1.70° ± 2.50°; F=25.1, p<0.01) (
Figure 5A). A planned contrast analysis identified differences during both fellow and amblyopic eye viewing conditions between controls versus patients without nystagmus, controls versus patients with nystagmus no FMN, controls versus patients with FMN (Helmert contrasts were significant at p<0.01). Unlike the BCEA, significant differences were observed between patients without nystagmus versus those with nystagmus (p<0.01), and patients without nystagmus no FMN and patients with FMN (p<0.01) during both fellow and amblyopic eye viewing conditions.
When comparing the composite amplitude between different types of amblyopia, the composite amplitude of the fellow eye was greater in amblyopic patients during FEV compared to controls irrespective of the type of amblyopia (controls: 0.50° ± 0.27°, anisometropic: 0.56° ± 0.38°, mixed/strabismic: 0.71° ± 0.62°; F=48.3, p<0.01). A greater increase of composite amplitude of the amblyopic eye was seen during amblyopic eye viewing condition irrespective of the type of amblyopia (controls: 0.54° ± 0.30°, anisometropic: 1.09° ± 1.34°, mixed/strabismic: 1.50° ± 2.10°; F=40.6, p<0.01). A planned contrast analysis identified differences between controls and fellow eye and controls and amblyopic eye of anisometropic amblyopia (p<0.01). Similar differences were seen with planned contrast analysis with greater amplitude of the fellow and amblyopic eye of strabismic/mixed amblyopia patients compared to controls (p<0.01). We also found a greater increase in amplitude of the fellow and amblyopic eye of mixed/strabismic amblyopia patients compared to anisometropia patients (p<0.01, p<0.01).
A two-way between-subjects ANCOVA was carried out to examine the effects of type and waveform on the amplitude of the fellow eye obtained during fellow eye viewing condition. There was a statistically significant main effect of the type (F=75.4, p<0.01) and waveform (F=28.5, p<0.01) on amplitude of the fellow eye whilst controlling for visual acuity and stereopsis. The covariates acuity (F=2.7, p=0.10) and stereopsis (F=0.1, p=0.80) did not have any significant effects on the amplitude of the fellow eye. A similar analysis was done for the amplitude of the amblyopic eye obtained during amblyopic eye viewing condition. There was a statistically significant main effect of the type (F=14.2, p<0.01) and waveform (F=13.06, p<0.01) on amplitude of the amblyopic eye whilst controlling for visual acuity and stereopsis. The covariates acuity (F=28.8, p<0.01) and stereopsis (F=53.3, p<0.01) showed significant effects on the amplitude of the amblyopic eye.
Eye position variance during inter-saccadic drifts and slow phase velocity
We found an increase in the eye position variance of the fellow eye of amblyopic patients (controls: 0.01° ± 0.03°, no nystagmus: 0.01° ± 0.05°, nystagmus no FMN: 0.01° ± 0.04°, FMN: 0.04° ± 0.09°; F=11.6, p<0.01). A planned contrast analysis showed an increase in variance of slow phases in patients with FMN compared to the other groups (p<0.01). An increase was also seen in amblyopic eye across all fixation eye movement waveforms (controls: 0.01° ± 0.06°, no nystagmus: 0.04° ± 0.25°, nystagmus no FMN: 0.09° ± 0.40°, FMN: 0.21° ± 0.69°; F=23.4, p<0.01) (
Figure 5B). A planned contrast analysis identified differences between controls and patients without nystagmus, controls and patients with nystagmus without FMN, controls and patients with FMN, patients with and without nystagmus, and patients with and without FMN (Helmert contrasts were significant at p<0.01).
When comparing the eye position variance between groups of type of amblyopia, the eye position variance of the fellow eye was greater in amblyopic patients during FEV compared to controls (controls: 0.01° ± 0.03°, anisometropic: 0.01° ± 0.02°, mixed/strabismic: 0.02° ± 0.08°; F=24.3, p<0.01). A planned contrast analysis identified differences between controls and fellow eye of patients with mixed/strabismic amblyopia, and patients with anisometropia and those with mixed/strabismic amblyopia (all Helmert contrasts were significant at p<0.01). A greater increase of eye position variance of the amblyopic eye was seen during amblyopic eye viewing condition irrespective of the type of amblyopia (controls: 0.01° ± 0.06°, anisometropic: 0.03° ± 0.10°, mixed/strabismic: 0.14° ± 0.58°; F=33.6, p<0.01). A planned contrast analysis identified differences between controls and amblyopic eye of anisometropic amblyopia (p=0.03) and controls and amblyopic eye of mixed/strabismic amblyopia (p<0.01) and anisometropic and mixed/strabismic amblyopia (p<0.01).
A two-way between-subjects ANCOVA was carried out to examine the effects of type and waveform on the eye position variance of the fellow eye obtained during fellow eye viewing condition. We found a statistically significant main effect of type (F=13.4, p<0.01) and waveform (F=16.7, p<0.01) on eye position variance of the fellow eye (F=65.8, p<0.01) whilst controlling for visual acuity and stereopsis. The covariates acuity (F=9.3, p=0.02) and stereopsis (F=3.7, p=0.05) also had significant effects on the eye position variance of the fellow eye. A similar analysis was done for eye position variance of the amblyopic eye obtained during amblyopic eye viewing condition. There was a statistically significant main effect of the waveform (F=7.4, p<0.01) but not the type of amblyopia (F=2.6, p=0.1) whilst controlling for visual acuity and stereopsis. The covariates stereopsis (F=24.0, p<0.01) but not the visual acuity of the amblyopic eye (F=0.2, p=0.64) had significant effects on the eye position variance of the amblyopic eye.
Eye velocity during inter-saccadic drifts and slow phase velocity
When comparing the eye velocity between groups of eye movement waveforms, the velocity was greater under both fellow eye viewing (controls: 0.63°/s ± 0.66°/s, no nystagmus: 0.71°/s ± 0.71°/s, nystagmus no FMN: 0.68°/s ± 0.68°/s, FMN: 2.50°/s ± 3.50°/s; F=66.1, p<0.01) and amblyopic eye viewing (controls: 0.76°/s ± 0.65°/s, no nystagmus: 1.03°/s ± 2.0°/s, nystagmus no FMN: 1.20°/s ± 1.80°/s, FMN: 3.60°/s ± 6.90°/s; F=53.0, p<0.01) of amblyopic patients (
Figure 5C). A planned contrast analysis identified differences during both fellow and amblyopic eye viewing conditions between controls versus patients without nystagmus (p<0.01) and controls versus patients with FMN (p<0.01). Unlike the BCEA, differences were observed between patients without nystagmus versus those with nystagmus, and patients without nystagmus no FMN and patients with FMN during both fellow and amblyopic eye viewing conditions (all Helmert contrasts were significant at p<0.01).
When comparing the eye velocity between groups of type of amblyopia, the eye velocity of the fellow eye was greater in amblyopic patients during FEV compared to controls irrespective of the type of amblyopia (controls: 0.63°/s ± 0.66°/s, anisometropic: 0.67°/s ± 0.74°/s, mixed/strabismic: 1.50°/s ± 2.50°/s; F=85.4, p<0.01). A greater increase of eye velocity of the amblyopic eye was seen during amblyopic eye viewing condition irrespective of the type of amblyopia (controls: 0.67°/s ± 0.65°/s, anisometropic: 0.84°/s ± 1.06°/s, mixed/strabismic: 2.40°/s ± 5.30°/s; F=78.4, p<0.01). A planned contrast analysis did not identify any difference between controls and anisometropic amblyopia under fellow eye or amblyopic eye viewing condition (p=0.20). However, strabismic/mixed amblyopia patients had significantly higher eye velocity of the fellow and amblyopic eye of compared to controls (both Helmert contrasts were significant at p<0.01).
A two-way between-subjects ANCOVA was carried out to examine the effects of type and waveform on the eye velocity of the fellow eye under fellow eye viewing condition while controlling for visual acuity and stereopsis. There was a statistically significant main effect of type (F=83.3, p<0.01) and waveform (F=165.3, p<0.01) on the velocity of the fellow eye. In addition, significant effects of the covariates, visual acuity (F=120.9, p<0.01) and stereopsis (F=4.1, p=0.04) on the velocity of the fellow eye were observed. There was a statistically significant main effect of waveform (F=31.4, p<0.01) and type (F=3.2, p=0.07) on the velocity of the amblyopic eye while controlling for visual acuity and stereopsis. In addition, there was a significant effect of covariate stereopsis (F=58.2, p<0.01) but not the visual acuity (F=2.8, p=0.08) on the eye velocity of the amblyopic eye.
Disconjugacy of Microsaccades and Quick Phases of Nystagmus
We have shown that patients with medium and large angle strabismus have an increase in the disconjugacy of fixational saccades in patients without nystagmus and quick phase of patients with nystagmus (
Shaikh et al., 2016). In the current paper, we wanted to investigate the effects of presence of microstrabismus, severity of amblyopia and binocular function deficits on binocular coordination of the fixation eye movements. Thus, we analyzed the disconjugacy (difference in amplitude of the two eyes) of the fixational saccades in patients without nystagmus and quick phases in patients with nystagmus (
Figure 6).
Both eyes viewing
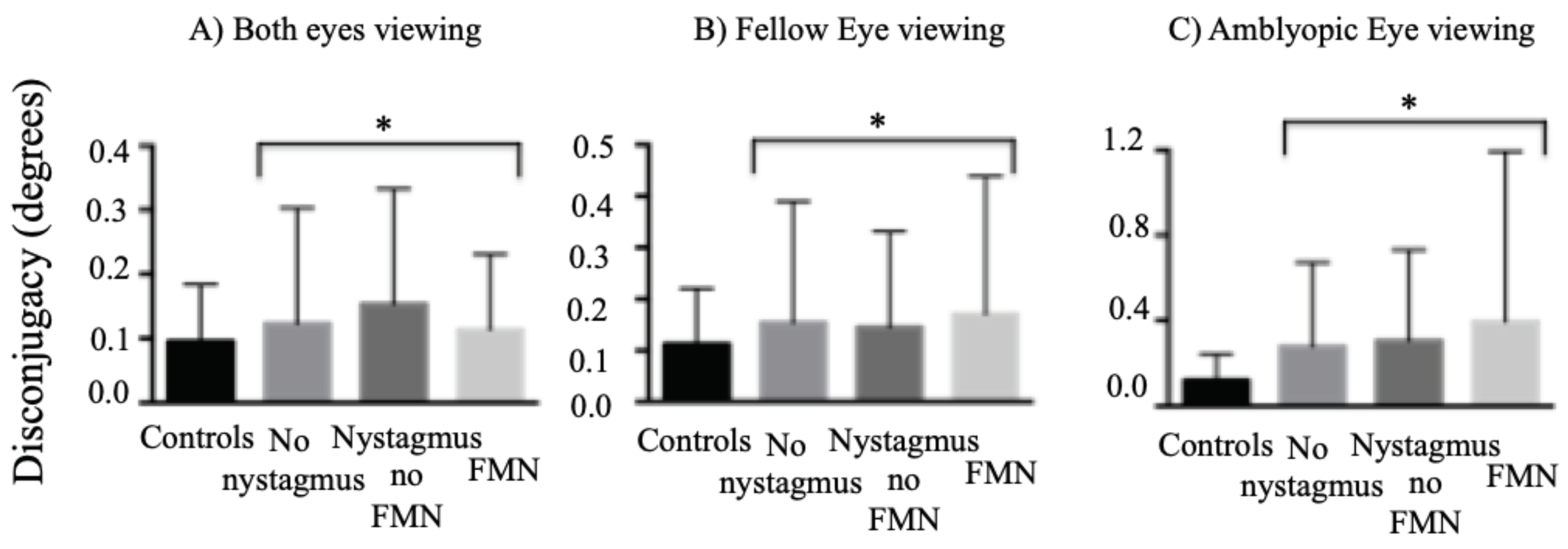
We found an increase in the disconjugacy of amplitude of fixational saccades in patients without nystagmus and quick phases in patients with nystagmus compared to controls (controls: 0.09° ± 0.08°, no nystagmus: 0.12° ± 0.17°, nystagmus no FMN: 0.15° ± 0.17°, FMN: 0.11° ± 0.11°; F=7.8, p<0.01) during both eyes viewing conditions (
Figure 6A). A planned contrast analysis identified differences between controls and patients without nystagmus (p=0.01), controls and patients with nystagmus no FMN (p<0.01), and control and patients with FMN (p=0.02). No differences were seen between patients without nystagmus and patients with nystagmus (p=0.39). There was a difference with quick phases of patients with FMN being more disconjugate compared to patients with nystagmus without FMN (p=0.04).
We also evaluated the disconjugacy as a function of type of amblyopia during both eyes viewing conditions. The disconjugacy of fixational saccades and quick phases of nystagmus was greater in both anisometropic and mixed/strabismic amblyopic patients compared to controls (controls: 0.09° ± 0.08°, anisometropic amblyopia: 0.13° ± 0.21°, mixed/strabismic amblyopia: 0.12° ± 0.13°; F=10.1, p<0.01). A planned contrast analysis identified differences between controls and patients with anisometropia (p=0.01) and controls and patients with mixed/strabismic amblyopia (p<0.01). No difference in disconjugacy was observed between anisometropic versus mixed/strabismic amblyopia in the fellow eye (p=0.95) and amblyopic eye (p=0.43). A two-way between-subjects ANCOVA was carried out to examine the effects of type and waveform on the disconjugacy obtained under both eyes viewing conditions while controlling for visual acuity and stereopsis. No statistically significant differences were seen between type (p=0.3) and waveform (p=0.06) on the disconjugacy.
Fellow Eye Viewing
When comparing the composite disconjugacy during fellow eye viewing condition (
Figure 6B), the disconjugacy was greater in amblyopic patients across all eye movement waveforms compared to controls (controls: 0.11° ± 0.10°, no nystagmus: 0.15° ± 0.23°, nystagmus no FMN: 0.14° ± 0.18°, FMN: 0.17° ± 0.26°; F=16.3, p<0.01). A planned contrast analysis identified differences between controls and patients without nystagmus (p<0.01), controls and patients with nystagmus no FMN (p<0.01) and controls versus patients with FMN (p<0.01). No differences were observed between patients without nystagmus versus those with nystagmus (p=0.70). Patients with FMN had greater disconjugacy during fellow eye viewing condition than patients with nystagmus no FMN.
When comparing the composite disconjugacy across type of amblyopia, the disconjugacy was greater for anisometropic and strabismic/mixed amblyopia patients compared to controls (controls: 0.11° ± 0.10°, anisometropic amblyopia: 0.13° ± 0.20°, mixed/strabismic amblyopia: 0.16° ± 0.24°; F=27.0, p<0.01). A planned contrast analysis identified differences between controls and anisometropic amblyopia (p=0.03), controls and mixed/strabismic amblyopia (p<0.01) and anisometropic and mixed/strabismic amblyopia (p<0.01).
A two-way between-subjects ANCOVA was carried out to examine the effects of type and waveform on disconjugacy obtained during fellow eye viewing condition. There was a statistically significant effect of type of amblyopia (F=4.8, p=0.01) but not the waveform (F=1.7, p=0.17) while controlling for visual acuity and stereopsis. As predicted per our hypothesis, there was a significant effect of the covariate stereopsis (F=8.5, p<0.01) but not visual acuity of the fellow eye (F=0.5, p=0.46).
Amblyopic Eye Viewing
When comparing the composite disconjugacy during amblyopic eye viewing condition (
Figure 6C), the disconjugacy was greater in amblyopic patients across all eye movement waveforms compared to controls (controls: 0.12° ± 0.12°, no nystagmus: 0.27° ± 0.39°, nystagmus no FMN: 0.29° ± 0.40°, FMN: 0.39° ± 0.80°; F=68.3, p<0.01). A planned contrast analysis identified differences between controls and patients without nystagmus, controls and patients with nystagmus no FMN and controls versus patients with FMN. Significant differences were observed between patients without nystagmus versus those with nystagmus and patients with nystagmus with and without FMN (Helmert contrasts were significant at p<0.01).
When comparing the composite disconjugacy across type of amblyopia, the disconjugacy was greater for anisometropic and strabismic/mixed amblyopia patients compared to controls (controls: 0.12° ± 0.12°, anisometropia: 0.19° ± 0.32°, mixed/strabismic: 0.36° ± 0.63°; F=98.2, p<0.01). A planned contrast analysis identified differences between controls and anisometropic amblyopia, controls and mixed/strabismic amblyopia, and anisometropic and mixed/strabismic amblyopia (Helmert contrasts were significant at p<0.01).
A two-way between-subjects ANCOVA was carried out to examine the effects of type and waveform on disconjugacy obtained under amblyopic eye viewing condition. There was a statistically significant effect of waveform of fixation eye movements (F=5.6, p<0.01) and type of amblyopia (F=25.0, p<0.01) while controlling for visual acuity and stereopsis. In addition, the covariates, stereopsis (F=42.2, p<0.01) and visual acuity of the amblyopic eye (F=6.3, p=0.01) showed significant effects.
Correlation between drift velocity and fixational saccade amplitude in controls and patients without nystagmus and quick and slow phase in patients with nystagmus
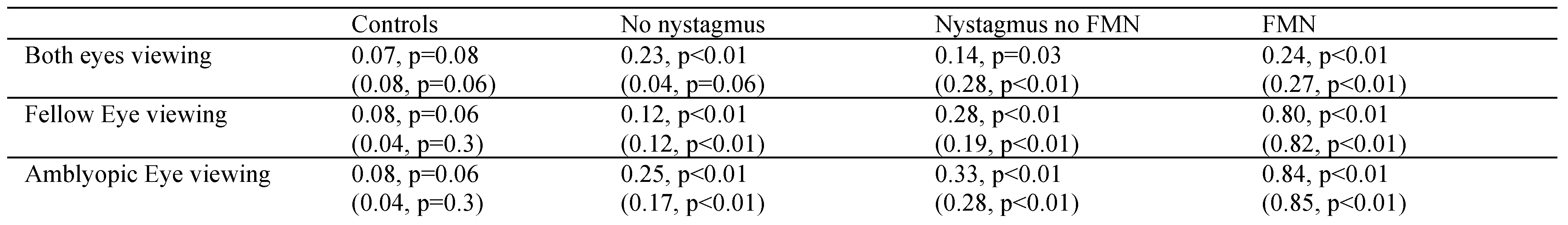
A Spearman correlation was performed between drift velocity and subsequent fixational saccade amplitude of the corresponding eye in patients without nystagmus and slow phase followed by the subsequent quick phase in patients with nystagmus. The greatest correlation coefficient (Spearman’s rho) values were seen under AEV, followed by FEV and then BEV in patients with FMN followed by nystagmus no FMN. In patients without nystagmus, the correlation coefficients were greater during AEV and BEV and least during FEV. Nevertheless, in all amblyopes this correlation between slow and fast eye movements was quite robust. A similar but much weaker correlation was also seen in control subjects (
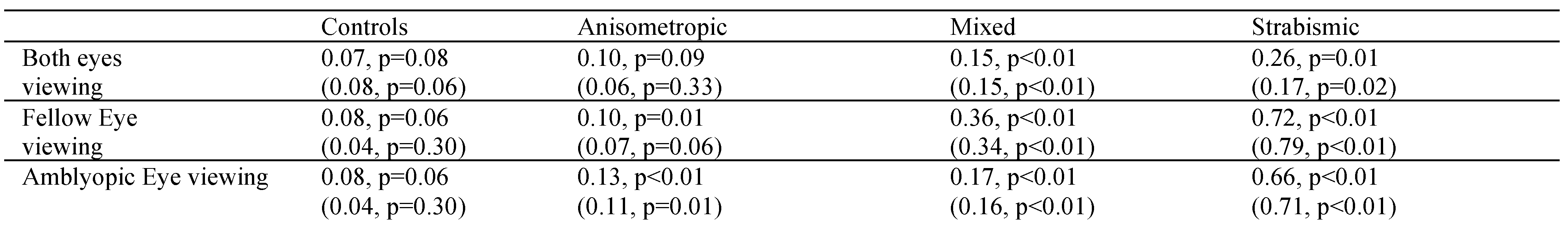
Table 3). We also explored a similar correlation as a function of type of amblyopia (
Table 4). The strabismic amblyopes had greatest correlation followed by mixed and then anisometropic amblyopia (
Table 4).