Mangosteen Pericarp Extract Supplementation Boosts Antioxidant Status via Rebuilding Gut Microbiota to Attenuate Motor Deficit in 6-OHDA-Induced Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Preparation of MP
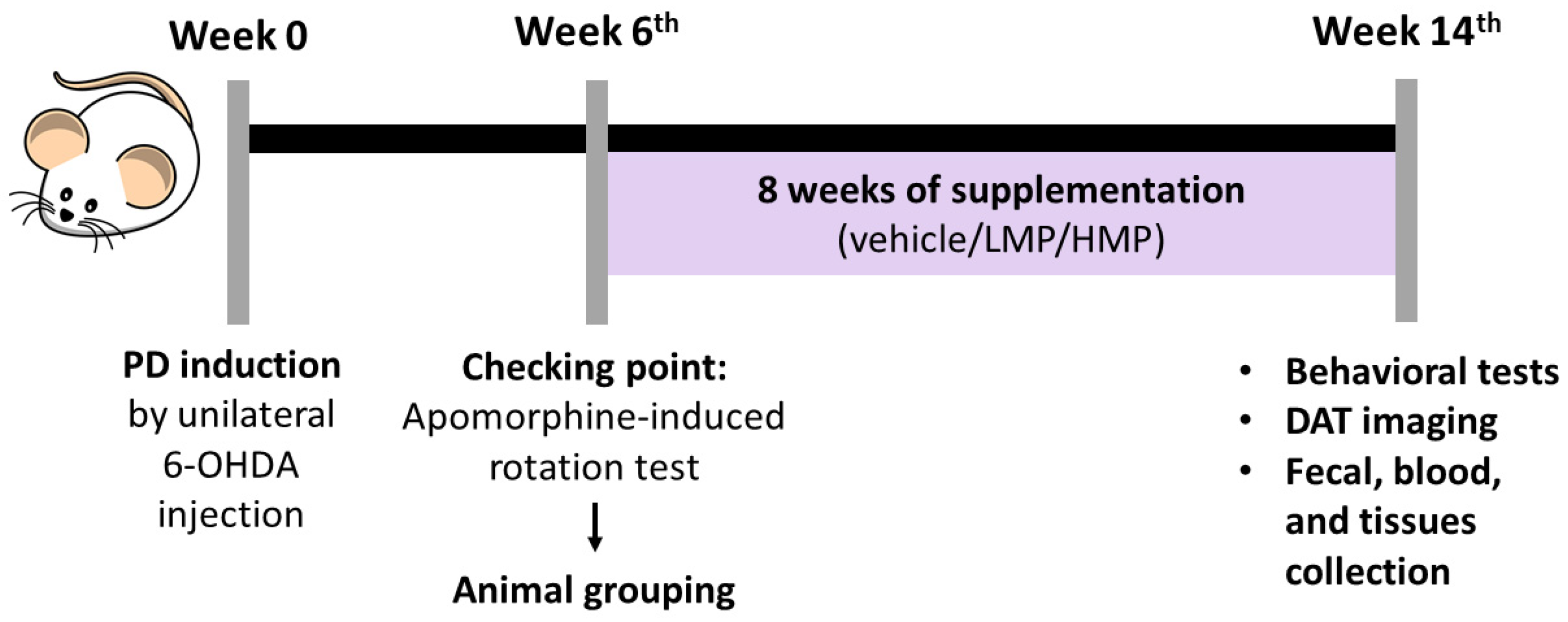
2.2. Animals and Study Design
2.3. Unilateral 6-OHDA Injection
2.4. BW Gain, Intake of Food and Water, and Food Conversion Efficiency (FCE)
2.5. Motor Deficits Assessment
2.5.1. Behavioral Tests
2.5.2. [18F]FE-PE2I PET for Dopamine Transporter (DAT) Imaging
2.6. Assessment of Antioxidant Defense Mechanisms in the Brain and Muscles
2.6.1. Quantification of Reactive Oxygen Species (ROS) Levels
2.6.2. Analysis of Antioxidant Gene Expression by Reverse-Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
2.7. Evaluation of Systemic Antioxidant Activity and Inflammatory Markers Using an Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Analysis of Mitochondrial Function and Mitochondrial Gene Expression in the Brain and Muscles
2.8.1. Seahorse Bioscience XFe24 Extracellular Flux Analysis
2.8.2. RT-qPCR Analysis of Mitochondrial Gene Expression
2.9. Evaluation of Fecal Microbiota Composition
2.9.1. Bacterial DNA Isolation and 16S rRNA Amplicon Sequencing
2.9.2. Bioinformatic Analysis
2.10. Statistical Analysis
3. Results
3.1. LMP and HMP Supplementations Do Not Affect BW Gain, Food Intake, FCE, or Water Intake
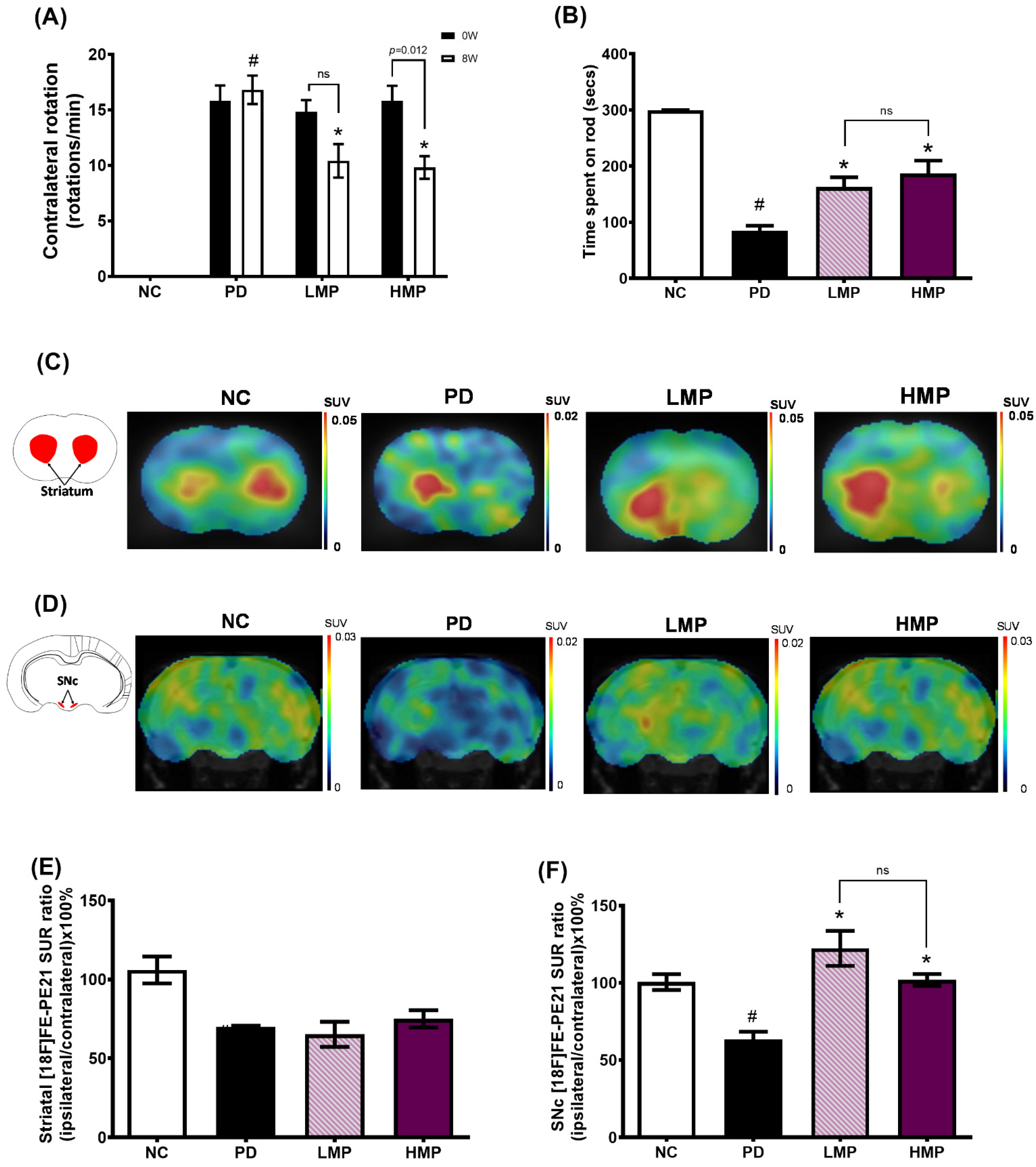
3.2. Supplementation with LMP and HMP Improves Motor Function in a PD-like Condition
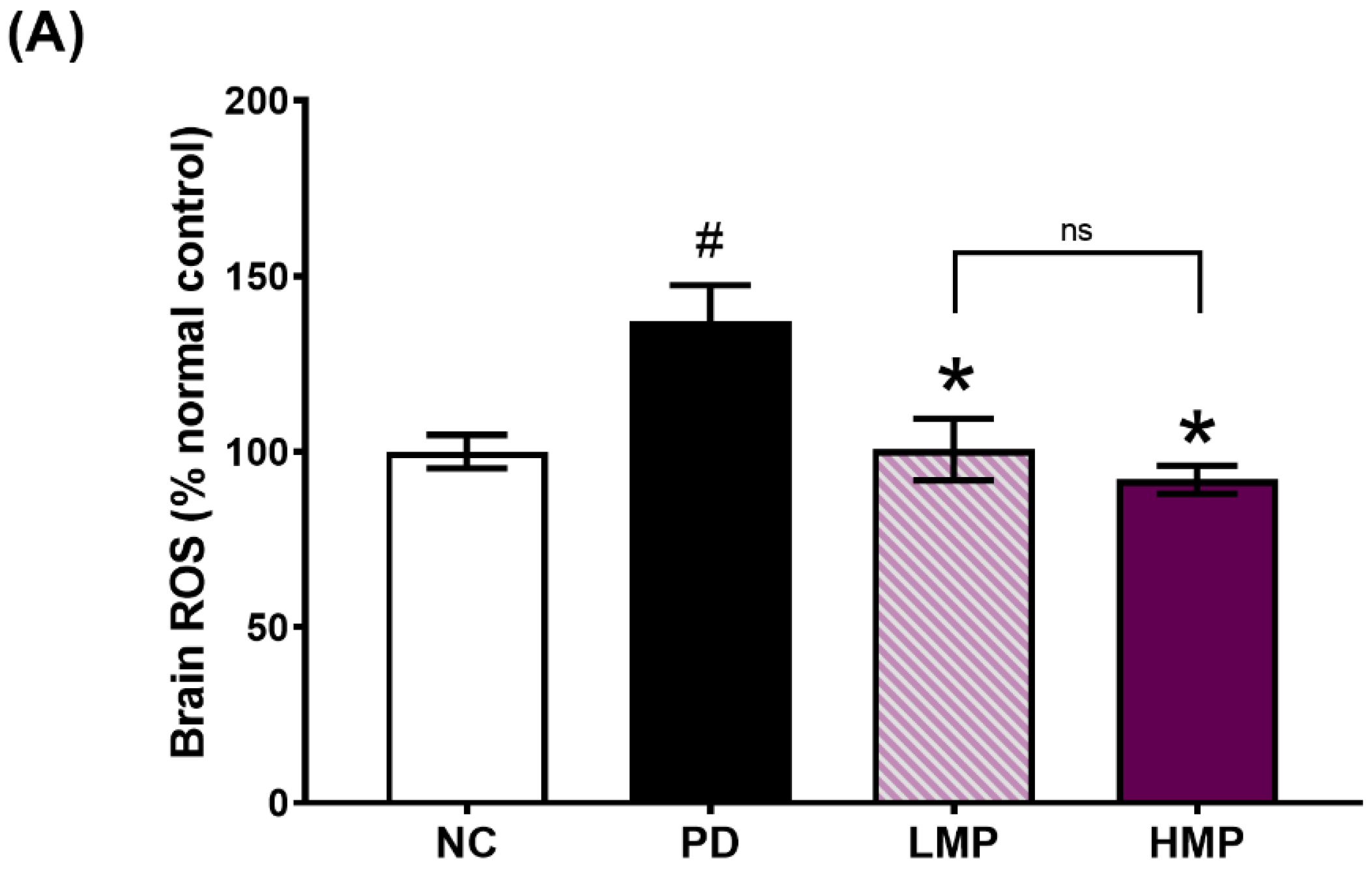
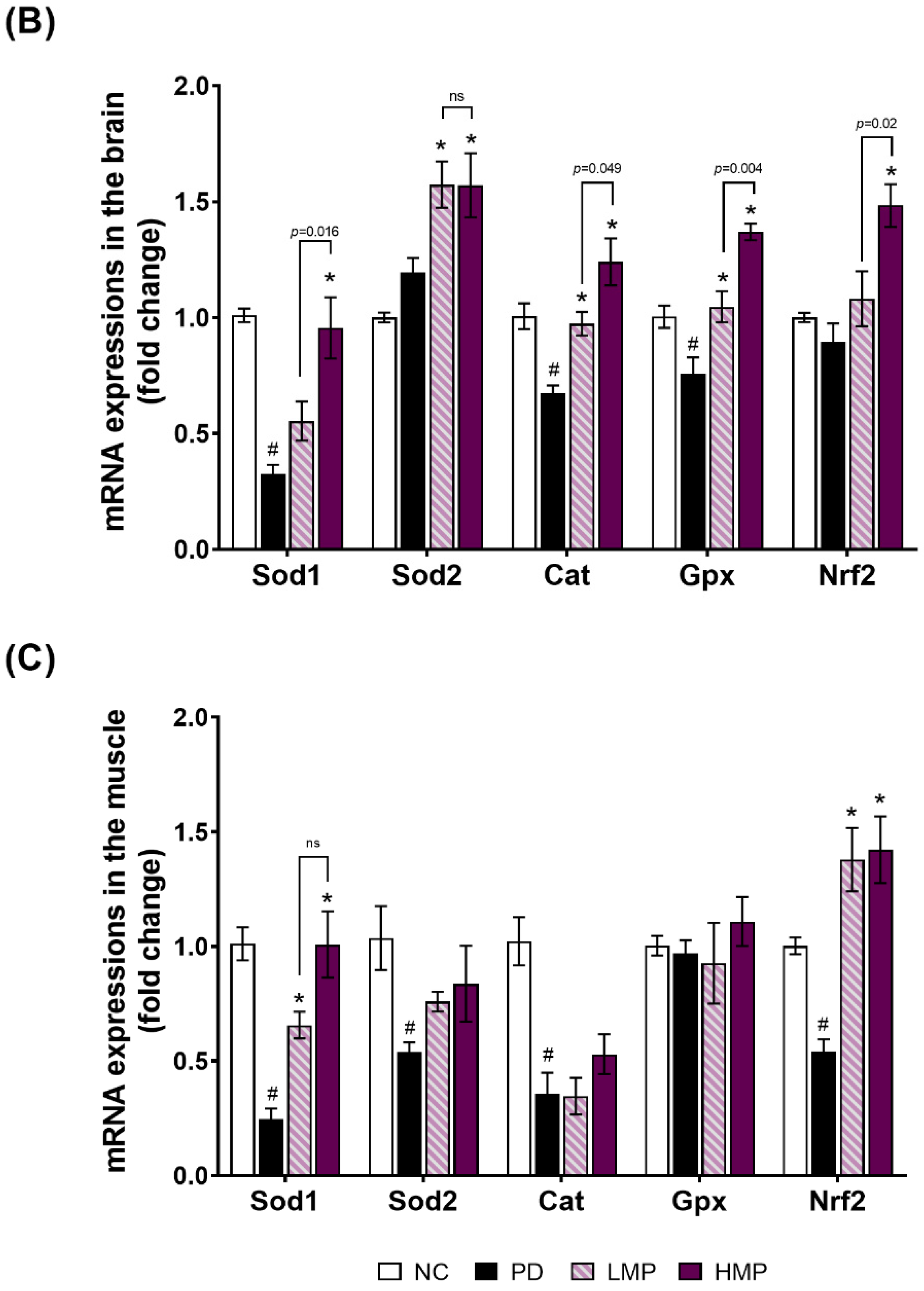
3.3. Supplementation with LMP and HMP Decreases Brain ROS Levels, Increase Expression of Antioxidant Genes, and Reduce Plasma Inflammatory Cytokines
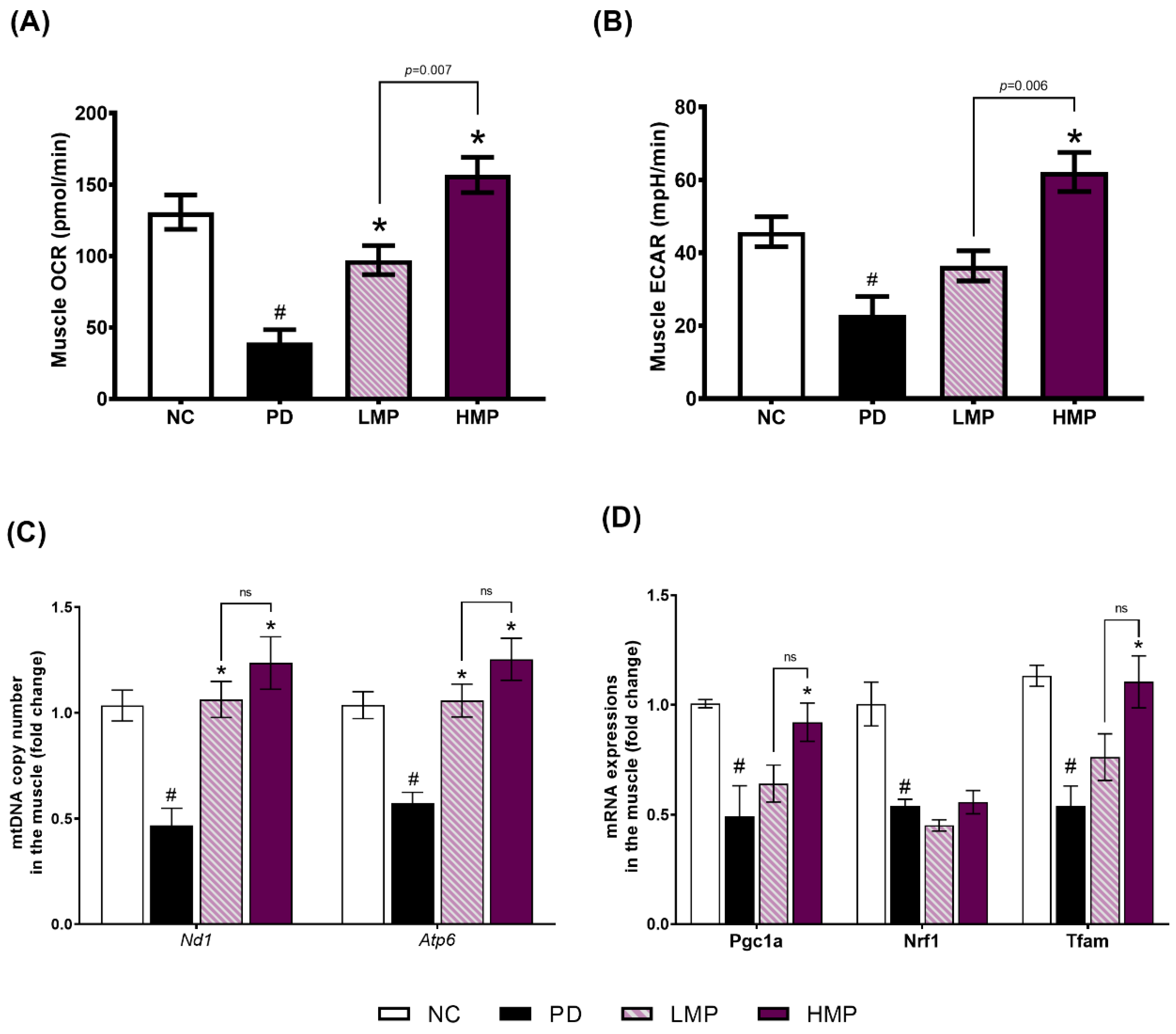
3.4. Effects of LMP and HMP on Mitochondrial Function, mtDNA Copy Number and Mitochondrial Biogenesis in Muscle
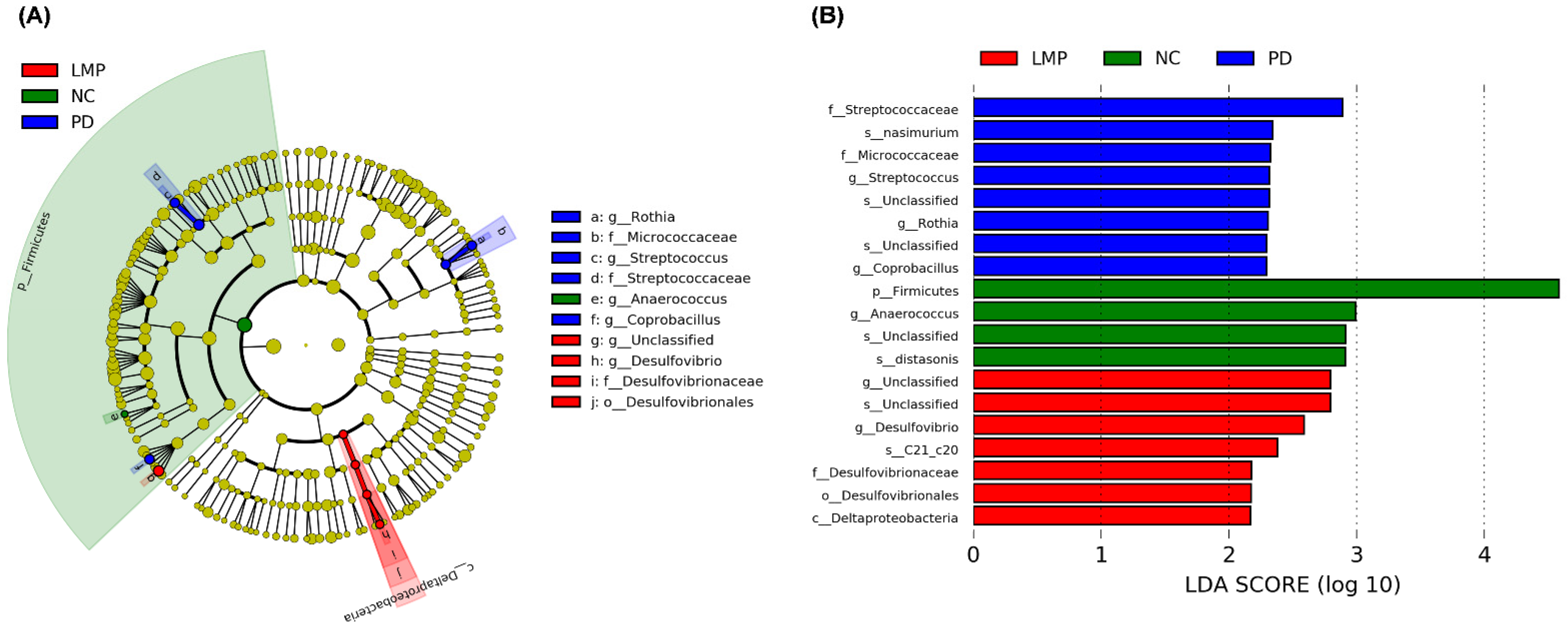
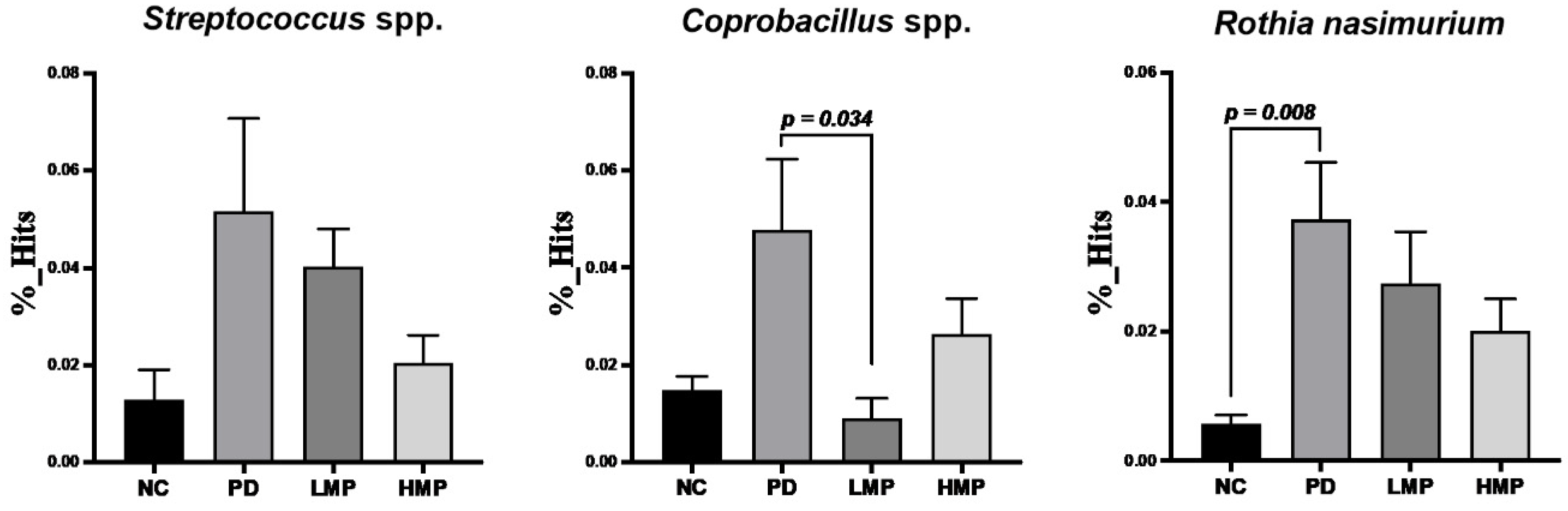
3.5. Long-Term Supplementation with LMP and HMP Alters the Gut Microbiota Profile in the PD-like Rat Model
4. Discussion
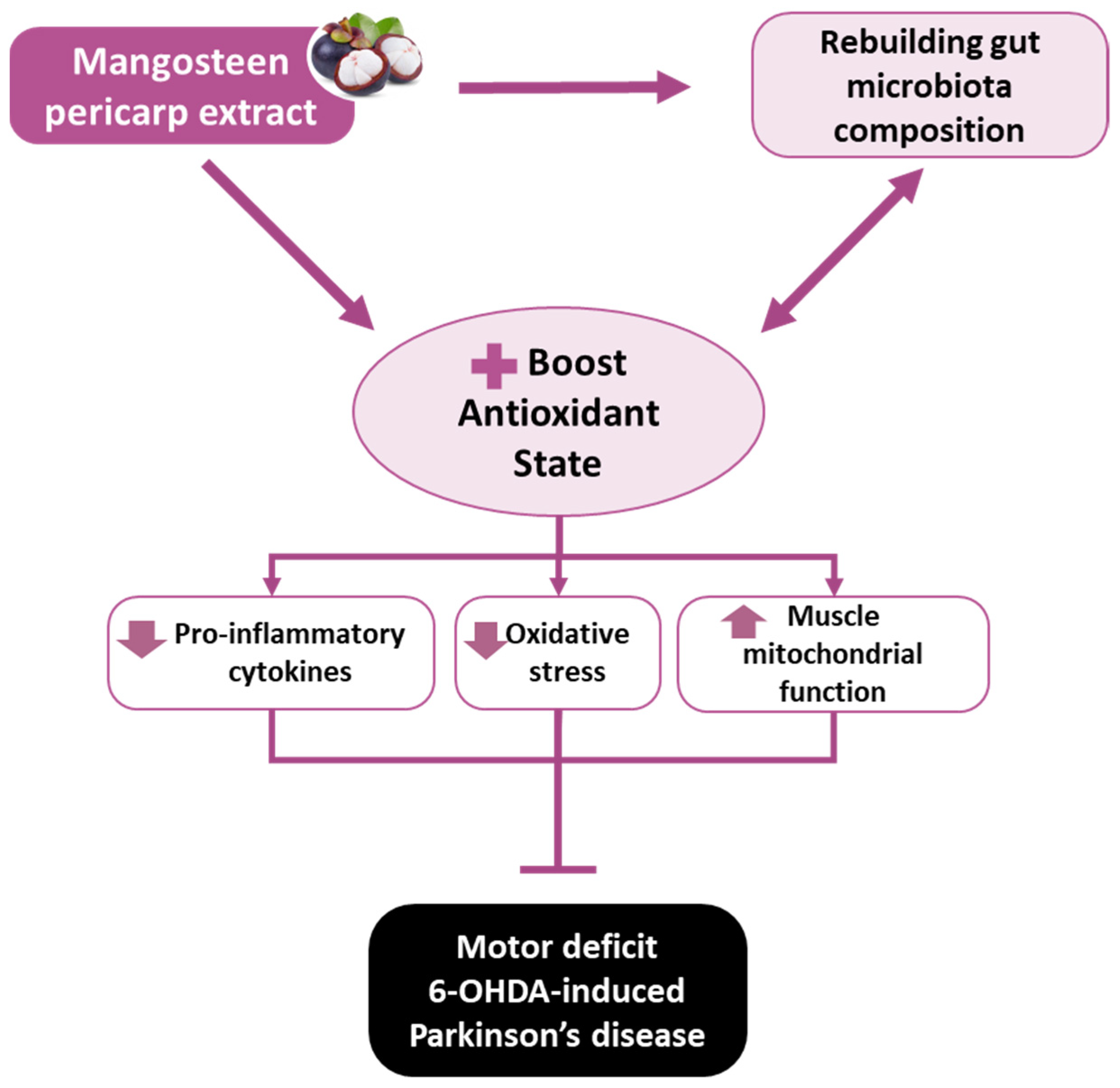
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ou, Z.; Pan, J.; Tang, S.; Duan, D.; Yu, D.; Nong, H.; Wang, Z. Global trends in the incidence, prevalence, and years lived with disability of Parkinson’s disease in 204 countries/territories from 1990 to 2019. Front. Public Health 2021, 9, 776847. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Manna, J.; Dunbar, G.L. Current understanding of the molecular mechanisms in Parkinson’s disease: Targets for potential treatments. Transl. Neurodegener. 2017, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.F.; Shen, Y.Q. Dysbiosis of gut microbiota and microbial metabolites in Parkinson’s Disease. Ageing Res. Rev. 2018, 45, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Henchcliffe, C.; Beal, M.F. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat. Clin. Pract. Neurol. 2008, 4, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Motherwell, M.S. The impact of reactive oxygen species and genetic mitochondrial mutations in Parkinson’s disease. Gene 2013, 532, 18–23. [Google Scholar] [CrossRef]
- Nagatsu, T.; Mogi, M.; Ichinose, H.; Togari, A. Changes in cytokines and neurotrophins in Parkinson’s disease. J. Neural. Transm. Suppl. 2000, 277–290. [Google Scholar] [CrossRef]
- Pajares, M.; Ana, I.R.; Manda, G.; Bosca, L.; Cuadrado, A. Inflammation in Parkinson’s disease: Mechanisms and therapeutic implications. Cells 2020, 9, 1687. [Google Scholar] [CrossRef]
- Schapira, A.H.; Cooper, J.M.; Dexter, D.; Jenner, P.; Clark, J.B.; Marsden, C.D. Mitochondrial complex I deficiency in Parkinson’s disease. Lancet 1989, 1, 1269. [Google Scholar] [CrossRef]
- Flones, I.H.; Fernandez-Vizarra, E.; Lykouri, M.; Brakedal, B.; Skeie, G.O.; Miletic, H.; Lilleng, P.K.; Alves, G.; Tysnes, O.B.; Haugarvoll, K.; et al. Neuronal complex I deficiency occurs throughout the Parkinson’s disease brain, but is not associated with neurodegeneration or mitochondrial DNA damage. Acta Neuropathol. 2018, 135, 409–425. [Google Scholar] [CrossRef]
- Keeney, P.M.; Xie, J.; Capaldi, R.A.; Bennett, J.P., Jr. Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J. Neurosci. 2006, 26, 5256–5264. [Google Scholar] [CrossRef]
- Unger, M.M.; Spiegel, J.; Dillmann, K.U.; Grundmann, D.; Philippeit, H.; Burmann, J.; Fassbender, K.; Schwiertz, A.; Schafer, K.H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism. Relat. Disord. 2016, 32, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.R.; Chesselet, M.F. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog. Neurobiol. 2013, 106–107, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Verstreken, P.; Ly, C.V.; Venken, K.J.; Koh, T.W.; Zhou, Y.; Bellen, H.J. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron 2005, 47, 365–378. [Google Scholar] [CrossRef]
- Tait, S.W.; Green, D.R. Mitochondrial regulation of cell death. Cold Spring Harb. Perspect. Biol. 2013, 5, a008706. [Google Scholar] [CrossRef] [PubMed]
- Mann, V.M.; Cooper, J.M.; Daniel, S.E.; Srai, K.; Jenner, P.; Marsden, C.D.; Schapira, A.H. Complex I, iron, and ferritin in Parkinson’s disease substantia nigra. Ann. Neurol. 1994, 36, 876–881. [Google Scholar] [CrossRef]
- Cho, C.H.; Kim, E.A.; Kim, J.; Choi, S.Y.; Yang, S.J.; Cho, S.W. N-Adamantyl-4-methylthiazol-2-amine suppresses amyloid beta-induced neuronal oxidative damage in cortical neurons. Free Radic. Res. 2016, 50, 678–690. [Google Scholar] [CrossRef]
- Di Pietro, V.; Lazzarino, G.; Amorini, A.M.; Tavazzi, B.; D’Urso, S.; Longo, S.; Vagnozzi, R.; Signoretti, S.; Clementi, E.; Giardina, B.; et al. Neuroglobin expression and oxidant/antioxidant balance after graded traumatic brain injury in the rat. Free Radic. Biol. Med. 2014, 69, 258–264. [Google Scholar] [CrossRef]
- Federico, A.; Cardaioli, E.; Da Pozzo, P.; Formichi, P.; Gallus, G.N.; Radi, E. Mitochondria, oxidative stress and neurodegeneration. J. Neurol. Sci. 2012, 322, 254–262. [Google Scholar] [CrossRef]
- Holper, L.; Ben-Shachar, D.; Mann, J.J. Multivariate meta-analyses of mitochondrial complex I and IV in major depressive disorder, bipolar disorder, schizophrenia, Alzheimer disease, and Parkinson disease. Neuropsychopharmacology 2019, 44, 837–849. [Google Scholar] [CrossRef]
- Winkler-Stuck, K.; Kirches, E.; Mawrin, C.; Dietzmann, K.; Lins, H.; Wallesch, C.W.; Kunz, W.S.; Wiedemann, F.R. Re-evaluation of the dysfunction of mitochondrial respiratory chain in skeletal muscle of patients with Parkinson’s disease. J. Neural Transm. 2005, 112, 499–518. [Google Scholar] [CrossRef]
- Li, W.; Wu, X.; Hu, X.; Wang, T.; Liang, S.; Duan, Y.; Jin, F.; Qin, B. Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features. Sci. China Life Sci. 2017, 60, 1223–1233. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Green, S.J.; Engen, P.A.; Voigt, R.M.; Naqib, A.; Forsyth, C.B.; Mutlu, E.; Shannon, K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015, 30, 1351–1360. [Google Scholar] [CrossRef]
- Hill-Burns, E.M.; Debelius, J.W.; Morton, J.T.; Wissemann, W.T.; Lewis, M.R.; Wallen, Z.D.; Peddada, S.D.; Factor, S.A.; Molho, E.; Zabetian, C.P.; et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov. Disord. 2017, 32, 739–749. [Google Scholar] [CrossRef]
- Johnson, M.E.; Stringer, A.; Bobrovskaya, L. Rotenone induces gastrointestinal pathology and microbiota alterations in a rat model of Parkinson’s disease. Neurotoxicology 2018, 65, 174–185. [Google Scholar] [CrossRef]
- Hasegawa, S.; Goto, S.; Tsuji, H.; Okuno, T.; Asahara, T.; Nomoto, K.; Shibata, A.; Fujisawa, Y.; Minato, T.; Okamoto, A.; et al. Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson’s disease. PLoS ONE 2015, 10, e0142164. [Google Scholar] [CrossRef]
- Maeda, Y.; Kurakawa, T.; Umemoto, E.; Motooka, D.; Ito, Y.; Gotoh, K.; Hirota, K.; Matsushita, M.; Furuta, Y.; Narazaki, M.; et al. Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. Arthritis Rheumatol. 2016, 68, 2646–2661. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Srivastav, S.; Neupane, S.; Bhurtel, S.; Katila, N.; Maharjan, S.; Choi, H.; Hong, J.T.; Choi, D.Y. Probiotics mixture increases butyrate, and subsequently rescues the nigral dopaminergic neurons from MPTP and rotenone-induced neurotoxicity. J. Nutr. Biochem. 2019, 69, 73–86. [Google Scholar] [CrossRef]
- Toribio-Mateas, M. Harnessing the power of microbiome assessment tools as part of neuroprotective nutrition and lifestyle medicine interventions. Microorganisms 2018, 6, 35. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Pedraza-Chaverri, J.; Cardenas-Rodriguez, N.; Orozco-Ibarra, M.; Perez-Rojas, J.M. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem. Toxicol. 2008, 46, 3227–3239. [Google Scholar] [CrossRef]
- Ovalle-Magallanes, B.; Eugenio-Perez, D.; Pedraza-Chaverri, J. Medicinal properties of mangosteen (Garcinia mangostana L.): A comprehensive update. Food Chem. Toxicol. 2017, 109, 102–122. [Google Scholar] [CrossRef]
- Fu, C.; Loo, A.E.; Chia, F.P.; Huang, D. Oligomeric proanthocyanidins from mangosteen pericarps. J. Agric. Food Chem. 2007, 55, 7689–7694. [Google Scholar] [CrossRef]
- Parkhe, A.; Parekh, P.; Nalla, L.V.; Sharma, N.; Sharma, M.; Gadepalli, A.; Kate, A.; Khairnar, A. Protective effect of alpha mangostin on rotenone induced toxicity in rat model of Parkinson’s disease. Neurosci. Lett. 2020, 716, 134652. [Google Scholar] [CrossRef]
- Catorce, M.; Acero, G.; Pedraza-Chaverri, J.; Fragoso, G.; Govezensky, T.; Gevorkian, G. Alpha-mangostin attenuates brain inflammation induced by peripheral lipopolysaccharide administration in C57BL/6J mice. J. Neuroimmunol. 2016, 297, 20–27. [Google Scholar] [CrossRef]
- Sundaram, B.M.; Gopalakrishnan, C.; Subramanian, S.; Shankaranarayanan, D.; Kameswaran, L. Antimicrobial activities of Garcinia mangostana. Planta Med. 1983, 48, 59–60. [Google Scholar] [CrossRef]
- Weecharangsan, W.; Opanasopit, P.; Sukma, M.; Ngawhirunpat, T.; Sotanaphun, U.; Siripong, P. Antioxidative and neuroprotective activities of extracts from the fruit hull of mangosteen (Garcinia mangostana Linn.). Med. Princ. Pract. 2006, 15, 281–287. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Li, P.; Yang, Z.; Tang, B.; Zhang, Q.; Chen, Z.; Zhang, J.; Wei, J.; Sun, L.; Yan, J. Identification of xanthones from the mangosteen pericarp that inhibit the growth of Ralstonia solanacearum. ACS Omega 2020, 5, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Tsao, S.P.; Nurrahma, B.A.; Kumar, R.; Wu, C.H.; Yeh, T.H.; Chiu, C.C.; Lee, Y.P.; Liao, Y.C.; Huang, C.H.; Yeh, Y.T.; et al. Probiotic enhancement of antioxidant capacity and alterations of gut microbiota composition in 6-hydroxydopamin-induced Parkinson’s disease rats. Antioxidants 2021, 10, 1823. [Google Scholar] [CrossRef]
- Storch, A.; Ludolph, A.C.; Schwarz, J. Dopamine transporter: Involvement in selective dopaminergic neurotoxicity and degeneration. J. Neural Transm. 2004, 111, 1267–1286. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kerstens, V.S.; Fazio, P.; Sundgren, M.; Matheson, G.J.; Franzen, E.; Halldin, C.; Cervenka, S.; Svenningsson, P.; Varrone, A. Reliability of dopamine transporter PET measurements with [(18)F]FE-PE2I in patients with Parkinson’s disease. EJNMMI Res. 2020, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Varrone, A.; Steiger, C.; Schou, M.; Takano, A.; Finnema, S.J.; Guilloteau, D.; Gulyas, B.; Halldin, C. In vitro autoradiography and in vivo evaluation in cynomolgus monkey of [18F]FE-PE2I, a new dopamine transporter PET radioligand. Synapse 2009, 63, 871–880. [Google Scholar] [CrossRef]
- Nurrahma, B.A.; Tsao, S.P.; Wu, C.H.; Yeh, T.H.; Hsieh, P.S.; Panunggal, B.; Huang, H.Y. Probiotic supplementation facilitates recovery of 6-OHDA-induced motor deficit via improving mitochondrial function and energy metabolism. Front. Aging Neurosci. 2021, 13, 668775. [Google Scholar] [CrossRef]
- Schwarting, R.K.; Huston, J.P. The unilateral 6-hydroxydopamine lesion model in behavioral brain research. Analysis of functional deficits, recovery and treatments. Prog. Neurobiol. 1996, 50, 275–331. [Google Scholar] [CrossRef]
- Bang, J.I.; Jung, I.S.; Song, Y.S.; Park, H.S.; Moon, B.S.; Lee, B.C.; Kim, S.E. PET imaging of dopamine transporters with [(18)F]FE-PE2I: Effects of anti-Parkinsonian drugs. Nucl. Med. Biol. 2016, 43, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Berger, A. How does it work? Positron emission tomography. BMJ 2003, 326, 1449. [Google Scholar] [CrossRef]
- Palermo, G.; Ceravolo, R. Molecular Imaging of the Dopamine Transporter. Cells 2019, 8, 872. [Google Scholar] [CrossRef]
- He, J.; Zhu, G.; Wang, G.; Zhang, F. Oxidative stress and neuroinflammation potentiate each other to promote progression of dopamine neurodegeneration. Oxid. Med. Cell Longev. 2020, 2020, 6137521. [Google Scholar] [CrossRef]
- Shukla, V.; Mishra, S.K.; Pant, H.C. Oxidative stress in neurodegeneration. Adv. Pharmacol. Sci. 2011, 2011, 572634. [Google Scholar] [CrossRef]
- Perier, C.; Tieu, K.; Guegan, C.; Caspersen, C.; Jackson-Lewis, V.; Carelli, V.; Martinuzzi, A.; Hirano, M.; Przedborski, S.; Vila, M. Complex I deficiency primes Bax-dependent neuronal apoptosis through mitochondrial oxidative damage. Proc. Natl. Acad. Sci. USA 2005, 102, 19126–19131. [Google Scholar] [CrossRef] [PubMed]
- Kunikowska, G.; Jenner, P. Alterations in m-RNA expression for Cu,Zn-superoxide dismutase and glutathione peroxidase in the basal ganglia of MPTP-treated marmosets and patients with Parkinson’s disease. Brain Res. 2003, 968, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Poirier, J.; Dea, D.; Baccichet, A.; Thiffault, C. Superoxide dismutase expression in Parkinson’s disease. Ann. N. Y. Acad. Sci. 1994, 738, 116–120. [Google Scholar] [CrossRef]
- Boll, M.C.; Alcaraz-Zubeldia, M.; Montes, S.; Rios, C. Free copper, ferroxidase and SOD1 activities, lipid peroxidation and NO(x) content in the CSF. A different marker profile in four neurodegenerative diseases. Neurochem. Res. 2008, 33, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Ihara, Y.; Chuda, M.; Kuroda, S.; Hayabara, T. Hydroxyl radical and superoxide dismutase in blood of patients with Parkinson’s disease: Relationship to clinical data. J. Neurol. Sci. 1999, 170, 90–95. [Google Scholar] [CrossRef]
- Torsdottir, G.; Sveinbjornsdottir, S.; Kristinsson, J.; Snaedal, J.; Johannesson, T. Ceruloplasmin and superoxide dismutase (SOD1) in Parkinson’s disease: A follow-up study. J. Neurol. Sci. 2006, 241, 53–58. [Google Scholar] [CrossRef]
- In, S.; Hong, C.W.; Choi, B.; Jang, B.G.; Kim, M.J. Inhibition of mitochondrial clearance and Cu/Zn-SOD activity enhance 6-hydroxydopamine-induced neuronal apoptosis. Mol. Neurobiol. 2016, 53, 777–791. [Google Scholar] [CrossRef]
- Trist, B.G.; Davies, K.M.; Cottam, V.; Genoud, S.; Ortega, R.; Roudeau, S.; Carmona, A.; De Silva, K.; Wasinger, V.; Lewis, S.J.G.; et al. Amyotrophic lateral sclerosis-like superoxide dismutase 1 proteinopathy is associated with neuronal loss in Parkinson’s disease brain. Acta Neuropathol. 2017, 134, 113–127. [Google Scholar] [CrossRef]
- Jaisin, Y.; Ratanachamnong, P.; Kuanpradit, C.; Khumpum, W.; Suksamrarn, S. Protective effects of gamma-mangostin on 6-OHDA-induced toxicity in SH-SY5Y cells. Neurosci. Lett. 2018, 665, 229–235. [Google Scholar] [CrossRef]
- Janhom, P.; Dharmasaroja, P. Neuroprotective effects of alpha-mangostin on MPP(+)-induced apoptotic cell death in neuroblastoma SH-SY5Y cells. J. Toxicol. 2015, 2015, 919058. [Google Scholar] [CrossRef]
- Hao, X.M.; Li, L.D.; Duan, C.L.; Li, Y.J. Neuroprotective effect of alpha-mangostin on mitochondrial dysfunction and alpha-synuclein aggregation in rotenone-induced model of Parkinson’s disease in differentiated SH-SY5Y cells. J. Asian Nat. Prod. Res. 2017, 19, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Su, T.; Qiu, X.; Mao, P.; Xu, Y.; Hu, Z.; Zhang, Y.; Zheng, X.; Xie, P.; Liu, Q. Protective effect of alpha-mangostin against oxidative stress induced-retinal cell death. Sci. Rep. 2016, 6, 21018. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Chung, P.C.; Owaga, E.E.; Tsai, I.J.; Wang, P.Y.; Tsai, J.I.; Yeh, T.S.; Hsieh, R.H. Alpha-mangostin from mangosteen (Garcinia mangostana Linn.) pericarp extract reduces high fat-diet induced hepatic steatosis in rats by regulating mitochondria function and apoptosis. Nutr. Metab 2016, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Valadez, B.; Lugo-Huitron, R.; Valdivia-Cerda, V.; Miranda-Ramirez, L.R.; Perez-De La Cruz, V.; Gonzalez-Cuahutencos, O.; Rivero-Cruz, I.; Mata, R.; Santamaria, A.; Pedraza-Chaverri, J. The natural xanthone alpha-mangostin reduces oxidative damage in rat brain tissue. Nutr. Neurosci. 2009, 12, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, W.; Ling, J.; Jiang, C. Alpha-mangostin inhibits alpha-synuclein-induced microglial neuroinflammation and neurotoxicity. Cell Mol. Neurobiol. 2016, 36, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Thull, U.; Testa, B. Screening of unsubstituted cyclic compounds as inhibitors of monoamine oxidases. Biochem. Pharmacol. 1994, 47, 2307–2310. [Google Scholar] [CrossRef]
- Ohishi, N.; Suzuki, T.; Ogasawara, T.; Yagi, K. Xanthone derivatives as inhibitors for monoamine oxidase. J. Mol. Catal. B Enzym. 2000, 10, 291–294. [Google Scholar] [CrossRef]
- Zhang, Z.; Hamada, H.; Gerk, P.M. Selectivity of dietary phenolics for inhibition of human monoamine oxidases A and B. Biomed. Res. Int. 2019, 2019, 8361858. [Google Scholar] [CrossRef]
- Youdim, M.B.; Edmondson, D.; Tipton, K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006, 7, 295–309. [Google Scholar] [CrossRef]
- Youdim, M.B.; Lavie, L. Selective MAO-A and B inhibitors, radical scavengers and nitric oxide synthase inhibitors in Parkinson’s disease. Life Sci. 1994, 55, 2077–2082. [Google Scholar] [CrossRef]
- Magnusen, A.F.; Hatton, S.L.; Rani, R.; Pandey, M.K. Genetic Defects and Pro-inflammatory Cytokines in Parkinson’s Disease. Front. Neurol. 2021, 12, 636139. [Google Scholar] [CrossRef] [PubMed]
- Adu, T.S.; Mabandla, M.V. Effects of bromelain on motor responses following intra-medial forebrain bundle 6-OHDA injection in rat model of parkinsonism. Metab. Brain Dis. 2019, 34, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Blin, O.; Desnuelle, C.; Rascol, O.; Borg, M.; Peyro Saint Paul, H.; Azulay, J.P.; Bille, F.; Figarella, D.; Coulom, F.; Pellissier, J.F.; et al. Mitochondrial respiratory failure in skeletal muscle from patients with Parkinson’s disease and multiple system atrophy. J. Neurol. Sci. 1994, 125, 95–101. [Google Scholar] [CrossRef]
- Cardellach, F.; Marti, M.J.; Fernandez-Sola, J.; Marin, C.; Hoek, J.B.; Tolosa, E.; Urbano-Marquez, A. Mitochondrial respiratory chain activity in skeletal muscle from patients with Parkinson’s disease. Neurology 1993, 43, 2258–2262. [Google Scholar] [CrossRef]
- Bindoff, L.A.; Birch-Machin, M.A.; Cartlidge, N.E.; Parker, W.D., Jr.; Turnbull, D.M. Respiratory chain abnormalities in skeletal muscle from patients with Parkinson’s disease. J. Neurol. Sci. 1991, 104, 203–208. [Google Scholar] [CrossRef]
- Saiki, S.; Hatano, T.; Fujimaki, M.; Ishikawa, K.I.; Mori, A.; Oji, Y.; Okuzumi, A.; Fukuhara, T.; Koinuma, T.; Imamichi, Y.; et al. Decreased long-chain acylcarnitines from insufficient beta-oxidation as potential early diagnostic markers for Parkinson’s disease. Sci. Rep. 2017, 7, 7328. [Google Scholar] [CrossRef]
- Di Martino, S.; Tramonti, C.; Unti, E.; Del Gamba, C.; Bonuccelli, U.; Rossi, B.; Ceravolo, R.; Chisari, C. Aerobic rehabilitation program for improving muscle function in Parkinson’s disease. Restor. Neurol. Neurosci. 2018, 36, 13–20. [Google Scholar] [CrossRef]
- Peker, N.; Donipadi, V.; Sharma, M.; McFarlane, C.; Kambadur, R. Loss of Parkin impairs mitochondrial function and leads to muscle atrophy. Am. J. Physiol. Cell Physiol. 2018, 315, C164–C185. [Google Scholar] [CrossRef]
- Tsai, H.H.; Chen, C.W.; Yu, P.L.; Lin, Y.L.; Hsieh, R.H. Mangosteen pericarp components alleviate progression of prostatic hyperplasia and mitochondrial dysfunction in rats. Sci. Rep. 2020, 10, 322. [Google Scholar] [CrossRef]
- Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Rosaria Lauro, M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; Pandey, U.; Wicke, T.; Sixel-Doring, F.; Janzen, A.; Sittig-Wiegand, E.; Trenkwalder, C.; Oertel, W.H.; Mollenhauer, B.; Wilmes, P. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov. Disord. 2018, 33, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.A.B.; Aho, V.T.E.; Paulin, L.; Pekkonen, E.; Auvinen, P.; Scheperjans, F. Oral and nasal microbiota in Parkinson’s disease. Park. Relat. Disord. 2017, 38, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.K.; Nag, S.; Suh, K.N.; Gerald, A.E. Recurrent chronic ambulatory peritoneal dialysis-associated infection due to rothia dentocariosa. Can. J. Infect. Dis. Med. Microbiol. 2004, 15, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Clauwaert, M.; Druwe, P.; Depuydt, P. Meningitis in a patient with neutropenia due to Rothia mucilaginosa: A case report. J. Med. Case Rep. 2019, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Kelk, P.; Claesson, R.; Chen, C.; Sjostedt, A.; Johansson, A. IL-1beta secretion induced by Aggregatibacter (Actinobacillus) actinomycetemcomitans is mainly caused by the leukotoxin. Int. J. Med. Microbiol. 2008, 298, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Nyman, M.; Fak, F. Modulation of gut microbiota in rats fed high-fat diets by processing whole-grain barley to barley malt. Mol. Nutr. Food Res. 2015, 59, 2066–2076. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. The crosstalk between the gut microbiota and mitochondria during exercise. Front. Physiol. 2017, 8, 319. [Google Scholar] [CrossRef] [PubMed]



| Parameter | NC | PD | LMP | HMP |
|---|---|---|---|---|
| BW gain (g)/rat | 246.4 ± 37.2 | 236.4 ± 18.0 | 224.0 ± 18.7 | 227.7 ± 10.7 |
| Food intake (Kcal/day/rat) | 107.3 ± 7.7 | 105.0 ± 4.7 | 96.9 ± 4.5 | 97.1 ± 2.5 |
| FCE (%) | 225.3 ± 20.9 | 227.8 ± 22.0 | 231.5 ± 15.9 | 238.2 ± 17.7 |
| Water intake (mL)/day/rat | 44.7 ± 2.1 | 43.1 ± 3.6 | 44.8 ± 1.3 | 44.7 ± 2.4 |
| Motor Function Parameter | Correlation | Antioxidant Parameters |
|---|---|---|
| Time spent on rod (s) | + | Serum total SOD (r = 0.702; p < 0.0001), Brain Sod1 (r = 0.470; p = 0.018), Brain Cat (r = 0.511; p = 0.009) Muscle Sod1 (r = 0.610; p = 0.001), Muscle Sod2 (r = 0.417; p = 0.020), Muscle Cat (r = 0.518; p = 0.008) |
| − | Brain ROS level (r = −0.445; p = 0.026) |
| Parameters | NC | PD | LMP | HMP |
|---|---|---|---|---|
| Plasma IL-1β (pg/mL) | 382.5 ± 1.7 | 596.2 ± 6.7 a | 454.9 ± 2.5 b | 441.4 ± 3.0 b |
| Plasma IL-6 (pg/mL) | 100.9 ± 5.0 | 131.6 ± 4.3 a | 114.9 ± 6.2 | 108.2 ± 6.2 b |
| Plasma TNF-α (pg/mL) | 95.5 ± 2.1 | 130.9 ± 3.4 a | 112.6 ± 4.4 b | 101.9 ± 3.4 b |
| Parameter | Correlation | Bacterial Taxa | |
|---|---|---|---|
| Time spent on rod (s) | − | Streptococcus (r = −0.585; p = 0.002) | |
| Rothia (r = −0.663; p = 0.0003) | |||
| Rothia naismurium (r = −0.663; p = 0.0003) | |||
| Brain ROS level (% normal control) | + | Rothia (r = 0.438; p = 0.028) | |
| Rothia naismurium (r = 0.438; p = 0.028) | |||
| Brain antioxidant-related genes (fold change) | |||
| Sod1 | − | Sutterella (r = −0.459; p = 0.021) | |
| Aggregatibacter (r = −0.465; p = 0.019) | |||
| Rothia (r = −0.412; p = 0.041) | |||
| Rothia naismurium (r = −0.412; p = 0.041) | |||
| Aggregatibacter pneumotropica (r = −0.465; p = 0.019) | |||
| Sod2 | + | Rothia (r = 0.473; p = 0.017) | |
| Rothia naismurium (r = 0.473; p = 0.017) | |||
| Cat | − | Sutterella (r = −0.746; p < 0.0001) | |
| Aggregatibacter (r = −0.405; p = 0.045) | |||
| Streptococcus (r = −0.428; p = 0.033) | |||
| Aggregatibacter pneumotropica (r = −0.405; p = 0.045) | |||
| Gpx | + | Turicibacter (r = 0.417; p = 0.038) | |
| − | Prevotella (r = −0.446; p = 0.025) | ||
| Sutterella (r = −0.451; p = 0.024) | |||
| Streptococcus (r = −0.302; p = 0.142) | |||
| Nrf2 | − | Sutterella (r = −0.404; p = 0.045) | |
| Muscle antioxidant-related genes (fold change) | |||
| Sod1 | − | Lactococcus (r = −504; p = 0.010) | |
| Prevotella (r = −0.417; p = 0.038) | |||
| Aggregatibacter (r = −0.418; p = 0.037) | |||
| Streptococcus (r = −0.463; p = 0.020) | |||
| Rothia (r = −0.420; p = 0.037) | |||
| Rothia naismurium (r = −0.420; p = 0.037) | |||
| Aggregatibacter pneumotropica (r = −0.418; p = 0.037) | |||
| Cat | − | Lactococcus (r = −0.411; p = 0.041) | |
| Aggregatibacter (r = −0.450; p = 0.024) | |||
| Streptococcus (r = −0.455; p = 0.022) | |||
| Aggregatibacter pneumotropica (r = −0.45; p = 0.024) | |||
| Nrf2 | − | Sutterella (r = −0.540; p = 0.005) | |
| Serum total SOD (U/mL) | − | Prevotella (r = −0.409; p = 0.042) | |
| Muscle mitochondria-related genes (fold change) | |||
| Nd1 | − | Streptococcus (r = −0.468; p = 0.038) | |
| Rothia (r = −0.453; p = 0.045) | |||
| Sutterella (r = −0.447; p = 0.048) | |||
| Streptococcus spp. (r = −0.468; p = 0.038) | |||
| Rothia nasimurium (r = −0.453; p = 0.045) | |||
| Pgc1α | − | Streptococcus (r = −0.560; p = 0.010) | |
| Rothia (r = −0.544; p = 0.013) | |||
| Streptococcus spp. (r = −0.560; p = 0.010) | |||
| Rothia nasimurium (r = −0.544; p = 0.013) | |||
| Nrf1 | + | Coprococcus (r = 0.455; p = 0.044) | |
| − | Rothia (r = −0.464; p = 0.039) | ||
| Rothia nasimurium (r = −0.464; p = 0.039) | |||
| Tfam | − | Streptococcus (r = −0.629; p = 0.003) | |
| Rothia (r = −0.636; p = 0.002) | |||
| Sutterella (r = −0.516; p = 0.020) | |||
| Aggregatibacter (r = −0.528; p = 0.017) | |||
| Streptococcus spp. (r = −0.629; p = 0.003) | |||
| Rothia nasimurium (r = −0.636; p = 0.002) | |||
| Plasma inflammatory cytokines (pg/mL) | |||
| IL-1β | + | Streptococcus (r = 0.557; p = 0.011) | |
| Rothia (r = 0.708; p < 0.001) | |||
| Aggregatibacter (r = 0.563; p = 0.010) | |||
| Streptococcus spp. (r = 0.557; p = 0.011) | |||
| Rothia nasimurium (r = 0.708; p < 0.001) | |||
| IL-6 | + | Streptococcus (r = 0.602; p = 0.005) | |
| Rothia (r = 0.649; p = 0.002) | |||
| Sutterella (r = 0.585; p = 0.007) | |||
| Aggregatibacter (r = 0.544; p = 0.013) | |||
| Streptococcus spp. (r = 0.602; p = 0.005) | |||
| Rothia nasimurium (r = 0.649; p = 0.002) | |||
| Coprobacillus spp. (r = 0.580; p = 0.007) | |||
| TNF-α | + | Streptococcus (r = 0.513; p = 0.021) | |
| Rothia (r = 0.583; p = 0.007) | |||
| Aggregatibacter (r = 0.511; p = 0.021) | |||
| Streptococcus spp. (r = 0.513; p = 0.021) | |||
| Rothia nasimurium (r = 0.583; p = 0.007) | |||
| − | Lactobacillus (r = −0.465; p = 0.039) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurrahma, B.A.; Yeh, T.-H.; Hsieh, R.-H.; Tsao, S.-P.; Chen, C.-W.; Lee, Y.-P.; Pan, C.-H.; Huang, H.-Y. Mangosteen Pericarp Extract Supplementation Boosts Antioxidant Status via Rebuilding Gut Microbiota to Attenuate Motor Deficit in 6-OHDA-Induced Parkinson’s Disease. Antioxidants 2022, 11, 2396. https://doi.org/10.3390/antiox11122396
Nurrahma BA, Yeh T-H, Hsieh R-H, Tsao S-P, Chen C-W, Lee Y-P, Pan C-H, Huang H-Y. Mangosteen Pericarp Extract Supplementation Boosts Antioxidant Status via Rebuilding Gut Microbiota to Attenuate Motor Deficit in 6-OHDA-Induced Parkinson’s Disease. Antioxidants. 2022; 11(12):2396. https://doi.org/10.3390/antiox11122396
Chicago/Turabian StyleNurrahma, Bira Arumndari, Tu-Hsueh Yeh, Rong-Hong Hsieh, Shu-Ping Tsao, Chia-Wen Chen, Yen-Peng Lee, Chun-Hsu Pan, and Hui-Yu Huang. 2022. "Mangosteen Pericarp Extract Supplementation Boosts Antioxidant Status via Rebuilding Gut Microbiota to Attenuate Motor Deficit in 6-OHDA-Induced Parkinson’s Disease" Antioxidants 11, no. 12: 2396. https://doi.org/10.3390/antiox11122396
APA StyleNurrahma, B. A., Yeh, T.-H., Hsieh, R.-H., Tsao, S.-P., Chen, C.-W., Lee, Y.-P., Pan, C.-H., & Huang, H.-Y. (2022). Mangosteen Pericarp Extract Supplementation Boosts Antioxidant Status via Rebuilding Gut Microbiota to Attenuate Motor Deficit in 6-OHDA-Induced Parkinson’s Disease. Antioxidants, 11(12), 2396. https://doi.org/10.3390/antiox11122396






