Is the Gut Microbiome Implicated in the Excess Risk of Hypertension Associated with Obstructive Sleep Apnea? A Contemporary Review
Abstract
1. Introduction
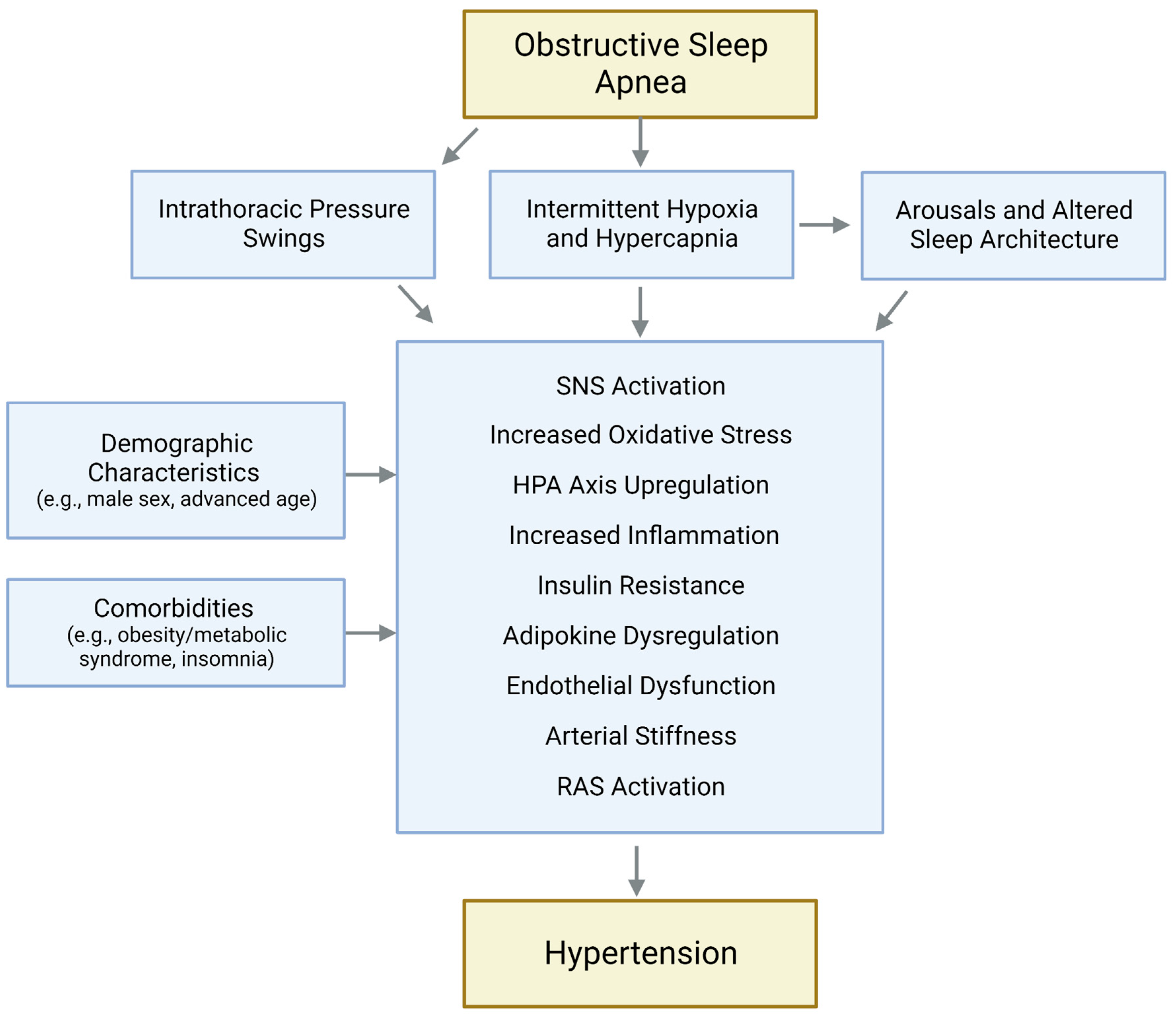
2. Effects of OSA on Blood Pressure
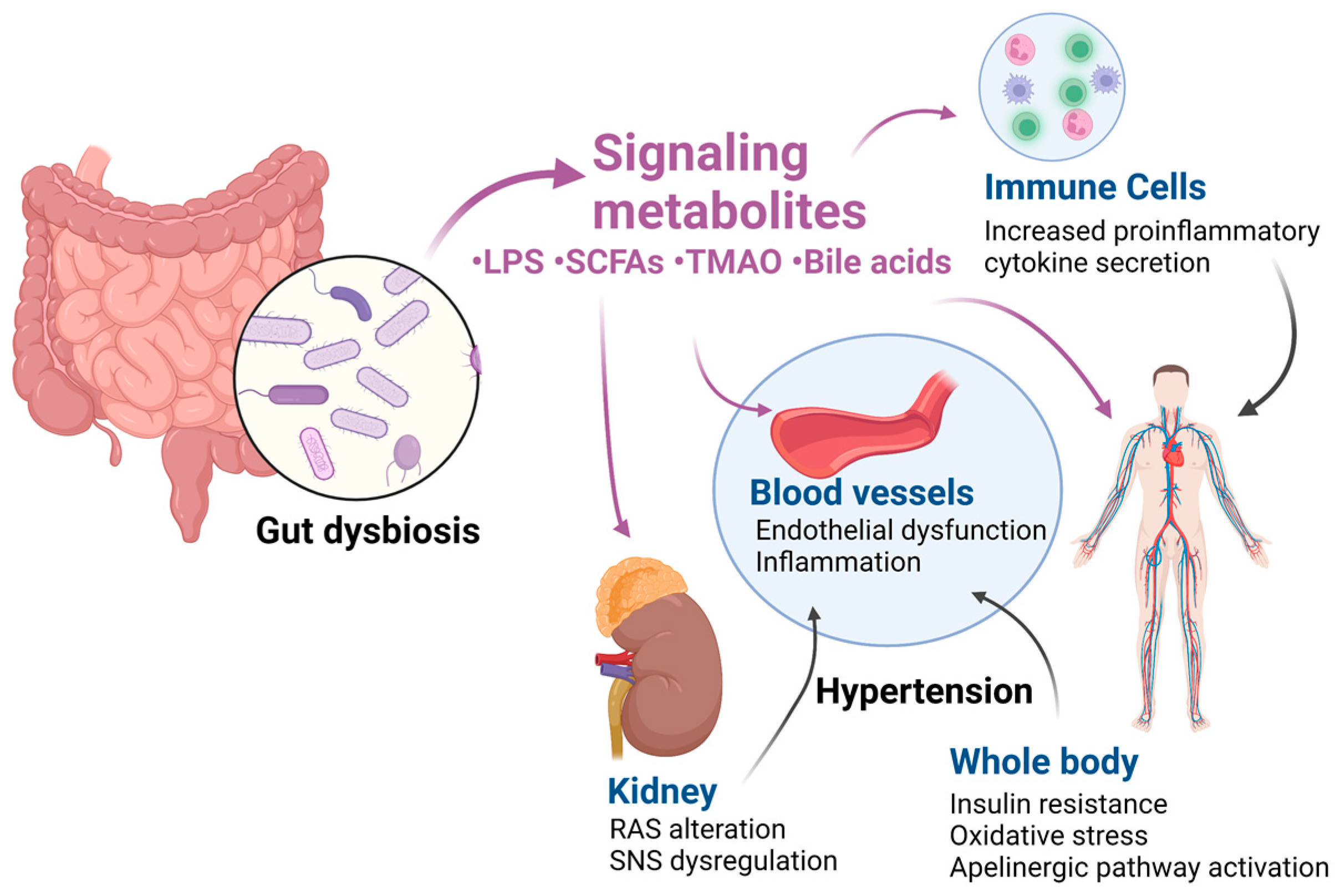
3. Effects of Gut Dysbiosis on Blood Pressure
4. Altered Gut Microbiome in OSA: Implications for Hypertension Risk
4.1. Clinical Studies
4.2. Preclinical Studies
5. Therapeutic Considerations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K. Obstructive sleep apnea and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2021, 144, e56–e67. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y. Heart disease and stroke statistics—2022 update: A report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Fond, G.; Boukouaci, W.; Chevalier, G.; Regnault, A.; Eberl, G.; Hamdani, N.; Dickerson, F.; Macgregor, A.; Boyer, L.; Dargel, A. The “psychomicrobiotic”: Targeting microbiota in major psychiatric disorders: A systematic review. Pathol. Biol. 2015, 63, 35–42. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [PubMed]
- Mancia, G.; De Backer, G.; Dominiczak, A.; Cifkova, R.; Fagard, R.; Germano, G.; Grassi, G.; Heagerty, A.M.; Kjeldsen, S.E.; Laurent, S. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2007, 28, 1462–1536. [Google Scholar] [CrossRef] [PubMed]
- Nieto, F.J.; Young, T.B.; Lind, B.K.; Shahar, E.; Samet, J.M.; Redline, S.; D’agostino, R.B.; Newman, A.B.; Lebowitz, M.D.; Pickering, T.G. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA 2000, 283, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Peppard, P.; Palta, M.; Hla, K.M.; Finn, L.; Morgan, B.; Skatrud, J. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch. Intern. Med. 1997, 157, 1746–1752. [Google Scholar] [CrossRef]
- Lavie, P.; Herer, P.; Hoffstein, V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: Population study. BMJ 2000, 320, 479–482. [Google Scholar] [CrossRef]
- Bixler, E.O.; Vgontzas, A.N.; Lin, H.-M.; Ten Have, T.; Leiby, B.E.; Vela-Bueno, A.; Kales, A. Association of hypertension and sleep-disordered breathing. Arch. Intern. Med. 2000, 160, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Huang, Y.; Peng, B.; Zhang, X.; Wu, Q.; Sang, Y.; Luo, Y.; Liu, X.; Chen, Q.; Tian, K. Relationship between obstructive sleep apnoea syndrome and essential hypertension: A dose–response meta-analysis. Sleep Med. 2018, 47, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Gonzaga, C.C.; Gaddam, K.K.; Ahmed, M.I.; Pimenta, E.; Thomas, S.J.; Harding, S.M.; Oparil, S.; Cofield, S.S.; Calhoun, D.A. Severity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertension. J. Clin. Sleep Med. 2010, 6, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Logan, A.G.; Perlikowski, S.M.; Mente, A.; Tisler, A.; Tkacova, R.; Niroumand, M.; Leung, R.S.; Bradley, T.D. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J. Hypertens. 2001, 19, 2271–2277. [Google Scholar] [CrossRef]
- Calhoun, D.A.; Nishizaka, M.K.; Zaman, M.A.; Harding, S.M. Aldosterone excretion among subjects with resistant hypertension and symptoms of sleep apnea. Chest 2004, 125, 112–117. [Google Scholar] [CrossRef]
- Cuspidi, C.; Tadic, M.; Sala, C.; Gherbesi, E.; Grassi, G.; Mancia, G. Blood pressure non-dipping and obstructive sleep apnea syndrome: A meta-analysis. J. Clin. Med. 2019, 8, 1367. [Google Scholar] [CrossRef]
- Seif, F.; Patel, S.R.; Walia, H.K.; Rueschman, M.; Bhatt, D.L.; Blumenthal, R.S.; Quan, S.F.; Gottlieb, D.J.; Lewis, E.F.; Patil, S.P. Obstructive sleep apnea and diurnal nondipping hemodynamic indices in patients at increased cardiovascular risk. J. Hypertens. 2014, 32, 267. [Google Scholar] [CrossRef]
- Mokhlesi, B.; Finn, L.A.; Hagen, E.W.; Young, T.; Hla, K.M.; Van Cauter, E.; Peppard, P.E. Obstructive sleep apnea during REM sleep and hypertension. results of the Wisconsin Sleep Cohort. Am. J. Respir. Crit. Care Med. 2014, 190, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Azarbarzin, A.; Wang, R.; Djonlagic, I.E.; Punjabi, N.M.; Zee, P.C.; Koo, B.B.; Soliman, E.Z.; Younes, M.; Redline, S. Association of novel measures of sleep disturbances with blood pressure: The Multi-Ethnic Study of Atherosclerosis. Thorax 2020, 75, 57–63. [Google Scholar] [CrossRef]
- Ren, R.; Covassin, N.; Yang, L.; Li, Y.; Zhang, Y.; Zhou, J.; Tan, L.; Li, T.; Li, X.; Wang, Y. Objective but not subjective short sleep duration is associated with hypertension in obstructive sleep apnea. Hypertension 2018, 72, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Li, Y.; Zhang, J.; Zhou, J.; Sun, Y.; Tan, L.; Li, T.; Wing, Y.-K.; Tang, X. Obstructive sleep apnea with objective daytime sleepiness is associated with hypertension. Hypertension 2016, 68, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Cowie, M.R.; Linz, D.; Redline, S.; Somers, V.K.; Simonds, A.K. Sleep disordered breathing and cardiovascular disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2021, 78, 608–624. [Google Scholar] [CrossRef]
- Venkataraman, S.; Vungarala, S.; Covassin, N.; Somers, V.K. Sleep apnea, hypertension and the sympathetic nervous system in the adult population. J. Clin. Med. 2020, 9, 591. [Google Scholar] [CrossRef]
- Salman, L.A.; Shulman, R.; Cohen, J.B. Obstructive sleep apnea, hypertension, and cardiovascular risk: Epidemiology, pathophysiology, and management. Curr. Cardiol. Rep. 2020, 22, 6. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J. Gut dysbiosis is linked to hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef]
- Silveira-Nunes, G.; Durso, D.F.; de Oliveira, L.R.A., Jr.; Cunha, E.H.M.; Maioli, T.U.; Vieira, A.T.; Speziali, E.; Corrêa-Oliveira, R.; Martins-Filho, O.A.; Teixeira-Carvalho, A. Hypertension is associated with intestinal microbiota dysbiosis and inflammation in a Brazilian population. Front. Pharmacol. 2020, 11, 258. [Google Scholar] [CrossRef]
- Yan, Q.; Gu, Y.; Li, X.; Yang, W.; Jia, L.; Chen, C.; Han, X.; Huang, Y.; Zhao, L.; Li, P. Alterations of the gut microbiome in hypertension. Front. Cell. Infect. Microbiol. 2017, 7, 381. [Google Scholar] [CrossRef]
- Kim, S.; Goel, R.; Kumar, A.; Qi, Y.; Lobaton, G.; Hosaka, K.; Mohammed, M.; Handberg, E.M.; Richards, E.M.; Pepine, C.J. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin. Sci. 2018, 132, 701–718. [Google Scholar] [CrossRef]
- Sun, S.; Lulla, A.; Sioda, M.; Winglee, K.; Wu, M.C.; Jacobs, D.R., Jr.; Shikany, J.M.; Lloyd-Jones, D.M.; Launer, L.J.; Fodor, A.A. Gut microbiota composition and blood pressure: The CARDIA study. Hypertension 2019, 73, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Palmu, J.; Salosensaari, A.; Havulinna, A.S.; Cheng, S.; Inouye, M.; Jain, M.; Salido, R.A.; Sanders, K.; Brennan, C.; Humphrey, G.C. Association between the gut microbiota and blood pressure in a population cohort of 6953 individuals. J. Am. Heart Assoc. 2020, 9, e016641. [Google Scholar] [CrossRef] [PubMed]
- Adnan, S.; Nelson, J.W.; Ajami, N.J.; Venna, V.R.; Petrosino, J.F.; Bryan, R.M., Jr.; Durgan, D.J. Alterations in the gut microbiota can elicit hypertension in rats. Physiol. Genom. 2017, 49, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.E.; Crawford, M.s.; Jasbi, P.; Fessler, S.; Sweazea, K.L. Lipopolysaccharide and the gut microbiota: Considering structural variation. FEBS Lett. 2022, 596, 849–875. [Google Scholar] [CrossRef] [PubMed]
- Tucureanu, M.M.; Rebleanu, D.; Constantinescu, C.A.; Deleanu, M.; Voicu, G.; Butoi, E.; Calin, M.; Manduteanu, I. Lipopolysaccharide-induced inflammation in monocytes/macrophages is blocked by liposomal delivery of Gi-protein inhibitor. Int. J. Nanomed. 2018, 13, 63. [Google Scholar] [CrossRef]
- Grylls, A.; Seidler, K.; Neil, J. Link between microbiota and hypertension: Focus on LPS/TLR4 pathway in endothelial dysfunction and vascular inflammation, and therapeutic implication of probiotics. Biomed. Pharmacother. 2021, 137, 111334. [Google Scholar] [CrossRef]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Pluznick, J.L. Microbial short-chain fatty acids and blood pressure regulation. Curr. Hypertens. Rep. 2017, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- González-Bosch, C.; Boorman, E.; Zunszain, P.A.; Mann, G.E. Short-chain fatty acids as modulators of redox signaling in health and disease. Redox Biol. 2021, 47, 102165. [Google Scholar] [CrossRef]
- Juraschek, S.P.; Bower, J.K.; Selvin, E.; Subash Shantha, G.P.; Hoogeveen, R.C.; Ballantyne, C.M.; Young, J.H. Plasma lactate and incident hypertension in the atherosclerosis risk in communities study. Am. J. Hypertens. 2015, 28, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Jansson, P.; Larsson, A.; Lönnroth, P. Relationship between blood pressure, metabolic variables and blood flow in obese subjects with or without non-insulin-dependent diabetes mellitus. Eur. J. Clin. Investig. 1998, 28, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jia, K.; Ren, Z.; Sun, J.; Pan, L.-L. PRMT5 critically mediates TMAO-induced inflammatory response in vascular smooth muscle cells. Cell Death Dis. 2022, 13, 299. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, R.A.; Kuliczkowski, W. Trimethylamine N-oxide in cardiovascular disease. Adv. Clin. Exp. Med. 2022, 31, 913–925. [Google Scholar] [CrossRef]
- Jiang, S.; Shui, Y.; Cui, Y.; Tang, C.; Wang, X.; Qiu, X.; Hu, W.; Fei, L.; Li, Y.; Zhang, S. Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II–induced hypertension. Redox Biol. 2021, 46, 102115. [Google Scholar] [CrossRef]
- Mell, B.; Jala, V.R.; Mathew, A.V.; Byun, J.; Waghulde, H.; Zhang, Y.; Haribabu, B.; Vijay-Kumar, M.; Pennathur, S.; Joe, B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol. Genom. 2015, 47, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Wilck, N.; Matus, M.G.; Kearney, S.M.; Olesen, S.W.; Forslund, K.; Bartolomaeus, H.; Haase, S.; Mähler, A.; Balogh, A.; Markó, L. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017, 551, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, Q.; Wu, H.; Tang, T.; Zhao, T.; Li, Z. The dysbiosis gut microbiota induces the alternation of metabolism and imbalance of Th17/Treg in OSA patients. Arch. Microbiol. 2022, 204, 217. [Google Scholar] [CrossRef]
- Ko, C.-Y.; Liu, Q.-Q.; Su, H.-Z.; Zhang, H.-P.; Fan, J.-M.; Yang, J.-H.; Hu, A.-K.; Liu, Y.-Q.; Chou, D.; Zeng, Y.-M. Gut microbiota in obstructive sleep apnea–hypopnea syndrome: Disease-related dysbiosis and metabolic comorbidities. Clin. Sci. 2019, 133, 905–917. [Google Scholar] [CrossRef]
- Ko, C.Y.; Fan, J.M.; Hu, A.K.; Su, H.Z.; Yang, J.H.; Huang, L.M.; Yan, F.R.; Zhang, H.P.; Zeng, Y.M. Disruption of sleep architecture in Prevotella enterotype of patients with obstructive sleep apnea-hypopnea syndrome. Brain Behav. 2019, 9, e01287. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, T.; Shao, C.; Gao, W.; Wang, M.; Dong, Y.; Wang, X.; Lu, F.; Li, D.; Tan, H. Obstructive sleep apnea is related to alterations in fecal microbiome and impaired intestinal barrier function. Sci. Rep. 2023, 13, 778. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhao, T.; Hu, H.; Zhang, W.; Hua, X. Association study of gut flora in coronary heart disease through high-throughput sequencing. BioMed Res. Int. 2017, 2017, 3796359. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, J.; Liu, H.; Tang, Y.; Zhan, Q.; Lai, W.; Ao, L.; Meng, X.; Ren, H.; Xu, D. The intestinal microbiota associated with cardiac valve calcification differs from that of coronary artery disease. Atherosclerosis 2019, 284, 121–128. [Google Scholar] [CrossRef]
- Bikov, A.; Szabo, H.; Piroska, M.; Kunos, L.; Szily, M.; Ligeti, B.; Makra, N.; Szabo, D.; Tarnoki, D.L.; Tarnoki, A.D. Gut Microbiome in Patients with Obstructive Sleep Apnoea. Appl. Sci. 2022, 12, 2007. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; Iglesias-Aguirre, C.E.; Meoro, A.; Selma, M.V.; Espín, J.C. There is no distinctive gut microbiota signature in the metabolic syndrome: Contribution of cardiovascular disease risk factors and associated medication. Microorganisms 2020, 8, 416. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, F.; Shen, Y.; Chen, Y.; Ma, J. Sleep apnea is associated with the increase of certain genera of Ruminococcaceae and Lachnospiraceae in the gut microbiome of hypertensive patients. Expert Rev. Respir. Med. 2022, 16, 1247–1256. [Google Scholar] [CrossRef]
- Lu, D.; Xu, S.; Dai, P.; Wu, L.; Zhang, H.; Zhou, B. Gut microbiota in hypertensive patients with versus without obstructive sleep apnea. J. Clin. Hypertens. 2022, 24, 1598–1605. [Google Scholar] [CrossRef]
- Ko, C.-Y.; Su, H.-Z.; Zhang, L.; Zeng, Y.-M. Disturbances of the gut microbiota, sleep architecture, and mTOR signaling pathway in patients with severe obstructive sleep apnea-associated hypertension. Int. J. Hypertens. 2021, 2021, 9877053. [Google Scholar] [CrossRef]
- Sciarretta, S.; Forte, M.; Frati, G.; Sadoshima, J. New insights into the role of mTOR signaling in the cardiovascular system. Circ. Res. 2018, 122, 489–505. [Google Scholar] [CrossRef]
- Zierer, J.; Jackson, M.A.; Kastenmüller, G.; Mangino, M.; Long, T.; Telenti, A.; Mohney, R.P.; Small, K.S.; Bell, J.T.; Steves, C.J. The fecal metabolome as a functional readout of the gut microbiome. Nat. Genet. 2018, 50, 790–795. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, P.; Lin, J.; Han, C.; Jiao, J.; Zuo, K.; Chen, M.; Yang, X.; Cai, J.; Jiang, H. Characterization of fecal metabolome changes in patients with obstructive sleep apnea. J. Clin. Sleep Med. 2022, 18, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Indias, I.; Torres, M.; Montserrat, J.M.; Sanchez-Alcoholado, L.; Cardona, F.; Tinahones, F.J.; Gozal, D.; Poroyko, V.A.; Navajas, D.; Queipo-Ortuño, M.I. Intermittent hypoxia alters gut microbiota diversity in a mouse model of sleep apnoea. Eur. Respir. J. 2015, 45, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Ericsson, A.; Qiao, Z.; Almendros, I.; Farré, R.; Gozal, D. Circulating exosomes and gut microbiome induced insulin resistance in mice exposed to intermittent hypoxia: Effects of physical activity. EBioMedicine 2021, 64, 103208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, H.; Niu, Y.; Yang, X.; Li, Z.; Wang, K.; Bi, H.; Pang, X. Chronic intermittent hypoxia induces gut microbial dysbiosis and infers metabolic dysfunction in mice. Sleep Med. 2022, 91, 84–92. [Google Scholar] [CrossRef]
- Wu, J.; Sun, X.; Wu, Q.; Li, H.; Li, L.; Feng, J.; Zhang, S.; Xu, L.; Li, K.; Li, X. Disrupted intestinal structure in a rat model of intermittent hypoxia. Mol. Med. Rep. 2016, 13, 4407–4413. [Google Scholar] [CrossRef]
- Kameyama, K.; Itoh, K. Intestinal colonization by a Lachnospiraceae bacterium contributes to the development of diabetes in obese mice. Microbes Environ. 2014, 29, 427–430. [Google Scholar] [CrossRef]
- Muñiz Pedrogo, D.A.; Chen, J.; Hillmann, B.; Jeraldo, P.; Al-Ghalith, G.; Taneja, V.; Davis III, J.M.; Knights, D.; Nelson, H.; Faubion, W.A. An increased abundance of Clostridiaceae characterizes arthritis in inflammatory bowel disease and rheumatoid arthritis: A cross-sectional study. Inflamm. Bowel Dis. 2019, 25, 902–913. [Google Scholar] [CrossRef]
- Tripathi, A.; Melnik, A.V.; Xue, J.; Poulsen, O.; Meehan, M.J.; Humphrey, G.; Jiang, L.; Ackermann, G.; McDonald, D.; Zhou, D. Intermittent hypoxia and hypercapnia, a hallmark of obstructive sleep apnea, alters the gut microbiome and metabolome. Msystems 2018, 3, e00020-18. [Google Scholar] [CrossRef]
- Xue, J.; Allaband, C.; Zhou, D.; Poulsen, O.; Martino, C.; Jiang, L.; Tripathi, A.; Elijah, E.; Dorrestein, P.C.; Knight, R. Influence of intermittent hypoxia/hypercapnia on atherosclerosis, gut microbiome, and metabolome. Front. Physiol. 2021, 12, 663950. [Google Scholar] [CrossRef] [PubMed]
- Badran, M.; Khalyfa, A.; Ericsson, A.C.; Puech, C.; McAdams, Z.; Bender, S.B.; Gozal, D. Gut microbiota mediate vascular dysfunction in a murine model of sleep apnoea: Effect of probiotics. Eur. Respir. J. 2023, 61, 2200002. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Xue, K.; Gao, F.; Li, C.; Fang, H. Elevated reactivity of Apelin inhibited renal fibrosis induced by chronic intermittent hypoxia. Arch. Biochem. Biophys. 2021, 711, 109021. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, R.; Kappel, B.A.; Carnevale, D.; Cavalera, M.; Mavilio, M.; Arisi, I.; Fardella, V.; Cifelli, G.; Casagrande, V.; Rizza, S. TIMP3 interplays with apelin to regulate cardiovascular metabolism in hypercholesterolemic mice. Mol. Metab. 2015, 4, 741–752. [Google Scholar] [CrossRef]
- Pitkin, S.L.; Maguire, J.J.; Bonner, T.I.; Davenport, A.P. International Union of Basic and Clinical Pharmacology. LXXIV. Apelin receptor nomenclature, distribution, pharmacology, and function. Pharmacol. Rev. 2010, 62, 331–342. [Google Scholar] [CrossRef]
- Geurts, L.; Lazarevic, V.; Derrien, M.; Everard, A.; Van Roye, M.; Knauf, C.; Valet, P.; Girard, M.; Muccioli, G.G.; François, P. Altered gut microbiota and endocannabinoid system tone in obese and diabetic leptin-resistant mice: Impact on apelin regulation in adipose tissue. Front. Microbiol. 2011, 2, 149. [Google Scholar] [CrossRef]
- Allaband, C.; Lingaraju, A.; Martino, C.; Russell, B.; Tripathi, A.; Poulsen, O.; Dantas Machado, A.C.; Zhou, D.; Xue, J.; Elijah, E. Intermittent hypoxia and hypercapnia alter diurnal rhythms of luminal gut microbiome and metabolome. Msystems 2021, 6, e00116-21. [Google Scholar] [CrossRef]
- Poroyko, V.A.; Carreras, A.; Khalyfa, A.; Khalyfa, A.A.; Leone, V.; Peris, E.; Almendros, I.; Gileles-Hillel, A.; Qiao, Z.; Hubert, N. Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci. Rep. 2016, 6, 35405. [Google Scholar] [CrossRef]
- Khannous-Lleiffe, O.; Willis, J.R.; Saus, E.; Cabrera-Aguilera, I.; Almendros, I.; Farré, R.; Gozal, D.; Farré, N.; Gabaldón, T. A mouse model suggests that heart failure and its common comorbidity sleep fragmentation have no synergistic impacts on the gut microbiome. Microorganisms 2021, 9, 641. [Google Scholar] [CrossRef]
- Triplett, J.; Ellis, D.; Braddock, A.; Roberts, E.; Ingram, K.; Perez, E.; Short, A.; Brown, D.; Hutzley, V.; Webb, C. Temporal and region-specific effects of sleep fragmentation on gut microbiota and intestinal morphology in Sprague Dawley rats. Gut Microbes 2020, 11, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Yuan, S.; Zhang, J. The interplay between sleep and gut microbiota. Brain Res. Bull. 2022, 180, 131–146. [Google Scholar] [CrossRef]
- Badran, M.; Khalyfa, A.; Ericsson, A.; Gozal, D. Fecal microbiota transplantation from mice exposed to chronic intermittent hypoxia elicits sleep disturbances in naïve mice. Exp. Neurol. 2020, 334, 113439. [Google Scholar] [CrossRef] [PubMed]
- Durgan, D.J.; Ganesh, B.P.; Cope, J.L.; Ajami, N.J.; Phillips, S.C.; Petrosino, J.F.; Hollister, E.B.; Bryan, R.M., Jr. Role of the gut microbiome in obstructive sleep apnea–induced hypertension. Hypertension 2016, 67, 469–474. [Google Scholar] [CrossRef]
- Wang, F.; Zou, J.; Xu, H.; Huang, W.; Zhang, X.; Wei, Z.; Li, X.; Liu, Y.; Zou, J.; Liu, F. Effects of chronic intermittent hypoxia and chronic sleep fragmentation on gut microbiome, serum metabolome, liver and adipose tissue morphology. Front. Endocrinol. 2022, 13, 820939. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, T.; Wu, H.; Shi, H.; Bai, J.; Zhao, W.; Jiang, D.; Jiang, X. Lactobacillus rhamnosus GG strain mitigated the development of obstructive sleep apnea-induced hypertension in a high salt diet via regulating TMAO level and CD4+ T cell induced-type I inflammation. Biomed. Pharmacother. 2019, 112, 108580. [Google Scholar] [CrossRef] [PubMed]
- Labarca, G.; Dreyse, J.; Drake, L.; Jorquera, J.; Barbe, F. Efficacy of continuous positive airway pressure (CPAP) in the prevention of cardiovascular events in patients with obstructive sleep apnea: Systematic review and meta-analysis. Sleep Med. Rev. 2020, 52, 101312. [Google Scholar] [CrossRef]
- Fava, C.; Dorigoni, S.; Dalle Vedove, F.; Danese, E.; Montagnana, M.; Guidi, G.C.; Narkiewicz, K.; Minuz, P. Effect of CPAP on blood pressure in patients with OSA/hypopnea: A systematic review and meta-analysis. Chest 2014, 145, 762–771. [Google Scholar] [CrossRef]
- Pengo, M.F.; Soranna, D.; Giontella, A.; Perger, E.; Mattaliano, P.; Schwarz, E.I.; Lombardi, C.; Bilo, G.; Zambon, A.; Steier, J. Obstructive sleep apnoea treatment and blood pressure: Which phenotypes predict a response? A systematic review and meta-analysis. Eur. Respir. J. 2020, 55, 1901945. [Google Scholar] [CrossRef]
- Labarca, G.; Schmidt, A.; Dreyse, J.; Jorquera, J.; Enos, D.; Torres, G.; Barbe, F. Efficacy of continuous positive airway pressure (CPAP) in patients with obstructive sleep apnea (OSA) and resistant hypertension (RH): Systematic review and meta-analysis. Sleep Med. Rev. 2021, 58, 101446. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Torres, M.; Sanchez-Alcoholado, L.; Cardona, F.; Almendros, I.; Gozal, D.; Montserrat, J.M.; Queipo-Ortuño, M.I.; Farré, R. Normoxic recovery mimicking treatment of sleep apnea does not reverse intermittent hypoxia-induced bacterial dysbiosis and low-grade endotoxemia in mice. Sleep 2016, 39, 1891–1897. [Google Scholar] [CrossRef] [PubMed]
- Robles-Vera, I.; Toral, M.; Romero, M.; Jiménez, R.; Sánchez, M.; Pérez-Vizcaíno, F.; Duarte, J. Antihypertensive effects of probiotics. Curr. Hypertens. Rep. 2017, 19, 26. [Google Scholar] [CrossRef]
- Khalesi, S.; Sun, J.; Buys, N.; Jayasinghe, R. Effect of probiotics on blood pressure: A systematic review and meta-analysis of randomized, controlled trials. Hypertension 2014, 64, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Robles-Vera, I.; Toral, M.; de la Visitación, N.; Sánchez, M.; Gómez-Guzmán, M.; Romero, M.; Yang, T.; Izquierdo-Garcia, J.L.; Jiménez, R.; Ruiz-Cabello, J. Probiotics prevent dysbiosis and the rise in blood pressure in genetic hypertension: Role of short-chain fatty acids. Mol. Nutr. Food Res. 2020, 64, 1900616. [Google Scholar] [CrossRef]
- Xu, H.; Wang, J.; Cai, J.; Feng, W.; Wang, Y.; Liu, Q.; Cai, L. Protective effect of Lactobacillus rhamnosus GG and its supernatant against myocardial dysfunction in obese mice exposed to intermittent hypoxia is associated with the activation of Nrf2 pathway. Int. J. Biol. Sci. 2019, 15, 2471. [Google Scholar] [CrossRef]
- Ganesh, B.P.; Nelson, J.W.; Eskew, J.R.; Ganesan, A.; Ajami, N.J.; Petrosino, J.F.; Bryan, M., Jr.; Durgan, D.J. Prebiotics, probiotics, and acetate supplementation prevent hypertension in a model of obstructive sleep apnea. Hypertension 2018, 72, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Nie, X.-L.; Zhang, J.-J. The effect of probiotics supplementation on blood pressure: A systemic review and meta-analysis. Lipids Health Dis. 2020, 19, 79. [Google Scholar] [CrossRef]
- Marques, F.Z.; Nelson, E.; Chu, P.-Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef]
- O’Donnell, J.A.; Zheng, T.; Meric, G.; Marques, F.Z. The gut microbiome and hypertension. Nat. Rev. Nephrol. 2023, 19, 153–167. [Google Scholar] [CrossRef]
- Lu, D.; Wang, J.; Zhang, H.; Shan, Q.; Zhou, B. Renal denervation improves chronic intermittent hypoxia induced hypertension and cardiac fibrosis and balances gut microbiota. Life Sci. 2020, 262, 118500. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munir, S.S.; Sert Kuniyoshi, F.H.; Singh, P.; Covassin, N. Is the Gut Microbiome Implicated in the Excess Risk of Hypertension Associated with Obstructive Sleep Apnea? A Contemporary Review. Antioxidants 2023, 12, 866. https://doi.org/10.3390/antiox12040866
Munir SS, Sert Kuniyoshi FH, Singh P, Covassin N. Is the Gut Microbiome Implicated in the Excess Risk of Hypertension Associated with Obstructive Sleep Apnea? A Contemporary Review. Antioxidants. 2023; 12(4):866. https://doi.org/10.3390/antiox12040866
Chicago/Turabian StyleMunir, Sanah S., Fatima H. Sert Kuniyoshi, Prachi Singh, and Naima Covassin. 2023. "Is the Gut Microbiome Implicated in the Excess Risk of Hypertension Associated with Obstructive Sleep Apnea? A Contemporary Review" Antioxidants 12, no. 4: 866. https://doi.org/10.3390/antiox12040866
APA StyleMunir, S. S., Sert Kuniyoshi, F. H., Singh, P., & Covassin, N. (2023). Is the Gut Microbiome Implicated in the Excess Risk of Hypertension Associated with Obstructive Sleep Apnea? A Contemporary Review. Antioxidants, 12(4), 866. https://doi.org/10.3390/antiox12040866







