Vascularization Strategies in 3D Cell Culture Models: From Scaffold-Free Models to 3D Bioprinting
Abstract
1. Introduction
2. Three-Dimensional Cell Cultures
2.1. Scaffold-Free 3D Cell Cultures
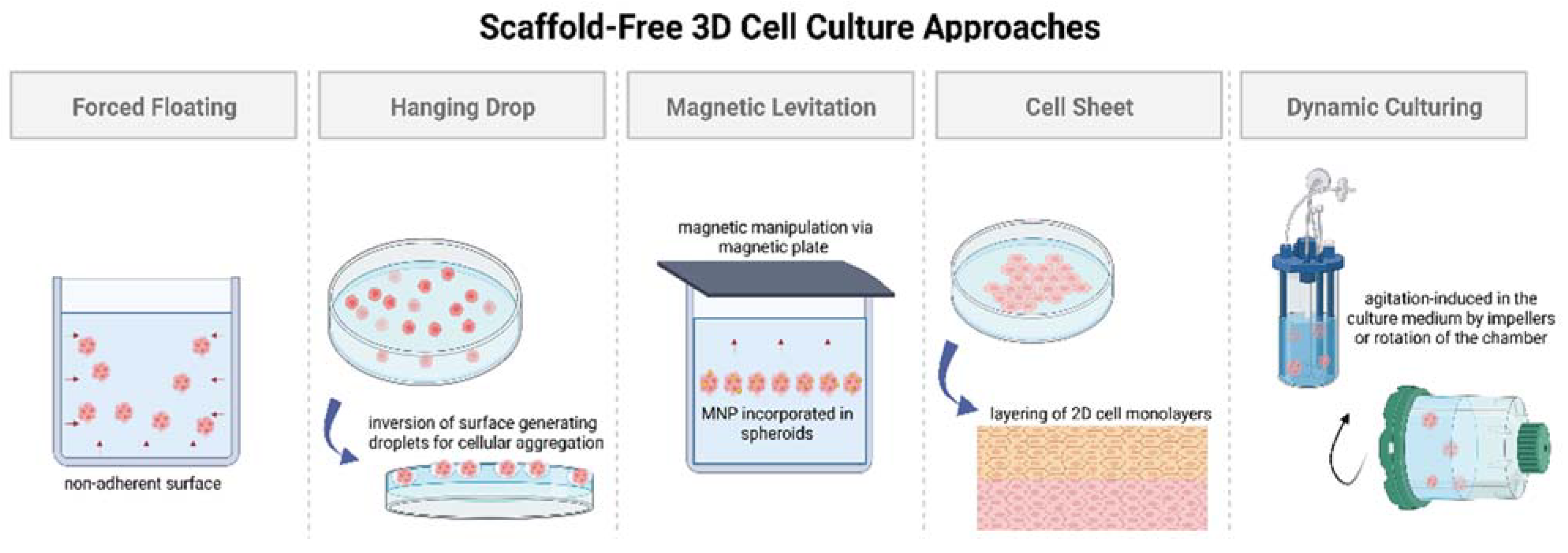
2.1.1. Static 3D Cell Culture
- The forced floating method, or liquid overlay method, employs uncoated low-adhesion plates or ultra-low attachment (ULA) plates coated with a hydrophilic polymer [34]. The negatively charged inert polymer coating reduces protein adsorption, inhibiting cellular attachment [35]. The ease of preparation and maintenance of forced floating cultures and the possibility of automation makes it suitable for high-throughput screening [32,34,36]. However, this method encounters issues with the variability of the size and shape of the spheroid models, increasing the difficulty in obtaining reproducible results [33].
- The hanging drop method involves the inversion of a culture plate containing a cell suspension to create droplets [37,38]. The inversion creates a free liquid–air interface where the micro-adhesive force from the substrate surface is higher than the weight of the accumulated cells in the droplet, resulting in spheroids [34,37,38]. Although this simplistic technique has shown around 90% reproducibility rate in the formation of multicellular tumor spheroids (MCTs), difficulties remain in medium exchange and its application in cell-based assays [32,37,38].
- Magnetic levitation is a suspension culture technology that aims to address the biodegradability issue surrounding porous scaffold and protein matrices in 3D cell cultures [38,39,40,41]. The technique involves the magnetic manipulation of bioinorganic hydrogel incorporated with magnetic nanoparticles (MNPs), such as magnetic iron oxide (Fe3O4, magnetite) and gold nanoparticles [39]. Incorporation is achieved via overnight incubation, and the cells are cultured by levitating them using a magnet placed above the plate [37]. Similar to the hanging drop method, magnetic manipulation directs the cells towards the air–liquid interface, taking advantage of the tendency of cells to aggregate and form spheroids. The magnetic levitation method results in spheroid formation within 16 h without requiring a specialized medium [42], making it suitable for high-throughput screening studies.
- Cell sheet engineering is a tissue-engineering approach towards scaffold-free 3D cell cultures that has showcased safety and efficacy in preclinical and clinical trials for developing implantable devices [43]. The manual gathering of suspended cells into 3D tissues eliminates the issues of gap junctions and unfavorable host responses towards biomaterials as observed in scaffold-based approaches [44]. The cell sheet engineering process involves building 3D tissues by layering 2D cellular monolayers known as “cell sheets” on a surface coated with a temperature-sensitive polymer [44]. The technique offers co-culturing and promotes the development of prevascularized networks through efficient cell–cell and cell–ECM interactions [43]. However, necrosis has still been observed in long-term cell cultures in the middle layers, especially when the tissue constructs go beyond a thickness greater than that of four-layered cell sheets (>100 μm), suggesting the need for vascularization due to insufficient ECM formation [44,45]. Achieving vascularization in cell sheets via co-culturing with endothelial cells (ECs), along with the incorporation of advanced bioreactor systems, is crucial for the creation of thicker cell sheet constructs [45].
| Static 3D Culturing Technique | Highlights | Limitations |
|---|---|---|
| Forced floating | Ease of preparation and maintenance, high throughput, and suitable for automation | Variability of size and shape of spheroids and difficulty in obtaining reproducible results |
| Hanging drop | Favorable reproducibility rate | Difficulties in medium exchange and incorporation with cell-based assays |
| Magnetic levitation | Uniformly shaped spheroids, no specialized medium, and high throughput | Requires specialized equipment and is a time-consuming process |
| Cell sheet engineering | Promotes prevascularization, and efficient cell–cell and cell–ECM interactions | Necrosis observed in larger constructs and sheets not necessarily replicating the native tissue microarchitecture |
2.1.2. Dynamic 3D Cell Culture
2.2. Scaffold-Based 3D Cell Cultures
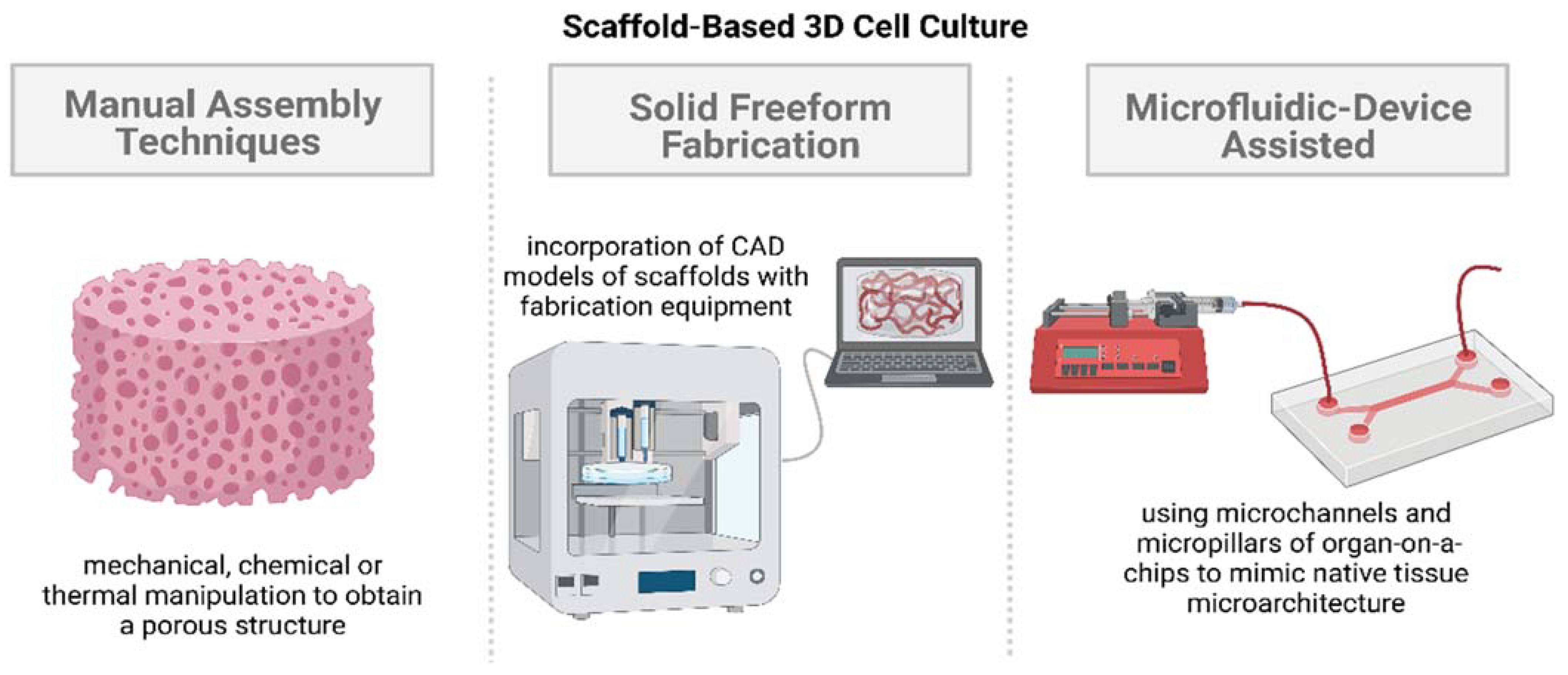
2.2.1. Manual Assembly Techniques
- Freeze-drying involves the creation of porous scaffolds through a controlled solvent sublimation process. The scaffold is frozen, and the embedded solvent, typically water, undergoes sublimation, leaving behind a porous scaffold structure [60]. The pore sizes, porosity, pore distribution, and connectivity of the scaffold are mainly influenced by the cooling rate and sublimation rate, controlled by altering the temperature and pressure conditions [56,61]. Cells are then seeded onto the porous freeze-dried scaffold and cultured, allowing the biological activities to be analyzed to obtain endpoint measurements.
- Gas foaming can be conducted in several ways, with the main principle involving the nucleation and growth of gas bubbles distributed throughout a polymer [62]. The conventional gas foaming technique involves the addition of a foaming agent, such as sodium bicarbonate, to a polymer in an acidic environment, producing an inert gas, such as carbon dioxide or nitrogen, at low or high pressure [62]. A porous scaffold is obtained as the dispersed gas is removed from the polymer, and cells are then seeded onto the structure and cultured to simulate the tissue microenvironment of interest. Gas foaming is a convenient technique for the fabrication of scaffolds with high porosity and interconnectivity [62]. However, the process has limited application due to the biocompatibility issues arising from the toxicity of the surfactant residue [62].
- Phase separation methodologies are also widely accepted for generating scaffolds showcasing ideal biomechanical properties, high porosity, and interconnectivity. Thermally induced phase separation (TIPS) is a common approach to the fabrication of scaffolds with a hierarchical pore structure using composite polymer matrix or inorganic filler foams [63]. The main principle of this technique is the separation of a homogeneous polymer solution (solid–liquid or liquid–liquid polymer solvent solution) into a polymer-rich phase and a polymerless phase via a change in temperature [63]. The ability to optimize the process parameters, such as the choice of polymer, solvent composition, temperature control, coarsening process, and the incorporation of inorganic particles, provides close control in the final structure of the scaffold, allowing accurate in vivo tissue microenvironment replication to be conducted [64].
- Another manual assembly scaffold-based cell culture approach that is popularly used to mimic bone marrow niche is the solvent-casting and particulate leaching technique (SCPL) [65]. The technique starts with the mixing of a polymer–solvent solution with an insoluble salt [65]. The mixture is heated to evaporate the solvent, leaving behind a salt–polymer composite, which is washed or submerged to leach out the salt to obtain a porous scaffold [65]. The SCPL technique is straightforward and does not require any special, expensive equipment to generate scaffolds with high porosity and interconnectivity but suffers from the scalability and limited bioactive properties of the resulting thin membranes [65,66].
- Shape-changing and self-folding processes to form 3D tubular systems from initial 2D structures have also started to get employed in 3D cell culture systems [67]. Self-rollable elastomeric films can consist of key surface topographical patterns, making possible cell encapsulation and application as tissue building blocks [67,68]. Two-dimensional layers constructed from hydrogels give greater control and customization of key parameters such as degree of swelling, network pore size, crosslinking degree, and stiffness [67]. The technique also permits the co-culturing of multiple cell types in the resulting folded 3D scaffold structure to be performed, giving rise to a hierarchical organisation and internal microvascularization, allowing the accurate replication of native tissues to be performed [67]. However, the overall rolling process can result in physical deformation and requires significant efforts to ensure repeatable results in terms of the final shape of the scaffold.
2.2.2. Solid Freeform Fabrication
- Electrospinning (ES) involves the alteration of an electric field to fabricate continuous thin micro-/nanofibers from microspheres [70]. The conventional ES procedure consists of a solution reservoir connected to a nozzle, high-voltage direct current source, a flow rate controller, and a grounded collector [69,70]. A microsphere is formed at the nozzle tip due to the difference in potential between the nozzle tip and the grounded collector, which gets stretched due to the change in the electric field, forming a conical shape called the Taylor cone [70]. The electrostatic force creates a liquid jet, resulting in a randomly oriented fibrous mat [70]. These continuous fibers form a large surface area-to-volume ratio, making the scaffold ideal for cell attachment, proliferation, and differentiation [70]. Control over process parameters such as solution concentration, nozzle tip and grounded collector distance, and applied voltage allows of the scaffold features to be adjusted to replicate the natural ECM of interest [69].
- Bioprinting is an additive manufacturing technique utilizing 3D printing principles to generate scaffolds using bioinks. Bioinks are synthesized based on the ECM characteristics of the native tissue of interest, consisting of biomaterials (hydrogels as base materials), active biomolecules, and even cells [71]. The ability to incorporate cells within the bioink allows the homogeneous distribution of cells in the scaffold structure to be obtained [71], making the process ideal for 3D cell culture studies. The scaffold structure is fabricated layer by layer based on the instructions outlined in the standard triangle language (STL) file obtained from the CAD model of the native ECM. The bioprinting of scaffolds can be conducted using three techniques: droplet-based, extrusion-based, or laser-based systems [71]. Laser-based systems, such as stereolithography, digital light processing, and two-photon polymerization bioprinting, utilize photo-crosslinking, allowing the accurate, controlled fabrication of in vivo-like vascular structures to be performed owing to the high resolution (≤20 μm [71]) compared with extrusion-based bioprinting. Each bioprinting technique has its own sets of advantages and disadvantages, but the ideal technique is chosen based on the tissue of interest and on whether the spatial resolution of the technique can accurately replicate the tissue microenvironment or not [71].
- Multilayer scaffolds requiring substantial mechanical integrity can also be fabricated using a high-power laser-based system called selective laser sintering (SLS). SLS allows structurally complex scaffolds with controlled pore sizes, porosity, and topology to be fabricated [72]. The main principle of SLS is the fusion of powders (bio-ceramics) based on a CAD model of the scaffold. Conventional SLS is carried out using a carbon dioxide laser, which increases the temperature at the focal point, causing the powder to melt and fuse together [72]. Each layer is scanned by the laser, and the powder bed is lowered by one-layer thickness; the process is repeated to create a multilayer porous structure [72]. SLS has gained popularity over the years in bone tissue engineering, owing to the greater control in fabricating constructs with tunable mechanical properties, interconnected macropores and micropores to achieve vascularization, and sustaining a high density of cells [73].
2.2.3. Microfluidic-Device-Assisted Systems
3. Vascularization Strategies in 3D Cell Culture Models
3.1. Spheroid-Based 3D Cell Culture Models

3.2. Bioprinting-Based 3D Cell Culture Models

3.3. Microfluidic-Device-Based 3D Cell Culture Models
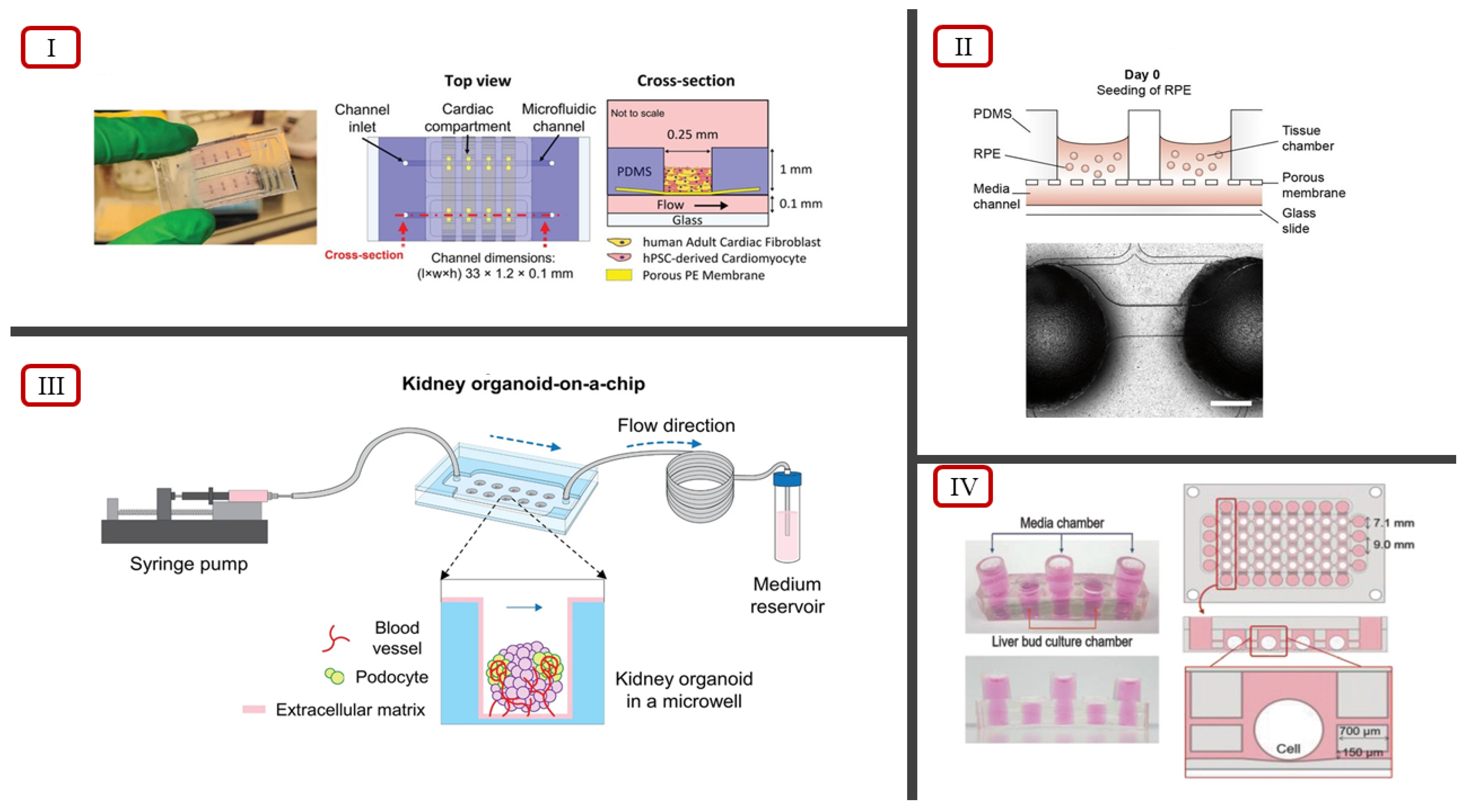
| Three-Dimensional Cell Culture Model | Vascularization Approach | Highlights | References |
|---|---|---|---|
| Spheroid-based | Incorporation of collagen/fibrin hydrogels with MSC/HUVEC spheroids | Enhanced functionality due to the presence of vascularized networks | [79] |
| Culturing SVF-derived cells in EGM2 using forced floating cell culture method | Presence of dense and highly organized vascular networks, showcasing morphology similar to that of in vivo vasculature | [81] | |
| Co-culturing β cells and ECs using magnetic levitation method | Heterogeneous distribution of cells, distinguishable CD31 expression, and significant stimulation of basal insulin secretion | [83] | |
| Seeding HUVECs, hTMSCs, and ADSCs on a micro-patterned hydrogel surface | Six-fold increase in CD31 expression for harvested spheroids, allowing them to be used as building blocks for constructing complex 3D microtissues | [84] | |
| Incorporating 2D cell monolayer of HUVECs with MG-63 spheroids cultured using hanging drop technique | Enhanced vascularization from increased VEGF expression | [85] | |
| Bioprinting-based | Seeding MCTSs on bioprinted blood vessel layer using a cell-ladened bioink with HUVECs and LFs in GAF hydrogel | Significant vascularization and accurate anti-cancer drug treatment results; coherent with results in mice cells | [86] |
| Fusing stem cells and organoids through bioprinting constraints, making them self-organizing building blocks | Ability to showcase multicellular self-organization and control over printing parameters to replicate native ECM | [87] | |
| Dual extrusion head bioprinter, utilizing two bioinks: parenchymal bioink 1 and non-parenchymal bioink 2 | Exhibited similar physiological and metabolic properties, as well as highlighted the need of using primary cell lines for accurate modelling | [88] | |
| Preset extrusion bioprinting, where preset cartridge mimics the structure of a human hepatic lobule | Increased drug resistance and higher levels of albumin, MPR2, and CD31 relative to non-engineered models | [89] | |
| Dynamic flow-based 3D vascularized tumor model consisting of central vasculature and perfusion chamber | Significant angiogenesis and successful perfusion for a physiologically relevant drug and immunotherapy screening platform | [90] | |
| Microfluidic-device-based | Multicellular spheroids using ECs and LFs seeded into fibrin–collagen hydrogel embedded within a microfluidic device | Showcased increased cellular migration, allowing accurate modeling and the studying of cancer metastasis to be performed | [91] |
| Kidney organoid on-a-chip system involving a PDMS chip and organoids cultured in microwells under dynamic flow conditions | Cultured organoids showed increased vascularization and maturation | [92] | |
| Retina on-a-chip system involving a layered microfluidic chip and perfusion through connections via microchannels | First in vitro system to replicate key in vivo physiological features by showcasing interactions between photoreceptors and retinal pigment epithelium | [93] | |
| Bi-compartmental, monolithic heart-on-a-chip device capable of 3D carbon electrodes integration for electrical pacing | Accurate recapitulation of native cardiac tissue via electromechanical stimulations, endothelial monolayer, and selective perfusion | [94] | |
| Mimicking native ECM utilizing a decellularized liver ECM hydrogel in a microfluidic device | Increased drug sensitivity, and mature and functional hepatic state; high-throughput drug screening platform, and capable of multiorgan model studies | [95] |
4. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hay, M.; Thomas, D.W.; Craighead, J.L.; Economides, C.; Rosenthal, J. Clinical Development Success Rates for Investigational Drugs. Nat. Biotechnol. 2014, 32, 40–51. [Google Scholar] [CrossRef]
- Booij, T.H.; Price, L.S.; Danen, E.H.J. 3D Cell-Based Assays for Drug Screens: Challenges in Imaging, Image Analysis, and High-Content Analysis. SLAS Discov. 2019, 24, 615–627. [Google Scholar] [CrossRef]
- Gurski, L.A.; Petrelli, N.J.; Jia, X.; Farach-Carson, M.C. 3D Matrices for Anti-Cancer Drug Testing and Development. Oncol. Issues 2010, 25, 20–25. [Google Scholar] [CrossRef]
- Gurski, L.A.; Jha, A.K.; Zhang, C.; Jia, X.; Farach-Carson, M.C. Hyaluronic Acid-Based Hydrogels as 3D Matrices for in Vitro Evaluation of Chemotherapeutic Drugs Using Poorly Adherent Prostate Cancer Cells. Biomaterials 2009, 30, 6076–6085. [Google Scholar] [CrossRef]
- Feder-Mengus, C.; Ghosh, S.; Reschner, A.; Martin, I.; Spagnoli, G.C. New Dimensions in Tumor Immunology: What Does 3D Culture Reveal? Trends Mol. Med. 2008, 14, 333–340. [Google Scholar] [CrossRef]
- Görlach, A.; Herter, P.; Hentschel, H.; Frosch, P.J.; Acker, H. Effects of nIFN Beta and rIFN Gamma on Growth and Morphology of Two Human Melanoma Cell Lines: Comparison between Two- and Three-Dimensional Culture. Int. J. Cancer 1994, 56, 249–254. [Google Scholar] [CrossRef]
- Santini, M.T.; Rainaldi, G.; Romano, R.; Ferrante, A.; Clemente, S.; Motta, A.; Indovina, P.L. MG-63 Human Osteosarcoma Cells Grown in Monolayer and as Three-Dimensional Tumor Spheroids Present a Different Metabolic Profile: A (1)H NMR Study. FEBS Lett. 2004, 557, 148–154. [Google Scholar] [CrossRef]
- Valente, K.P.; Khetani, S.; Kolahchi, A.R.; Sanati-Nezhad, A.; Suleman, A.; Akbari, M. Microfluidic Technologies for Anticancer Drug Studies. Drug Discov. Today 2017, 22, 1654–1670. [Google Scholar] [CrossRef]
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in Tissue Engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef]
- Lovett, M.; Lee, K.; Edwards, A.; Kaplan, D.L. Vascularization Strategies for Tissue Engineering. Tissue Eng. Part B Rev. 2009, 15, 353–370. [Google Scholar] [CrossRef]
- Novosel, E.C.; Kleinhans, C.; Kluger, P.J. Vascularization Is the Key Challenge in Tissue Engineering. Adv. Drug Deliv. Rev. 2011, 63, 300–311. [Google Scholar] [CrossRef]
- Malda, J.; Rouwkema, J.; Martens, D.E.; Le Comte, E.P.; Kooy, F.K.; Tramper, J.; van Blitterswijk, C.A.; Riesle, J. Oxygen Gradients in Tissue-Engineered PEGT/PBT Cartilaginous Constructs: Measurement and Modeling. Biotechnol. Bioeng. 2004, 86, 9–18. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, Z.; Xiao, L.; Shi, T.; Xiao, H.; Wang, Y.; Li, Y.; Xue, F.; Zeng, W. Review on the Vascularization of Organoids and Organoids-on-a-Chip. Front. Bioeng. Biotechnol. 2021, 9, 637048. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a Dish: Modeling Development and Disease Using Organoid Technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef]
- Satpathy, A.; Datta, P.; Wu, Y.; Ayan, B.; Bayram, E.; Ozbolat, I.T. Developments with 3D Bioprinting for Novel Drug Discovery. Expert Opin. Drug Discov. 2018, 13, 1115–1129. [Google Scholar] [CrossRef] [PubMed]
- Laschke, M.W.; Menger, M.D. Prevascularization in Tissue Engineering: Current Concepts and Future Directions. Biotechnol. Adv. 2016, 34, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Koledova, Z. 3D Cell Culture: Methods and Protocols; Springer Science + Business Media: New York, NY, USA, 2017; ISBN 978-1-4939-7019-3. [Google Scholar]
- Haycock, J.W. 3D Cell Culture; Methods in Molecular Biology, Ed.; Humana Press: Totowa, NJ, USA, 2011; Volume 695, ISBN 978-1-60761-983-3. [Google Scholar]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef]
- Murray Walker, D.; Boey, G.; McDonald, L.A. The Pathology of Oral Cancer. Pathology 2003, 35, 376–383. [Google Scholar] [CrossRef]
- Bissell, M.J.; Radisky, D. Putting Tumours in Context. Nat. Rev. Cancer 2001, 1, 46–54. [Google Scholar] [CrossRef]
- Wiseman, B.S.; Werb, Z. Stromal Effects on Mammary Gland Development and Breast Cancer. Science 2002, 296, 1046–1049. [Google Scholar] [CrossRef]
- Sośniak, J.; Opiela, J. 3D Cell Culture Technology—A New Insight Into in vitro Research—A Review. Ann. Anim. Sci. 2021, 21, 1257–1273. [Google Scholar] [CrossRef]
- Cannon, T.M.; Shah, A.T.; Skala, M.C. Autofluorescence Imaging Captures Heterogeneous Drug Response Differences between 2D and 3D Breast Cancer Cultures. Biomed. Opt. Express 2017, 8, 1911–1925. [Google Scholar] [CrossRef]
- Stoker, A.W.; Streuli, C.H.; Martins-Green, M.; Bissell, M.J. Designer Microenvironments for the Analysis of Cell and Tissue Function. Curr. Opin. Cell Biol. 1990, 2, 864–874. [Google Scholar] [CrossRef]
- Carrel, A. On the permanent life of tissues outside of the organism. J. Exp. Med. 1912, 15, 516–528. [Google Scholar] [CrossRef]
- Verjans, E.-T.; Doijen, J.; Luyten, W.; Landuyt, B.; Schoofs, L. Three-Dimensional Cell Culture Models for Anticancer Drug Screening: Worth the Effort? J. Cell. Physiol. 2018, 233, 2993–3003. [Google Scholar] [CrossRef]
- Härmä, V.; Schukov, H.-P.; Happonen, A.; Ahonen, I.; Virtanen, J.; Siitari, H.; Åkerfelt, M.; Lötjönen, J.; Nees, M. Quantification of Dynamic Morphological Drug Responses in 3D Organotypic Cell Cultures by Automated Image Analysis. PLoS ONE 2014, 9, e96426. [Google Scholar] [CrossRef]
- Lin, R.-Z.; Chang, H.-Y. Recent Advances in Three-Dimensional Multicellular Spheroid Culture for Biomedical Research. Biotechnol. J. 2008, 3, 1172–1184. [Google Scholar] [CrossRef]
- Mehta, G.; Hsiao, A.Y.; Ingram, M.; Luker, G.D.; Takayama, S. Opportunities and Challenges for Use of Tumor Spheroids as Models to Test Drug Delivery and Efficacy. J. Control. Release 2012, 164, 192–204. [Google Scholar] [CrossRef]
- Strese, S.; Fryknäs, M.; Larsson, R.; Gullbo, J. Effects of Hypoxia on Human Cancer Cell Line Chemosensitivity. BMC Cancer 2013, 13, 331. [Google Scholar] [CrossRef]
- Mapanao, A.K.; Voliani, V. Three-Dimensional Tumor Models: Promoting Breakthroughs in Nanotheranostics Translational Research. Appl. Mater. Today 2020, 19, 100552. [Google Scholar] [CrossRef]
- Breslin, S.; O’Driscoll, L. Three-Dimensional Cell Culture: The Missing Link in Drug Discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef]
- Amaral, R.L.F.; Miranda, M.; Marcato, P.D.; Swiech, K. Comparative Analysis of 3D Bladder Tumor Spheroids Obtained by Forced Floating and Hanging Drop Methods for Drug Screening. Front. Physiol. 2017, 8, 605. [Google Scholar] [CrossRef]
- Nath, S.; Devi, G.R. Three-Dimensional Culture Systems in Cancer Research: Focus on Tumor Spheroid Model. Pharmacol. Ther. 2016, 163, 94–108. [Google Scholar] [CrossRef]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-Based Drug Screen: Considerations and Practical Approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef]
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-Dimensional Cell Culture: A Powerful Tool in Tumor Research and Drug Discovery. Oncol. Lett. 2017, 14, 6999–7010. [Google Scholar] [CrossRef]
- Kelm, J.M.; Timmins, N.E.; Brown, C.J.; Fussenegger, M.; Nielsen, L.K. Method for Generation of Homogeneous Multicellular Tumor Spheroids Applicable to a Wide Variety of Cell Types. Biotechnol. Bioeng. 2003, 83, 173–180. [Google Scholar] [CrossRef]
- Souza, G.R.; Molina, J.R.; Raphael, R.M.; Ozawa, M.G.; Stark, D.J.; Levin, C.S.; Bronk, L.F.; Ananta, J.S.; Mandelin, J.; Georgescu, M.-M.; et al. Three-Dimensional Tissue Culture Based on Magnetic Cell Levitation. Nat. Nanotechnol. 2010, 5, 291–296. [Google Scholar] [CrossRef]
- Petersen, O.W.; Rønnov-Jessen, L.; Howlett, A.R.; Bissell, M.J. Interaction with Basement Membrane Serves to Rapidly Distinguish Growth and Differentiation Pattern of Normal and Malignant Human Breast Epithelial Cells. Proc. Natl. Acad. Sci. USA 1992, 89, 9064–9068. [Google Scholar] [CrossRef]
- Mikos, A.G.; Herring, S.W.; Ochareon, P.; Elisseeff, J.; Lu, H.H.; Kandel, R.; Schoen, F.J.; Toner, M.; Mooney, D.; Atala, A.; et al. Engineering Complex Tissues. Tissue Eng. 2006, 12, 3307–3339. [Google Scholar] [CrossRef]
- Haisler, W.L.; Timm, D.M.; Gage, J.A.; Tseng, H.; Killian, T.C.; Souza, G.R. Three-Dimensional Cell Culturing by Magnetic Levitation. Nat. Protoc. 2013, 8, 1940–1949. [Google Scholar] [CrossRef]
- De Pieri, A.; Rochev, Y.; Zeugolis, D.I. Scaffold-Free Cell-Based Tissue Engineering Therapies: Advances, Shortfalls and Forecast. Npj Regen. Med. 2021, 6, 18. [Google Scholar] [CrossRef]
- Kobayashi, J.; Akiyama, Y.; Yamato, M.; Shimizu, T.; Okano, T. Design of Temperature-Responsive Cell Culture Surfaces for Cell Sheet-Based Regenerative Therapy and 3D Tissue Fabrication. Adv. Exp. Med. Biol. 2018, 1078, 371–393. [Google Scholar] [CrossRef]
- Takahashi, H.; Okano, T. Thermally-Triggered Fabrication of Cell Sheets for Tissue Engineering and Regenerative Medicine. Adv. Drug Deliv. Rev. 2019, 138, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B. Three-Dimensional Tissue Culture Models in Cancer Biology. Semin. Cancer Biol. 2005, 15, 365–377. [Google Scholar] [CrossRef]
- Goodwin, T.J.; Prewett, T.L.; Wolf, D.A.; Spaulding, G.F. Reduced Shear Stress: A Major Component in the Ability of Mammalian Tissues to Form Three-Dimensional Assemblies in Simulated Microgravity. J. Cell. Biochem. 1993, 51, 301–311. [Google Scholar] [CrossRef]
- Zwezdaryk, K.J.; Warner, J.A.; Machado, H.L.; Morris, C.A.; Höner zu Bentrup, K. Rotating Cell Culture Systems for Human Cell Culture: Human Trophoblast Cells as a Model. J. Vis. Exp. 2012, 59, e3367. [Google Scholar] [CrossRef]
- Yourek, G.; McCormick, S.M.; Mao, J.J.; Reilly, G.C. Shear Stress Induces Osteogenic Differentiation of Human Mesenchymal Stem Cells. Regen. Med. 2010, 5, 713–724. [Google Scholar] [CrossRef]
- Koh, B.; Sulaiman, N.; Fauzi, M.B.; Law, J.X.; Ng, M.H.; Idrus, R.B.H.; Yazid, M.D. Three Dimensional Microcarrier System in Mesenchymal Stem Cell Culture: A Systematic Review. Cell Biosci. 2020, 10, 75. [Google Scholar] [CrossRef]
- Malda, J.; Frondoza, C.G. Microcarriers in the Engineering of Cartilage and Bone. Trends Biotechnol. 2006, 24, 299–304. [Google Scholar] [CrossRef]
- Aishwarya, P.; Agrawal, G.; Sally, J.; Ravi, M. Dynamic Three-Dimensional Cell-Culture Systems for Enhanced in Vitro Applications. Curr. Sci. 2022, 122, 149. [Google Scholar] [CrossRef]
- Cai, H.; Ao, Z.; Hu, L.; Moon, Y.; Wu, Z.; Lu, H.-C.; Kim, J.; Guo, F. Acoustofluidic Assembly of 3D Neurospheroids to Model Alzheimer’s Disease. Analyst 2020, 145, 6243–6253. [Google Scholar] [CrossRef]
- Kang, Y.; Li, D.; Kalams, S.A.; Eid, J.E. DC-Dielectrophoretic Separation of Biological Cells by Size. Biomed. Microdevices 2008, 10, 243–249. [Google Scholar] [CrossRef]
- Ze, Y.; Li, Y.; Huang, L.; Shi, Y.; Li, P.; Gong, P.; Lin, J.; Yao, Y. Biodegradable Inks in Indirect Three-Dimensional Bioprinting for Tissue Vascularization. Front. Bioeng. Biotechnol. 2022, 10, 856398. [Google Scholar] [CrossRef]
- Carletti, E.; Motta, A.; Migliaresi, C. Scaffolds for Tissue Engineering and 3D Cell Culture. Methods Mol. Biol. 2011, 695, 17–39. [Google Scholar] [CrossRef]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.D.P. 3D Cell Culture Systems: Advantages and Applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef]
- Sun, M.; Liu, A.; Yang, X.; Gong, J.; Yu, M.; Yao, X.; Wang, H.; He, Y. 3D Cell Culture—Can It Be As Popular as 2D Cell Culture? Adv. NanoBiomed Res. 2021, 1, 2000066. [Google Scholar] [CrossRef]
- Sackett, S.D.; Tremmel, D.M.; Ma, F.; Feeney, A.K.; Maguire, R.M.; Brown, M.E.; Zhou, Y.; Li, X.; O’Brien, C.; Li, L.; et al. Extracellular Matrix Scaffold and Hydrogel Derived from Decellularized and Delipidized Human Pancreas. Sci. Rep. 2018, 8, 10452. [Google Scholar] [CrossRef]
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Three-Dimensional Cell Culture Systems as an in Vitro Platform for Cancer and Stem Cell Modeling. World J. Stem Cells 2019, 11, 1065–1083. [Google Scholar] [CrossRef]
- Grenier, J.; Duval, H.; Barou, F.; Lv, P.; David, B.; Letourneur, D. Mechanisms of Pore Formation in Hydrogel Scaffolds Textured by Freeze-Drying. Acta Biomater. 2019, 94, 195–203. [Google Scholar] [CrossRef]
- Dehghani, F.; Annabi, N. Engineering Porous Scaffolds Using Gas-Based Techniques. Curr. Opin. Biotechnol. 2011, 22, 661–666. [Google Scholar] [CrossRef]
- Conoscenti, G.; Carrubba, V.L.; Brucato, V. A Versatile Technique to Produce Porous Polymeric Scaffolds: The Thermally Induced Phase Separation (TIPS) Method. Arch. Chem. Res. 2017, 1. [Google Scholar] [CrossRef]
- Akbarzadeh, R.; Yousefi, A.-M. Effects of Processing Parameters in Thermally Induced Phase Separation Technique on Porous Architecture of Scaffolds for Bone Tissue Engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1304–1315. [Google Scholar] [CrossRef]
- Sola, A.; Bertacchini, J.; D’Avella, D.; Anselmi, L.; Maraldi, T.; Marmiroli, S.; Messori, M. Development of Solvent-Casting Particulate Leaching (SCPL) Polymer Scaffolds as Improved Three-Dimensional Supports to Mimic the Bone Marrow Niche. Mater. Sci. Eng. C 2019, 96, 153–165. [Google Scholar] [CrossRef]
- Zhu, N.; Che, X. Biofabrication of Tissue Scaffolds. In Advances in Biomaterials Science and Biomedical Applications; Pignatello, R., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-1051-4. [Google Scholar]
- Vannozzi, L.; Yasa, I.C.; Ceylan, H.; Menciassi, A.; Ricotti, L.; Sitti, M. Self-Folded Hydrogel Tubes for Implantable Muscular Tissue Scaffolds. Macromol. Biosci. 2018, 18, 1700377. [Google Scholar] [CrossRef]
- Vannozzi, L.; Lucantonio, A.; Castillo, A.; De Simone, A.; Ricotti, L. Modeling Self-Rollable Elastomeric Films for Building Bioinspired Hierarchical 3D Structures. Int. J. Mol. Sci. 2022, 23, 8467. [Google Scholar] [CrossRef]
- Sampath Kumar, T.S.; Yogeshwar Chakrapani, V. Electrospun 3D Scaffolds for Tissue Regeneration. Adv. Exp. Med. Biol. 2018, 1078, 29–47. [Google Scholar] [CrossRef]
- Hong, J.; Yeo, M.; Yang, G.H.; Kim, G. Cell-Electrospinning and Its Application for Tissue Engineering. Int. J. Mol. Sci. 2019, 20, 6208. [Google Scholar] [CrossRef]
- Rider, P.; Kačarević, Ž.P.; Alkildani, S.; Retnasingh, S.; Barbeck, M. Bioprinting of Tissue Engineering Scaffolds. J. Tissue Eng. 2018, 9, 2041731418802090. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, J.Y.; Cho, D.-W. Solid Free-Form Fabrication Technology and Its Application to Bone Tissue Engineering. Int. J. Stem Cells 2010, 3, 85–95. [Google Scholar] [CrossRef]
- Yeong, W.Y.; Sudarmadji, N.; Yu, H.Y.; Chua, C.K.; Leong, K.F.; Venkatraman, S.S.; Boey, Y.C.F.; Tan, L.P. Porous Polycaprolactone Scaffold for Cardiac Tissue Engineering Fabricated by Selective Laser Sintering. Acta Biomater. 2010, 6, 2028–2034. [Google Scholar] [CrossRef]
- Li, X.J.; Valadez, A.V.; Zuo, P.; Nie, Z. Microfluidic 3D Cell Culture: Potential Application for Tissue-Based Bioassays. Bioanalysis 2012, 4, 1509–1525. [Google Scholar] [CrossRef] [PubMed]
- Tomasina, C.; Bodet, T.; Mota, C.; Moroni, L.; Camarero-Espinosa, S. Bioprinting Vasculature: Materials, Cells and Emergent Techniques. Materials 2019, 12, 2701. [Google Scholar] [CrossRef] [PubMed]
- Vailhé, B.; Vittet, D.; Feige, J.J. In Vitro Models of Vasculogenesis and Angiogenesis. Lab. Investig. 2001, 81, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Hauser, P.V.; Chang, H.-M.; Nishikawa, M.; Kimura, H.; Yanagawa, N.; Hamon, M. Bioprinting Scaffolds for Vascular Tissues and Tissue Vascularization. Bioengineering 2021, 8, 178. [Google Scholar] [CrossRef] [PubMed]
- West, J.; Moon, J. Vascularization of Engineered Tissues: Approaches to Promote Angiogenesis in Biomaterials. Curr. Top. Med. Chem. 2008, 8, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.N.; Hospodiuk, M.; Ozbolat, I.T. Synergistic Interplay between Human MSCs and HUVECs in 3D Spheroids Laden in Collagen/Fibrin Hydrogels for Bone Tissue Engineering. Acta Biomater. 2019, 95, 348–356. [Google Scholar] [CrossRef]
- Liu, L.; Shi, G.-P. CD31: Beyond a Marker for Endothelial Cells. Cardiovasc. Res. 2012, 94, 3–5. [Google Scholar] [CrossRef]
- Muller, S.; Ader, I.; Creff, J.; Leménager, H.; Achard, P.; Casteilla, L.; Sensebé, L.; Carrière, A.; Deschaseaux, F. Human Adipose Stromal-Vascular Fraction Self-Organizes to Form Vascularized Adipose Tissue in 3D Cultures. Sci. Rep. 2019, 9, 7250. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Alitalo, K.; Allen, E.; Anisimov, A.; Aplin, A.C.; Auerbach, R.; Augustin, H.G.; Bates, D.O.; van Beijnum, J.R.; Bender, R.H.F.; et al. Consensus Guidelines for the Use and Interpretation of Angiogenesis Assays. Angiogenesis 2018, 21, 425–532. [Google Scholar] [CrossRef]
- Urbanczyk, M.; Zbinden, A.; Layland, S.L.; Duffy, G.; Schenke-Layland, K. Controlled Heterotypic Pseudo-Islet Assembly of Human β-Cells and Human Umbilical Vein Endothelial Cells Using Magnetic Levitation. Tissue Eng. Part A 2020, 26, 387–399. [Google Scholar] [CrossRef]
- Kim, E.M.; Lee, Y.B.; Kim, S.; Park, J.; Lee, J.; Kim, S.W.; Park, H.; Shin, H. Fabrication of Core-Shell Spheroids as Building Blocks for Engineering 3D Complex Vascularized Tissue. Acta Biomater. 2019, 100, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Chaddad, H.; Kuchler-Bopp, S.; Fuhrmann, G.; Gegout, H.; Ubeaud-Sequier, G.; Schwinté, P.; Bornert, F.; Benkirane-Jessel, N.; Idoux-Gillet, Y. Combining 2D Angiogenesis and 3D Osteosarcoma Microtissues to Improve Vascularization. Exp. Cell Res. 2017, 360, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Kim, S.; Chen, Z.; Shin, H.K.; Lee, S.-Y.; Moon, H.E.; Paek, S.H.; Park, S. 3D Bioprinted Vascularized Tumour for Drug Testing. Int. J. Mol. Sci. 2020, 21, 2993. [Google Scholar] [CrossRef] [PubMed]
- Brassard, J.A.; Nikolaev, M.; Hübscher, T.; Hofer, M.; Lutolf, M.P. Recapitulating Macro-Scale Tissue Self-Organization through Organoid Bioprinting. Nat. Mater. 2021, 20, 22–29. [Google Scholar] [CrossRef]
- Janani, G.; Priya, S.; Dey, S.; Mandal, B.B. Mimicking Native Liver Lobule Microarchitecture In Vitro with Parenchymal and Non-Parenchymal Cells Using 3D Bioprinting for Drug Toxicity and Drug Screening Applications. ACS Appl. Mater. Interfaces 2022, 14, 10167–10186. [Google Scholar] [CrossRef]
- Kang, D.; Hong, G.; An, S.; Jang, I.; Yun, W.; Shim, J.; Jin, S. Bioprinting of Multiscaled Hepatic Lobules within a Highly Vascularized Construct. Small 2020, 16, 1905505. [Google Scholar] [CrossRef]
- Dey, M.; Kim, M.H.; Nagamine, M.; Dogan, M.; Kozhaya, L.; Unutmaz, D.; Ozbolat, I.T. 3D Bioprinted Perfusable and Vascularized Breast Tumor Model for Dynamic Screening of Chemotherapeutics and CAR-T Cells. bioRxiv 2022. [Google Scholar] [CrossRef]
- Sano, E.; Mori, C.; Nashimoto, Y.; Yokokawa, R.; Kotera, H.; Torisawa, Y.-S. Engineering of Vascularized 3D Cell Constructs to Model Cellular Interactions through a Vascular Network. Biomicrofluidics 2018, 12, 042204. [Google Scholar] [CrossRef]
- Lee, H.N.; Choi, Y.Y.; Kim, J.W.; Lee, Y.S.; Choi, J.W.; Kang, T.; Kim, Y.K.; Chung, B.G. Effect of Biochemical and Biomechanical Factors on Vascularization of Kidney Organoid-on-a-Chip. Nano Converg. 2021, 8, 35. [Google Scholar] [CrossRef]
- Achberger, K.; Probst, C.; Haderspeck, J.; Bolz, S.; Rogal, J.; Chuchuy, J.; Nikolova, M.; Cora, V.; Antkowiak, L.; Haq, W.; et al. Merging Organoid and Organ-on-a-Chip Technology to Generate Complex Multi-Layer Tissue Models in a Human Retina-on-a-Chip Platform. eLife 2019, 8, e46188. [Google Scholar] [CrossRef]
- Vivas, A.; IJspeert, C.; Pan, J.Y.; Vermeul, K.; van den Berg, A.; Passier, R.; Keller, S.S.; van der Meer, A.D. Generation and Culture of Cardiac Microtissues in a Microfluidic Chip with a Reversible Open Top Enables Electrical Pacing, Dynamic Drug Dosing and Endothelial Cell Co-Culture. Adv. Mater. Technol. 2022, 7, 2101355. [Google Scholar] [CrossRef]
- Jin, Y.; Kim, J.; Lee, J.S.; Min, S.; Kim, S.; Ahn, D.-H.; Kim, Y.-G.; Cho, S.-W. Vascularized Liver Organoids Generated Using Induced Hepatic Tissue and Dynamic Liver-Specific Microenvironment as a Drug Testing Platform. Adv. Funct. Mater. 2018, 28, 1801954. [Google Scholar] [CrossRef]
- Dellaquila, A.; Le Bao, C.; Letourneur, D.; Simon-Yarza, T. In Vitro Strategies to Vascularize 3D Physiologically Relevant Models. Adv. Sci. 2021, 8, 2100798. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anthon, S.G.; Valente, K.P. Vascularization Strategies in 3D Cell Culture Models: From Scaffold-Free Models to 3D Bioprinting. Int. J. Mol. Sci. 2022, 23, 14582. https://doi.org/10.3390/ijms232314582
Anthon SG, Valente KP. Vascularization Strategies in 3D Cell Culture Models: From Scaffold-Free Models to 3D Bioprinting. International Journal of Molecular Sciences. 2022; 23(23):14582. https://doi.org/10.3390/ijms232314582
Chicago/Turabian StyleAnthon, Shamapto Guha, and Karolina Papera Valente. 2022. "Vascularization Strategies in 3D Cell Culture Models: From Scaffold-Free Models to 3D Bioprinting" International Journal of Molecular Sciences 23, no. 23: 14582. https://doi.org/10.3390/ijms232314582
APA StyleAnthon, S. G., & Valente, K. P. (2022). Vascularization Strategies in 3D Cell Culture Models: From Scaffold-Free Models to 3D Bioprinting. International Journal of Molecular Sciences, 23(23), 14582. https://doi.org/10.3390/ijms232314582






