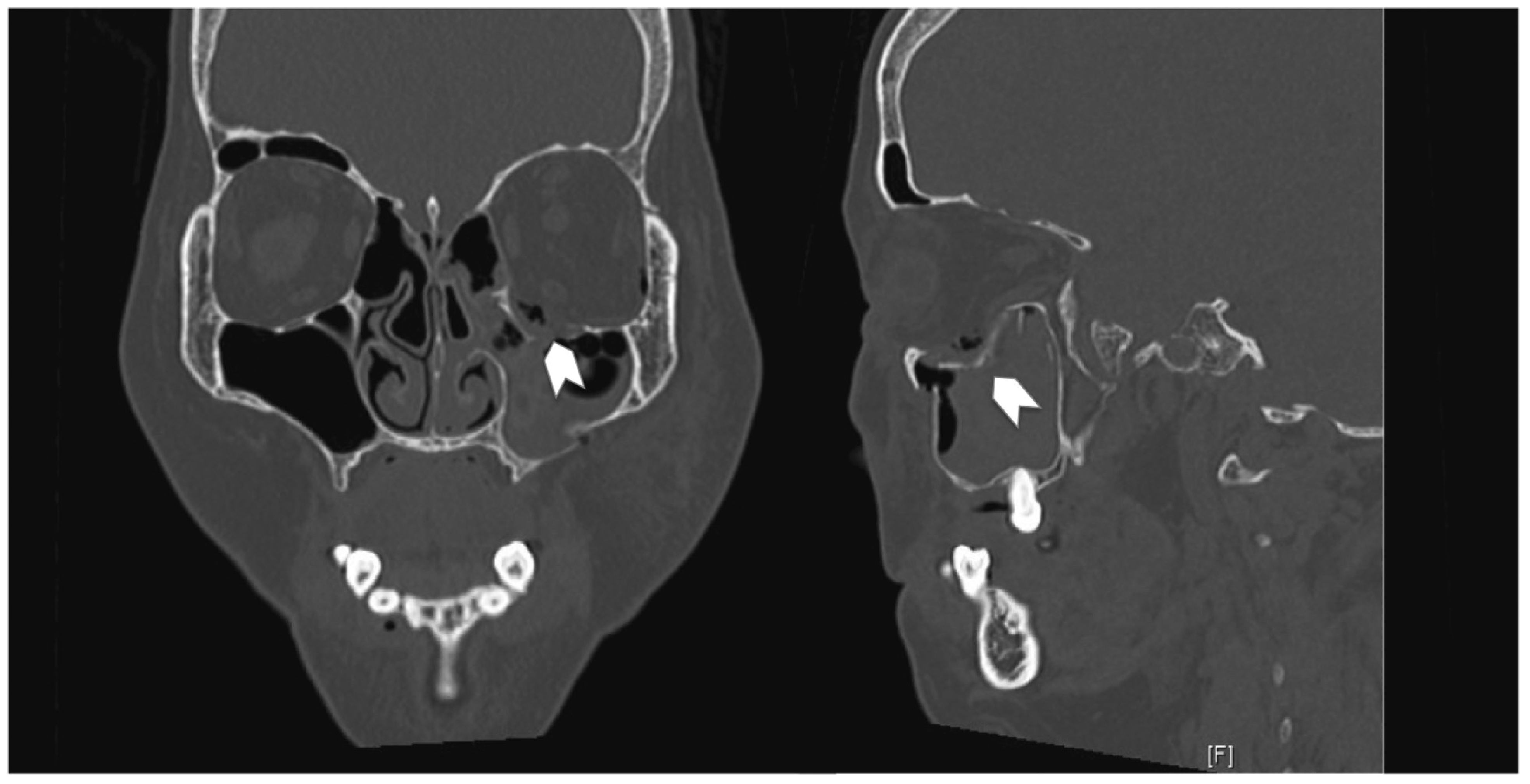
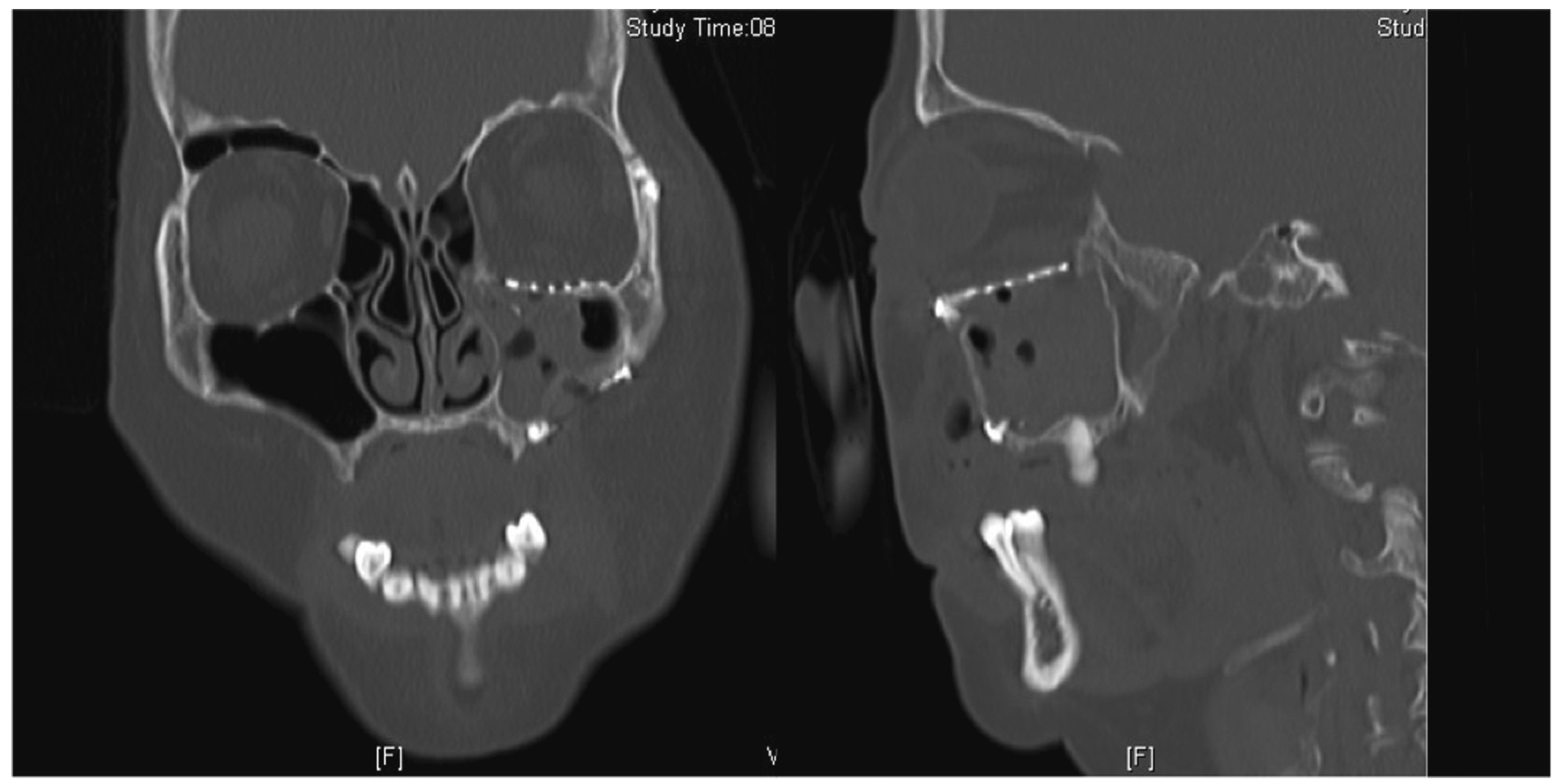
Patients with large orbital floor defects, defined in our institution as larger than 2 cm
2 on a computed tomographic (CT) scan, or who have symptoms of enophthalmos or entrapment, will require surgical intervention to reposition the herniated tissue, restore orbital volume, and reconstruct the orbital floor defect. [
3] This includes the use of autogenous materials such as bone grafts, or more frequently alloplastic materials that are either resorbable (polyglactin based) or nonresorbable (titanium mesh). [
4] Proper restoration of orbital contents, the orbital volume, and the bone orbit continuity usually results in an excellent surgical outcome for the patient regardless of the material chosen for reconstruction. However, we present two cases of orbital adherence syndrome after the use of titanium mesh implants, resulting in significant restriction of upward gaze despite anatomical placement of the implants on the postoperative CT scan. We also delineate the management of these patients and the resultant treatment outcomes.
Discussion
Orbital floor reconstruction is a commonly undertaken surgical procedure in our trauma center, whether in isolation in pure orbital blowouts or in conjunction with the fixation of accompanying facial fractures. The objectives for orbital floor reconstruction are to replace the herniated orbital contents, reconstruct the orbital floor defect, and to reconstitute the anatomically correct orbital volume. This serves to relieve symptoms of entrapment or incarceration of periorbita, as well as prevent sequelae such as enophthalmos and dystopia. The transconjunctival approach was used, as it causes less cicatricial ectropion and lid retraction. [
5]
Reconstruction of the orbital floor may be performed using a variety of materials, broadly divided into biological and alloplastic materials. Autologous bone has been the “gold standard” biomaterial for reconstruction of bony defects in the craniofacial region; however, it suffers from unpredictable resorption and delayed enophthalmos; others include autologous cartilage, fascia, allografts, and xenografts. [
2,
4,
6] Increasingly, there has been a shift toward using alloplastic materials for orbital floor reconstruction; it has the advantage of easy availability without donor site morbidity and decreased operative time. These materials take the form of metals, biological ceramics, resorbable and nonresorbable polymers, and composite implants. [
4,
6,
7]
In the selection of the appropriate alloplast, several factors are pertinent: size of the orbital defect, the ability of the implant to recreate and maintain the normal anatomy, its biologic behavior, and radiopacity. [
6,
7] Dubois et al. have suggested an algorithm based on defect size. [
6] Most materials including resorbable polymers are suitable for smaller defects, although polydioxanone degradation has been shown to be associated with thick scar formation and granulomas, and poly-L-lactic acid with persistence of the implant. Larger, high-complexity defects require more rigid materials that provide support to orbital contents and are capable of being contoured. Titanium and porous polyethylene (PE) are two preferred alloplastic materials.[
6,
7] The main disadvantage of PE lies in its radiolucency, which precludes postoperative evaluation of the implant position.
In our center, the titanium mesh is the alloplastic implant of choice if a permanent implant is desired; if an absorbable implant is required, the use of Osteomesh (polycaprolactone; Osteopore International, Singapore) or Rapidsorb (DePuy Synthes, Johnson & Johnson) is usually chosen. Titanium is preferred, despite its known propensity for inciting fibrosis, [
8] as it possesses sufficient rigidity for the support of orbital contents, easy deformability to allow for adaptation to each individual’s anatomy, [
5] is highly biocompatible, has a low extrusion rate, and displays good osseointegration. [
7,
8]
Titanium may be more useful in comminuted fractures where it can secure and/or bridge across multiple bone fragments. [
6] The fibrogenic response of titanium that results in orbital adherence syndrome manifests itself in the form of fibrous band ingrowth through the pores in the mesh (Case 1). This then tethers down the periorbita, hindering globe movement in the upward direction. It may also be accompanied by cicatricial eyelid retraction (Case 2).
Le Guéhennec demonstrated that increasing surface irregularities and roughness of the titanium implant resulted in greater bone-to-implant contact, leading to faster osseointegration and stronger adhesion between implant and host. [
9] As osseointegration is regarded as immune-modulated inflammatory process, the study of Lee and Nunery on orbital adherence syndrome attributed the extensive fibrotic reaction surrounding the mesh implants to the titanium implant itself. [
10] They also theorized that the concomitant use of titanium orbital floor implants anchored to the infraorbital rim and titanium orbital rim plates resulted in greater surface irregularities and exposure of the orbit and eyelid to titanium, resulting in greater fibrosis.
In the 30 cases of orbital fractures performed in our institution over the past year (May 2014 to May 2015) with simultaneous use of titanium mesh and orbital rim implants, only 2 cases were complicated by orbital adherence syndrome (6.67%). Due to the early manifestations of symptoms (<1week) in Case 1, we postulate that another mechanism apart from fibrotic adhesions incited by the titanium implant was responsible for the tethering, as the cicatricial bands would not have been well formed within this time frame to result in significant orbital tethering. As orbital adherence syndrome does not occur in the vast majority of our patients who undergo orbital floor reconstruction with a titanium mesh implant, we postulate that there are certain predisposing risk factors, the significant disruption of periorbital fat after a comminuted orbital floor fracture and the nonmeticulous dissection resulting in periorbital fat disruption.
Pretrauma, the intact periorbita, namely, the periosteum, Lockwood ligament, and the periorbital fat, acts in tandem as an intervening layer between the globe, extraocular muscles, and the bony orbit. This allows for a smooth gliding interface. The periorbital fat is further held intact by the ligaments of Koornneef, which extend throughout the extraocular fat as thin bands dividing the fat into smaller compartments. These ligaments run from the bony orbit to the extraocular muscle fascia and Tenon capsule, maintaining the shape of the soft tissue. The disruption of the periorbita in an orbital floor fracture exposes these small fat components to the orbital mesh. These small fat strands then fall through the pores in the titanium mesh and become incarcerated (similar to an inguinal hernia) due to swelling. Owing to the attachments of the periorbita and fat strands to the muscle fascia via the Koornneef ligaments, this results in tethering of the muscle, manifesting clinically as gaze restriction in the early postoperative period. Subsequently, fat necrosis occurs which incites an inflammatory response; this is exacerbated by the titanium implant material, which results in fibrous ingrowth through the mesh. This leads to persistence or even worsening of symptoms such as diplopia as time passes.
The cases presented by Lee and Nunery [
10] and Kersey et al [
11] were referral cases from other surgeons, and as such, they may not have had the benefit of witnessing the early manifestations of this syndrome. In both case series, a majority of the cases managed operatively underwent removal of the implant, with isolated eyelid retraction repair for two of the eight cases in the study of Lee and Nunery. The mean time to reoperation was 5.8 and 7.1 months, respectively. In this patient (Case 1), the revision operation was performed early, where the orbital floor defect had yet to be sufficiently bridged with bony ingrowth; hence the titanium implant was left in situ. Instead, a smooth Suprafoil (S. Jackson Inc., Alexandria, VA) sheet was placed on top of the titanium implant, thereby creating a smooth gliding plane on the mesh, reducing traction of the mesh on the periorbita, and minimizing the contact of the orbital fat with the titanium implant, yet maintaining orbital support. Suprafoil alone lacks sufficient rigidity to adequately support the orbital contents in their anatomical position. This also prevents small pockets of periorbital fat from herniating through the mesh pores. As can be seen from the negative forced-duction test intraoperatively and the improvement of gaze restriction postoperation, this interposing layer was sufficient to prevent further adhesions.
Thus, the authors propose that in the primary operation, should there be severe disruption of the periorbita with the presence of multiple small fat lobules, we would consider a preemptive onlay alloplastic sheet on the titanium mesh. In cases with generally intact periorbita, the titanium mesh alone would suffice. Composite implants such as a titanium-PE composite implant (Medpor Titan, Stryker, Kalamazoo, MI) have been used to exploit the advantages of each material while minimizing its detrimental side effects [
12]; however, this is not available to us locally.
Conversely, the patient in Case 2 was managed successfully with nonsurgical methods. In the study of Kersey et al., all operatively managed cases were noted to have an improvement in globe movement on forced-duction test. [
11] There was no mention of the forced-duction test for the conservatively managed patients, or in the study of Lee and Nunery. [
10] We hypothesize that patients with a negative forced-duction test (Case 2) are likely to have cicatricial scarring and fibrous ingrowth into the orbital mesh to a lesser degree as compared with patients with a positive forced-duction test. This could indicate greater success with conservative measures, which would involve massage, steroid, or HA injections. In this subset of patients, a higher threshold for operative management might be prudent while awaiting temporal improvement. The authors acknowledge that while scar massage therapy is widely used especially by physical therapists, [
13] there are little randomized control trials to determine its efficacy, with even less evidence available on eyelid cicatricial scarring. [
14,
15] Although vigorous prolonged lid massage might cause a loose lower lid resulting in late ectropion or entropion, it has been shown to improve the outcome of eyelid scars and was initiated in Case 2 as part of the initial management for lid retraction from scarring. [
16,
17]
In case 2, the lower eyelid retraction and tugging sensation was likely due to a volume contraction in the posterior lamella rather than actual shortening. [
18,
19] The HA filler acts to restore the volume of the posterior lamella and resulted in relief of lower eyelid retraction and the tugging sensation. A temporary filler is adequate, as HA fillers last at least 9 months or more in the eyelids. Goldberg et al. found that there was still residual effect of the HA filler even at 6 months. [
19] While this necessitates repeated treatments, this method is acceptable in patients who may be more inclined toward a less invasive approach.
On occasion, there can be persistent diplopia following orbital floor surgery. Apart from incomplete reduction of herniated orbital contents and entrapment of the periorbita by the implant, an important consideration is the existing trauma to the extraocular muscles, in particular the inferior rectus. This can occur due to the original trauma or surgery, resulting in fibrotic restriction of the muscle. [
20] This is not mutually exclusive with orbital adherence syndrome, and may often only be diagnosed after all the other causes have been excluded.
Inherent limitations of this report include its retrospective nature and the small numbers, which would inevitably lead to a degree of bias. Unfortunately, due to the infrequent presentation of orbital adherence syndrome, all published studies on this topic are retrospective, with the exact pathogenesis poorly understood. The two largest case series available are by Lee and Nunery10 and Kersey et al.11; as they recruited cases that were operated on by other surgeons, we believe that they saw a delayed spectrum of this condition. While more cases would lend credence to this study, it may not be possible to add to these case numbers in the near future. Certainly surgical technique could play a causative role; however, blunt dissection was always performed in an attempt to reduce damage to the periorbita. There have been no further cases of orbital adherence syndrome, as the senior surgeon (H.S.) has modified his technique. Certainly further research is required to establish whether the degree of periorbital soft-tissue disruption with the use of titanium implants has a higher complication rate compared with other materials.
Despite the rare complication of orbital adherence syndrome, the authors believe that titanium implants remain a useful option especially in the reconstruction of large, comminuted orbital floor defects. In select cases where there is greater disruption of the periorbita and exposure of the orbital fat, it may be prudent to insert a smooth interposing alloplastic sheet concomitantly in the initial surgery as a preventive measure. In smaller floor defects requiring less rigid support, the authors have moved toward using porous polymer implants alone. Treatment for orbital adherence syndrome is surgical adhesiolysis, with removal of the implants, or placement of a smooth permanent interface sheet in the early recovery period. Patients with a negative forced-duction test would benefit from a trial of conservative management before considering surgical adhesiolysis.