Natriuretic Peptides—New Targets for Neurocontrol of Blood Pressure via Baroreflex Afferent Pathway
Abstract
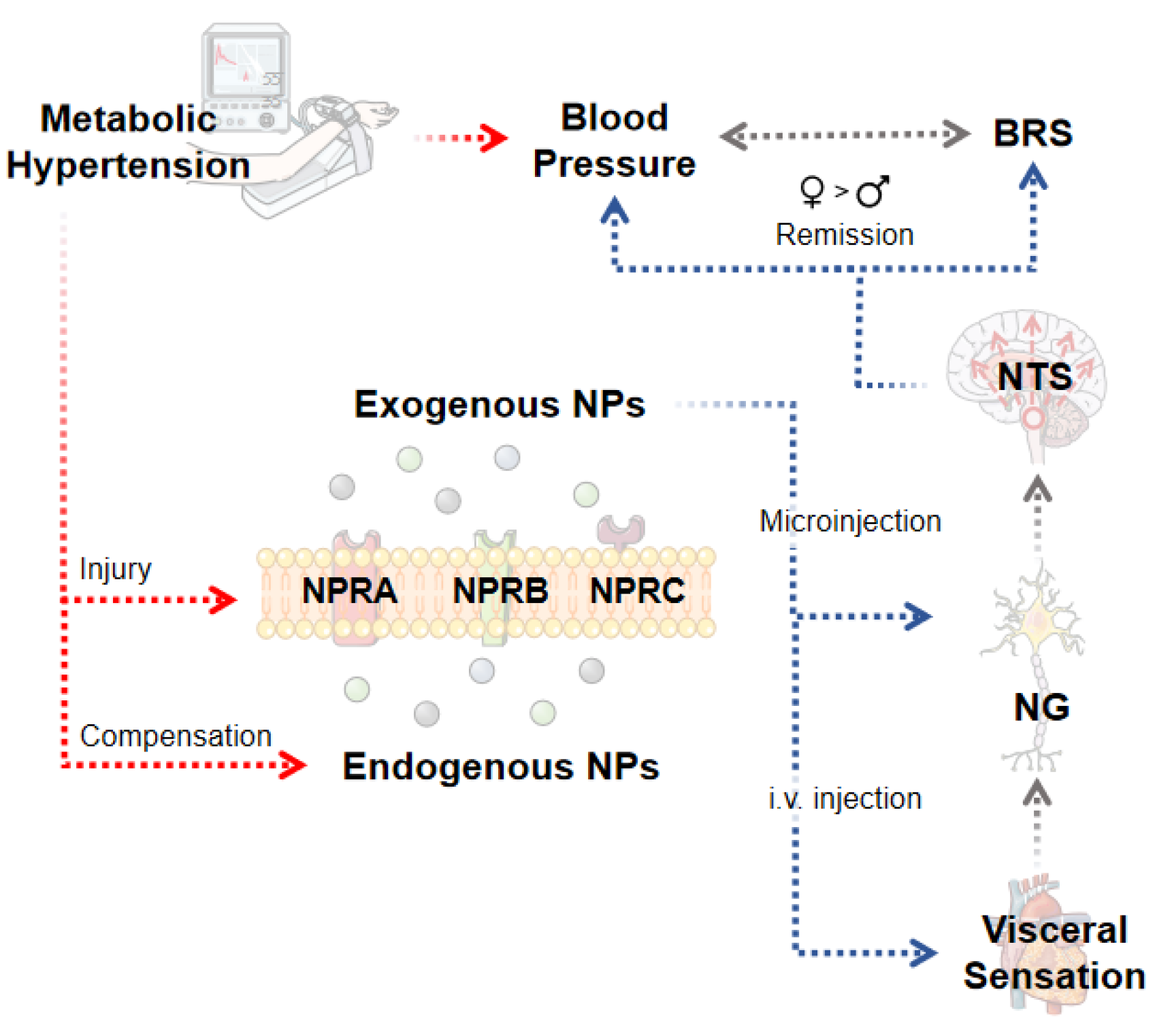
1. Introduction
2. Results
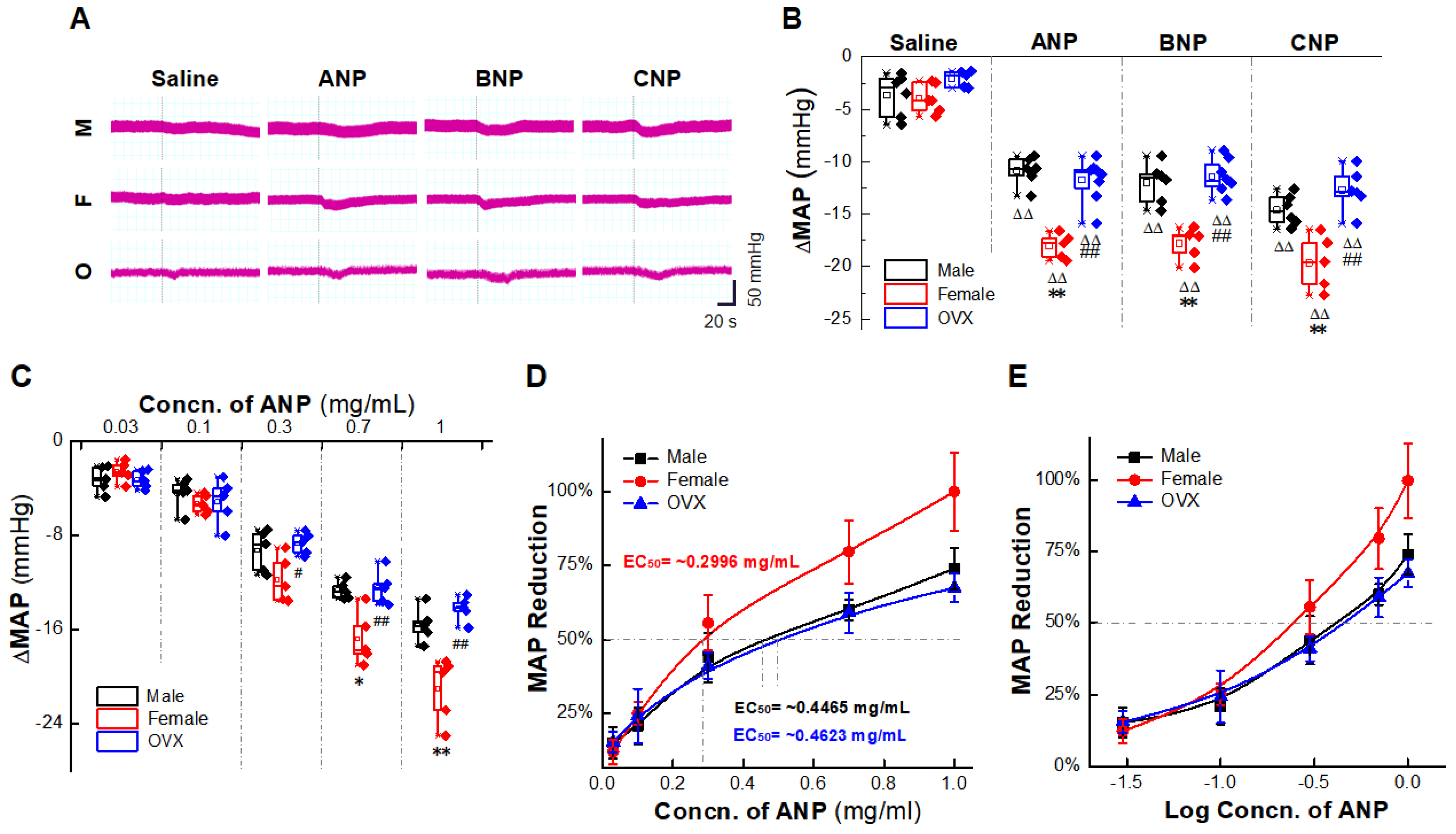
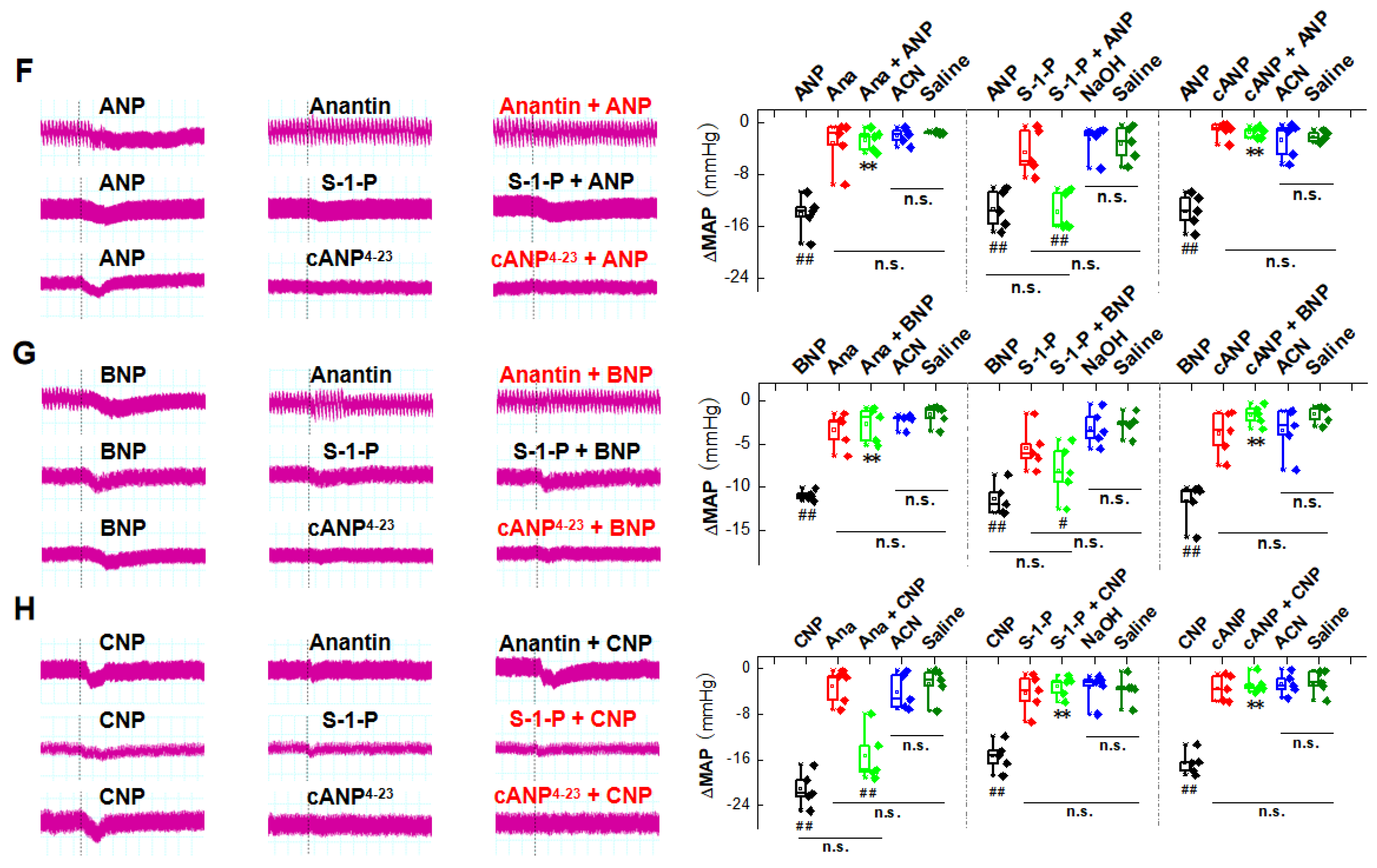
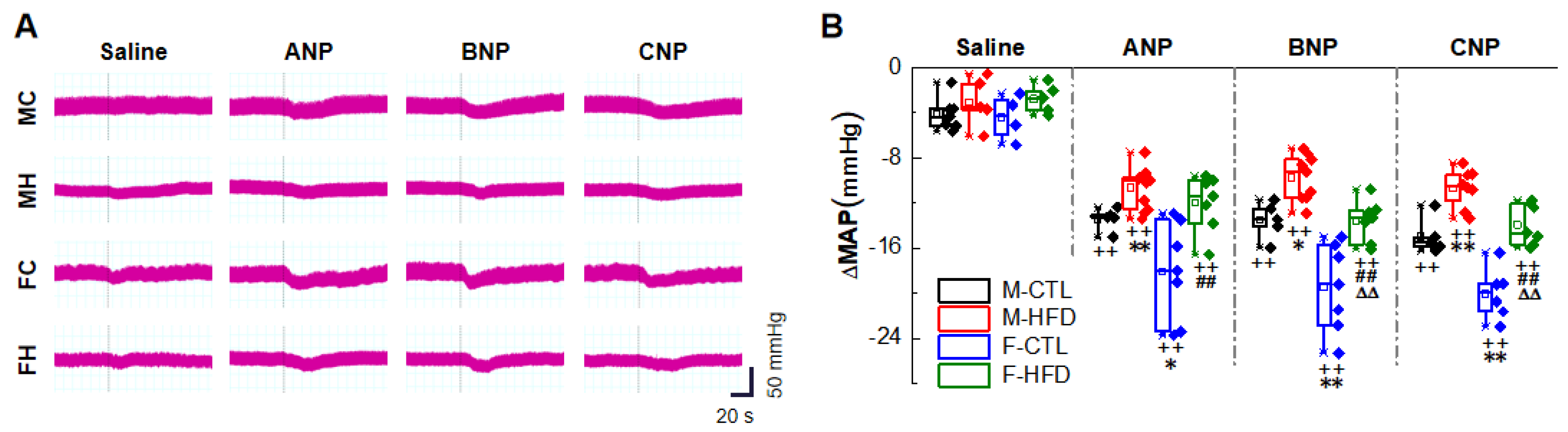
2.1. Natriuretic Peptides-Mediated BP Reduction by NG Microinjection under Physiological Condition
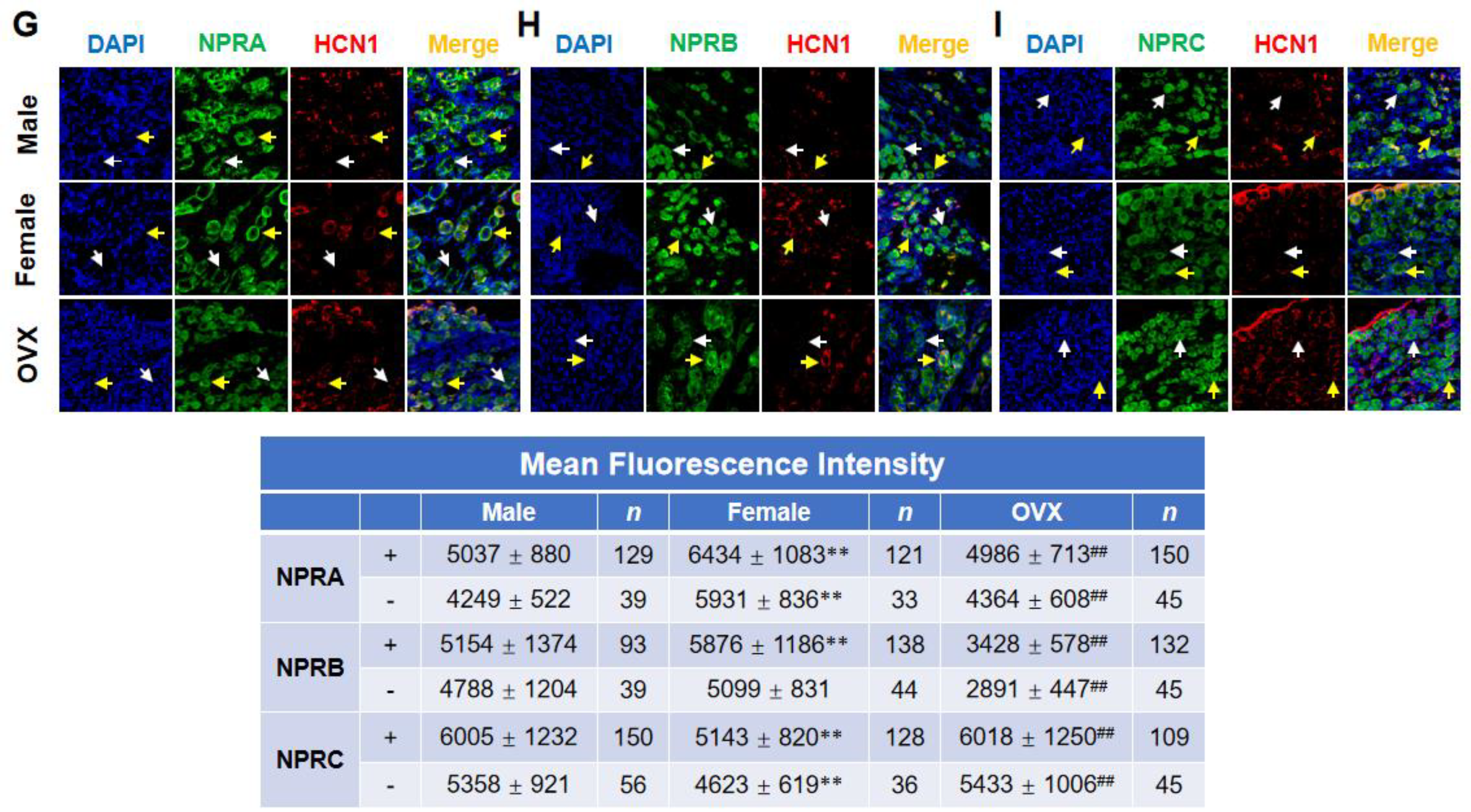
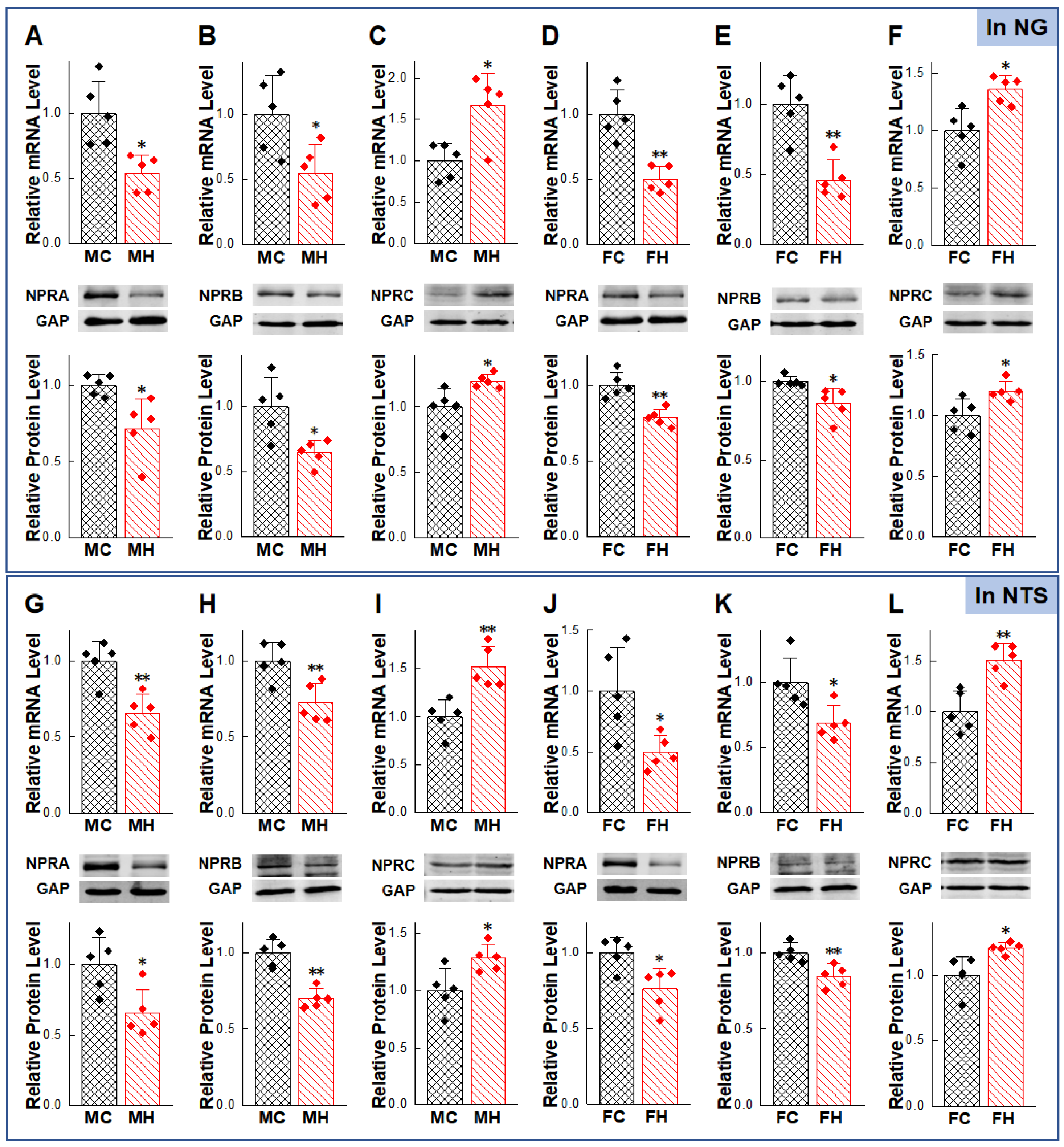
2.2. Gender-Differentiated Distribution of NPRs in Adult Male, Age-Matched Female, and Ovariectomized Female Rats
2.3. Anti-Hypertensive Effects of NPs on Hypertensive Condition of HFD Rat Models
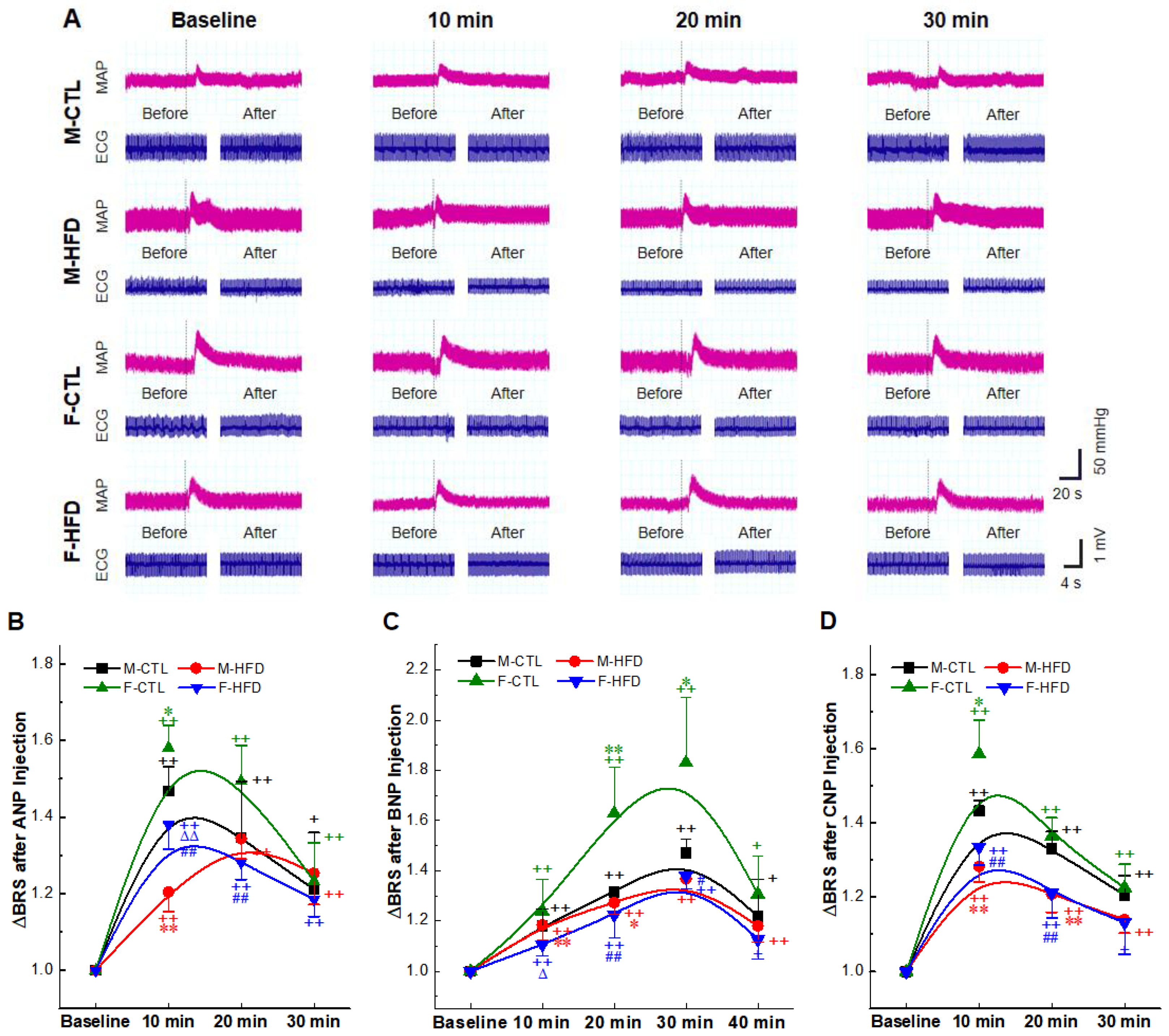
2.4. BRS Enhancement Effect of NPs Intravenous Injection
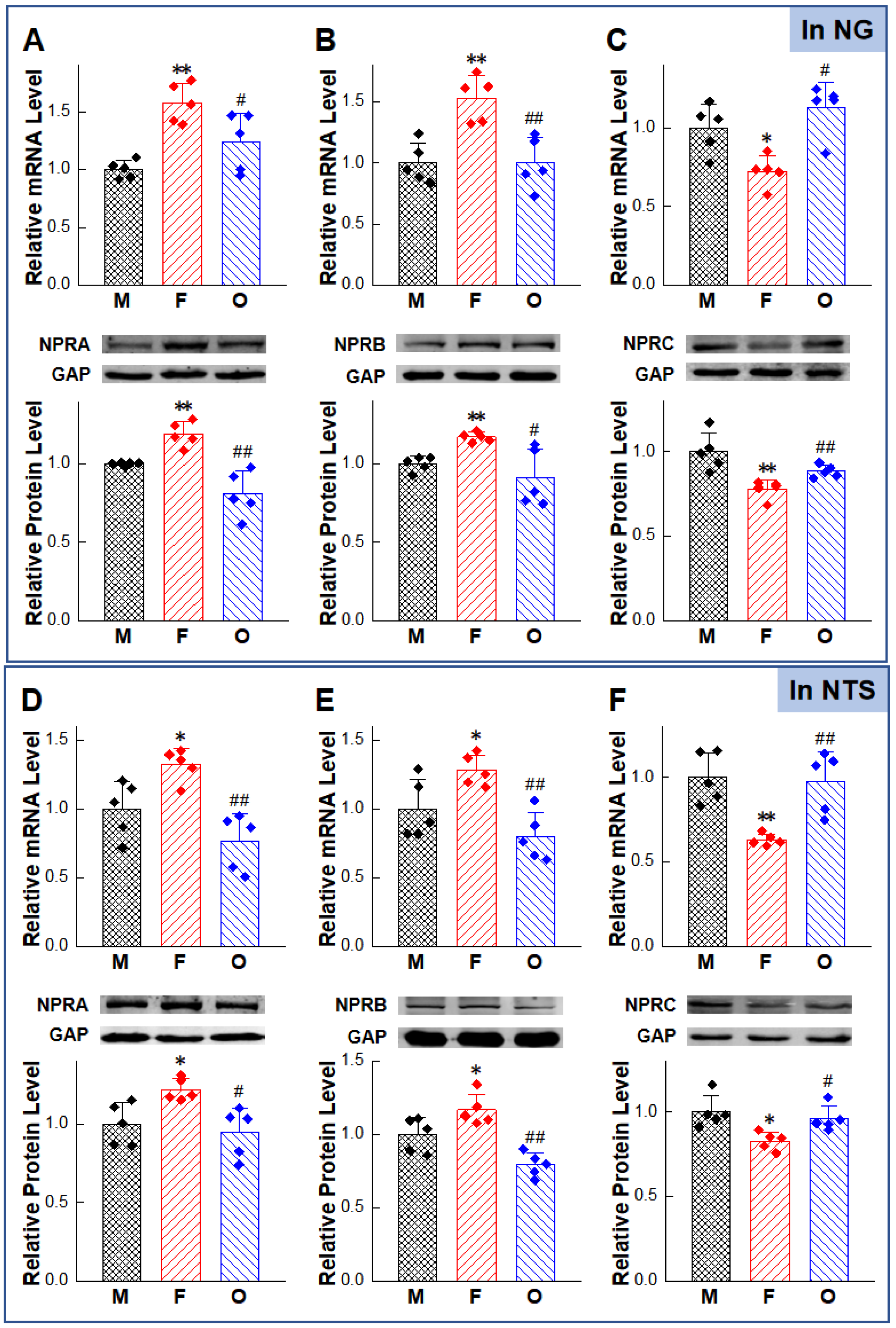
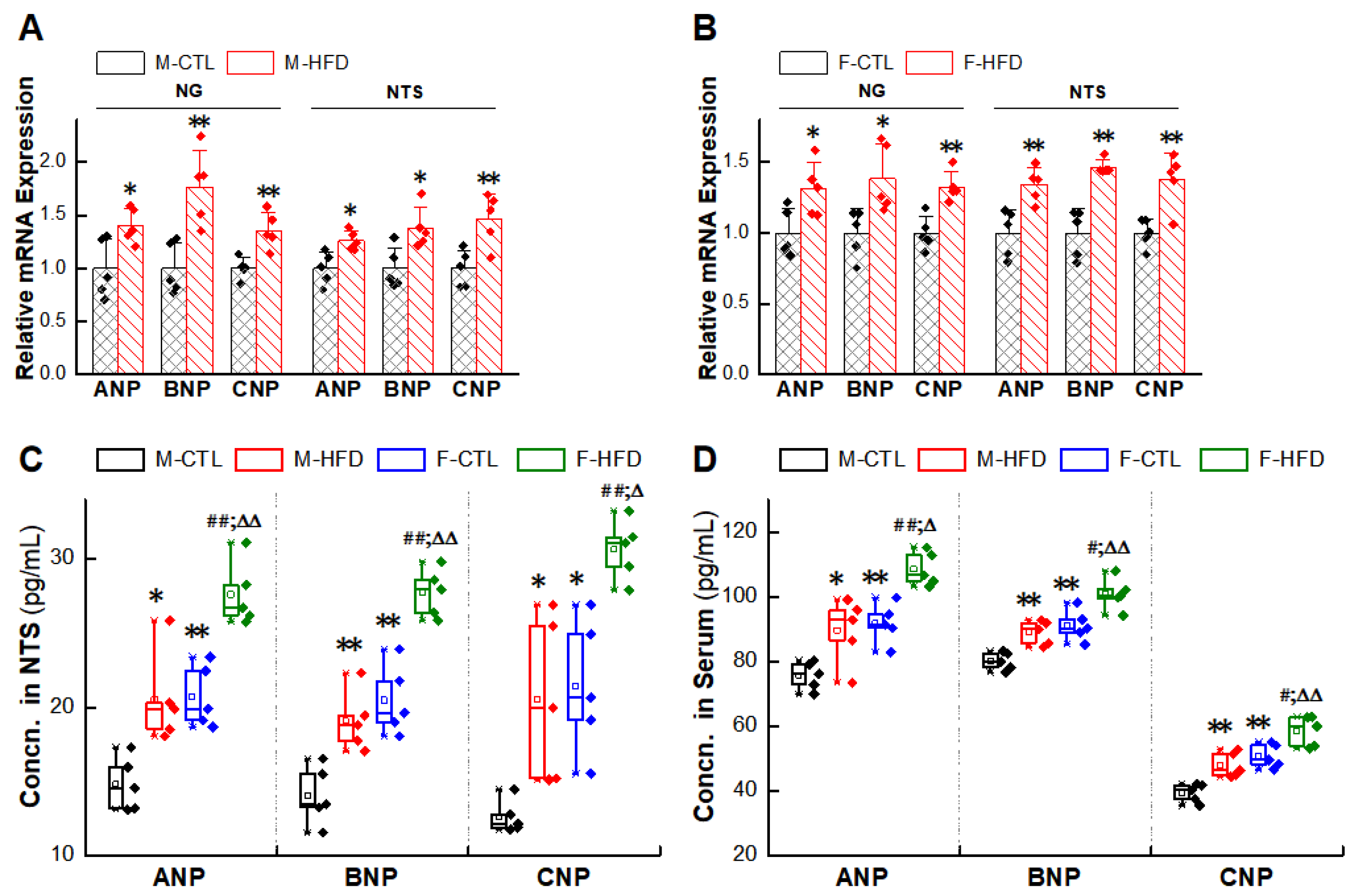
2.5. Abnormal Expression of NPs/NPRs in the NG and NTS of HFD Rat Models
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals
4.3. Systolic Blood Pressure Measurements
4.4. Surgical Procedures of Ovariectomy
4.5. High Fructose-Drinking Induced Hypertension Model
4.6. Nodose Ganglion Microinjection
4.7. Acute Intravenous NPs Injections and Baroreflex Sensitivity Detection
4.8. Tissue Preparation of NG and NTS
4.9. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
4.10. Western Blot
4.11. Immunohistochemical Analysis
4.12. Enzyme Linked Immunosorbent Assay (ELISA)
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Misono, K.S.; Philo, J.S.; Arakawa, T.; Ogata, C.M.; Qiu, Y.; Ogawa, H.; Young, H.S. Structure, signaling mechanism and regulation of the natriuretic peptide receptor guanylate cyclase. FEBS J. 2011, 278, 1818–1829. [Google Scholar] [CrossRef] [PubMed]
- De Bold, A.J. Atrial natriuretic factor: A hormone produced by the heart. Science 1985, 230, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Sudoh, T.; Kangawa, K.; Minamino, N.; Matsuo, H. A new natriuretic peptide in porcine brain. Nature 1988, 332, 78–81. [Google Scholar] [CrossRef]
- Sudoh, T.; Minamino, N.; Kangawa, K.; Matsuo, H. C-type natriuretic peptide (CNP): A new member of natriuretic peptide family identified in porcine brain. Biochem. Biophys. Res. Commun. 1990, 168, 863–870. [Google Scholar] [CrossRef]
- Goetze, J.P.; Bruneau, B.G.; Ramos, H.R.; Ogawa, T.; de Bold, M.K.; de Bold, A.J. Cardiac natriuretic peptides. Nat. Rev. Cardiol. 2020, 17, 698–717. [Google Scholar] [CrossRef] [PubMed]
- Potter, L.R.; Yoder, A.R.; Flora, D.R.; Antos, L.K.; Dickey, D.M. Natriuretic peptides: Their structures, receptors, physiologic functions and therapeutic applications. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; Volume 191, pp. 341–366. [Google Scholar]
- Nakagawa, Y.; Nishikimi, T.; Kuwahara, K. Atrial and brain natriuretic peptides: Hormones secreted from the heart. Peptides 2019, 111, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Renew, J.R.; Cyrille, N.; Elyahu, A.Y.; Ramakrishna, H. B-Natriuretic Peptide Pathway Modulation for the Management of Heart Failure with Reduced Ejection Fraction. J. Cardiothorac. Vasc. Anesth. 2018, 32, 1500–1506. [Google Scholar] [CrossRef]
- Buttgereit, J.; Shanks, J.; Li, D.; Hao, G.; Athwal, A.; Langenickel, T.H.; Wright, H.; da Costa Goncalves, A.C.; Monti, J.; Plehm, R.; et al. C-type natriuretic peptide and natriuretic peptide receptor B signalling inhibits cardiac sympathetic neurotransmission and autonomic function. Cardiovasc. Res. 2016, 112, 637–644. [Google Scholar] [CrossRef]
- Santhekadur, P.K.; Kumar, D.P.; Seneshaw, M.; Mirshahi, F.; Sanyal, A.J. The multifaceted role of natriuretic peptides in metabolic syndrome. Biomed. Pharmacother. 2017, 92, 826–835. [Google Scholar] [CrossRef]
- Verboven, K.; Hansen, D.; Jocken, J.W.E.; Blaak, E.E. Natriuretic peptides in the control of lipid metabolism and insulin sensitivity. Obes. Rev. 2017, 18, 1243–1259. [Google Scholar] [CrossRef]
- Coue, M.; Moro, C. Natriuretic peptide control of energy balance and glucose homeostasis. Biochimie 2016, 124, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Moro, C. Natriuretic peptides and fat metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Das, S.R.; Drazner, M.H.; Dries, D.L.; Vega, G.L.; Stanek, H.G.; Abdullah, S.M.; Canham, R.M.; Chung, A.K.; Leonard, D.; Wians, F.H., Jr.; et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: Results from the Dallas Heart Study. Circulation 2005, 112, 2163–2168. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Levy, D.; Benjamin, E.J.; Leip, E.P.; Wilson, P.W.; Vasan, R.S. Impact of obesity on plasma natriuretic peptide levels. Circulation 2004, 109, 594–600. [Google Scholar] [CrossRef]
- Hamada, M.; Shigematsu, Y.; Takezaki, M.; Ikeda, S.; Ogimoto, A. Plasma levels of atrial and brain natriuretic peptides in apparently healthy subjects: Effects of sex, age, and hemoglobin concentration. Int. J. Cardiol. 2017, 228, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Maffei, S.; Del Ry, S.; Prontera, C.; Clerico, A. Increase in circulating levels of cardiac natriuretic peptides after hormone replacement therapy in postmenopausal women. Clin. Sci. 2001, 101, 447–453. [Google Scholar] [CrossRef]
- Komukai, K.; Mochizuki, S.; Yoshimura, M. Gender and the renin-angiotensin-aldosterone system. Fundam. Clin. Pharmacol. 2010, 24, 687–698. [Google Scholar] [CrossRef]
- Lam, C.S.; Cheng, S.; Choong, K.; Larson, M.G.; Murabito, J.M.; Newton-Cheh, C.; Bhasin, S.; McCabe, E.L.; Miller, K.K.; Redfield, M.M.; et al. Influence of sex and hormone status on circulating natriuretic peptides. J. Am. Coll. Cardiol. 2011, 58, 618–626. [Google Scholar] [CrossRef]
- Glisic, M.; Rojas, L.Z.; Asllanaj, E.; Vargas, K.G.; Kavousi, M.; Ikram, M.A.; Fauser, B.; Laven, J.S.E.; Muka, T.; Franco, O.H. Sex steroids, sex hormone-binding globulin and levels of N-terminal pro-brain natriuretic peptide in postmenopausal women. Int. J. Cardiol. 2018, 261, 189–195. [Google Scholar] [CrossRef]
- De Lemos, J.A.; Das, S.R. Closing the Book on Androgens and Natriuretic Peptides. J. Am. Coll. Cardiol. 2019, 73, 1297–1299. [Google Scholar] [CrossRef]
- Walther, T.; Stepan, H. C-type natriuretic peptide in reproduction, pregnancy and fetal development. J. Endocrinol. 2004, 180, 17–22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaufmann, H.; Norcliffe-Kaufmann, L.; Palma, J.A. Baroreflex Dysfunction. N. Engl. J. Med. 2020, 382, 163–178. [Google Scholar] [CrossRef] [PubMed]
- La Rovere, M.T.; Pinna, G.D.; Raczak, G. Baroreflex sensitivity: Measurement and clinical implications. Ann. Noninvasive Electrocardiol. 2008, 13, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Zhang, Y.; Cheng, Y.Q.; Zhang, J.M.; Liu, H.Q.; Wang, W.Z.; Mehta, J.L.; Xiong, Z.G.; Su, D.F.; Liu, A.J. Metabolic syndrome emerges after artificial selection for low baroreflex sensitivity. CNS Neurosci. Ther. 2018, 24, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Ogoh, S. Sex differences in baroreflex function in health and disease. J. Physiol. Sci. 2019, 69, 851–859. [Google Scholar] [CrossRef]
- Santa Cruz Chavez, G.C.; Li, B.Y.; Glazebrook, P.A.; Kunze, D.L.; Schild, J.H. An afferent explanation for sexual dimorphism in the aortic baroreflex of rat. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H910–H921. [Google Scholar] [CrossRef]
- Ito, S.; Ohtsuki, S.; Katsukura, Y.; Funaki, M.; Koitabashi, Y.; Sugino, A.; Murata, S.; Terasaki, T. Atrial natriuretic peptide is eliminated from the brain by natriuretic peptide receptor-C-mediated brain-to-blood efflux transport at the blood-brain barrier. J. Cereb. Blood Flow Metab. 2011, 31, 457–466. [Google Scholar] [CrossRef]
- Koebele, S.V.; Bimonte-Nelson, H.A. Modeling menopause: The utility of rodents in translational behavioral endocrinology research. Maturitas 2016, 87, 5–17. [Google Scholar] [CrossRef]
- Douard, V.; Ferraris, R.P. The role of fructose transporters in diseases linked to excessive fructose intake. J. Physiol. 2013, 591, 401–414. [Google Scholar] [CrossRef]
- Maeda, M.; Mizuno, Y.; Wakita, M.; Yamaga, T.; Nonaka, K.; Shin, M.C.; Shoudai, K.; Akaike, N. Potent and direct presynaptic modulation of glycinergic transmission in rat spinal neurons by atrial natriuretic peptide. Brain Res. Bull. 2013, 99, 19–26. [Google Scholar] [CrossRef]
- Abbey-Hosch, S.E.; Cody, A.N.; Potter, L.R. Sphingosine-1-phosphate inhibits C-type natriuretic peptide activation of guanylyl cyclase B (GC-B/NPR-B). Hypertension 2004, 43, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.A.; Elesgaray, R.; Balaszczuk, A.M.; Arranz, C. Role of NPR-C natriuretic receptor in nitric oxide system activation induced by atrial natriuretic peptide. Regul. Pept. 2006, 135, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.H.; Yang, X.L. Natriuretic peptides and their receptors in the central nervous system. Prog. Neurobiol. 2008, 84, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Ermirio, R.; Ruggeri, P.; Cogo, C.E.; Molinari, C.; Calaresu, F.R. Neuronal and cardiovascular responses to ANF microinjected into the solitary nucleus. Am. J. Physiol. 1989, 256 Pt 2, R577–R582. [Google Scholar] [CrossRef]
- McKitrick, D.J.; Calaresu, F.R. Cardiovascular responses to microinjection of ANF into dorsal medulla of rats. Am. J. Physiol. 1988, 255 Pt 2, R182–R187. [Google Scholar] [CrossRef]
- Yang, R.H.; Jin, H.K.; Wyss, J.M.; Chen, Y.F.; Oparil, S. Pressor effect of blocking atrial natriuretic peptide in nucleus tractus solitarii. Hypertension 1992, 19, 198–205. [Google Scholar] [CrossRef]
- Pandey, K.N. Biology of natriuretic peptides and their receptors. Peptides 2005, 26, 901–932. [Google Scholar] [CrossRef]
- Pandey, K.N. Guanylyl cyclase/natriuretic peptide receptor-A signaling antagonizes phosphoinositide hydrolysis, Ca2+ release, and activation of protein kinase C. Front. Mol. Neurosci. 2014, 7, 75. [Google Scholar] [CrossRef]
- Lu, X.L.; Xu, W.X.; Yan, Z.Y.; Qian, Z.; Xu, B.; Liu, Y.; Han, L.M.; Gao, R.C.; Li, J.N.; Yuan, M.; et al. Subtype identification in acutely dissociated rat nodose ganglion neurons based on morphologic parameters. Int. J. Biol. Sci. 2013, 9, 716–727. [Google Scholar] [CrossRef][Green Version]
- Li, B.Y.; Qiao, G.F.; Feng, B.; Zhao, R.B.; Lu, Y.J.; Schild, J.H. Electrophysiological and neuroanatomical evidence of sexual dimorphism in aortic baroreceptor and vagal afferents in rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1301–R1310. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, X.; Liu, S.Z.; Song, D.X.; Wu, D.; Guan, J.; Wang, L.Q.; Li, J.N.; Lu, X.L.; Guo, T.Z.; et al. KCa1.1-mediated frequency-dependent central and peripheral neuromodulation via Ah-type baroreceptor neurons located within nodose ganglia and nucleus of solitary tract of female rats. Int. J. Cardiol. 2015, 185, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Qiao, G.F.; Li, B.Y.; Lu, Y.J.; Fu, Y.L.; Schild, J.H. 17Beta-estradiol restores excitability of a sexually dimorphic subset of myelinated vagal afferents in ovariectomized rats. Am. J. Physiol. Cell Physiol. 2009, 297, C654–C664. [Google Scholar] [CrossRef] [PubMed]
- He, J.L.; Zhao, M.; Xia, J.J.; Guan, J.; Liu, Y.; Wang, L.Q.; Song, D.X.; Qu, M.Y.; Zuo, M.; Wen, X.; et al. FGF21 ameliorates the neurocontrol of blood pressure in the high fructose-drinking rats. Sci. Rep. 2016, 6, 29582. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, D.; Qu, M.Y.; He, J.L.; Yuan, M.; Zhao, M.; Wang, J.X.; He, J.; Wang, L.Q.; Guo, X.J.; et al. Neuropeptide Y-mediated sex- and afferent-specific neurotransmissions contribute to sexual dimorphism of baroreflex afferent function. Oncotarget 2016, 7, 66135–66148. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Zhou, J.Y.; Zhou, Y.H.; Wu, D.; He, J.L.; Han, L.M.; Liang, X.B.; Wang, L.Q.; Lu, X.L.; Chen, H.; et al. Unique Expression of Angiotensin Type-2 Receptor in Sex-Specific Distribution of Myelinated Ah-Type Baroreceptor Neuron Contributing to Sex-Dimorphic Neurocontrol of Circulation. Hypertension 2016, 67, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Jalal, D.I.; Smits, G.; Johnson, R.J.; Chonchol, M. Increased fructose associates with elevated blood pressure. J. Am. Soc. Nephrol. 2010, 21, 1543–1549. [Google Scholar] [CrossRef]
- Chen, L.; Caballero, B.; Mitchell, D.C.; Loria, C.; Lin, P.H.; Champagne, C.M.; Elmer, P.J.; Ard, J.D.; Batch, B.C.; Anderson, C.A.; et al. Reducing consumption of sugar-sweetened beverages is associated with reduced blood pressure: A prospective study among United States adults. Circulation 2010, 121, 2398–2406. [Google Scholar] [CrossRef]
- Brown, C.M.; Dulloo, A.G.; Yepuri, G.; Montani, J.P. Fructose ingestion acutely elevates blood pressure in healthy young humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R730–R737. [Google Scholar] [CrossRef]
- Tran, L.T.; Yuen, V.G.; McNeill, J.H. The fructose-fed rat: A review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol. Cell. Biochem. 2009, 332, 145–159. [Google Scholar] [CrossRef]
- Xu, C.M.; Yang, T.X. New advances in renal mechanisms of high fructose-induced salt-sensitive hypertension. Sheng Li Xue Bao 2018, 70, 581–590. [Google Scholar]
- Lipov, E.; Ritchie, E.C. A review of the use of stellate ganglion block in the treatment of PTSD. Curr. Psychiatry Rep. 2015, 17, 599. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Ponikowski, P.; Mitrovic, V.; Peacock, W.F.; Filippatos, G. Ularitide for the treatment of acute decompensated heart failure: From preclinical to clinical studies. Eur. Heart J. 2015, 36, 715–723. [Google Scholar] [CrossRef]
- Asakura, M.; Jiyoong, K.; Minamino, T.; Shintani, Y.; Asanuma, H.; Kitakaze, M.; Investigators, J.W. Rationale and design of a large-scale trial using atrial natriuretic peptide (ANP) as an adjunct to percutaneous coronary intervention for ST-segment elevation acute myocardial infarction: Japan-Working groups of acute myocardial infarction for the reduction of Necrotic Damage by ANP (J-WIND-ANP). Circ. J. 2004, 68, 95–100. [Google Scholar]
- Fajardo, J.; Heywood, J.T.; Patterson, J.H.; Adams, K.; Chow, S.L. Natriuretic peptides for the treatment of acute heart failure: A focus on nesiritide in recent clinical trials. Expert Rev. Cardiovasc. Ther. 2015, 13, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Yandle, T.G.; Richards, A.M.; Nicholls, M.G.; Cuneo, R.; Espiner, E.A.; Livesey, J.H. Metabolic clearance rate and plasma half life of alpha-human atrial natriuretic peptide in man. Life Sci. 1986, 38, 1827–1833. [Google Scholar] [PubMed]
- Hunt, P.J.; Richards, A.M.; Espiner, E.A.; Nicholls, M.G.; Yandle, T.G. Bioactivity and metabolism of C-type natriuretic peptide in normal man. J. Clin. Endocrinol. Metab. 1994, 78, 1428–1435. [Google Scholar]
- Holmes, S.J.; Espiner, E.A.; Richards, A.M.; Yandle, T.G.; Frampton, C. Renal, endocrine, and hemodynamic effects of human brain natriuretic peptide in normal man. J. Clin. Endocrinol. Metab. 1993, 76, 91–96. [Google Scholar]
- Qiao, G.F.; Qian, Z.; Sun, H.L.; Xu, W.X.; Yan, Z.Y.; Liu, Y.; Zhou, J.Y.; Zhang, H.C.; Wang, L.J.; Pan, X.D.; et al. Remodeling of hyperpolarization-activated current, Ih, in Ah-type visceral ganglion neurons following ovariectomy in adult rats. PLoS ONE 2013, 8, e71184. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Cui, Y.; Zhang, Q.; Li, Q.; Cheng, M.; Sun, J.; Cui, C.; Fan, X.; Li, B. Natriuretic Peptides—New Targets for Neurocontrol of Blood Pressure via Baroreflex Afferent Pathway. Int. J. Mol. Sci. 2022, 23, 13619. https://doi.org/10.3390/ijms232113619
Li X, Cui Y, Zhang Q, Li Q, Cheng M, Sun J, Cui C, Fan X, Li B. Natriuretic Peptides—New Targets for Neurocontrol of Blood Pressure via Baroreflex Afferent Pathway. International Journal of Molecular Sciences. 2022; 23(21):13619. https://doi.org/10.3390/ijms232113619
Chicago/Turabian StyleLi, Xinyu, Yali Cui, Qing Zhang, Qingyuan Li, Mengxing Cheng, Jie Sun, Changpeng Cui, Xiongxiong Fan, and Baiyan Li. 2022. "Natriuretic Peptides—New Targets for Neurocontrol of Blood Pressure via Baroreflex Afferent Pathway" International Journal of Molecular Sciences 23, no. 21: 13619. https://doi.org/10.3390/ijms232113619
APA StyleLi, X., Cui, Y., Zhang, Q., Li, Q., Cheng, M., Sun, J., Cui, C., Fan, X., & Li, B. (2022). Natriuretic Peptides—New Targets for Neurocontrol of Blood Pressure via Baroreflex Afferent Pathway. International Journal of Molecular Sciences, 23(21), 13619. https://doi.org/10.3390/ijms232113619






