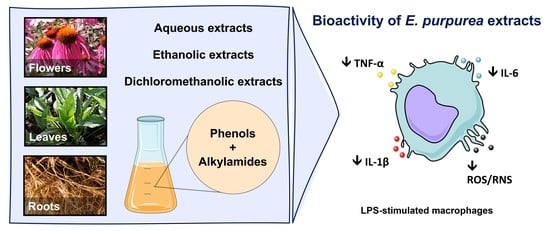
On the Bioactivity of Echinacea purpurea Extracts to Modulate the Production of Inflammatory Mediators
Abstract
1. Introduction
2. Results
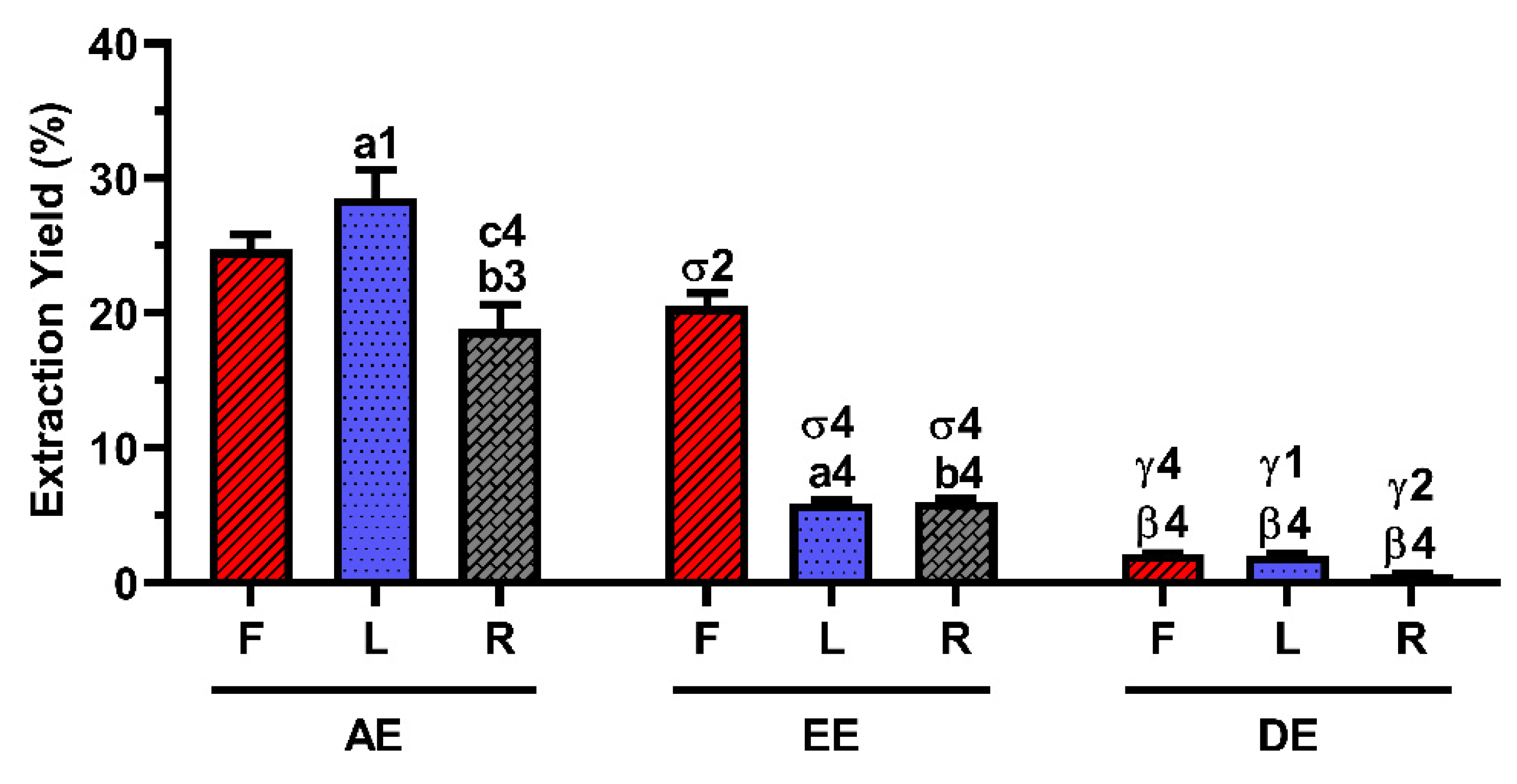
2.1. Extraction Yield
2.2. Composition of the E. purpurea Extracts
2.3. Cytotoxicity of the E. purpurea Extracts
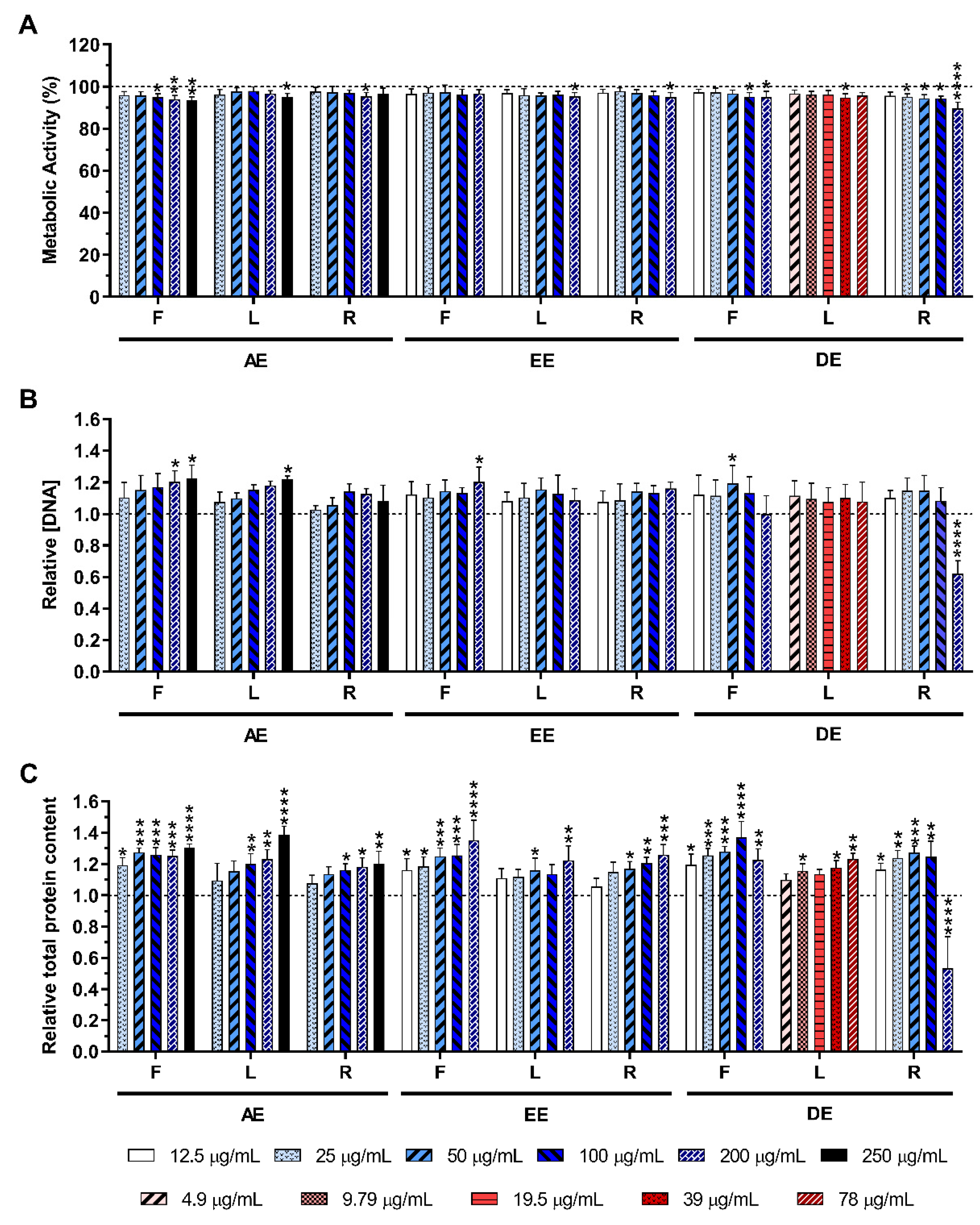
2.3.1. Non-Stimulated Macrophages
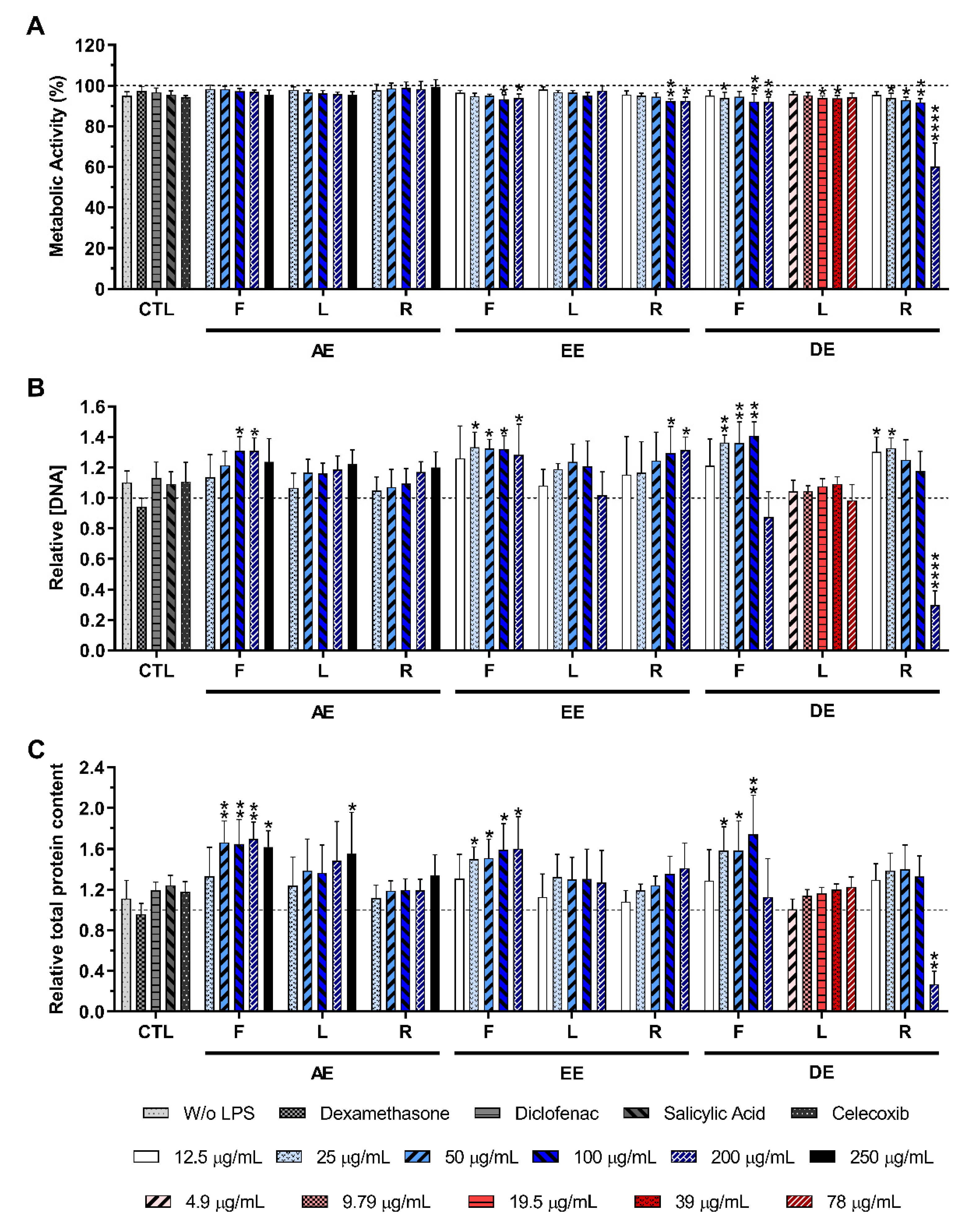
2.3.2. LPS-Stimulated Macrophages
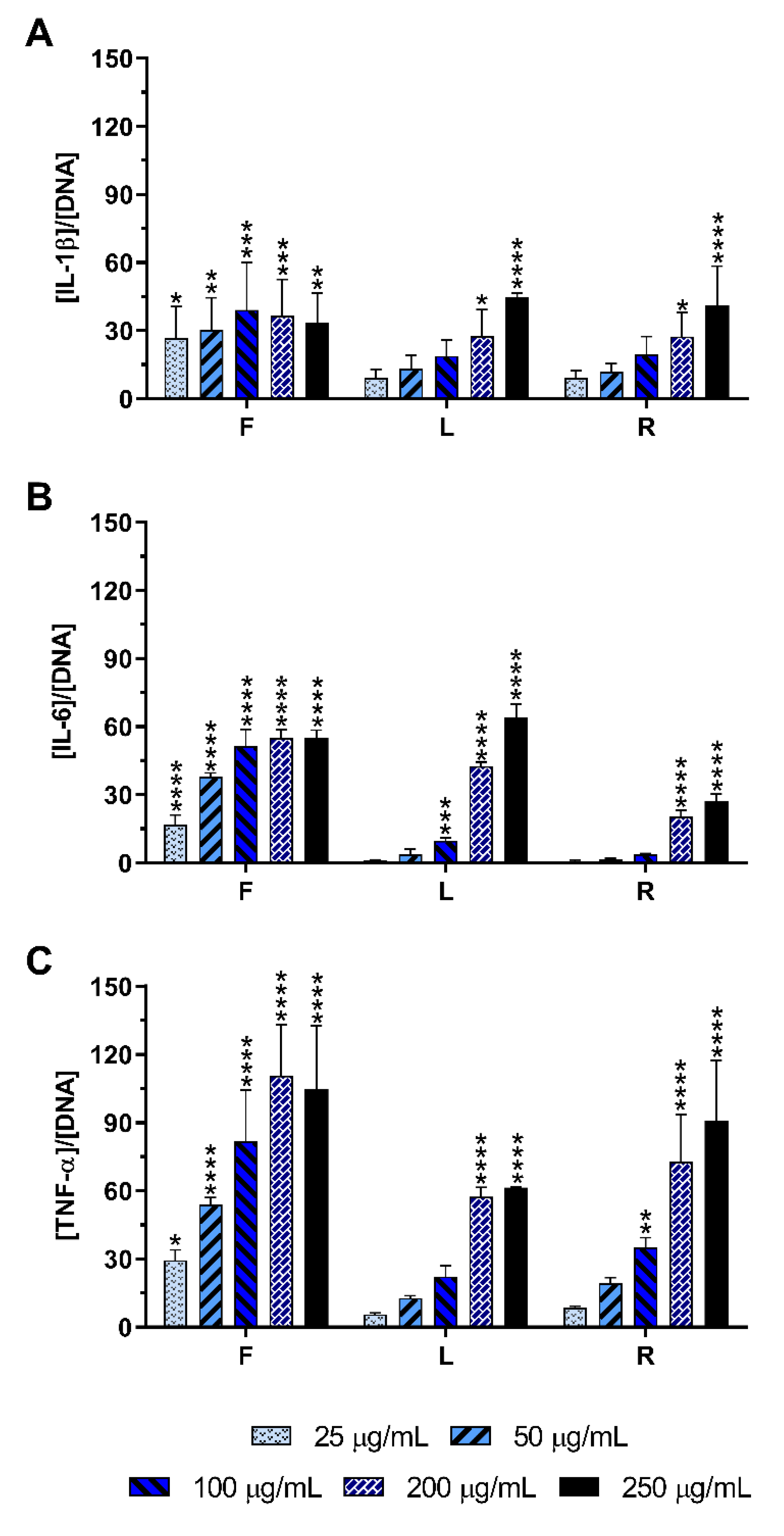
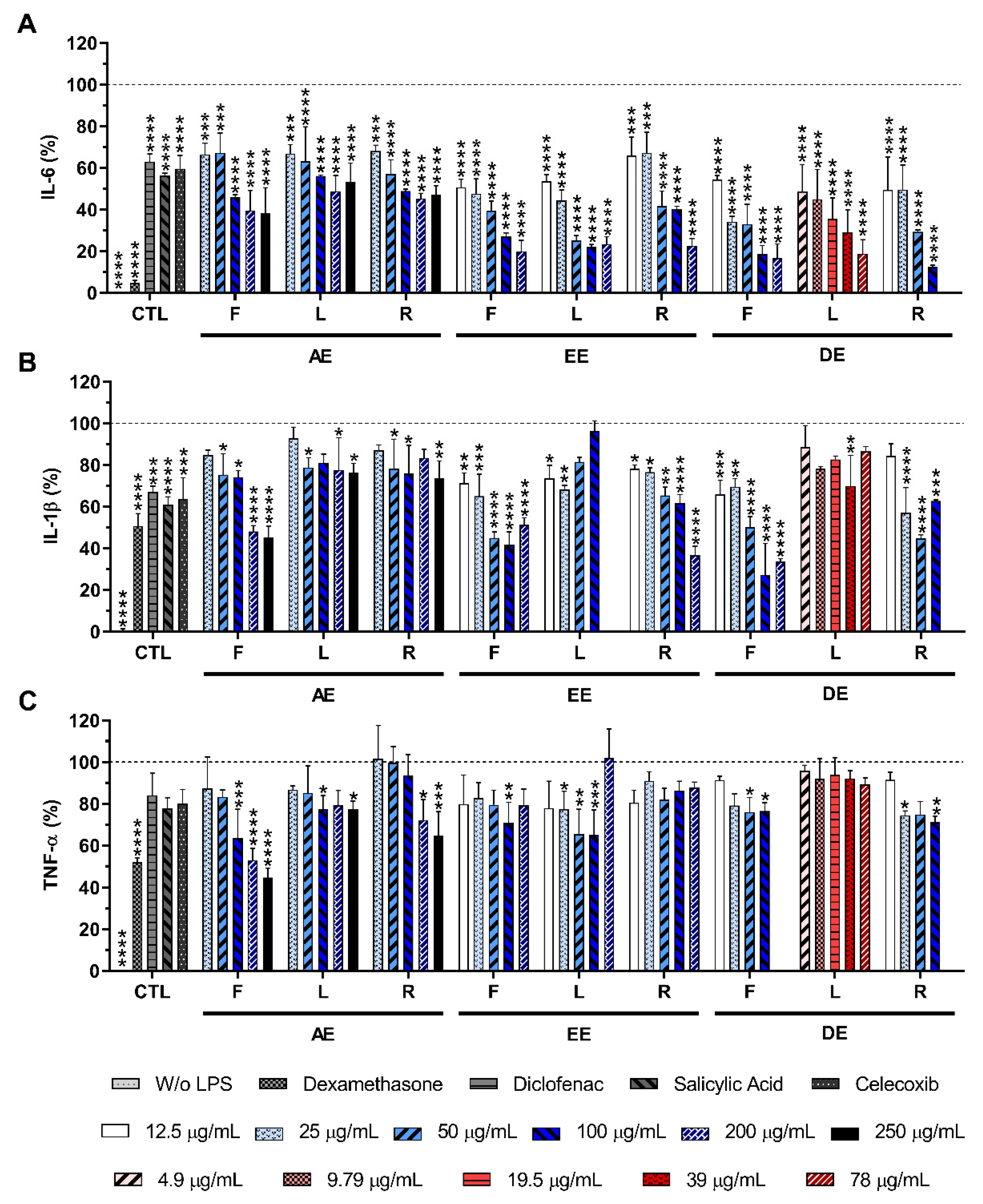
2.4. Effect of E. purpurea Extracts on Cytokine Production
2.4.1. Non-Stimulated Macrophages
2.4.2. LPS-Stimulated Macrophages
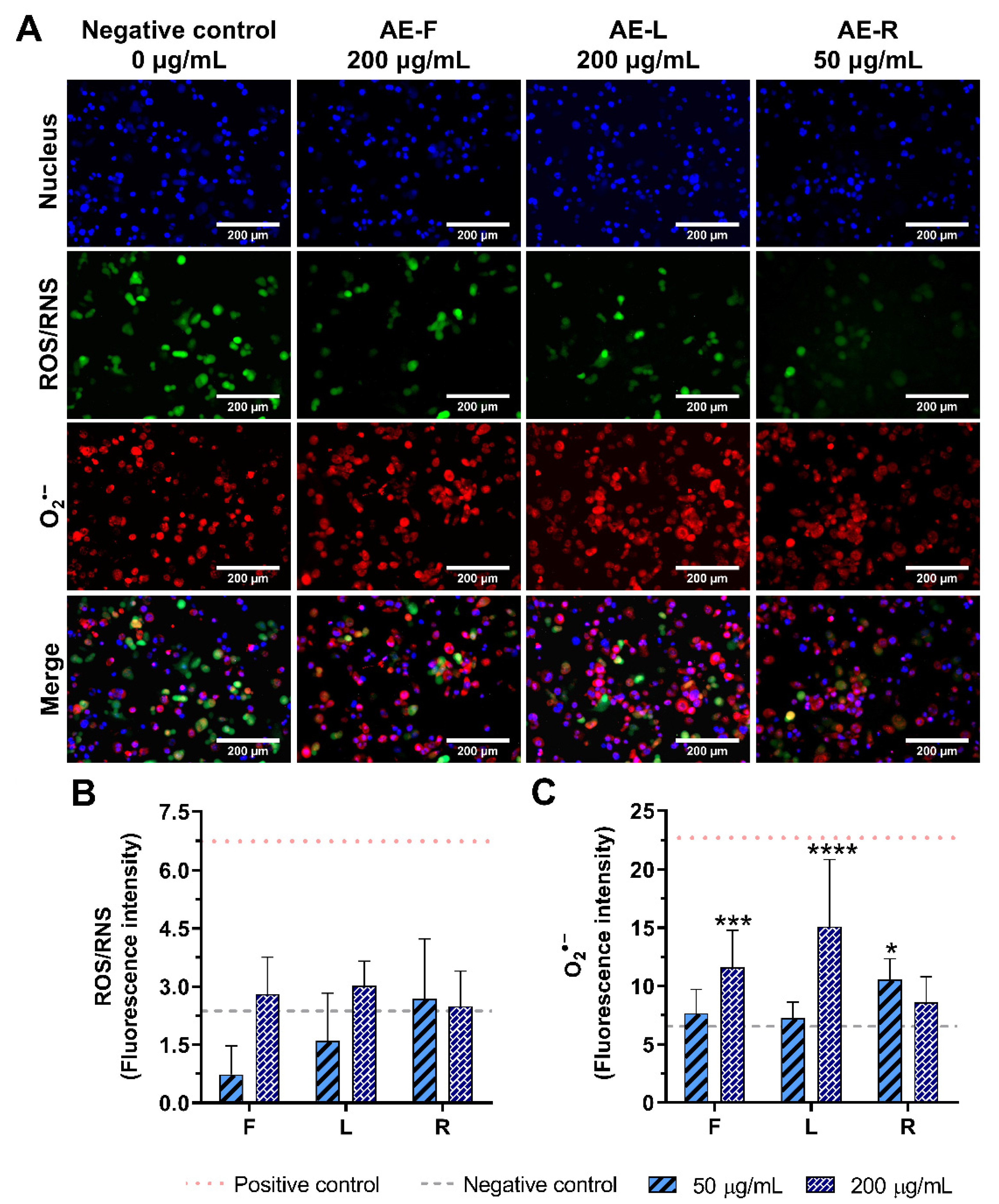
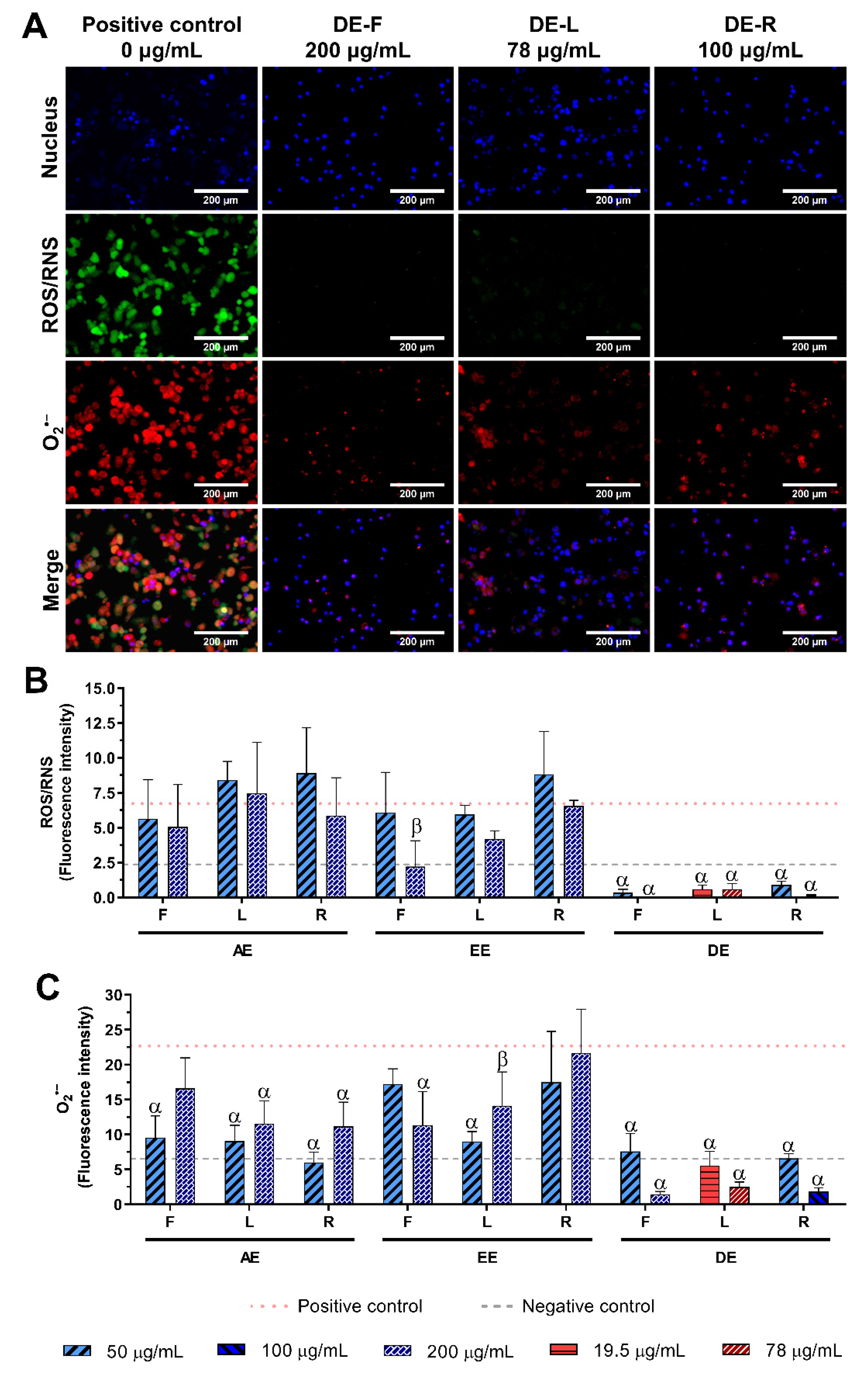
2.5. Effect of E. purpurea Extracts on ROS/RNS Generation
2.5.1. Non-Stimulated Macrophages
2.5.2. LPS-Stimulated Macrophages
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Bioactive Compounds Extraction
4.3. Characterization of E. purpurea Extracts Composition
4.3.1. Preparation of E. purpurea Extracts and Standards
4.3.2. LC-HRMS Analysis
4.4. E. purpurea Extract Solutions
4.5. Pro-Inflammatory Activity Evaluation
4.6. Anti-Inflammatory Activity Evaluation
4.7. Metabolic Activity, DNA Quantification, and Total Protein Content
4.8. Cytokine Quantification
4.9. Cellular ROS/RNS/O2•− Detection Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428. [Google Scholar] [CrossRef]
- Meng, F.; Lowell, C.A. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. J. Exp. Med. 1997, 185, 1661–1670. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Faustman, D.; Davis, M. TNF receptor 2 and disease: Autoimmunity and regenerative medicine. Front. Immunol. 2013, 4, 478. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Lamacchia, C.; Palmer, G. IL-1 pathways in inflammation and human diseases. Nat. Rev. Rheumatol. 2010, 6, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Dan Dunn, J.; Alvarez, L.A.J.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef]
- Chitnis, T.; Weiner, H.L. CNS inflammation and neurodegeneration. J. Clin. Investig. 2017, 127, 3577–3587. [Google Scholar] [CrossRef] [PubMed]
- Abou-Raya, S.; Abou-Raya, A.; Naim, A.; Abuelkheir, H. Chronic inflammatory autoimmune disorders and atherosclerosis. Ann. N. Y. Acad. Sci. 2007, 1107, 56–67. [Google Scholar] [CrossRef]
- Steinmeyer, J. Pharmacological basis for the therapy of pain and inflammation with nonsteroidal anti-inflammatory drugs. Arthritis Res. Ther. 2000, 2, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. How corticosteroids control inflammation: Quintiles prize lecture 2005. Br. J. Pharmacol. 2006, 148, 245–254. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Misery, L.; Naeyaert, S.; Taylor, P.C. Biological therapies in immune-mediated inflammatory diseases: Can biosimilars reduce access inequities? Front. Pharmacol. 2019, 10, 279. [Google Scholar] [CrossRef]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef]
- Poetker, D.M.; Reh, D.D. A comprehensive review of the adverse effects of systemic corticosteroids. Otolaryngol. Clin. N. Am. 2010, 43, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Boyman, O.; Comte, D.; Spertini, F. Adverse reactions to biologic agents and their medical management. Nat. Rev. Rheumatol. 2014, 10, 612. [Google Scholar] [CrossRef]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef]
- Kindscher, K. Ethnobotany of purple coneflower (Echinacea angustifolia, Asteraceae) and other Echinacea species. Econ. Bot. 1989, 43, 498–507. [Google Scholar] [CrossRef]
- Karsch-Völk, M.; Barrett, B.; Kiefer, D.; Bauer, R.; Ardjomand-Woelkart, K.; Linde, K. Echinacea for preventing and treating the common cold. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Wu, Y.; Wang, B.; Chen, X.; Xu, X.; Chen, H.; Li, W.; Xu, X. Echinacea pupurea extracts promote murine dendritic cell maturation by activation of JNK, p38 MAPK and NF-κB pathways. Dev. Comp. Immunol. 2017, 73, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.; Wang, Y.; Wu, Y.; Chen, H.; Zheng, S.; Li, Y.; Xu, X.; Li, W. Echinacea purpurea extract polarizes M1 macrophages in murine bone marrow-derived macrophages through the activation of JNK. J. Cell. Biochem. 2017, 118, 2664–2671. [Google Scholar] [CrossRef] [PubMed]
- Sasagawa, M.; Cech, N.B.; Gray, D.E.; Elmer, G.W.; Wenner, C.A. Echinacea alkylamides inhibit interleukin-2 production by Jurkat T cells. Int. Immunopharmacol. 2006, 6, 1214–1221. [Google Scholar] [CrossRef]
- Matthias, A.; Banbury, L.; Stevenson, L.M.; Bone, K.M.; Leach, D.N.; Lehmann, R.P. Alkylamides from echinacea modulate induced immune responses in macrophages. Immunol. Investig. 2007, 36, 117–130. [Google Scholar] [CrossRef]
- Zhang, H.; Lang, W.; Wang, S.; Li, B.; Li, G.; Shi, Q. Echinacea polysaccharide alleviates LPS-induced lung injury via inhibiting inflammation, apoptosis and activation of the TLR4/NF-κB signal pathway. Int. Immunopharmacol. 2020, 88, 106974. [Google Scholar] [CrossRef]
- Šutovská, M.; Capek, P.; Kazimierová, I.; Pappová, L.; Jošková, M.; Matulová, M.; Fraňová, S.; Pawlaczyk, I.; Gancarz, R. Echinacea complex—Chemical view and anti-asthmatic profile. J. Ethnopharmacol. 2015, 175, 163–171. [Google Scholar] [CrossRef]
- Cheng, Z.-Y.; Sun, X.; Liu, P.; Lin, B.; Li, L.-Z.; Yao, G.-D.; Huang, X.-X.; Song, S.-J. Sesquiterpenes from Echinacea purpurea and their anti-inflammatory activities. Phytochemistry 2020, 179, 112503. [Google Scholar] [CrossRef]
- Benson, J.M.; Pokorny, A.J.; Rhule, A.; Wenner, C.A.; Kandhi, V.; Cech, N.B.; Shepherd, D.M. Echinacea purpurea extracts modulate murine dendritic cell fate and function. Food Chem. Toxicol. 2010, 48, 1170–1177. [Google Scholar] [CrossRef]
- Yildiz, E.; Karabulut, D.; Yesil-Celiktas, O. A bioactivity based comparison of Echinacea purpurea extracts obtained by various processes. J. Supercrit. Fluids 2014, 89, 8–15. [Google Scholar] [CrossRef]
- Pires, C.; Martins, N.; Carvalho, A.M.; Barros, L.; Ferreira, I.C.F.R. Phytopharmacologic preparations as predictors of plant bioactivity: A particular approach to Echinacea purpurea (L.) Moench antioxidant properties. Nutrition 2016, 32, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Fast, D.J.; Balles, J.A.; Scholten, J.D.; Mulder, T.; Rana, J. Echinacea purpurea root extract inhibits TNF release in response to Pam3Csk4 in a phosphatidylinositol-3-kinase dependent manner. Cell. Immunol. 2015, 297, 94–99. [Google Scholar] [CrossRef]
- Thomsen, M.O.; Christensen, L.P.; Grevsen, K. Harvest strategies for optimization of the content of bioactive alkamides and caffeic acid derivatives in aerial parts and in roots of Echinacea purpurea. J. Agric. Food Chem. 2018, 66, 11630–11639. [Google Scholar] [CrossRef] [PubMed]
- Clifford, L.J.; Nair, M.G.; Rana, J.; Dewitt, D.L. Bioactivity of alkamides isolated from Echinacea purpurea (L.) Moench. Phytomedicine 2002, 9, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Todd, D.A.; Gulledge, T.V.; Britton, E.R.; Oberhofer, M.; Leyte-Lugo, M.; Moody, A.N.; Shymanovich, T.; Grubbs, L.F.; Juzumaite, M.; Graf, T.N.; et al. Ethanolic Echinacea purpurea extracts contain a mixture of cytokine-suppressive and cytokine-inducing compounds, including some that originate from endophytic bacteria. PLoS ONE 2015, 10, e0124276. [Google Scholar] [CrossRef]
- Tsai, Y.-L.; Chiou, S.-Y.; Chan, K.-C.; Sung, J.-M.; Lin, S.-D. Caffeic acid derivatives, total phenols, antioxidant and antimutagenic activities of Echinacea purpurea flower extracts. LWT Food Sci. Technol. 2012, 46, 169–176. [Google Scholar] [CrossRef]
- Stanisavljević, I.; Stojičević, S.; Veličković, D.; Veljković, V.; Lazić, M. Antioxidant and antimicrobial activities of Echinacea (Echinacea purpurea L.) extracts obtained by classical and ultrasound extraction. Chinese J. Chem. Eng. 2009, 17, 478–483. [Google Scholar] [CrossRef]
- Hu, C.; Kitts, D.D. Studies on the antioxidant activity of Echinacea root extract. J. Agric. Food Chem. 2000, 48, 1466–1472. [Google Scholar] [CrossRef]
- Kotowska, D.; El-Houri, R.B.; Borkowski, K.; Petersen, R.K.; Frette, X.C.; Wolber, G.; Grevsen, K.; Christensen, K.B.; Christensen, L.P.; Kristiansen, K. Isomeric C12-alkamides from the roots of Echinacea purpurea improve basal and insulin-dependent glucose uptake in 3T3-L1 adipocytes. Planta Med. 2014, 80, 1712–1720. [Google Scholar] [CrossRef]
- Gradišar, H.; Keber, M.M.; Pristovšek, P.; Jerala, R. MD-2 as the target of curcumin in the inhibition of response to LPS. J. Leukoc. Biol. 2007, 82, 968–974. [Google Scholar] [CrossRef]
- Perrin-Cocon, L.; Aublin-Gex, A.; Sestito, S.E.; Shirey, K.A.; Patel, M.C.; André, P.; Blanco, J.C.; Vogel, S.N.; Peri, F.; Lotteau, V. TLR4 antagonist FP7 inhibits LPS-induced cytokine production and glycolytic reprogramming in dendritic cells, and protects mice from lethal influenza infection. Sci. Rep. 2017, 7, 40791. [Google Scholar] [CrossRef] [PubMed]
- Cech, N.B.; Eleazer, M.S.; Shoffner, L.T.; Crosswhite, M.R.; Davis, A.C.; Mortenson, A.M. High performance liquid chromatography/electrospray ionization mass spectrometry for simultaneous analysis of alkamides and caffeic acid derivatives from Echinacea purpurea extracts. J. Chromatogr. A 2006, 1103, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Spelman, K.; Wetschler, M.H.; Cech, N.B. Comparison of alkylamide yield in ethanolic extracts prepared from fresh versus dry Echinacea purpurea utilizing HPLC–ESI-MS. J. Pharm. Biomed. Anal. 2009, 49, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Pellati, F.; Epifano, F.; Contaldo, N.; Orlandini, G.; Cavicchi, L.; Genovese, S.; Bertelli, D.; Benvenuti, S.; Curini, M.; Bertaccini, A.; et al. Chromatographic methods for metabolite profiling of virus- and phytoplasma-infected plants of Echinacea purpurea. J. Agric. Food Chem. 2011, 59, 10425–10434. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.O.; Fretté, X.C.; Christensen, K.B.; Christensen, L.P.; Grevsen, K. Seasonal variations in the concentrations of lipophilic compounds and phenolic acids in the roots of Echinacea purpurea and Echinacea pallida. J. Agric. Food Chem. 2012, 60, 12131–12141. [Google Scholar] [CrossRef]
- He, X.; Lin, L.; Bernart, M.W.; Lian, L. Analysis of alkamides in roots and achenes of Echinacea purpurea by liquid chromatography–electrospray mass spectrometry. J. Chromatogr. A 1998, 815, 205–211. [Google Scholar] [CrossRef]
- Mudge, E.; Lopes-Lutz, D.; Brown, P.; Schieber, A. Analysis of alkylamides in Echinacea plant materials and dietary supplements by ultrafast liquid chromatography with diode array and mass spectrometric detection. J. Agric. Food Chem. 2011, 59, 8086–8094. [Google Scholar] [CrossRef]
- Bauer, R.; Remiger, P. TLC and HPLC analysis of alkamides in Echinacea drugs. Planta Med. 1989, 55, 367–371. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Zhai, Z.; Solco, A.; Wu, L.; Wurtele, E.S.; Kohut, M.L.; Murphy, P.A.; Cunnick, J.E. Echinacea increases arginase activity and has anti-inflammatory properties in RAW 264.7 macrophage cells, indicative of alternative macrophage activation. J. Ethnopharmacol. 2009, 122, 76–85. [Google Scholar] [CrossRef]
- Gulledge, T.V.; Collette, N.M.; Mackey, E.; Johnstone, S.E.; Moazami, Y.; Todd, D.A.; Moeser, A.J.; Pierce, J.G.; Cech, N.B.; Laster, S.M. Mast cell degranulation and calcium influx are inhibited by an Echinacea purpurea extract and the alkylamide dodeca-2E,4E-dienoic acid isobutylamide. J. Ethnopharmacol. 2018, 212, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Chiao, M.-T.; Yen, P.-J.; Huang, W.-C.; Hou, C.-C.; Chien, S.-C.; Yeh, K.-C.; Yang, W.-C.; Shyur, L.-F.; Yang, N.-S. Modulatory effects of Echinacea purpurea extracts on human dendritic cells: A cell- and gene-based study. Genomics 2006, 88, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Staniforth, V.; Chiao, M.-T.; Hou, C.-C.; Wu, H.-M.; Yeh, K.-C.; Chen, C.-H.; Hwang, P.-I.; Wen, T.-N.; Shyur, L.-F.; et al. Genomics and proteomics of immune modulatory effects of a butanol fraction of echinacea purpurea in human dendritic cells. BMC Genom. 2008, 9, 479. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.-Y.; Wang, W.-H.; Wang, B.-X.; Aravindaram, K.; Hwang, P.-I.; Wu, H.-M.; Yang, N.-S. Stimulatory effect of Echinacea purpurea extract on the trafficking activity of mouse dendritic cells: Revealed by genomic and proteomic analyses. BMC Genom. 2010, 11, 612. [Google Scholar] [CrossRef] [PubMed]
- Cech, N.B.; Kandhi, V.; Davis, J.M.; Hamilton, A.; Eads, D.; Laster, S.M. Echinacea and its alkylamides: Effects on the influenza A-induced secretion of cytokines, chemokines, and PGE₂ from RAW 264.7 macrophage-like cells. Int. Immunopharmacol. 2010, 10, 1268–1278. [Google Scholar] [CrossRef]
- Hou, R.; Xu, T.; Li, Q.; Yang, F.; Wang, C.; Huang, T.; Hao, Z. Polysaccharide from Echinacea purpurea reduce the oxidant stress in vitro and in vivo. Int. J. Biol. Macromol. 2020, 149, 41–50. [Google Scholar] [CrossRef]
- Hou, C.-C.; Chen, C.-H.; Yang, N.-S.; Chen, Y.-P.; Lo, C.-P.; Wang, S.-Y.; Tien, Y.-J.; Tsai, P.-W.; Shyur, L.-F. Comparative metabolomics approach coupled with cell- and gene-based assays for species classification and anti-inflammatory bioactivity validation of Echinacea plants. J. Nutr. Biochem. 2010, 21, 1045–1059. [Google Scholar] [CrossRef]
- Hou, C.-C.; Huang, C.-C.; Shyur, L.-F. Echinacea alkamides prevent lipopolysaccharide/D-galactosamine-induced acute hepatic injury through JNK pathway-mediated HO-1 expression. J. Agric. Food Chem. 2011, 59, 11966–11974. [Google Scholar] [CrossRef]
- Raduner, S.; Majewska, A.; Chen, J.-Z.; Xie, X.-Q.; Hamon, J.; Faller, B.; Altmann, K.-H.; Gertsch, J. Alkylamides from Echinacea are a new class of cannabinomimetics. Cannabinoid type 2 receptor-dependent and -independent immunomodulatory effects. J. Biol. Chem. 2006, 281, 14192–14206. [Google Scholar] [CrossRef]
- Gertsch, J.; Schoop, R.; Kuenzle, U.; Suter, A. Echinacea alkylamides modulate TNF-alpha gene expression via cannabinoid receptor CB2 and multiple signal transduction pathways. FEBS Lett. 2004, 577, 563–569. [Google Scholar] [CrossRef]
- Binns, S.E.; Livesey, J.F.; Arnason, J.T.; Baum, B.R. Phytochemical variation in Echinacea from roots and flowerheads of wild and cultivated populations. J. Agric. Food Chem. 2002, 50, 3673–3687. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Azmin, S.N.H.; Abdul Manan, Z.; Wan Alwi, S.R.; Chua, L.S.; Mustaffa, A.A.; Yunus, N.A. Herbal processing and extraction technologies. Sep. Purif. Rev. 2016, 45, 305–320. [Google Scholar] [CrossRef]
- Mølgaard, P.; Johnsen, S.; Christensen, P.; Cornett, C. HPLC method validated for the simultaneous analysis of cichoric acid and alkamides in Echinacea purpurea plants and products. J. Agric. Food Chem. 2003, 51, 6922–6933. [Google Scholar] [CrossRef]
- Lin, S.-D.; Sung, J.-M.; Chen, C.-L. Effect of drying and storage conditions on caffeic acid derivatives and total phenolics of Echinacea purpurea grown in Taiwan. Food Chem. 2011, 125, 226–231. [Google Scholar] [CrossRef]
- Kim, H.-O.; Durance, T.D.; Scaman, C.H.; Kitts, D.D. Retention of caffeic acid derivatives in dried Echinacea purpurea. J. Agric. Food Chem. 2000, 48, 4182–4186. [Google Scholar] [CrossRef]
- Bohlmann, F.; Hoffmann, H. Further amides from Echinacea purpurea. Phytochemistry 1983, 22, 1173–1175. [Google Scholar] [CrossRef]
- Głowniak, K.; Zgórka, G.; Kozyra, M. Solid-phase extraction and reversed-phase high-performance liquid chromatography of free phenolic acids in some Echinacea species. J. Chromatogr. A 1996, 730, 25–29. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Lepojević, I.; Lepojević, Ž.; Pavlić, B.; Ristić, M.; Zeković, Z.; Vidović, S. Solid-liquid and high-pressure (liquid and supercritical carbon dioxide) extraction of Echinacea purpurea L. J. Supercrit. Fluids 2017, 119, 159–168. [Google Scholar] [CrossRef]
- Coelho, J.; Barros, L.; Dias, M.I.; Finimundy, T.C.; Amaral, J.S.; Alves, M.J.; Calhelha, R.C.; Santos, P.F.; Ferreira, I.C.F.R. Echinacea purpurea (L.) Moench: Chemical characterization and bioactivity of its extracts and fractions. Pharmaceuticals 2020, 13, 125. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [PubMed]
- Marciano, B.E.; Holland, S.M. Primary immunodeficiency diseases: Current and emerging therapeutics. Front. Immunol. 2017, 8, 937. [Google Scholar] [CrossRef]
- Fonseca, F.N.; Papanicolaou, G.; Lin, H.; Lau, C.B.S.; Kennelly, E.J.; Cassileth, B.R.; Cunningham-Rundles, S. Echinacea purpurea (L.) Moench modulates human T-cell cytokine response. Int. Immunopharmacol. 2014, 19, 94–102. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, T.; Tao, T.; Yang, J.; Chang, Y.; Wang, M.; Kim, L.; Qu, L.; Cassady, J.; Scalzo, R.; et al. Macrophage activating effects of new alkamides from the roots of Echinacea species. J. Nat. Prod. 2005, 68, 773–776. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.; Vreeburg, R.A.M.; Savelkoul, H.F.J.; Wichers, H.J. Transcription profiles of LPS-stimulated THP-1 monocytes and macrophages: A tool to study inflammation modulating effects of food-derived compounds. Food Funct. 2010, 1, 254–261. [Google Scholar] [CrossRef]
- DeForge, L.E.; Remick, D.G. Kinetics of TNF, IL-6, and IL-8 gene expression in LPS-stimulated human whole blood. Biochem. Biophys. Res. Commun. 1991, 174, 18–24. [Google Scholar] [CrossRef]
- Barber, A.E.; Coyle, S.M.; Fischer, E.; Smith, C.; van der Poll, T.; Shires, G.T.; Lowry, S.F. Influence of hypercortisolemia on soluble tumor necrosis factor receptor II and interleukin-1 receptor antagonist responses to endotoxin in human beings. Surgery 1995, 118, 406–411. [Google Scholar] [CrossRef]
- Van Deventer, S.J.; Buller, H.R.; ten Cate, J.W.; Aarden, L.A.; Hack, C.E.; Sturk, A. Experimental endotoxemia in humans: Analysis of cytokine release and coagulation, fibrinolytic, and complement pathways. Blood 1990, 76, 2520–2526. [Google Scholar] [CrossRef]
- Granowitz, E.V.; Poutsiaka, D.D.; Cannon, J.G.; Wolff, S.M.; Dinarello, C.A.; Santos, A.A.; Wilmore, D.W. Production of interleukin-1-receptor antagonist during experimental endotoxaemia. Lancet 1991, 338, 1423–1424. [Google Scholar] [CrossRef]
- Beckman, J.S. Oxidative damage and tyrosine nitration from peroxynitrite. Chem. Res. Toxicol. 1996, 9, 836–844. [Google Scholar] [CrossRef]
- Vieira, S.F.; Ferreira, H.; Neves, N.M. Antioxidant and anti-Inflammatory activities of cytocompatible Salvia officinalis extracts: A comparison between traditional and soxhlet extraction. Antioxidants 2020, 9, 1157. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.C.; Cunha, C.; Carvalho, A.; Ferreira, H.; Neves, N.M. Interleukin-6 neutralization by antibodies immobilized at the surface of polymeric nanoparticles as a therapeutic strategy for arthritic diseases. ACS Appl. Mater. Interfaces 2018, 10, 13839–13850. [Google Scholar] [CrossRef] [PubMed]
- Olival, A.; Vieira, S.F.; Gonçalves, V.M.F.; Cunha, C.; Tiritan, M.E.; Carvalho, A.; Reis, R.L.; Ferreira, H.; Neves, N.M. Erythrocyte-derived liposomes for the treatment of inflammatory diseases. J. Drug Target. 2022, 30, 873–883. [Google Scholar] [CrossRef]
- Loureiro, D.R.P.; Magalhães, Á.F.; Soares, J.X.; Pinto, J.; Azevedo, C.M.G.; Vieira, S.; Henriques, A.; Ferreira, H.; Neves, N.; Bousbaa, H.; et al. Yicathins B and C and analogues: Total synthesis, lipophilicity and biological activities. ChemMedChem 2020, 15, 749–755. [Google Scholar] [CrossRef]
- Guedes, M.; Vieira, S.F.; Reis, R.L.; Ferreira, H.; Neves, N.M. Fishroesomes as carriers with antioxidant and anti-inflammatory bioactivities. Biomed. Pharmacother. 2021, 140, 111680. [Google Scholar] [CrossRef]
| Compound | AE | EE | DE | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F | L | R | F | L | R | F | L | R | |
| Phenols/acids | |||||||||
| Malic Acid | X | X | X | X | X | X | - | X | X |
| Vanillic acid | X | X | - | X | - | - | - | - | - |
| Protocatechuic acid | X | X | - | X | X | - | - | - | - |
| Caftaric acid | X | X | - | X | X | X | - | X | - |
| Chlorogenic acid | - | - | - | X | - | X | - | - | - |
| Quinic acid | - | - | - | - | - | X | - | - | - |
| Vanillin | - | - | - | - | X | - | - | - | - |
| Caffeic acid | - | - | - | X | X | X | X | X | X |
| Benzoic acid | X | X | X | X | X | X | X | X | X |
| Cynarin | - | - | - | - | - | - | - | - | - |
| Echinacoside | - | - | - | - | - | - | - | - | - |
| p-coumaric acid | X | X | - | X | - | X | - | - | X |
| Chicoric acid | X | X | X | X | X | X | X | X | - |
| Rutin | - | - | - | X | - | - | - | - | - |
| Quercetin | - | - | - | X | - | - | - | - | - |
| Alkylamides | |||||||||
| Dodeca-2E,4Z,10E-triene-8-ynoic acid isobutylamide | X | - | X | X | - | X | X | - | X |
| Dodeca-2E,4Z,10Z-triene-8-ynoic acid isobutylamide | X | - | X | X | - | X | X | - | X |
| Dodeca-2,4,10-triene-8-ynoic acid isobutylamide (isomer 1) | - | - | - | - | - | - | X | - | X |
| Dodeca-2E,4E,10Z-triene-8-ynoic acid isobutylamide | - | - | X | X | - | X | X | - | X |
| Dodeca-2Z,4E,10Z-triene-8-ynoic acid isobutylamide | - | - | X | - | - | X | - | - | X |
| Dodeca-2E,4E,10E-triene-8-ynoic acid isobutylamide | X | - | - | X | X | X | X | X | X |
| Undeca-2E,4Z-diene-8,10-diynoic acid isobutylamide | X | - | X | X | X | X | X | X | X |
| Undeca-2E/Z-ene-8,10-diynoic acid isobutylamide | X | - | - | X | - | - | X | - | - |
| Undeca-2Z,4E-diene-8,10-diynoic acid isobutylamide | - | - | X | - | - | X | - | - | X |
| Undeca-2E/Z,4Z/E-diene-8,10-diynoic acid 2-methylbutylamide | - | - | - | - | - | - | - | - | - |
| Pentadeca-2E,9Z-diene-12,14-diynoic acid 2-hydroxyisobutylamide | - | - | - | X | X | - | X | X | - |
| Dodeca-2E,4Z-diene-8,10-diynoic acid isobutylamide | X | - | X | X | - | X | X | X | X |
| Undeca-2E,4E-diene-8,10-diynoic acid isobutylamide | - | - | - | - | - | X | - | - | X |
| Dodeca-2Z,4E-diene-8,10-diynoic acid isobutylamide | - | - | X | - | - | X | - | - | - |
| Dodeca-2E-ene-8,10-diynoic acid isobutylamide | X | - | - | X | X | X | X | X | X |
| Trideca-2E,7Z-diene-10,12-diynoic acid isobutylamide | X | - | X | X | - | X | X | - | X |
| Dodeca-2,4-diene-8,10-diynoic acid 2-methylbutylamide | - | - | X | X | - | X | X | - | X |
| Dodeca-2Z,4Z,10Z-triene-8-ynoic acid isobutylamide 1 | - | - | X | - | - | X | - | - | X |
| Trideca-2E,7Z-diene-10,12-diynoic acid 2-methylbutylamide | X | - | - | X | - | X | X | - | X |
| Dodeca-2E,4E,8Z,10E/Z-tetraenoic acid isobutylamide | X | - | X | X | X | X | X | X | X |
| Dodeca-2E,4Z,10E-triene-8-ynoic acid 2-methylbutylamide OR Dodeca-2E-ene-8,10-diynoic acid 2-methylbutylamide | X | - | X | X | - | X | X | - | X |
| Dodeca-2E,4E,8Z-trienoic acid isobutylamide (isomer 1) | - | - | - | X | - | - | X | - | - |
| Dodeca-2E,4E-dienoic acid isobutylamide (isomer 1) | - | - | - | - | X | - | - | - | - |
| Pentadeca-2E,9Z-diene-12,14-diynoic acid isobutylamide | - | - | X | X | X | X | X | X | X |
| Dodeca-2E,4E,8Z-trienoic acid isobutylamide | X | - | X | X | - | X | X | - | X |
| Trideca-2Z,7Z-diene-10,12-diynoic acid 2-methylbutylamide | - | - | - | - | - | X | - | - | X |
| Dodeca-2E,4E,8Z,10E/Z-tetraenoic acid 2-methylbutylamide | X | - | - | X | - | X | X | - | X |
| Hexadeca-2E,9Z-diene-12,14-diynoic acid isobutylamide | - | - | - | - | - | X | - | - | X |
| Dodeca-2E,4E,8Z-trienoic acid isobutylamide (isomer 2) | - | - | - | - | - | - | - | - | X |
| Dodeca-2E,4E-dienoic acid isobutylamide | X | - | X | X | X | X | X | X | X |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, S.F.; Gonçalves, V.M.F.; Llaguno, C.P.; Macías, F.; Tiritan, M.E.; Reis, R.L.; Ferreira, H.; Neves, N.M. On the Bioactivity of Echinacea purpurea Extracts to Modulate the Production of Inflammatory Mediators. Int. J. Mol. Sci. 2022, 23, 13616. https://doi.org/10.3390/ijms232113616
Vieira SF, Gonçalves VMF, Llaguno CP, Macías F, Tiritan ME, Reis RL, Ferreira H, Neves NM. On the Bioactivity of Echinacea purpurea Extracts to Modulate the Production of Inflammatory Mediators. International Journal of Molecular Sciences. 2022; 23(21):13616. https://doi.org/10.3390/ijms232113616
Chicago/Turabian StyleVieira, Sara F., Virgínia M. F. Gonçalves, Carmen P. Llaguno, Felipe Macías, Maria Elizabeth Tiritan, Rui L. Reis, Helena Ferreira, and Nuno M. Neves. 2022. "On the Bioactivity of Echinacea purpurea Extracts to Modulate the Production of Inflammatory Mediators" International Journal of Molecular Sciences 23, no. 21: 13616. https://doi.org/10.3390/ijms232113616
APA StyleVieira, S. F., Gonçalves, V. M. F., Llaguno, C. P., Macías, F., Tiritan, M. E., Reis, R. L., Ferreira, H., & Neves, N. M. (2022). On the Bioactivity of Echinacea purpurea Extracts to Modulate the Production of Inflammatory Mediators. International Journal of Molecular Sciences, 23(21), 13616. https://doi.org/10.3390/ijms232113616