Regulation of the Key Epithelial Cancer Suppressor miR-124 Function by Competing Endogenous RNAs
Abstract
1. Introduction
2. Protein-Coding Target Genes of miR-124 and Their Role in the Biological Processes Involved in the Carcinogenesis of Epithelial Tumors
3. Long Non-Coding RNAs in Dysregulation of miR-124 Target Genes in Epithelial Cancers
4. The Circular Long Non-Coding RNAs in Dysregulation of miR-124 Target Genes in Epithelial Cancers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Romano, G.; Veneziano, D.; Acunzo, M.; Croce, C.M. Small non-coding RNA and cancer. Carcinogenesis 2017, 38, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Sheikhvatan, M.; Chaichian, S.; Moazzami, B. A systematic review and bioinformatics study on genes and micro-RNAs involving the transformation of endometriosis into ovarian cancer. MicroRNA 2020, 9, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wang, X.; Guo, X.; Ji, J.; Lou, G.; Zhao, J.; Zhou, W.; Guo, M.; Zhang, M.; Li, C.; et al. MicroRNA-124: An emerging therapeutic target in cancer. Cancer Med. 2019, 8, 5638–5650. [Google Scholar] [CrossRef] [PubMed]
- Moghadasi, M.; Alivand, M.; Fardi, M.; Moghadam, K.S.; Solali, S. Emerging molecular functions of microRNA-124: Cancer pathology and therapeutic implications. Pathol. Res. Pract. 2020, 216, 152827. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, L.; Zhang, J.; Chen, H.; Fan, J.; Wang, K.; Luo, J.; Chen, Z.; Meng, Z.; Liu, L. Methylation-mediated silencing of the miR-124 genes facilitates pancreatic cancer progression and metastasis by targeting Rac1. Oncogene 2014, 33, 514–524. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, W.K.; Lee, E.B.; Son, J.W.; Kim, D.S.; Park, J.Y. Combined effect of metastasis-related microRNA, miR-34 and miR-124 family, methylation on prognosis of non-small-cell lung cancer. Clin. Lung Cancer 2017, 18, e13–e20. [Google Scholar] [CrossRef]
- Shahmohamadnejad, S.; Nouri Ghonbalani, Z.; Tahbazlahafi, B.; Panahi, G.; Meshkani, R.; Emami Razavi, A.; Shokri Afra, H.; Khalili, E. Aberrant methylation of miR-124 upregulates DNMT3B in colorectal cancer to accelerate invasion and migration. Arch. Physiol. Biochem. 2020, 128, 1503–1509. [Google Scholar] [CrossRef]
- Razavi, Z.S.; Tajiknia, V.; Majidi, S.; Ghandali, M.; Mirzaei, H.R.; Rahimian, N.; Hamblin, M.R.; Mirzaei, H. Gynecologic cancers and non-coding RNAs: Epigenetic regulators with emerging roles. Crit. Rev. Oncol. Hematol. 2021, 157, 103192. [Google Scholar] [CrossRef]
- Iwakawa, H.O.; Tomari, Y. Life of RISC: Formation, action, and degradation of RNA-induced silencing complex. Mol. Cell 2022, 82, 30–43. [Google Scholar] [CrossRef]
- Qian, X.; Zhao, J.; Yeung, P.Y.; Zhang, Q.C.; Kwok, C.K. Revealing lncRNA structures and interactions by sequencing-based approaches. Trends Biochem. Sci. 2019, 44, 33–52. [Google Scholar] [CrossRef]
- Bhan, A.; Soleimani, M.; Mandal, S.S. Long noncoding RNA and cancer: A new paradigm. Cancer Res. 2017, 77, 3965–3981. [Google Scholar] [CrossRef] [PubMed]
- Tay, Y.; Rinn, J.; Pandolfi, P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature 2014, 505, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Yu, H.; Zhang, X.; Song, D. Crosstalk between the lncRNA UCA1 and microRNAs in cancer. FEBS Lett. 2019, 593, 1901–1914. [Google Scholar] [CrossRef]
- Braga, E.A.; Fridman, M.V.; Moscovtsev, A.A.; Filippova, E.A.; Dmitriev, A.A.; Kushlinskii, N.E. LncRNAs in ovarian cancer progression, metastasis, and main pathways: ceRNA and alternative mechanisms. Int. J. Mol. Sci. 2020, 21, 8855. [Google Scholar] [CrossRef]
- Sheng, R.; Li, X.; Wang, Z.; Wang, X. Circular RNAs and their emerging roles as diagnostic and prognostic biomarkers in ovarian cancer. Cancer Lett. 2020, 473, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, S.; Yan, J.; Sun, M.Z.; Greenaway, F.T. The potential role of miR-124-3p in tumorigenesis and other related diseases. Mol. Biol. Rep. 2021, 48, 3579–3591. [Google Scholar] [CrossRef]
- Feng, Y.; Jiang, W.; Zhao, W.; Lu, Z.; Gu, Y.; Dong, Y. miR-124 regulates liver cancer stem cells expansion and sorafenib resistance. Exp. Cell Res. 2020, 394, 112162. [Google Scholar] [CrossRef]
- Torrejon, B.; Cristobal, I.; Rojo, F.; Garcia-Foncillas, J. Caveolin-1 is markedly downregulated in patients with early-stage colorectal cancer. World J. Surg. 2017, 41, 2625–2630. [Google Scholar] [CrossRef]
- Hu, X.X.; Feng, J.; Huang, X.W.; Lu, P.Z.; Wang, Z.X.; Dai, H.Q.; Deng, J.H.; Ye, X.P.; Peng, T.; Hooi, S.C.; et al. Histone deacetylases up-regulate C/EBPalpha expression through reduction of miR-124-3p and miR-25 in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2019, 514, 1009–1016. [Google Scholar] [CrossRef]
- Majid, A.; Wang, J.; Nawaz, M.; Abdul, S.; Ayesha, M.; Guo, C.; Liu, Q.; Liu, S.; Sun, M.Z. miR-124-3p suppresses the invasiveness and metastasis of hepatocarcinoma cells via targeting CRKL. Front. Mol. Biosci. 2020, 7, 223. [Google Scholar] [CrossRef]
- Cao, J.; Qiu, J.; Wang, X.; Lu, Z.; Wang, D.; Feng, H.; Li, X.; Liu, Q.; Pan, H.; Han, X.; et al. Identification of microRNA-124 in regulation of Hepatocellular carcinoma through BIRC3 and the NF-kappaB pathway. J. Cancer 2018, 9, 3006–3015. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Jiang, F.; Zhuang, H.; Chu, Y.; Zhang, F.; Wang, C. MicroRNA miR-124-3p suppresses proliferation and epithelial-mesenchymal transition of hepatocellular carcinoma via ARRDC1 (arrestin domain containing 1). Bioengineered 2022, 13, 8255–8265. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Cui, Y.; You, Q.; Lu, Y.; Zhang, J. MicroRNA124 negatively regulates chloride intracellular channel 1 to suppress the migration and invasion of liver cancer cells. Oncol. Rep. 2019, 42, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhang, W.; Wu, Y.; Wan, P.; Guo, Q.; Zhang, Y. miR124 inhibits cell growth through targeting IQGAP1 in colorectal cancer. Mol. Med. Rep. 2018, 18, 5270–5278. [Google Scholar] [CrossRef]
- Hu, D.; Li, M.; Su, J.; Miao, K.; Qiu, X. Dual-targeting of miR-124-3p and ABCC4 promotes sensitivity to adriamycin in breast cancer cells. Genet. Test. Mol, Biomark. 2019, 23, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Loginov, V.I.; Pronina, I.V.; Filippova, E.A.; Burdennyy, A.M.; Lukina, S.S.; Kazubskaya, T.P.; Uroshlev, L.A.; Fridman, M.V.; Brovkina, O.I.; Apanovich, N.V.; et al. Aberrant methylation of 20 miRNA genes specifically involved in various steps of ovarian carcinoma spread: From primary tumors to peritoneal macroscopic metastases. Int. J. Mol. Sci. 2022, 23, 1300. [Google Scholar] [CrossRef]
- Pronina, I.V.; Uroshlev, L.A.; Moskovtsev, A.A.; Zaichenko, D.M.; Filippova, E.A.; Fridman, M.V.; Burdennyy, A.M.; Loginov, V.I.; Kazubskaya, T.P.; Kushlinskii, N.E.; et al. Dysregulation of lncRNA-miRNA-mRNA Interactome as a marker of metastatic process in ovarian cancer. Biomedicines 2022, 10, 824. [Google Scholar] [CrossRef]
- Ji, H.; Sang, M.; Liu, F.; Ai, N.; Geng, C. miR-124 regulates EMT based on ZEB2 target to inhibit invasion and metastasis in triple-negative breast cancer. Pathol. Res. Pract. 2019, 215, 697–704. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, Y.; Li, L. miR-124 and miR-203 synergistically inactivate EMT pathway via coregulation of ZEB2 in clear cell renal cell carcinoma (ccRCC). J. Transl. Med. 2020, 18, 69. [Google Scholar] [CrossRef]
- Shi, P.; Chen, C.; Li, X.; Wei, Z.; Liu, Z.; Liu, Y. MicroRNA124 suppresses cell proliferation and invasion of triple negative breast cancer cells by targeting STAT3. Mol. Med. Rep. 2019, 19, 3667–3675. [Google Scholar] [CrossRef]
- Wu, D.H.; Liang, H.; Lu, S.N.; Wang, H.; Su, Z.L.; Zhang, L.; Ma, J.Q.; Guo, M.; Tai, S.; Yu, S. miR-124 Suppresses pancreatic ductal adenocarcinoma growth by regulating monocarboxylate transporter 1-mediated cancer lactate metabolism. Cell. Physiol. Biochem. 2018, 50, 924–935. [Google Scholar] [CrossRef]
- Qi, Z.; Zhang, T.; Song, L.; Fu, H.; Luo, H.; Wu, J.; Zhao, S.; Zhang, T.; Guo, L.; Jin, L.; et al. PRAS40 hyperexpression promotes hepatocarcinogenesis. eBioMedicine 2020, 51, 102604. [Google Scholar] [CrossRef]
- Roshani Asl, E.; Rasmi, Y.; Baradaran, B. MicroRNA-124-3p suppresses PD-L1 expression and inhibits tumorigenesis of colorectal cancer cells via modulating STAT3 signaling. J. Cell. Physiol. 2021, 236, 7071–7087. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Y.; Lin, Y.; Lin, S.; Gao, J.; Chen, Z.; Chen, S. MicroRNA-124 and microRNA-378 inhibit the proliferation and invasion of colorectal cancer by upregulating KiSS1. Transl. Cancer Res. 2020, 9, 2838–2846. [Google Scholar] [CrossRef]
- Huang, T.; Zhou, Y.; Zhang, J.; Wong, C.C.; Li, W.; Kwan, J.S.H.; Yang, R.; Chan, A.K.Y.; Dong, Y.; Wu, F.; et al. SRGAP1, a crucial target of miR-340 and miR-124, functions as a potential oncogene in gastric tumorigenesis. Oncogene 2018, 37, 1159–1174. [Google Scholar] [CrossRef]
- Tian, Y.; Tian, Y.; Tu, Y.; Zhang, G.; Zeng, X.; Lin, J.; Ai, M.; Mao, Z.; Zheng, R.; Yuan, Y. microRNA-124 inhibits stem-like properties and enhances radiosensitivity in nasopharyngeal carcinoma cells via direct repression of expression of JAMA. J. Cell. Mol. Med. 2020, 24, 9533–9544. [Google Scholar] [CrossRef]
- Zeng, B.; Zhang, X.; Zhao, J.; Wei, Z.; Zhu, H.; Fu, M.; Zou, D.; Feng, Y.; Luo, H.; Lei, Y. The role of DNMT1/hsa-miR-124-3p/BCAT1 pathway in regulating growth and invasion of esophageal squamous cell carcinoma. BMC Cancer 2019, 19, 609. [Google Scholar] [CrossRef]
- Yang, W.; Cui, G.; Ding, M.; Yang, M.; Dai, D. MicroRNA-124-3p.1 promotes cell proliferation through Axin1-dependent Wnt signaling pathway and predicts a poor prognosis of triple-negative breast cancer. J. Clin. Lab. Anal. 2020, 34, e23266. [Google Scholar] [CrossRef]
- Ma, X.; Ning, S. Cyanidin-3-glucoside attenuates the angiogenesis of breast cancer via inhibiting STAT3/VEGF pathway. Phytother. Res. PTR 2019, 33, 81–89. [Google Scholar] [CrossRef]
- Li, S.; Mei, Z.; Hu, H.B.; Zhang, X. The lncRNA MALAT1 contributes to non-small cell lung cancer development via modulating miR-124/STAT3 axis. J. Cell. Physiol. 2018, 233, 6679–6688. [Google Scholar] [CrossRef]
- Shang, H.; Zhang, H.; Ren, Z.; Zhao, H.; Zhang, Z.; Tong, J. Characterization of the potential role of NTPCR in epithelial ovarian cancer by integrating transcriptomic and metabolomic analysis. Front. Genet. 2021, 12, 695245. [Google Scholar] [CrossRef]
- Zu, L.; Xue, Y.; Wang, J.; Fu, Y.; Wang, X.; Xiao, G.; Hao, M.; Sun, X.; Wang, Y.; Fu, G.; et al. The feedback loop between miR-124 and TGF-beta pathway plays a significant role in non-small cell lung cancer metastasis. Carcinogenesis 2016, 37, 333–343. [Google Scholar] [CrossRef]
- Tian, Y.; Ma, R.; Sun, Y.; Liu, H.; Zhang, H.; Sun, Y.; Liu, L.; Li, Y.; Song, L.; Gao, P. SP1-activated long noncoding RNA lncRNA GCMA functions as a competing endogenous RNA to promote tumor metastasis by sponging miR-124 and miR-34a in gastric cancer. Oncogene 2020, 39, 4854–4868. [Google Scholar] [CrossRef]
- Hussen, B.M.; Honarmand Tamizkar, K.; Hidayat, H.J.; Taheri, M.; Ghafouri-Fard, S. The role of circular RNAs in the development of hepatocellular carcinoma. Pathol. Res. Pract. 2021, 223, 153495. [Google Scholar] [CrossRef]
- Yuan, L.; Li, S.; Zhou, Q.; Wang, D.; Zou, D.; Shu, J.; Huang, Y. MiR-124 inhibits invasion and induces apoptosis of ovarian cancer cells by targeting programmed cell death 6. Oncol. Lett. 2017, 14, 7311–7317. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; Zhang, X.; Li, Z.; Wang, Q.; Shi, Y.; Jiang, X.; Sun, X. The miR-124-3p/neuropilin-1 axis contributes to the proliferation and metastasis of triple-negative breast cancer cells and co-activates the TGF-beta pathway. Front. Oncol. 2021, 11, 654672. [Google Scholar] [CrossRef]
- Singh, R.; Som, A. Common miRNAs, candidate genes and their interaction network across four subtypes of epithelial ovarian cancer. Bioinformation 2021, 17, 748–759. [Google Scholar] [CrossRef]
- Abedi, Z.; MotieGhader, H.; Hosseini, S.S.; Sheikh Beig Goharrizi, M.A.; Masoudi-Nejad, A. mRNA-miRNA bipartite networks reconstruction in different tissues of bladder cancer based on gene co-expression network analysis. Sci. Rep. 2022, 12, 5885. [Google Scholar] [CrossRef]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef]
- Karreth, F.A.; Pandolfi, P.P. ceRNA cross-talk in cancer: When ce-bling rivalries go awry. Cancer Discov. 2013, 3, 1113–1121. [Google Scholar] [CrossRef]
- Chan, J.J.; Tay, Y. Noncoding RNA:RNA regulatory networks in cancer. Int. J. Mol. Sci. 2018, 19, 1310. [Google Scholar] [CrossRef]
- Ye, J.; Li, J.; Zhao, P. Roles of ncRNAs as ceRNAs in gastric cancer. Genes 2021, 12, 1036. [Google Scholar] [CrossRef]
- Soutschek, M.; Gross, F.; Schratt, G.; Germain, P.L. scanMiR: A biochemically-based toolkit for versatile and efficient microRNA target prediction. Bioinformatics 2022, 38, 2466–2473. [Google Scholar] [CrossRef]
- Rincon-Riveros, A.; Morales, D.; Rodriguez, J.A.; Villegas, V.E.; Lopez-Kleine, L. Bioinformatic tools for the analysis and prediction of ncRNA interactions. Int. J. Mol. Sci. 2021, 22, 11397. [Google Scholar] [CrossRef]
- Li, Z.; Qin, X.; Bian, W.; Li, Y.; Shan, B.; Yao, Z.; Li, S. Exosomal lncRNA ZFAS1 regulates esophageal squamous cell carcinoma cell proliferation, invasion, migration and apoptosis via microRNA-124/STAT3 axis. J. Exp. Clin. Cancer Res. CR 2019, 38, 477. [Google Scholar] [CrossRef]
- Xue, Y.; Diao, M.; Lyu, J.; Li, K.; He, L.; Chen, J.; Li, X. Long noncoding RNAs PTPRG antisense RNA 1 targets cyclin D1 to facilitate cell proliferation in lung adenocarcinoma. Cancer Biother. Radiopharm. ahead of print. 2021. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Y.; Liu, L.; Yang, W.; Zhang, Q. HNF1A-AS1 regulates cell migration, invasion and glycolysis via modulating miR-124/MYO6 in colorectal cancer cells. OncoTarg. Ther. 2020, 13, 1507–1518. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, F.; Tan, F.; Tang, L.; Du, Z.; Mou, J.; Zhou, G.; Yuan, C. HNF1A-AS1: A tumor-associated long non-coding RNA. Curr. Pharm. Des. 2022, 28, 1720–1729. [Google Scholar] [CrossRef]
- Pan, Y.; Wu, Y.; Hu, J.; Shan, Y.; Ma, J.; Ma, H.; Qi, X.; Jia, L. Long noncoding RNA HOTAIR promotes renal cell carcinoma malignancy through alpha-2, 8-sialyltransferase 4 by sponging microRNA-124. Cell Prolif. 2018, 51, e12507. [Google Scholar] [CrossRef]
- Xiong, L.; Tang, Y.; Tang, J.; Liu, Z.; Wang, X. Downregulation of lncRNA HOTTIP suppresses the proliferation, migration, and invasion of oral tongue squamous cell carcinoma by regulation of HMGA2-mediated Wnt/beta-catenin pathway. Cancer Biother. Radiopharm. 2020, 35, 720–730. [Google Scholar] [CrossRef]
- Zhang, W.L.; Zhao, Y.N.; Shi, Z.Z.; Gu, G.Y.; Cong, D.; Wei, C.; Bai, Y.S. HOXA11-AS promotes the migration and invasion of hepatocellular carcinoma cells by inhibiting miR-124 expression by binding to EZH2. Human Cell 2019, 32, 504–514. [Google Scholar] [CrossRef]
- You, L.; Wu, Q.; Xin, Z.; Zhong, H.; Zhou, J.; Jiao, L.; Song, X.; Ying, B. The long non-coding RNA HOXA11-AS activates ITGB3 expression to promote the migration and invasion of gastric cancer by sponging miR-124-3p. Cancer Cell Int. 2021, 21, 576. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Peng, W.; Jiang, H.; Sha, H.; Li, J. LncRNA HOXA11-AS promotes proliferation and invasion by targeting miR-124 in human non-small cell lung cancer cells. Tumour Biol. 2017, 39, 1010428317721440. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.Y.; Qiao, T.Y.; Jin, H.; Liu, L.L.; Zheng, M.D.; Wang, Z.L. LncRNA KCNQ1OT1 contributes to the cisplatin resistance of tongue cancer through the KCNQ1OT1/miR-124-3p/TRIM14 axis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 200–212. [Google Scholar] [CrossRef]
- Li, Y.; Yan, J.; Wang, Y.; Wang, C.; Zhang, C.; Li, G. LINC00240 promotes gastric cancer cell proliferation, migration and EMT via the miR-124-3p / DNMT3B axis. Cell Biochem. Funct. 2020, 38, 1079–1088. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zhang, J.; Liang, H. Natural killer T cell cytotoxic activity in cervical cancer is facilitated by the LINC00240/microRNA-124-3p/STAT3/MICA axis. Cancer Lett. 2020, 474, 63–73. [Google Scholar] [CrossRef]
- Huang, H.G.; Tang, X.L.; Huang, X.S.; Zhou, L.; Hao, Y.G.; Zheng, Y.F. Long noncoding RNA LINC00511 promoted cell proliferation and invasion via regulating miR-124-3p/EZH2 pathway in gastric cancer. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4232–4245. [Google Scholar] [CrossRef]
- Sun, C.B.; Wang, H.Y.; Han, X.Q.; Liu, Y.N.; Wang, M.C.; Zhang, H.X.; Gu, Y.F.; Leng, X.G. LINC00511 promotes gastric cancer cell growth by acting as a ceRNA. World J. Gastrointest. Oncol. 2020, 12, 394–404. [Google Scholar] [CrossRef]
- Zheng, K.; Zhang, T.K. LncRNA LINC00963 promotes proliferation and migration through the miR-124-3p/FZD4 pathway in colorectal cancer. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7634–7644. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, C.; Zhu, Y.; Han, H. LINC01410 promotes cell proliferation and migration of cholangiocarcinoma through modulating miR-124-3p/SMAD5 axis. J. Gene Med. 2020, 22, e3162. [Google Scholar] [CrossRef]
- Li, H.; Guo, X.; Li, Q.; Ran, P.; Xiang, X.; Yuan, Y.; Dong, T.; Zhu, B.; Wang, L.; Li, F.; et al. Long non-coding RNA 1308 promotes cell invasion by regulating the miR-124/ADAM 15 axis in non-small-cell lung cancer cells. Cancer Manag. Res. 2018, 10, 6599–6609. [Google Scholar] [CrossRef]
- Zhang, H.R.; Wu, S.Y.; Fu, Z.X. LncRNA-cCSC1 promotes cell proliferation of colorectal cancer through sponging miR-124-3p and upregulating CD44. Biochem. Biophys. Res. Commun. 2021, 557, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Wang, Y.; Yin, F. MALAT1/miR-124/Capn4 axis regulates proliferation, invasion and EMT in nasopharyngeal carcinoma cells. Cancer Biol. Ther. 2017, 18, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Shao, F.; Wu, Q.; Zhang, X.; Xu, D.; Qian, K.; Xie, Y.; Wang, S.; Xu, N.; Wang, Y.; et al. miR-124 downregulation leads to breast cancer progression via LncRNA-MALAT1 regulation and CDK4/E2F1 signal activation. Oncotarget 2016, 7, 16205–16216. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Cui, H.; Xu, W. Hydrogen inhibits the proliferation and migration of gastric cancer cells by modulating lncRNA MALAT1/miR-124-3p/EZH2 axis. Cancer Cell Int. 2021, 21, 70. [Google Scholar] [CrossRef] [PubMed]
- Jiao, D.; Li, Z.; Zhu, M.; Wang, Y.; Wu, G.; Han, X. LncRNA MALAT1 promotes tumor growth and metastasis by targeting miR-124/foxq1 in bladder transitional cell carcinoma (BTCC). Am. J. Cancer Res. 2018, 8, 748–760. [Google Scholar] [PubMed]
- Liu, S.; Song, L.; Zeng, S.; Zhang, L. MALAT1-miR-124-RBG2 axis is involved in growth and invasion of HR-HPV-positive cervical cancer cells. Tumour Biol. 2016, 37, 633–640. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Peng, F.; Li, W.; Jiang, Y. Interaction of lncRNA-MALAT1 and miR-124 regulates HBx-induced cancer stem cell properties in HepG2 through PI3K/Akt signaling. J. Cell. Biochem. 2019, 120, 2908–2918. [Google Scholar] [CrossRef]
- Cui, R.J.; Fan, J.L.; Lin, Y.C.; Pan, Y.J.; Liu, C.; Wan, J.H.; Wang, W.; Jiang, Z.Y.; Zheng, X.L.; Tang, J.B.; et al. miR-124-3p availability is antagonized by LncRNA-MALAT1 for Slug-induced tumor metastasis in hepatocellular carcinoma. Cancer Med. 2019, 8, 6358–6369. [Google Scholar] [CrossRef]
- Du, M.; Chen, W.; Zhang, W.; Tian, X.K.; Wang, T.; Wu, J.; Gu, J.; Zhang, N.; Lu, Z.W.; Qian, L.X.; et al. TGF-β regulates the ERK/MAPK pathway independent of the SMAD pathway by repressing miRNA-124 to increase MALAT1 expression in nasopharyngeal carcinoma. Biomed. Pharmacother. 2018, 99, 688–696. [Google Scholar] [CrossRef]
- Liu, X.; Liang, Y.; Song, R.; Yang, G.; Han, J.; Lan, Y.; Pan, S.; Zhu, M.; Liu, Y.; Wang, Y.; et al. Long non-coding RNA NEAT1-modulated abnormal lipolysis via ATGL drives hepatocellular carcinoma proliferation. Mol. Cancer 2018, 17, 90. [Google Scholar] [CrossRef]
- Cheng, N.; Guo, Y. Long noncoding RNA NEAT1 promotes nasopharyngeal carcinoma progression through regulation of miR-124/NF-kappaB pathway. OncoTargets Ther. 2017, 10, 5843–5853. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhou, N.; Zhao, P. Expression level of NEAT1 differentiates benign and malignant thyroid nodules by regulating NEAT1/miR9/PTEN and NEAT1/miR124/PDCD6 signalling. Int. J. Mol. Med. 2020, 46, 1661–1670. [Google Scholar] [CrossRef]
- Pang, Y.; Wu, J.; Li, X.; Wang, C.; Wang, M.; Liu, J.; Yang, G. NEAT1/miR124/STAT3 feedback loop promotes breast cancer progression. Int. J. Oncol. 2019, 55, 745–754. [Google Scholar] [CrossRef]
- Tang, L.X.; Chen, G.H.; Li, H.; He, P.; Zhang, Y.; Xu, X.W. Long non-coding RNA OGFRP1 regulates LYPD3 expression by sponging miR-124-3p and promotes non-small cell lung cancer progression. Biochem. Biophys. Res. Commun. 2018, 505, 578–585. [Google Scholar] [CrossRef]
- Yan, K.; Hou, L.; Liu, T.; Jiao, W.; Ma, Q.; Fang, Z.; Zhang, S.; Song, D.; Liu, J.; Gao, X.; et al. lncRNA OGFRP1 functions as a ceRNA to promote the progression of prostate cancer by regulating SARM1 level via miR-124-3p. Aging 2020, 12, 8880–8892. [Google Scholar] [CrossRef]
- Li, L.; Ma, Y.; Maerkeya, K.; Reyanguly, D.; Han, L. LncRNA OIP5-AS1 regulates the Warburg effect through miR-124-5p/IDH2/HIF-1alpha pathway in cervical cancer. Front. Cell Dev. Biol. 2021, 9, 655018. [Google Scholar] [CrossRef]
- Xie, C.; Zhang, L.Z.; Chen, Z.L.; Zhong, W.J.; Fang, J.H.; Zhu, Y.; Xiao, M.H.; Guo, Z.W.; Zhao, N.; He, X.; et al. A hMTR4-PDIA3P1-miR-125/124-TRAF6 regulatory axis and its function in NF kappa B signaling and chemoresistance. Hepatology 2020, 71, 1660–1677, Erratum in Hepatology 2020, 72, 367. [Google Scholar] [CrossRef]
- Shen, Z.; Wu, Y.; He, G. Long non-coding RNA PTPRG-AS1/microRNA-124-3p regulates radiosensitivity of nasopharyngeal carcinoma via the LIM Homeobox 2-dependent Notch pathway through competitive endogenous RNA mechanism. Bioengineered 2022, 13, 8208–8225. [Google Scholar] [CrossRef]
- Hu, Y.Z.; Hu, Z.L.; Liao, T.Y.; Li, Y.; Pan, Y.L. LncRNA SND1-IT1 facilitates TGF-beta1-induced epithelial-to-mesenchymal transition via miR-124/COL4A1 axis in gastric cancer. Cell Death Discov. 2022, 8, 73. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Wang, X.Y.; Yang, Y.M.; Wu, M.H.; Yang, L.; Jiang, D.T.; Cai, H.; Peng, Y. LncRNA SNHG16 promotes colorectal cancer cell proliferation, migration, and epithelial-mesenchymal transition through miR-124-3p/MCP-1. Gene Ther. 2022, 29, 193–205. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Khoshbakht, T.; Taheri, M.; Shojaei, S. A review on the role of small nucleolar RNA host gene 6 long non-coding RNAs in the carcinogenic processes. Front. Cell Dev. Biol. 2021, 9, 741684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.H.; Liang, L.Z.; Liu, X.L.; Wu, J.N.; Su, K.; Chen, J.Y.; Zheng, Q.Y. LncRNA UCA1/miR-124 axis modulates TGFbeta1-induced epithelial-mesenchymal transition and invasion of tongue cancer cells through JAG1/Notch signaling. J. Cell. Biochem. 2019, 120, 10495–10504. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Wang, L.; Li, Y.; Chen, M.; He, W.; Qi, L. The long non-coding RNA XIST Interacted with MiR-124 to modulate bladder cancer growth, invasion and migration by targeting androgen receptor (AR). Cell. Physiol. Biochem. 2017, 43, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Cui, X.; Wang, X. Long noncoding RNA XIST increases the aggressiveness of laryngeal squamous cell carcinoma by regulating miR-124-3p/EZH2. Exp. Cell Res. 2019, 381, 172–178. [Google Scholar] [CrossRef]
- Gao, S.; Yu, J.; Shan, Z.; Zuo, L.; Huang, W.; Gan, L.; Tian, H. IncRNA XIST targets miR-124/JAG1 via CeRNA mechanism to facilitate the migration and proliferation of tongue squamous cell carcinoma. Clin. Lab. 2022, 68, 210325. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Gholipour, M.; Hussen, B.M.; Taheri, M. The impact of long non-coding RNAs in the pathogenesis of hepatocellular carcinoma. Front. Oncol. 2021, 11, 649107. [Google Scholar] [CrossRef]
- Lin, S.L.; Lin, Y.H.; Chi, H.C.; Lin, T.K.; Chen, W.J.; Yeh, C.T.; Lin, K.H. A novel long non-coding RNA-01488 suppressed metastasis and tumorigenesis by inducing miRNAs that reduce vimentin expression and ubiquitination of cyclin e. Cells 2020, 9, 1504. [Google Scholar] [CrossRef]
- Chai, Y.; Liu, J.; Zhang, Z.; Liu, L. HuR-regulated lncRNA NEAT1 stability in tumorigenesis and progression of ovarian cancer. Cancer Med. 2016, 5, 1588–1598. [Google Scholar] [CrossRef]
- Wu, X.; Ma, J.; Chen, J.; Huang, H. LncRNA CACS15 regulates tongue squamous cell carcinoma cell behaviors and predicts survival. BMC Oral Health 2019, 19, 231. [Google Scholar] [CrossRef]
- Du, Y.; Wei, N.; Hong, J.; Pan, W. Long non-coding RNASNHG17 promotes the progression of breast cancer by sponging miR-124-3p. Cancer Cell Int. 2020, 20, 40. [Google Scholar] [CrossRef]
- Wu, J.; Weng, Y.; He, F.; Liang, D.; Cai, L. LncRNA MALAT-1 competitively regulates miR-124 to promote EMT and development of non-small-cell lung cancer. Anti-Cancer Drugs 2018, 29, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Wang, Y.; Jiao, Y.; Cong, S.; Jiang, X.; Dong, L.; Zhang, G.; Xiao, D. LncRNA MALAT1 accelerates cervical carcinoma proliferation by suppressing miR-124 expression in cervical tumor cells. J. Oncol. 2021, 2021, 8836078. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Li, P.; Hei, Y.; Li, S.; Wang, J.; Lv, X.; Zhang, J. Long non-coding RNA-ZNF281 promotes cancer cell migration and invasion in gastric cancer via downregulation of microRNA-124. Oncol. Lett. 2020, 19, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Zhu, D.; Yin, B.; Ge, H.; Zhao, Y.; Huang, A.; Wang, X.; Cao, X.; Xia, N.; Qian, H. Expression of lncRNA NEAT1 in endometriosis and its biological functions in ectopic endometrial cells as mediated via miR-124-3p. Genes Genom. 2022, 44, 527–537. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Zhang, G. LncRNA DSCAM-AS1 negatively interacts with miR-124 to promote hepatocellular carcinoma proliferation. Crit. Rev. Eukaryot. Gene Express. 2022, 32, 1–8. [Google Scholar] [CrossRef]
- Tang, Z.; Wei, G.; Zhang, L.; Xu, Z. Signature microRNAs and long noncoding RNAs in laryngeal cancer recurrence identified using a competing endogenous RNA network. Mol. Med. Rep. 2019, 19, 4806–4818. [Google Scholar] [CrossRef]
- Bolha, L.; Ravnik-Glavac, M.; Glavac, D. Circular RNAs: Biogenesis, function, and a role as possible cancer biomarkers. Int. J. Genom. 2017, 2017, 6218353. [Google Scholar] [CrossRef]
- Cui, W.; Xue, J. Circular RNA DOCK1 downregulates microRNA-124 to induce the growth of human thyroid cancer cell lines. BioFactors 2020, 46, 591–599. [Google Scholar] [CrossRef]
- Yao, D.; Lin, S.; Chen, S.; Wang, Z. circHIPK3 regulates cell proliferation and migration by sponging microRNA-124 and regulating serine/threonine kinase 3 expression in esophageal squamous cell carcinoma. Bioengineered 2022, 13, 9767–9780. [Google Scholar] [CrossRef]
- Chen, G.; Shi, Y.; Liu, M.; Sun, J. circHIPK3 regulates cell proliferation and migration by sponging miR-124 and regulating AQP3 expression in hepatocellular carcinoma. Cell Death Dis. 2018, 9, 175. [Google Scholar] [CrossRef]
- Hu, H.; Wang, Y.; Qin, Z.; Sun, W.; Chen, Y.; Wang, J.; Wang, Y.; Nie, J.; Chen, L.; Cai, S.; et al. Regulation of MRP4 Expression by circHIPK3 via sponging miR-124-3p/miR-4524-5p in hepatocellular carcinoma. Biomedicines 2021, 9, 497. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Liu, Y.; Yang, H.; Hu, B. Breast cancer derived exosomes promoted angiogenesis of endothelial cells in microenvironment via circHIPK3/miR-124-3p/MTDH axis. Cell. Signal. 2022, 95, 110338. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Chen, W.; Li, Y.; He, J.; Wang, Y.; Yang, S.; Zhou, J. The novel circular RNA HIPK3 accelerates the proliferation and invasion of hepatocellular carcinoma cells by sponging the micro RNA-124 or micro RNA-506/pyruvate dehydrogenase kinase 2 axis. Bioengineered 2022, 13, 4717–4729. [Google Scholar] [CrossRef] [PubMed]
- Kai, D.; Yannian, L.; Yitian, C.; Dinghao, G.; Xin, Z.; Wu, J. Circular RNA HIPK3 promotes gallbladder cancer cell growth by sponging microRNA-124. Biochem. Biophys. Res. Commun. 2018, 503, 863–869. [Google Scholar] [CrossRef]
- Xu, Q.Y.; Xie, M.J.; Huang, J.; Wang, Z.W. Effect of circ MTHFD2 on resistance to pemetrexed in gastric cancer through regulating expression of miR-124. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10290–10299. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zhang, L.Y.; Du, W.Z. Circular RNA circ-PVT1 contributes to paclitaxel resistance of gastric cancer cells through the regulation of ZEB1 expression by sponging miR-124-3p. Biosci, Rep. 2019, 39, BSR20193045. [Google Scholar] [CrossRef]
- Sha, J.; Xia, L.; Han, Q.; Chi, C.; Zhu, Y.; Pan, J.; Huang, Y.; Xia, W.; Dong, B.; Xue, W.; et al. Downregulation of circ-TRPS1 suppressed prostatic cancer prognoses by regulating miR-124-3p/EZH2 axis-mediated stemness. Am. J. Cancer Res. 2020, 10, 4372–4385. [Google Scholar]
- Gao, C.; Xu, Y.J.; Qi, L.; Bao, Y.F.; Zhang, L.; Zheng, L. CircRNA VIM silence synergizes with sevoflurane to inhibit immune escape and multiple oncogenic activities of esophageal cancer by simultaneously regulating miR-124/PD-L1 axis. Cell Biol. Toxicol. 2021, 38, 825–845. [Google Scholar] [CrossRef]
- Chen, G.; Shi, Y.; Zhang, Y.; Sun, J. CircRNA_100782 regulates pancreatic carcinoma proliferation through the IL6-STAT3 pathway. OncoTarg. Ther. 2017, 10, 5783–5794. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.; Li, H.; Sun, Y.; Tong, X. Downregulation of hsa_circ_0026123 suppresses ovarian cancer cell metastasis and proliferation through the miR1243p/EZH2 signaling pathway. Int. J. Mol. Med. 2021, 47, 668–676. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Liu, Z.; Zhang, Y.; Chen, G.; Li, K.; Tang, K. CircRNA_0000502 promotes hepatocellular carcinoma metastasis and inhibits apoptosis through targeting microRNA-124. JBUON 2019, 24, 2402–2410. [Google Scholar] [PubMed]
- Fu, Y.; Sun, H. Biogenesis, cellular effects, and biomarker value of circHIPK3. Cancer Cell Int. 2021, 21, 256. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Wang, P.Y.; Su, D.F.; Liu, X. miRNA-124 in immune system and immune disorders. Front. Immunol. 2016, 7, 406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, H.; Chen, S.; Sun, D.; Zhang, D.; He, Y. Exosomal transfer of miR-124 inhibits normal fibroblasts to cancer-associated fibroblasts transition by targeting sphingosine kinase 1 in ovarian cancer. J. Cell. Biochem. 2019, 120, 13187–13201. [Google Scholar] [CrossRef]
- Zhou, Z.; Lv, J.; Wang, J.; Yu, H.; Lu, H.; Yuan, B.; Han, J.; Zhou, R.; Zhang, X.; Yang, X.; et al. Role of microRNA-124 as a prognostic factor in multiple neoplasms: A meta-analysis. Dis. Mark. 2019, 2019, 1654780. [Google Scholar] [CrossRef]
- Huang, J.; Gou, H.; Yao, J.; Yi, K.; Jin, Z.; Matsuoka, M.; Zhao, T. The noncanonical role of EZH2 in cancer. Cancer Sci. 2021, 112, 1376–1382. [Google Scholar] [CrossRef]
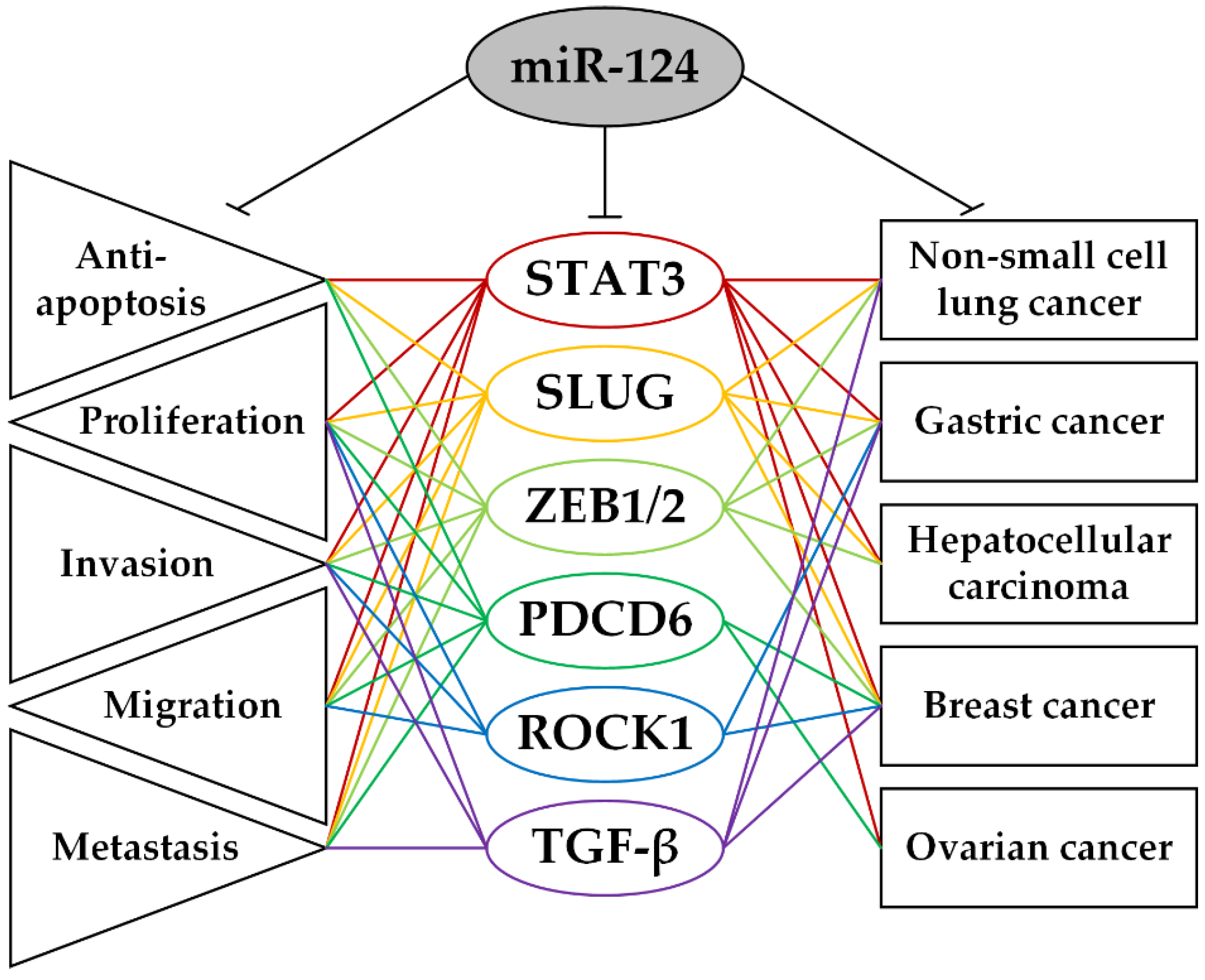
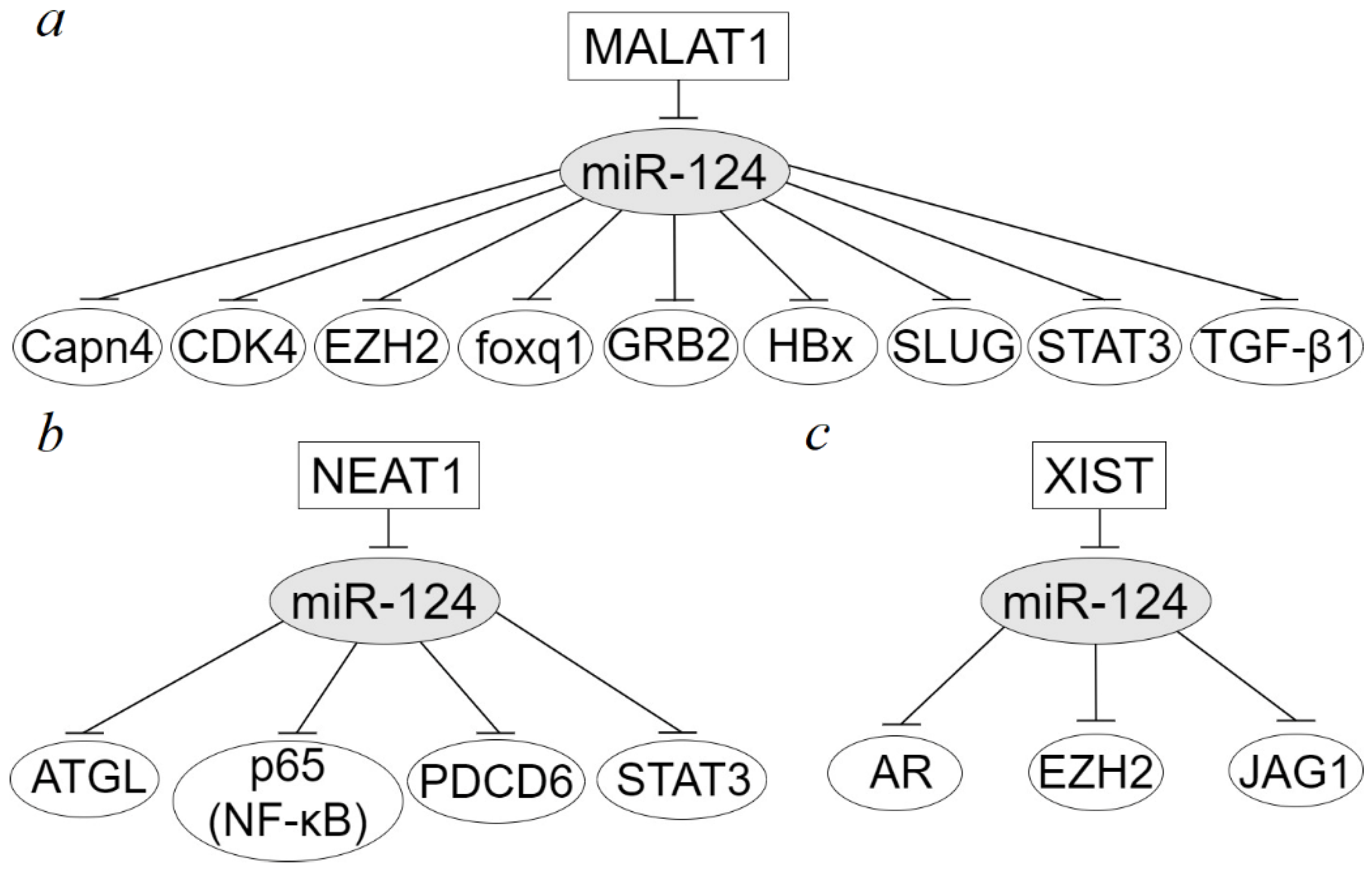
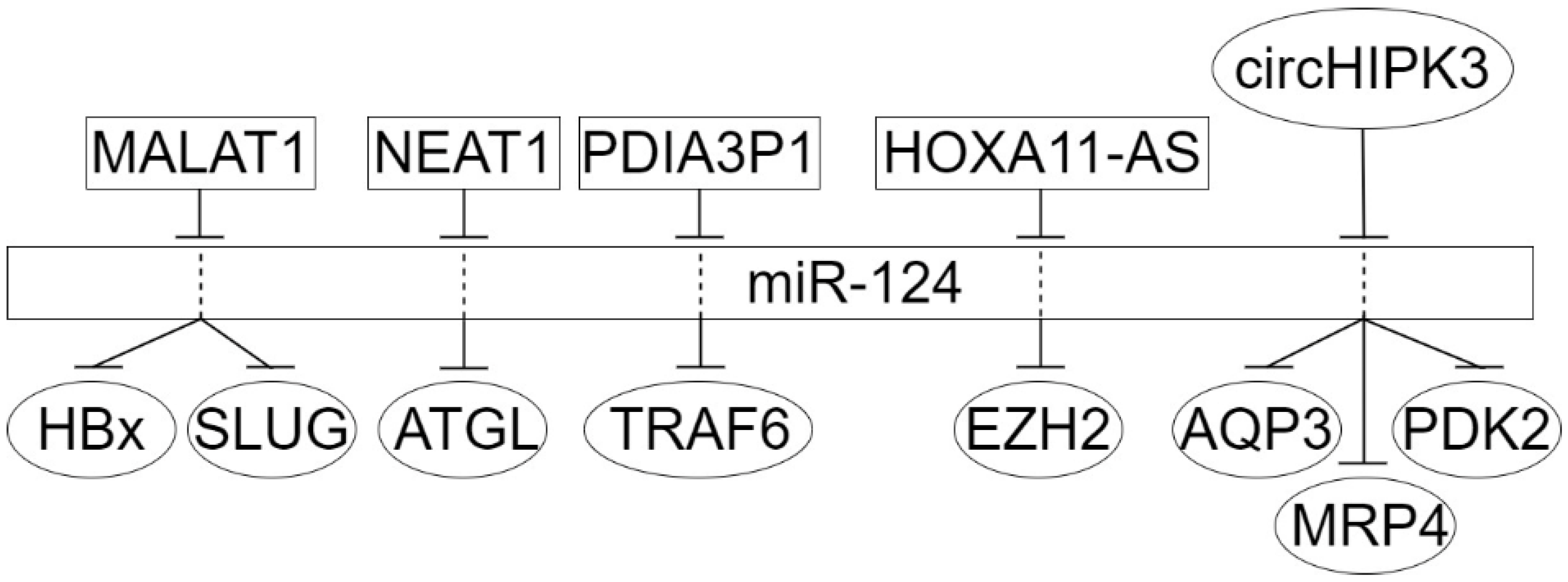


| LncRNA-Axis | Cancer | Methods of Analysis | Axis Functions | Ref. |
|---|---|---|---|---|
| HNF1A-AS1 /miR-124 /MYO6 | colorectal cancer (40 patients, 4 cancer cell lines) | qRT-PCR, luciferase, RIP assays, Western blot, Transwell assay, glycolysis assessment | promotes cell proliferation, migration, invasion, activates glycolysis | [57,58] |
| HOTAIR /miR-124 /ST8SIA4 | renal cell carcinoma (30 patients, 2 cancer cell lines) | qRT-PCR, luciferase assay, Western blot, FISH, Transwell assay, CCK-8, EdU, cell adhesion, colony formation, apoptosis, mouse xenografts | promotes proliferation, migration, invasion, decrease apoptosis in vitro; tumor growth and metastasis in vivo | [59] |
| HOTTIP /miR-124-3p /HMGA2 | oral tongue squamous cell carcinoma (60 patients, 4 cancer cell lines) | qRT-PCR, luciferase assay, Western blot, MTT, Transwell assays, mouse xenografts | promotes proliferation, migration, invasion, tumor growth in vivo, Wnt/b-catenin signaling pathway | [60] |
| HOXA11-AS /miR-124 /EZH2 | hepatocellular carcinoma (66 patients, 5 cancer cell lines) | qRT-PCR, cell transfection, loss-/ and gain-of-function, CHIP, RIP, Western blot, Transwell assay, Kaplan-Meier curve | mutual influence of triplet components, axis promotes migration, invasion, poor outcome | [61] |
| HOXA11-AS /miR-124-3p /ITGB3 | gastric cancer (40 patients, 3 cancer cell lines) | qRT-PCR, luciferase reporter assay, western blot, Wound-healing assay, xenografts in nude mice, Kaplan-Meier plotter | promotes cell proliferation, migration, invasion in vitro, growth in vivo, metastasis, shorter OS, PFS | [62] |
| HOXA11-AS /miR-124 /Sp1 | non-small cell lung cancer (78 patients, 4 cancer cell lines) | qRT-PCR, Western blot, luciferase reporter, RIP assays, cell proliferation, invasion assays | promotes invasion, proliferation, larger tumor size, lymph node metastasis | [63] |
| KCNQ1OT1 /miR-124-3p /TRIM14 | tongue squamous cell carcinoma (60 patients, 2 cancer cell lines) | qRT-PCR, dual-luciferase, RIP, RNA pull-down assays, Western blot, MTT, Transwell assays, xenografts | promotes migration, invasion, EMT, in vitro/in vivo, cisplatin resistance | [64] |
| LINC00240 /miR-124-3p /DNMT3B | gastric cancer (48 patients, 2 cancer cell lines) | qRT-PCR, luciferase, AGO2-RIP assays, Western blot, migration, invasion assays, EMT-markers, xenografts in nude mice | promotes cell proliferation, invasion, migration, EMT in vitro, tumor growth in vivo | [65] |
| LINC00240 /miR-124-3p /STAT3 | cervical cancer (167 patients, 5 cancer cell lines) | qRT-PCR, luciferase, RIP, RNA pull-down assays, Western blot, RNA FISH, CCK-8, Transwell, colony formation, tumor xenografts, cytotoxicity, T-cell conjugate assays | promotes cancer progression, cell proliferation, migration, invasion in vitro, in vivo; inhibits cytotoxicity of NKT cells via STAT3/MICA | [66] |
| LINC00511 /miR-124-3p /EZH2 | gastric cancer (80 patients, 4 cancer cell lines) | qRT-PCR, dual-luciferase assay, Western blot, CCK-8 assay, Transwell assay, Kaplan-Meier | promotes proliferation, invasion, migration in vitro, tumor growth, metastasis in vivo, lower OS | [67] |
| LINC00511 /miR-124-3p /PDK4 | gastric cancer (5 cancer cell lines) | qRT-PCR, luciferase, RIP, RNA pull-down assays, Western blot, caspase-3, CCK-8 assay, colony formation assay | promotes proliferation, inhibits apoptosis in vitro | [68] |
| LINC00963 /miR-124-3p /FZD4 | colorectal cancer (84 patients, 4 cancer cell lines) | qRT-PCR, dual-luciferase assay, Western blot, CCK-8, Transwell, radioimmunoprecipitation assays, Kaplan-Meier survival curves | promotes cell proliferation, migration, associated with high TNM stage, shorter 5-year survival time | [69] |
| LINC01410 /miR-124-3p /SMAD5 | cholangiocarcinoma (50 patients, 6 cancer cell lines) | qRT-PCR, luciferase, RNA pull-down, RIP, CCK8, colony formation, Transwell assays, Western blot | promotes cell proliferation, migration, invasion, colony formation ability, EMT | [70] |
| lnc-1308 /miR-124 /ADAM15 | non-small-cell lung cancer (40 patients, 4 cancer cell lines) | human lncRNA microarray assay, qRT-PCR, miRIP, luciferase reporter assays, immunoblotting | promotes cell proliferation, invasion, poorer patient outcome | [71] |
| lnc-cCSC1 /miR-124-3p /CD44 | colorectal cancer (4 cancer cell lines) | qRT-PCR, luciferase test, Western blot, CCK-8, colony formation, EdU staining, flow cytometry | promotes cell proliferation, inhibits apoptosis in vitro | [72] |
| MALAT1 /miR-124 /Capn4 | nasopharyngeal carcinoma (4 cancer cell lines) | qRT-PCR, target prediction, luciferase assay, loss/gain of function, western blot, MTT assay, Transwell chamber assay, EMT-related proteins expression | promotes proliferation, migration, invasion, EMT in vitro | [73] |
| MALAT1 /miR-124 /CDK4 | breast cancer (40 patients, 7 cancer cell lines) | qRT-PCR, dual-luciferase assay, Western blot, cell viability, cell cycle analyses, mouse xenografts, Kaplan-Meier curves | promotes proliferation, cell cycle progression, E2F1 signaling in vitro, tumor growth in vivo, poorer OS | [74] |
| MALAT1 /miR-124-3p /EZH2 | gastric cancer (2 cancer cell lines) | qRT-PCR, predicted binding sites for miR-124-3p, gain-/loss-of-function, Western blot, MTT assay, Would healing scratch assay, mouse xenografts | promotes cells proliferation, migration in vitro, cancer growth in vivo; H2 downregulated MALAT1, suppressed growth of cancer | [75] |
| MALAT1 /miR-124 /foxq1 | bladder transitional cell carcinoma (56 patients, 2 cancer cell lines) | qRT-PCR, luciferase assay, Western blot, cell proliferation assay, Transwell migration assay, invasion assay, tumor xenografts, Kaplan-Meier curves | promotes proliferation, EMT, migration, invasion in vitro, tumor growth, metastasis in vivo, shorter survival | [76] |
| MALAT1 /miR-124 /GRB2 | HR-HPV (+) cervical cancer (22 patients, 3 cancer cell lines) | qRT-PCR, predicted binding sites, dual luciferase assay, western blot, Transwell analysis, flow cytometry | promotes proliferation, migration, invasion, inhibits apoptosis in vitro | [77] |
| MALAT1 /miR-124 /HBx | hepatocellular carcinoma (20 patients, 1 cancer cell line) | qRT-PCR, dual-luciferase assay, Western blot, cell colony formation assay, mouse xenografts | promotes proliferation, migration, invasion in vitro, stemness, progression in vivo, PI3K/Akt signaling | [78] |
| MALAT1 /miR-124-3p /SLUG | hepatocellular carcinoma (30 patients, 2 cancer cell lines) | qRT-PCR, cDNA array, luciferase, RIP assays, Western blotting, scratch wound healing, Transwell chamber assays, mouse xenografts, Kaplan-Meier curves | promotes migration, invasion in vitro, tumor growth in vivo, poor differentiation, lower DFS | [79] |
| MALAT1 /miR-124 /STAT3 | non-small cell lung cancer (5 cancer cell lines) | qRT-PCR, RIP, RNA pull-down, dual-luciferase, Western blot, CCK8, colony formation, apoptosis assays | promotes proliferation, colony formation, inhibits apoptosis in vitro | [40] |
| MALAT1 /miR-124 /TGF-β1 | nasopharyngeal carcinoma (6-10B cell lines) | qRT-PCR, cell transfection, luciferase reporter assay, loss and gain of function, Western blot, cell counting Kit-8, cell wound healing assay, cell Matrigel invasion assay | promotes proliferation, migration, invasion, TGF-β signaling, SMAD pathway, ERK/MAPK pathway | [80] |
| NEAT1 /miR-124-3p /ATGL | hepatocellular carcinoma (40 patients, 4 cancer cell lines) | qRT-PCR, Western blot, immunohistochemistry, luciferase reporter assay, orthotopic mouse xenografts | promotes proliferation in vitro/in vivo, disrupt lipolysis, DAG+FFA/PPARα signaling | [81] |
| NEAT1 /miR-124 /p65 (NF-κB) | nasopharyngeal carcinoma (20 patients, 5 cancer cell lines) | qRT-PCR, luciferase, RIP, RNA pull-down assays, Western blot, CCK-8, Colony formation assays, Flow cytometry, xenograft mice | promotes proliferation, inhibits apoptosis in vitro, tumor growth in vivo, NF-κB signaling pathway | [82] |
| NEAT1 /miR-124 /PDCD6 | malignant and benign thyroid nodules (98 patients, 2 cancer cell lines) | qRT-PCR, luciferase assay, Western blot, immunohistochemistry, shRNA, ROC analysis | differs malignant from benign thyroid nodules; promotes EMT, cell migration, invasion in vitro | [83] |
| NEAT1 /miR-124 /STAT3 | breast cancer (31 patients, 3 cancer cell lines) | qRT-PCR, luciferase assay, western blot, MTT, colony formation assays, flow cytometry, cell cycle analysis | promotes proliferation, migration, cell cycle progression, inhibit apoptosis | [84] |
| OGFRP1 /miR-124-3p /LYPD3 | non-small cell lung cancer (120 patients, 5 cancer cell lines) | qRT-PCR, RIP, luciferase assays, colony formation, apoptosis assays, Western blot, migration and invasion assays, Kaplan-Meier curves | facilitates cell proliferation, migration, invasion, inhibits apoptosis in vitro; patient poor OS, DFS | [85] |
| OGFRP1 /miR-124-3p /SARM1, SAMD2 | prostate cancer (57 patients, 4 cancer cell lines) | qRT-PCR, luciferase, RIP assays, FISH, clone formation assay, Wound healing, Matrigel invasion, apoptosis analysis | promotes tumor growth, metastasis, inhibits apoptosis, associated with TNM stages, perineural invasion | [86] |
| OIP5-AS1 /miR-124-5p /IDH2 | cervical cancer (89 patients, 6 cancer cell lines) | qRT-PCR, luciferase assay, RIP assay, FISH, immunofluorescence, Western blot, cell proliferation assay, cell clone test, mouse xenografts | promotes cell proliferation, in vitro/in vivo, Warburg effect, HIF-1α-pathway, poor 5-years OS | [87] |
| hMTR4 /PDIA3P1 /miR-124-3p /TRAF6 | hepatocellular carcinoma (174 patients, 2 cell lines) | qRT-PCR, luciferase, RIP, RNA pull-down assays, gain-/loss-of-function, in vitro, mouse xenografts, Kaplan-Meier curves | promotes cell proliferation, chemoresistance in vitro/in vivo, NF-κB pathway, reduces RFS; hMTR4 degrades lncRNA PDIA3P1 | [88] |
| PTPRG-AS1 /miR-124-3p /CCND1 | lung adenocarcinoma (cell cultures) | qRT-PCR, RNA pulldown, luciferase, RIP assays, flow cytometry, FISH, mouse xenografts | promotes cell proliferation, cell cycle in vitro/in vivo | [56] |
| PTPRG-AS1 /miR-124-3 /LHX2 | nasopharyngeal carcinoma (61 patients, 5 cancer cell lines) | qRT-PCR, RNA pull-down, luciferase assays, Western blot; microarray, CCK-8, flow cytometry assays, site-directed mutagenesis | promotes NPC cell proliferation, reduces apoptosis, radiosensitivity; activates Notch pathway | [89] |
| SND1-IT1 /miR-124 /COL4A1 | gastric cancer (52 patients, 4 cancer cell lines) | qRT-PCR, luciferase assay, CCK-8, Transwell assays, immunoblotting, EMT-markers | promotes migration, invasion, TGF-β1-induced EMT, metastasis, poor outcomes | [90] |
| SNHG16 /miR-124-3p /MCP-1 | colorectal cancer (120 patients, 4 cancer cell lines) | qRT-PCR, luciferase, RIP, RNA pull down assays, Western blot, MTT, Wound healing, Transwell invasion assays, EMT-markers, mouse xenografts, Kaplan-Meier curves | promotes cell proliferation, migration, invasion, EMT in vitro, tumor growth, metastasis in vivo, reduces survival | [91,92] |
| SP1-GCMA /miR-124 /SLUG, SNAIL | gastric cancer (72 patients, 2 cancer cell lines) | qRT-PCR, ChIP-assay, luciferase, RIP assays, western-blot, RNA FISH, EMT-markers, xenografts in nude mice, Kaplan-Meier curves | SP1 activates GCMA via promoter; facilitates migration, invasion, EMT, metastasis in vitro/in vivo, worse OS, DFS | [43] |
| UCA1 /miR-124 /JAG1 | tongue cancer (67 patients, 2 cancer cell lines) | qRT-PCR, luciferase, RIP assays, immunoblotting, Transwell invasion assay, immunofluorescence staining, Kaplan-Meier curves | promotes TGFβ1-induced EMT, metastasis, Notch signaling, poorer OS | [93] |
| XIST /miR-124 /AR | bladder cancer (67 patients, 4 cancer cell lines) | bioinformatic analysis, qRT-PCR, luciferase assays, loss-of-function, MTT assay, Transwell assay, MMP9, MMP13 activity, Western blot | promotes proliferation, invasion, migration, tumor growth, metastasis; increases factors c-myc, MMP9, MMP13 | [94] |
| XIST /miR-124 /EZH2 | laryngeal squamous cell carcinoma (34 patients, 2 cancer cell lines) | qRT-PCR, luciferase assay, Western blot, lentiviral transfection, shRNA, cell and colonies counting, Transwell assay, tumor xenografts in nude mice | promotes proliferation, migration, invasion in vitro, tumor growth in vivo | [95] |
| XIST /miR-124 /JAG1 | tongue squamous cell carcinoma (cancer cell cultures) | qRT-PCR, luciferase assay, Western blot, Chip-seq analysis, CCK-8, scratch test | facilitates cell migration, proliferation in vitro | [96] |
| ZFAS1 /miR-124 /STAT3 | esophageal squamous cell carcinoma (136 patients, 5 cancer cell lines) | qRT-PCR, luciferase, RIP, RNA pull-down assays, Western blot, cell co-culture model, fluorescence-labeled exosomes, FISH, colony formation, Transwell assays, flow cytometry, scratch test, xenografts in nude mice | promotes proliferation, migration, invasion, inhibit apoptosis in vitro, ZFAS1-exo promotes tumor growth in nude mice | [55] |
| CircRNA-Axis | Cancer | Methods of Analysis | Axis Functions | Ref. |
|---|---|---|---|---|
| circDOCK1 /miR-124 /CCND1 | thyroid cancer, 25 patients, 2 cell lines | qRT-PCR, Transwell assays, overexpression of circDOCK1, miR-124 mimic, Western blots | cell migration, invasion, JAK/STAT/AMPK pathway | [109] |
| circHIPK3 /miR-124 /AKT3 | Esophageal squamous cell carcinoma, 32 patients, 4 cancer cell lines, 1 normal | qRT-PCR, knockdown of circHIPK3, miR-124 mimic, AKT silencing, colony formation assays, Transwell assay, bioinformatics tools and dual-luciferase reporter gene assay for all interactions, Western blot, circHIPK3 knockdown in xenografts | cancer cell proliferation, migration, EMT; tumor growth in xenografts | [110] |
| circHIPK3 /miR-124 /AQP3 | hepatocellular carcinoma, 50 patients, 5 tumor cell lines, 2 hepatocyte cell lines | qRT-PCR, CCK-8, Transwell assays, silencing circHIPK3, overexpression and knockdown of miR-124, overexpression and knockdown of AQP3, Sanger sequencing of circHIPK3, bioinformatics tools and luciferase reporter assay for circRNA-miR and miR-mRNA interaction, Western blots and immunohistochemistry, knockdown of circHIPK3 in xenografts | cell proliferation, migration, xenograft tumor growth | [111] |
| circHIPK3 /miR-124, miR-4524-5p /MRP4 | hepatocellular carcinoma, 19 patients, 4 tumor cell lines, primary hepatocyte cells | qRT-PCR, knockdown of circHIPK3, miR-124 and miR-4524-5p inhibitor and mimic, bioinformatics tools and dual-luciferase reporter gene assay for pairs circRNA-miRNA, circRIP, Western blots | multidrug resistance | [112] |
| circHIPK3 /miR-124 /MTDH | peripheral human endothelial cells (ECs) mediated by the breast cancer (BC) cells-derived exosomal circRNAs | cell viability and tube formation, bioinformatics tools and dual luciferase reporter assay, western blot, qPCR assays, knockdown and overexpression of CircHIPK3, rescue experiment in mice xenograft model | cell viability, angiogenesis, impact on the microenvironment | [113] |
| circHIPK3 /miR-124 /PDK2 | hepatocellular carcinoma, 30 patients, 4 tumor cell lines, 1 human hepatic cell line | qRT-PCR, CCK-8, EdU kit, Transwell assay, knockdown and overexpression of circHIPK3, miR-124 mimics, PDK2 overexpression plasmid, bioinformatics tools and dual-luciferase reporter gene assay for all interactions, Western blots, knockdown of circHIPK3 in xenografts | cell proliferation, invasion, xenograft tumor formation | [114] |
| circHIPK3 /miR-124 /ROCK1, CDK6 | gall bladder cancer, 3 tumor lines, primary cultures of tumor, normal cells | QRT-PCR, CCK-8 viability assay, BrdU ELISA assay, Clonogenicity assay, TUNEL apoptosis assay, knockdown and overexpression of circHIPK3, overexpression of miR-124, Western blots | cancer cell survival, proliferation, inhibition of apoptosis | [115] |
| circ MTHFD2 /miR-124 /FZD5, MDR-1 | gastric cancer, MGC-803 and MGC-803/MTA resistant cell model | qRT-PCR, screening of differentially expressed circRNAs, CCK-8, bioinformatics tool, microarray analysis, knockdown or overexpression of circ MTHFD2, miR-124 mimics transfection, luciferase reporter assay for circRNA-miRNA, Western blotting | resistance to pemetrexed (MTA) | [116] |
| circPVT1 /miR-124 /ZEB1 | gastric cancer, 30 PTX-sensitive and 30 PTX-resistant patients, 4 tumor cell lines, 1 normal gastric cell line | qRT-PCR, MTT assay, flow cytometry, Transwell assay, knockdown and overexpression of CircHIPK3 and ZEB1, mimic and anti-miR for miR-124, bioinformatics tools and dual-luciferase reporter gene assay for all interactions, Western blot, circPVT1 knockdown in xenografts | PTX resistance | [117] |
| circ-TRPS1 /miR-124 /EZH2 | prostate cancer, specimens from 80 patients, 3 tumor cell lines, xenografts | high-throughput sequencing, RT-qPCR, FISH, immunohistochemistry, tumor sphere formation assays, cell proliferation assays, colony formation assays, Transwell assays, knockdown of circ-TRPS1, miR-124 inhibition, EZH2 overexpression, bioinformatics tools, luciferase reporter assays for all interactions, Western blots, circ-TRPS1 knockdown in xenografts | cell proliferation and migration, metastasis | [118] |
| circ-VIM /miR-124 /PD-L1 | esophageal cancer, 20 patients, 4 EC cell lines, embryonic kidney 293T cell line, normal esophageal HET-1A cell line | RT-qPCR, CCK-8, wound healing, Transwell assays, LDH assay, CFSE staining, Annexin V/PI staining, histological analyses, knockdown of circ-VIM, mimic and anti-miR for miR-124, bioinformatic tools, luciferase reporter assays for all interactions, RNA immunoprecipitation, Western blots, xenografts | circ-VIM silence synergizes with sevoflurane reduces immune escape and multiple oncogenic activities | [119] |
| circRNA_100782 /miR-124 /IL6R, STAT3 | pancreatic ductal adeno-carcinoma, 2 tumor cell lines, 1 embryonic kidney cell line | qRT-PCR, colony formation assay, knockdown of circRNA_100782, mimic and anti-miR for miR-124, knockdown of STAT3, luciferase assay for circRNA-miR interaction, circRNA_100782 knockdown in xenografts | cell proliferation and colony formation | [120] |
| circ_0026123 /miR124 /EZH2 | ovarian cancer, 20 patients, 4 OC cell lines, 1 normal ovarian cell line | RT-qPCR, FISH, CCK-8 assay, Transwell assays, knockdown, bioinformatic tools, luciferase reporter assay, Western blots, xenografts | cell proliferation, migration, stemness | [121] |
| circ_0000502 /miR-124 | hepatocellular carcinoma, 40 patients, 6 HCC + 1 normal cell lines | qRT-PCR, CCK-8, Transwell assays, circ_0000502 knockdown, miR-124 overexpression, luciferase reporter gene assay for circRNA-miRNA pair | proliferation, invasion, migration, decrease in apoptosis | [122] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braga, E.A.; Fridman, M.V.; Burdennyy, A.M.; Filippova, E.A.; Loginov, V.I.; Pronina, I.V.; Dmitriev, A.A.; Kushlinskii, N.E. Regulation of the Key Epithelial Cancer Suppressor miR-124 Function by Competing Endogenous RNAs. Int. J. Mol. Sci. 2022, 23, 13620. https://doi.org/10.3390/ijms232113620
Braga EA, Fridman MV, Burdennyy AM, Filippova EA, Loginov VI, Pronina IV, Dmitriev AA, Kushlinskii NE. Regulation of the Key Epithelial Cancer Suppressor miR-124 Function by Competing Endogenous RNAs. International Journal of Molecular Sciences. 2022; 23(21):13620. https://doi.org/10.3390/ijms232113620
Chicago/Turabian StyleBraga, Eleonora A., Marina V. Fridman, Alexey M. Burdennyy, Elena A. Filippova, Vitaly I. Loginov, Irina V. Pronina, Alexey A. Dmitriev, and Nikolay E. Kushlinskii. 2022. "Regulation of the Key Epithelial Cancer Suppressor miR-124 Function by Competing Endogenous RNAs" International Journal of Molecular Sciences 23, no. 21: 13620. https://doi.org/10.3390/ijms232113620
APA StyleBraga, E. A., Fridman, M. V., Burdennyy, A. M., Filippova, E. A., Loginov, V. I., Pronina, I. V., Dmitriev, A. A., & Kushlinskii, N. E. (2022). Regulation of the Key Epithelial Cancer Suppressor miR-124 Function by Competing Endogenous RNAs. International Journal of Molecular Sciences, 23(21), 13620. https://doi.org/10.3390/ijms232113620







