Z. morio Hemolymph Relieves E. coli-Induced Mastitis by Inhibiting Inflammatory Response and Repairing the Blood–Milk Barrier
Abstract
1. Introduction
2. Results
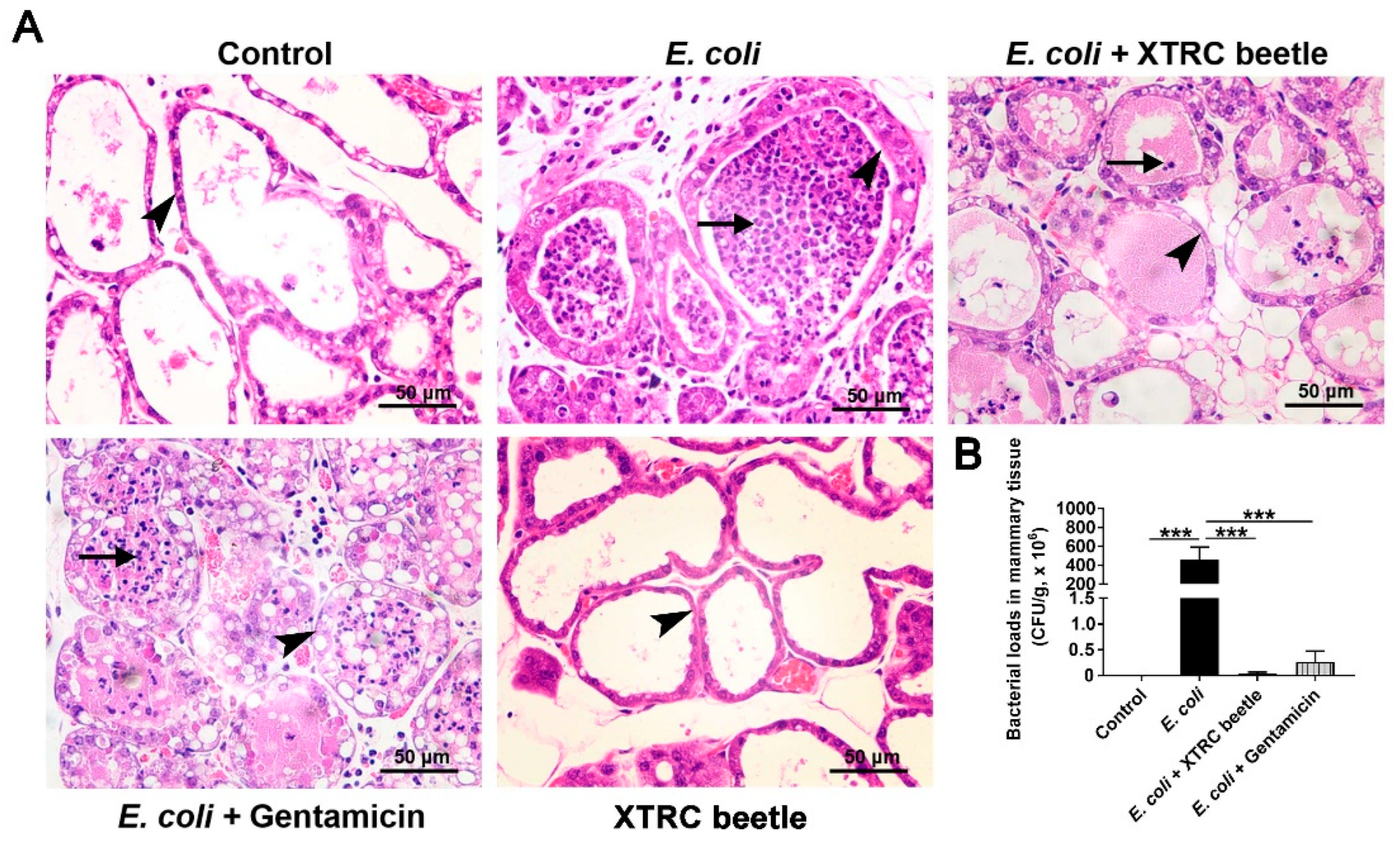
2.1. Z. morio Hemolymph Alleviates Pathological Injury of Mammary Gland in E. coli-Induced Mastitis
2.2. Z. morio Hemolymph Affects Peripheral Blood Parameters in E. coli-Induced Mastitis
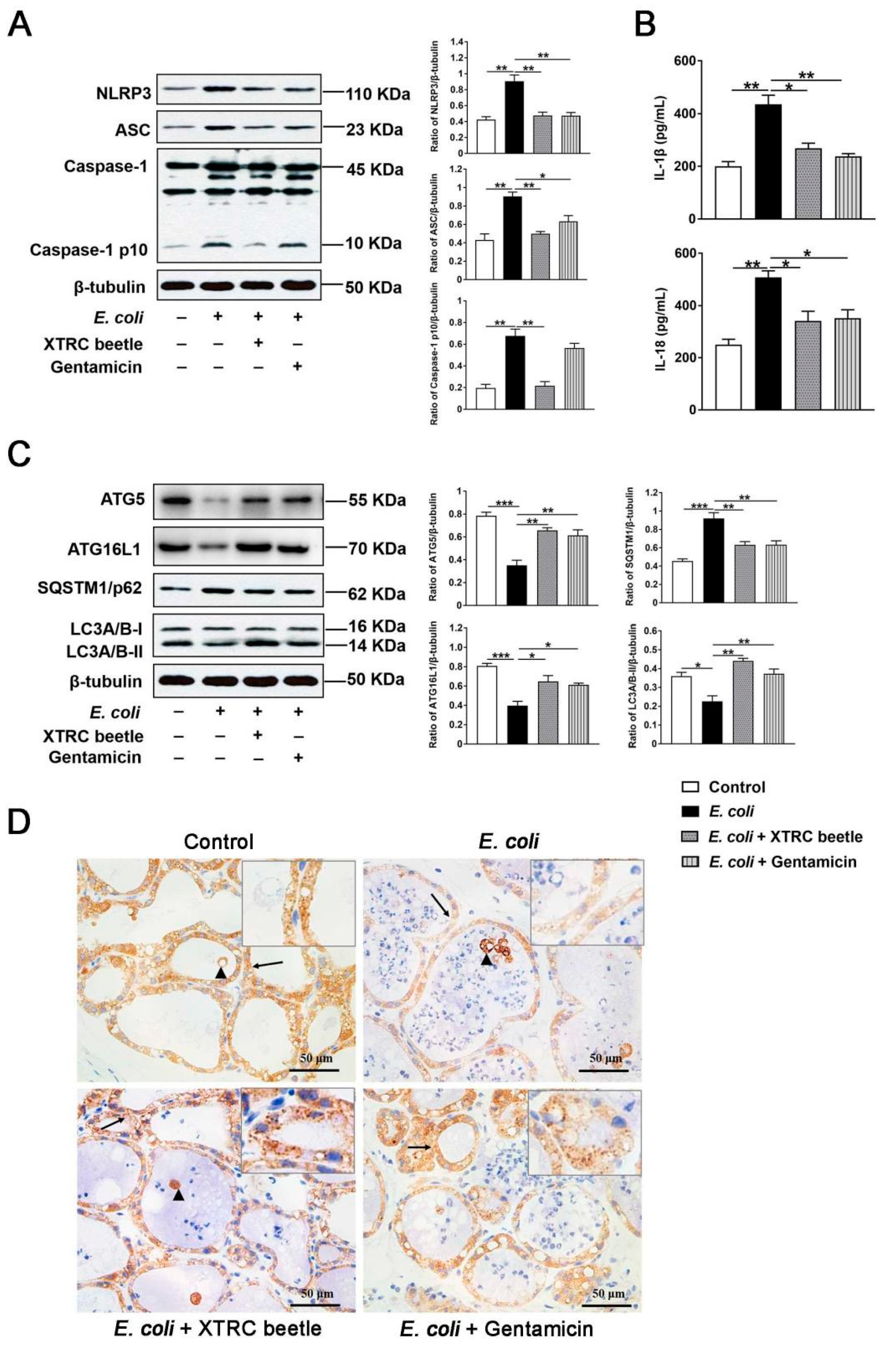
2.3. Z. morio Hemolymph Inhibits the NLRP3 Signaling Pathway and Promotes ATG5/ATG16L1-Mediated Autophagy Signaling Pathway in E. coli-Induced Mastitis
2.4. Z. morio Hemolymph Repairs the Blood–Milk Barrier Integrity in E. coli-Induced Mastitis
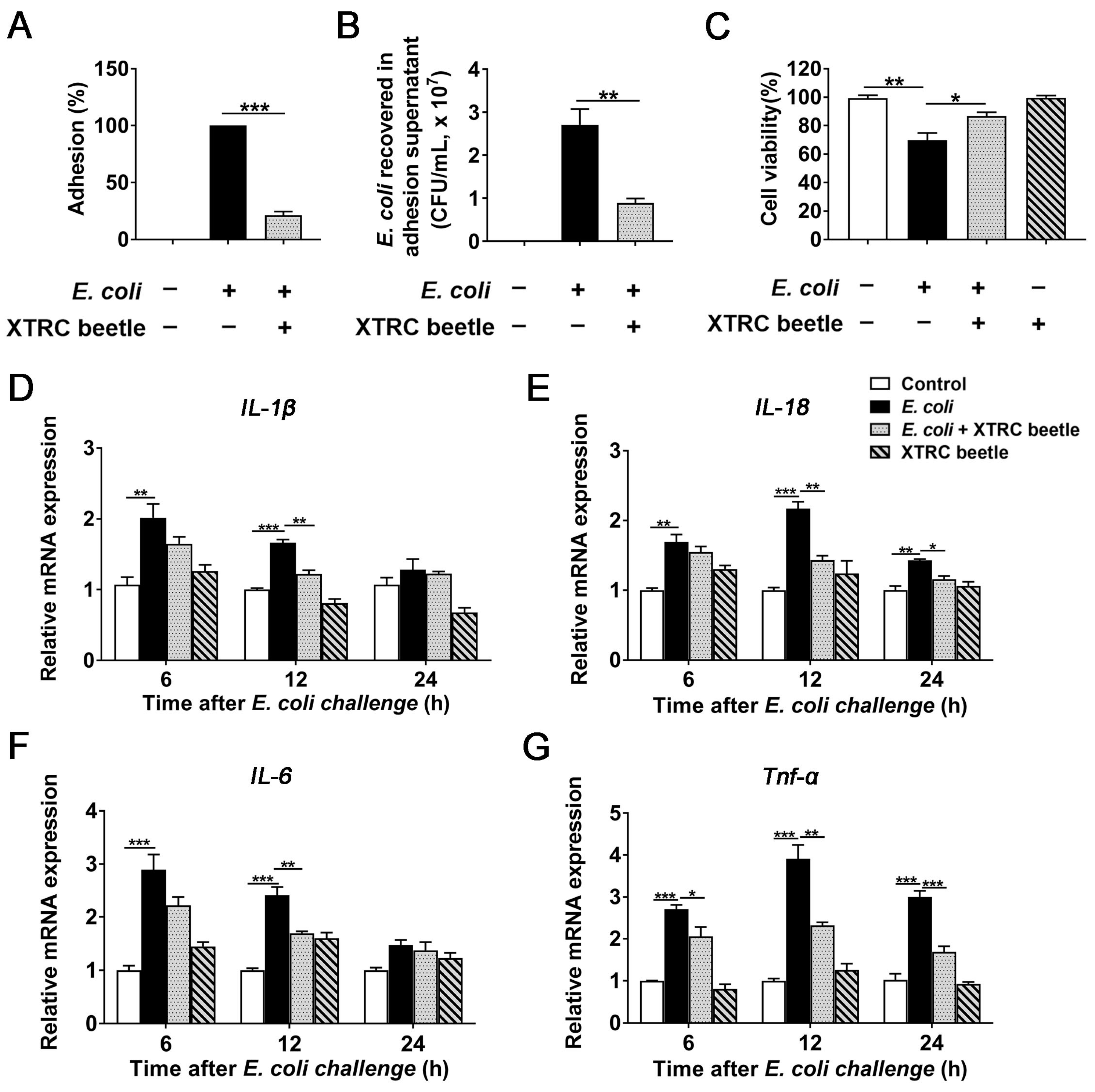
2.5. Z. morio Hemolymph Inhibits E. Coli-Induced Inflammatory Response in PMECs
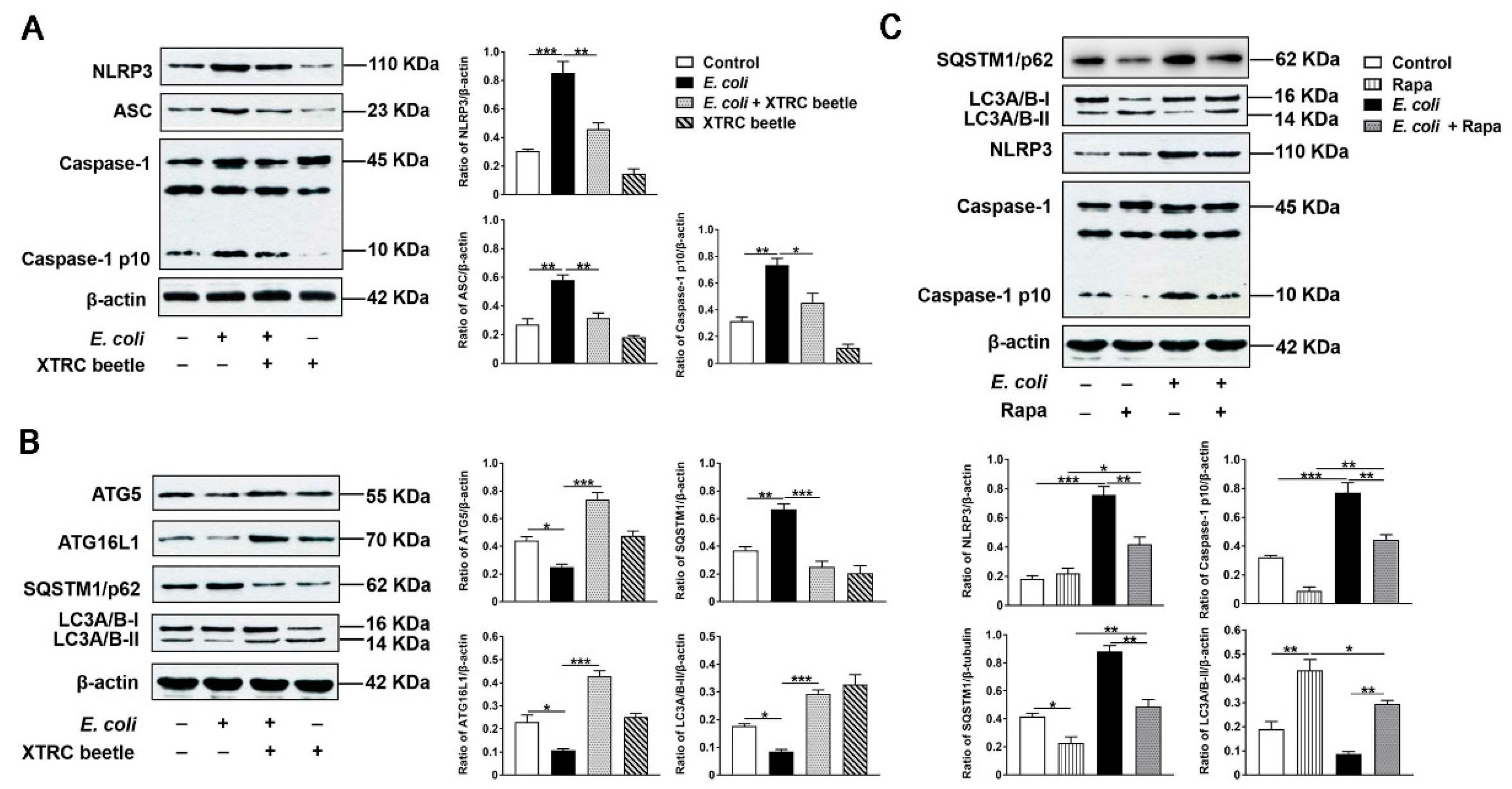
2.6. Z. morio Hemolymph Suppresses E. Coli-Induced Activation of NLRP3 and Inhibition of ATG5/ATG16L1-Mediated Autophagy Signaling Pathway in PMECs
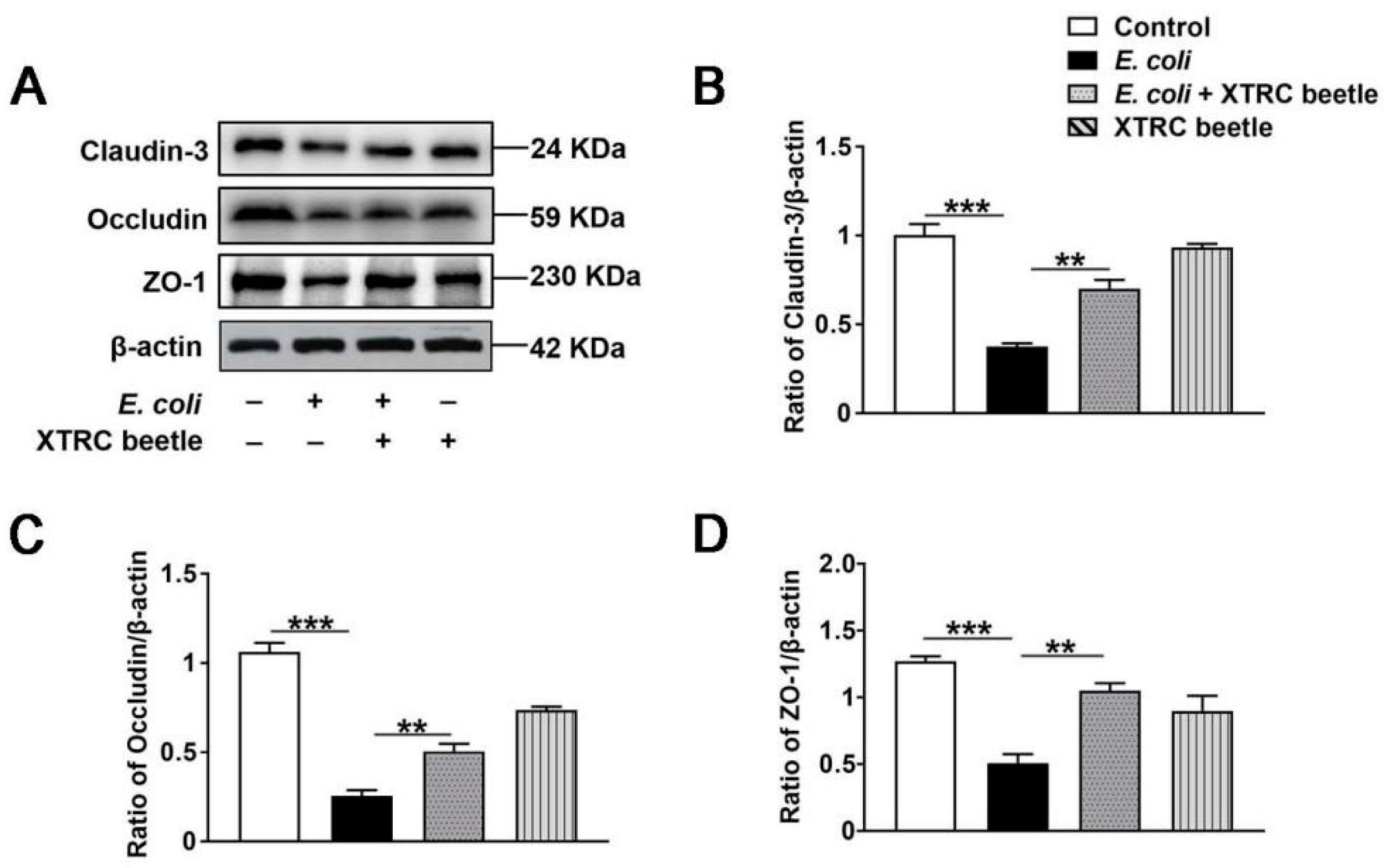
2.7. Z. morio Hemolymph Enhances the Protein Levels of Claudin3, Occludin and ZO-1 in PMECs
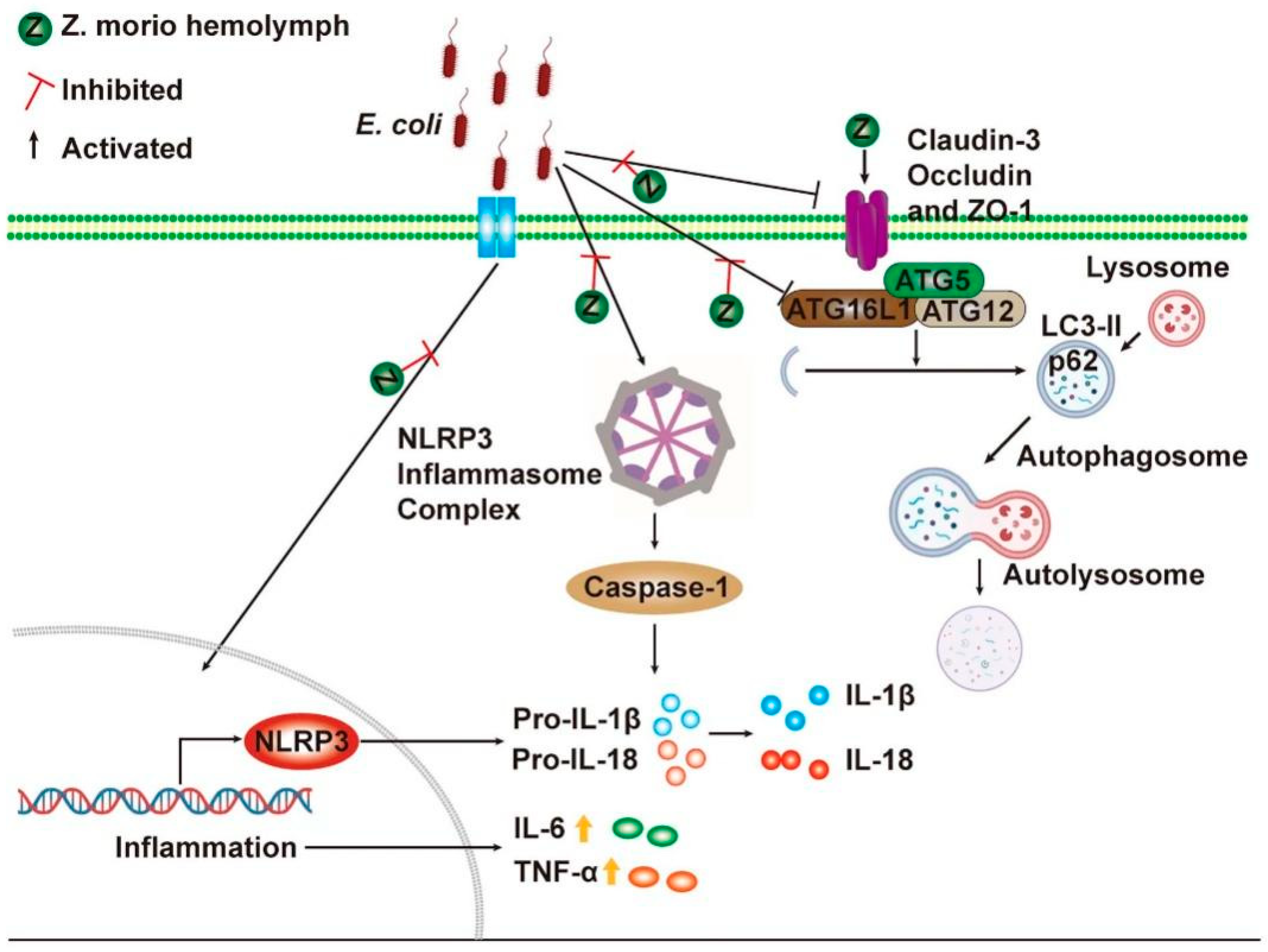
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Ethics Statement
4.3. Establishment of Mouse Mastitis Model
4.4. Bacteria Strains and Growth Conditions
4.5. Z. morio Immunization and Hemolymph Collection
4.6. Cell Culture and Treatments
4.7. Histologic Assessment
4.8. Determination of Bacterial Load in the Mammary Gland
4.9. Enzyme-Linked Immunosorbent Assay (ELISA)
4.10. Transmission Electron Microscopy (TEM)
4.11. Flow Cytometry
4.12. Immunohistochemistry
4.13. Adhesion Assay
4.14. Internalization Assay
4.15. Cell Viability
4.16. Real-Time Quantitative PCR
4.17. Western Blotting
4.18. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaeger, A.; Hadlich, F.; Kemper, N.; Lübke-Becker, A.; Muráni, E.; Wimmers, K.; Ponsuksili, S. MicroRNA expression profiling of porcine mammary epithelial cells after challenge with Escherichia coli in vitro. BMC Genom. 2017, 18, 660. [Google Scholar] [CrossRef] [PubMed]
- Niemi, J.K.; Bergman, P.; Ovaska, S.; Sevón-Aimonen, M.L.; Heinonen, M. Modeling the costs of postpartum dysgalactia syndrome and locomotory disorders on sow productivity and replacement. Front. Vet. Sci. 2017, 4, 181. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Jacobson, M.; Andersen, P.H.; Bækbo, P.; Cerón, J.J.; Dahl, J.; Escribano, D.; Jacobsen, S. Inflammatory markers before and after farrowing in healthy sows and in sows affected with postpartum dysgalactia syndrome. BMC Vet. Res. 2018, 14, 83. [Google Scholar]
- Jaeger, A.; Bardehle, D.; Oster, M.; Günther, J.; Muráni, E.; Ponsuksili, S.; Wimmers, K.; Kemper, N. Gene expression profiling of porcine mammary epithelial cells after challenge with Escherichia coli and Staphylococcus aureus in vitro. Vet. Res. 2015, 46, 50. [Google Scholar] [CrossRef]
- Sajjanar, B.; Trakooljul, N.; Wimmers, K.; Ponsuksili, S. DNA methylation analysis of porcine mammary epithelial cells reveals differentially methylated loci associated with immune response against Escherichia coli challenge. BMC Genom. 2019, 20, 623. [Google Scholar] [CrossRef]
- Zou, Y.J.; Xu, J.J.; Wang, X.; Zhu, Y.H.; Wang, J.F. Lactobacillus johnsonii L531 ameliorates Escherichia coli-induced cell damage via inhibiting NLRP3 inflammasome activity and promoting ATG5/ATG16L1-mediated autophagy in porcine mammary epithelial cells. Vet. Sci. 2020, 7, 112. [Google Scholar] [CrossRef]
- Marín, M.; Arroyo, R.; Espinosa-Martos, I.; Fernández, L.; Rodríguez, J.M. Identification of emerging human mastitis pathogens by MALDI-TOF and assessment of their antibiotic resistance patterns. Front. Microbiol. 2017, 8, 1258. [Google Scholar] [CrossRef]
- Bosch, T.C.G.; Zasloff, M. Antimicrobial peptides-or how our ancestors learned to control the microbiome. mBio 2021, 12, e0184721. [Google Scholar] [CrossRef]
- Wu, Q.; Patočka, J.; Kuča, K. Insect Antimicrobial Peptides, a Mini Review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef]
- Hollmann, A.; Martinez, M.; Maturana, P.; Semorile, L.C.; Maffia, P.C. Antimicrobial peptides: Interaction with model and biological membranes and synergism with chemical antibiotics. Front. Chem. 2018, 6, 204. [Google Scholar] [CrossRef]
- Krämer, J.; Lüddecke, T.; Marner, M.; Maiworm, E.; Eichberg, J.; Hardes, K.; Schäberle, T.F.; Vilcinskas, A. Antimicrobial, insecticidal and cytotoxic activity of linear venom peptides from the Pseudoscorpion Chelifer cancroides. Toxins 2022, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Diniz, L.C.L.; Miranda, A.; da Silva, P.I., Jr. Human antimicrobial peptide isolated from Triatoma infestans haemolymph, trypanosoma cruzi-transmitting vector. Front. Cell Infect. Microbiol. 2018, 8, 354. [Google Scholar] [CrossRef] [PubMed]
- Hultmark, D.; Steiner, H.; Rasmuson, T.; Boman, H.G. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur. J. Biochem. 1980, 106, 7–16. [Google Scholar] [CrossRef]
- Swanson, K.; Gorodetsky, S.; Good, L.; Davis, S.; Musgrave, D.; Stelwagen, K.; Farr, V.; Molenaar, A. Expression of a beta-defensin mRNA, lingual antimicrobial peptide, in bovine mammary epithelial tissue is induced by mastitis. Infect. Immun. 2004, 72, 7311–7314. [Google Scholar] [CrossRef]
- Du, M.Z.; Liu, X.D.; Xu, J.J.; Li, S.X.; Wang, S.H.; Zhu, Y.H.; Wang, J.F. Antimicrobial effect of Zophobas morio hemolymph against bovine mastitis pathogens. Microorganisms 2020, 8, 1488. [Google Scholar] [CrossRef] [PubMed]
- Dufies, O.; Doye, A.; Courjon, J.; Torre, C.; Michel, G.; Loubatier, C.; Jacquel, A.; Chaintreuil, P.; Majoor, A.; Guinamard, R.R.; et al. Escherichia coli Rho GTPase-activating toxin CNF1 mediates NLRP3 inflammasome activation via p21-activated kinases-1/2 during bacteraemia in mice. Nat. Microbiol. 2021, 6, 401–412. [Google Scholar] [CrossRef]
- Deets, K.A.; Vance, R.E. Inflammasomes and adaptive immune responses. Nat. Immunol. 2021, 22, 412–422. [Google Scholar] [CrossRef]
- Tweedell, R.E.; Malireddi, R.K.S. A comprehensive guide to studying inflammasome activation and cell death. Nat. Protoc. 2020, 15, 3284–3333. [Google Scholar] [CrossRef]
- Kesavardhana, S.; Kanneganti, T.D. Mechanisms governing inflammasome activation, assembly and pyroptosis induction. Int. Immunol. 2017, 29, 201–210. [Google Scholar] [CrossRef]
- Sharma, D.; Kanneganti, T.D. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J. Cell Biol. 2016, 213, 617–629. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Kuang, M.; Li, L.; Wang, Y.; Yang, F.; Wang, G. UFL1 modulates NLRP3 inflammasome activation and protects against pyroptosis in LPS-stimulated bovine mammary epithelial cells. Mol. Immunol. 2019, 112, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhu, Y.H.; Xu, J.; Liu, X.; Duan, C.; Wang, M.J.; Wang, J.F. Lactobacillus rhamnosus GR-1 ameliorates Escherichia coli-induced activation of NLRP3 and NLRC4 inflammasomes with differential requirement for ASC. Front. Microbiol. 2018, 9, 1661. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.S.; Shenderov, K.; Huang, N.N.; Kabat, J.; Abu-Asab, M.; Fitzgerald, K.A.; Sher, A.; Kehrl, J.H. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat. Immunol. 2012, 13, 255–263. [Google Scholar] [CrossRef]
- Saitoh, T.; Fujita, N.; Jang, M.H.; Uematsu, S.; Yang, B.G.; Satoh, T.; Omori, H.; Noda, T.; Yamamoto, N.; Komatsu, M.; et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 2008, 456, 264–268. [Google Scholar] [CrossRef]
- Akers, R.M.; Nickerson, S.C. Mastitis and its impact on structure and function in the ruminant mammary gland. J. Mammary Gland Biol. Neoplasia 2011, 16, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Wellnitz, O.; Zbinden, C.; Huang, X.; Bruckmaier, R.M. Short communication: Differential loss of bovine mammary epithelial barrier integrity in response to lipopolysaccharide and lipoteichoic acid. J. Dairy Sci. 2016, 99, 4851–4856. [Google Scholar] [CrossRef]
- Guo, W.J.; Liu, B.; Yin, Y.; Kan, X.C.; Gong, Q.; Li, Y.; Cao, Y.; Wang, J.; Xu, D.; Ma, H.; et al. Licochalcone A protects the blood-milk barrierintegrity and relieves the inflammatory response in LPS-induced mastitis. Front. Immunol. 2019, 10, 287. [Google Scholar] [CrossRef]
- Guo, W.J.; Li, W.; Su, Y.C.; Liu, S.; Kan, X.C.; Ran, X.; Cao, Y.; Fu, S.P.; Liu, J.X. GPR109A alleviate mastitis and enhances the blood milk barrier by activating AMPK/Nrf2 and autophagy. Int. J. Bio. Sci. 2021, 17, 4271–4284. [Google Scholar] [CrossRef]
- Jones, H.R.; Robb, C.T.; Perretti, M.; Rossi, A.G. The role of neutrophils in inflammation resolution. Semin. Immunol. 2016, 28, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Paape, M.; Mehrzad, J.; Zhao, X.; Detilleux, J.; Burvenich, C. Defense of the bovine mammary gland by polymorphonuclear neutrophil leukocytes. J. Mammary Gland Biol. Neoplasia 2002, 7, 109–121. [Google Scholar] [CrossRef]
- Mirsepasi-Lauridsen, H.C.; Vallance, B.A.; Krogfelt, K.A. Escherichia coli pathobionts associated with inflammatory bowel disease. Clin. Microbiol. Rev. 2019, 32, e00060-18. [Google Scholar] [CrossRef] [PubMed]
- Liew, P.X.; Kubes, P. The neutrophil’s role during health and disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef]
- Porcherie, A.; Cunha, P.; Trotereau, A.; Roussel, P.; Gilbert, F.B.; Rainard, P.; Germon, P. Repertoire of Escherichia coli agonists sensed by innate immunity receptors of the bovine udder and mammary epithelial cells. Vet. Res. 2012, 43, 14. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, V.A.K.; Zhao, Y.; Shao, F. Innate immunity to intracellular LPS. Nat. Immunol. 2019, 20, 527–533. [Google Scholar] [CrossRef]
- Jo, E.K.; Kim, J.K.; Shin, D.M.; Sasakawa, C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol. Immunol. 2016, 13, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Man, S.M.; Karki, R.; Sasai, M.; Place, D.E.; Kesavardhana, S.; Temirov, J.; Frase, S.; Zhu, Q.; Malireddi, R.K.S.; Kuriakose, T.; et al. IRGB10 liberates bacterial ligands for sensing by the AIM2 and Caspase-11-NLRP3 inflammasomes. Cell 2016, 167, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.H.; Berg, M.; Fossum, C.; Magnusson, U. Proinflammatory cytokine mRNA expression in mammary tissue of sows following intramammary inoculation with Escherichia coli. Vet. Immunol. Immunopathol. 2007, 116, 98–103. [Google Scholar] [CrossRef]
- Wall, S.K.; Hernández-Castellano, L.E.; Ahmadpour, A.; Bruckmaier, R.M.; Wellnitz, O. Differential glucocorticoid-induced closure of the blood-milk barrier during lipopolysaccharide- and lipoteichoic acid-induced mastitis in dairy cows. J. Dairy Sci. 2016, 99, 7544–7553. [Google Scholar] [CrossRef]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef]
- Shibutani, S.T.; Saitoh, T.; Nowag, H.; Münz, C.; Yoshimori, T. Autophagy and autophagy-related proteins in the immune system. Nat. Immunol. 2015, 16, 1014–1024. [Google Scholar] [CrossRef]
- Takahama, M.; Akira, S.; Saitoh, T. Autophagy limits activation of the inflammasomes. Immunol. Rev. 2018, 281, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Cadwell, K.; Liu, J.Y.; Brown, S.L.; Miyoshi, H.; Loh, J.; Lennerz, J.K.; Kishi, C.; Kc, W.; Carrero, J.A.; Hunt, S.; et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 2008, 456, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Tsugami, Y.; Matsunaga, K.; Suzuki, T.; Nishimura, T.; Kobayashi, K. Phytoestrogens Weaken the blood-milk barrier in lactating mammary epithelial cells by affecting tight junctions and cell viability. J. Agric. Food Chem. 2017, 65, 11118–11124. [Google Scholar] [CrossRef]
- Kessler, E.C.; Wall, S.K.; Hernandez, L.L.; Gross, J.J.; Bruckmaier, R.M. Short communication: Mammary gland tight junction permeability after parturition is greater in dairy cows with elevated circulating serotonin concentrations. J. Dairy Sci. 2019, 102, 1768–1774. [Google Scholar] [CrossRef]
- Dahanayaka, S.; Rezaei, R.; Porter, W.W.; Johnson, G.A.; Burghardt, R.C.; Bazer, F.W.; Hou, Y.Q.; Wu, Z.L.; Wu, G.Y. Technical note: Isolation and characterization of porcine mammary epithelial cells. J. Anim. Sci. 2015, 93, 5186–5193. [Google Scholar] [CrossRef]
- Ran, X.; Zhang, Y.; Yang, Y.X.; Hu, G.Q.; Liu, J.X.; Hou, S.; Guo, W.J.; Kan, X.C.; Fu, S.P. Dioscin improves pyroptosis in LPS-induced mice mastitis by activating AMPK/Nrf2 and inhibiting the NF-KappaB signaling pathway. Oxidative Med. Cell. Longev. 2020, 2020, 8845521. [Google Scholar] [CrossRef]


| Primers Name | Direction a | Sequence (5′→3′) | Accession Number |
|---|---|---|---|
| IL-1β | F | GGCCGCCAAGATATAACTGA | NM_214055 |
| R | GGACCTCTGGGTATGGCTTTC | ||
| IL-18 | F | GCTGCTGAACCGGAAGACAA | NM_213997.1 |
| R | AAACACGGCTTGATGTCCCT | ||
| IL-6 | F | GGGAAATGTCGAGGCTGTG | NM_214399 |
| R | AGGGGTGGTGGCTTTGTCT | ||
| Tnf-α | F | GCCCACGTTGTAGCCAATGTCAAA | NM_214022 |
| R | GTTGTCTTTCAGCTTCACGCCGTT | ||
| Gapdh | F | CCAGAACATCATCCCTGCTT | NM_001206359 |
| R | GTCCTCAGTGTAGCCCAGGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Y.; Wang, X.; Xu, J.; Wang, S.; Li, S.; Zhu, Y.; Wang, J. Z. morio Hemolymph Relieves E. coli-Induced Mastitis by Inhibiting Inflammatory Response and Repairing the Blood–Milk Barrier. Int. J. Mol. Sci. 2022, 23, 13279. https://doi.org/10.3390/ijms232113279
Zou Y, Wang X, Xu J, Wang S, Li S, Zhu Y, Wang J. Z. morio Hemolymph Relieves E. coli-Induced Mastitis by Inhibiting Inflammatory Response and Repairing the Blood–Milk Barrier. International Journal of Molecular Sciences. 2022; 23(21):13279. https://doi.org/10.3390/ijms232113279
Chicago/Turabian StyleZou, Yunjing, Xue Wang, Jiajia Xu, Shenghua Wang, Shuxian Li, Yaohong Zhu, and Jiufeng Wang. 2022. "Z. morio Hemolymph Relieves E. coli-Induced Mastitis by Inhibiting Inflammatory Response and Repairing the Blood–Milk Barrier" International Journal of Molecular Sciences 23, no. 21: 13279. https://doi.org/10.3390/ijms232113279
APA StyleZou, Y., Wang, X., Xu, J., Wang, S., Li, S., Zhu, Y., & Wang, J. (2022). Z. morio Hemolymph Relieves E. coli-Induced Mastitis by Inhibiting Inflammatory Response and Repairing the Blood–Milk Barrier. International Journal of Molecular Sciences, 23(21), 13279. https://doi.org/10.3390/ijms232113279







