Improving the Structural Changes, Electrophoretic Pattern, and Quality Attributes of Spent Hen Meat Patties by Using Kiwi and Pineapple Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Extracts Preparation
2.3. Preparation of Burger Ingredients
2.4. Marination Process
2.5. Burger Formulation, Processing, and Storage
2.6. Product Investigations
2.6.1. Quality Attributes
Measurement of Collagen Content and the Solubility of Collagen%
Measurement of Shear Force
Color Evaluation
Sensory Panel Analysis
2.6.2. Structural Examination
Light Microscope
Scanning Electron Microscope
2.6.3. Electrophoresis
2.7. Statistical Analysis
3. Results and Discussions
3.1. The Effect of Kiwi and Pineapple Extracts on the Quality Attributes of Spent Hen Meat Patties
3.1.1. The Consequence of Kiwi and Pineapple Extracts on the Collagen Content, Collagen Solubility %, and Shear Force of Spent Hen Meat Patties
3.1.2. The Effect of Kiwi and Pineapple Extracts on the Instrumental Color Values of Spent Hen Meat Patties
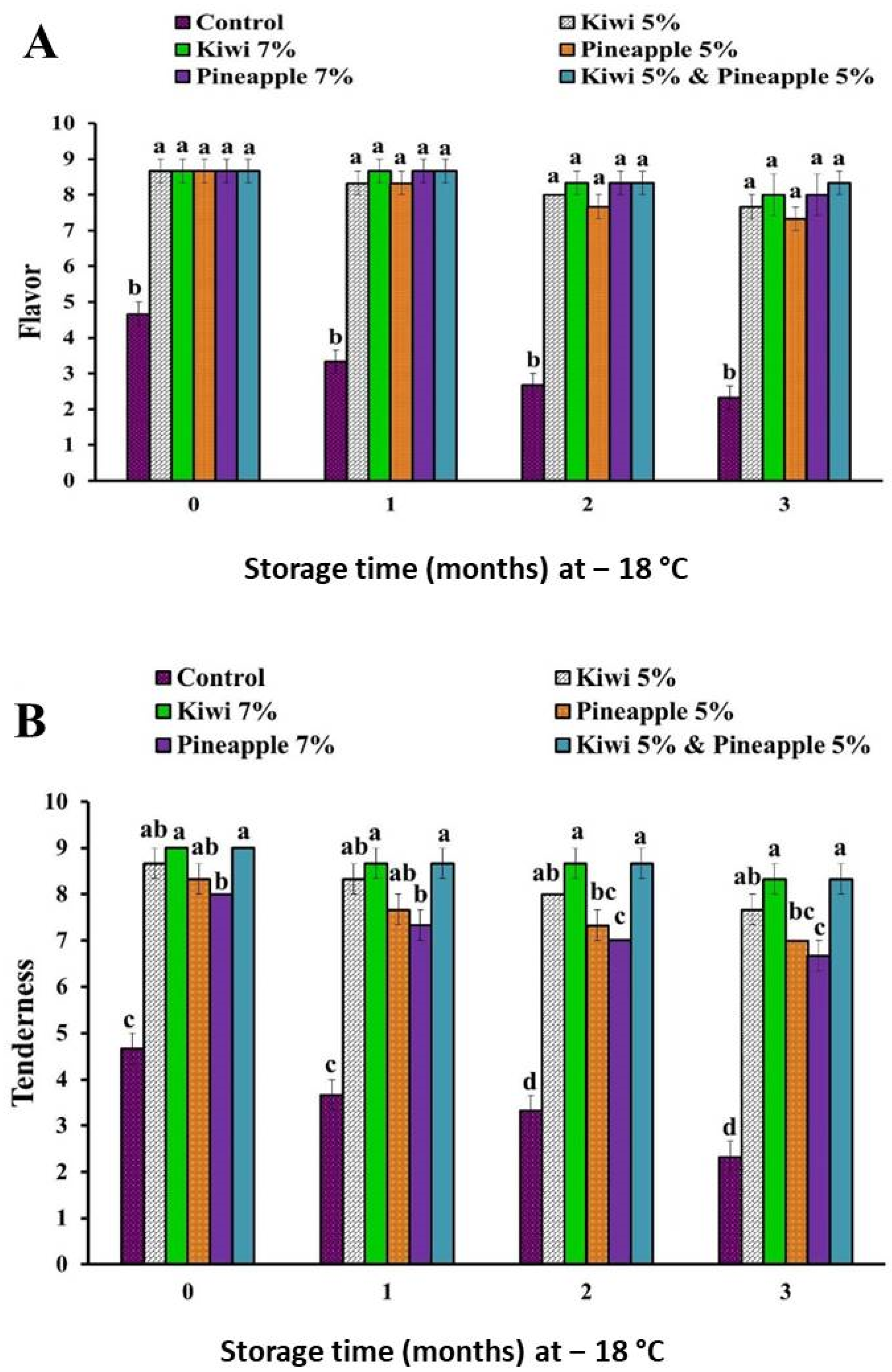
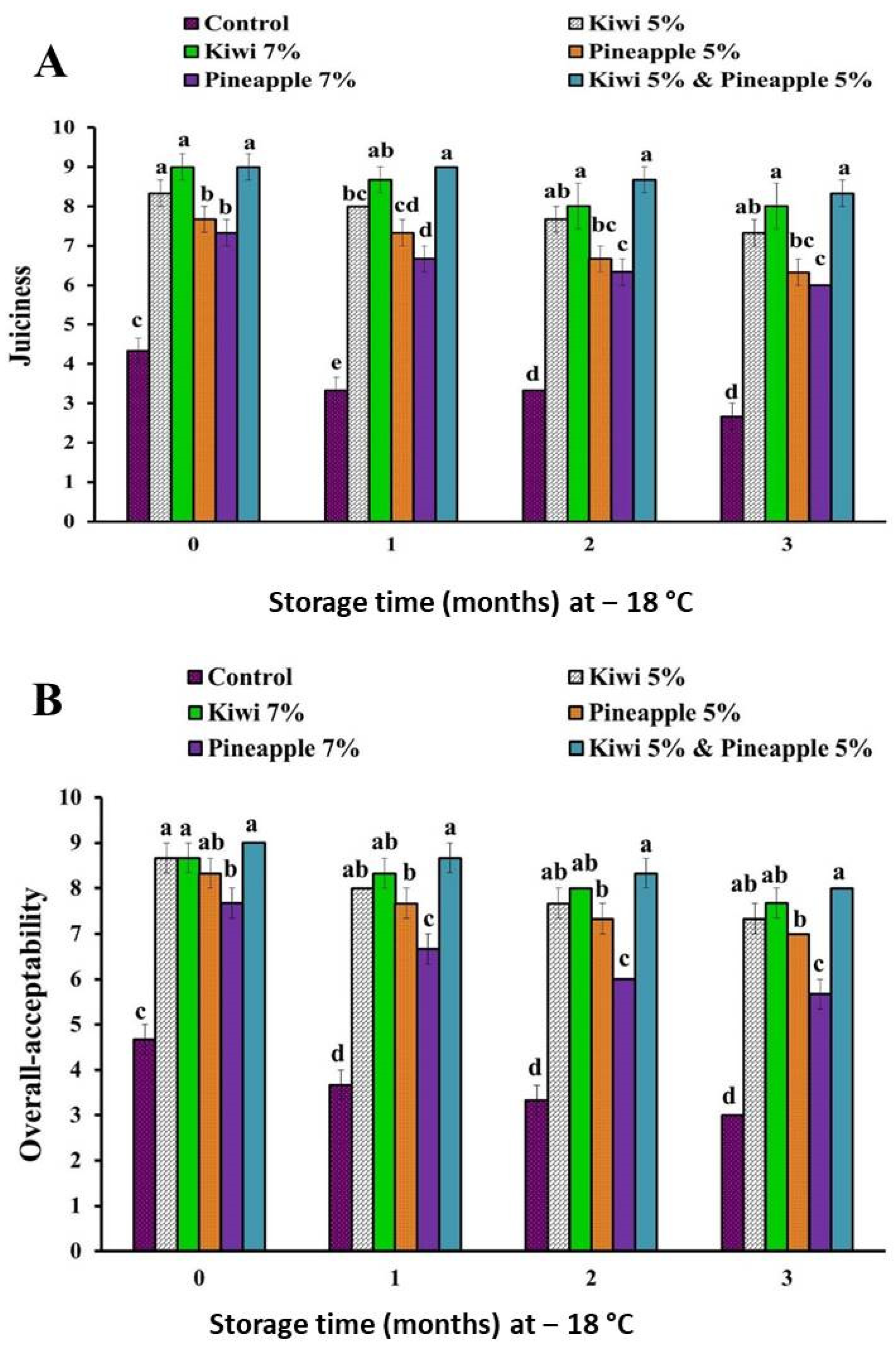
3.1.3. The Effect of Kiwi and Pineapple Extracts on the Sensory Attributes of Spent Hen Meat Patties
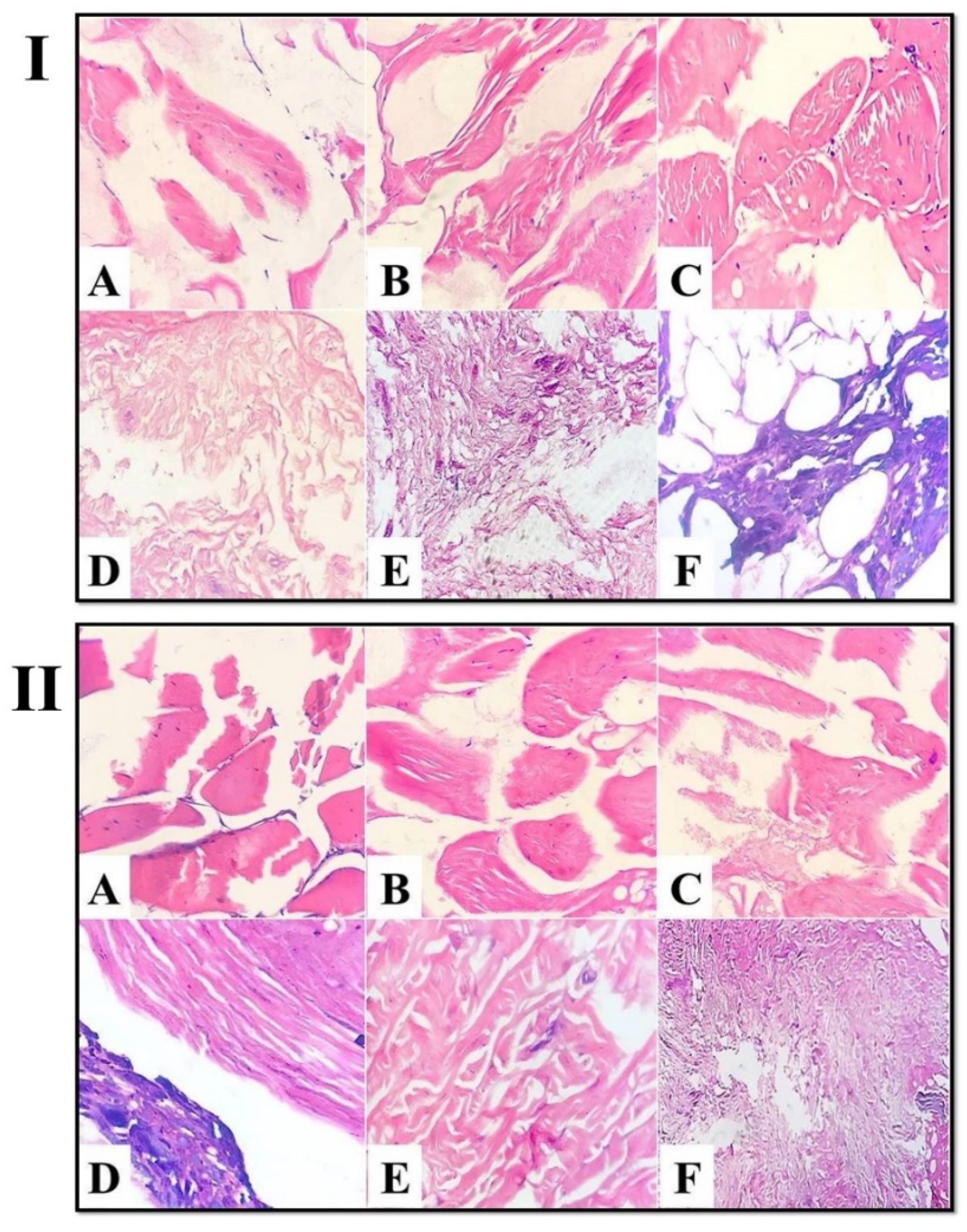
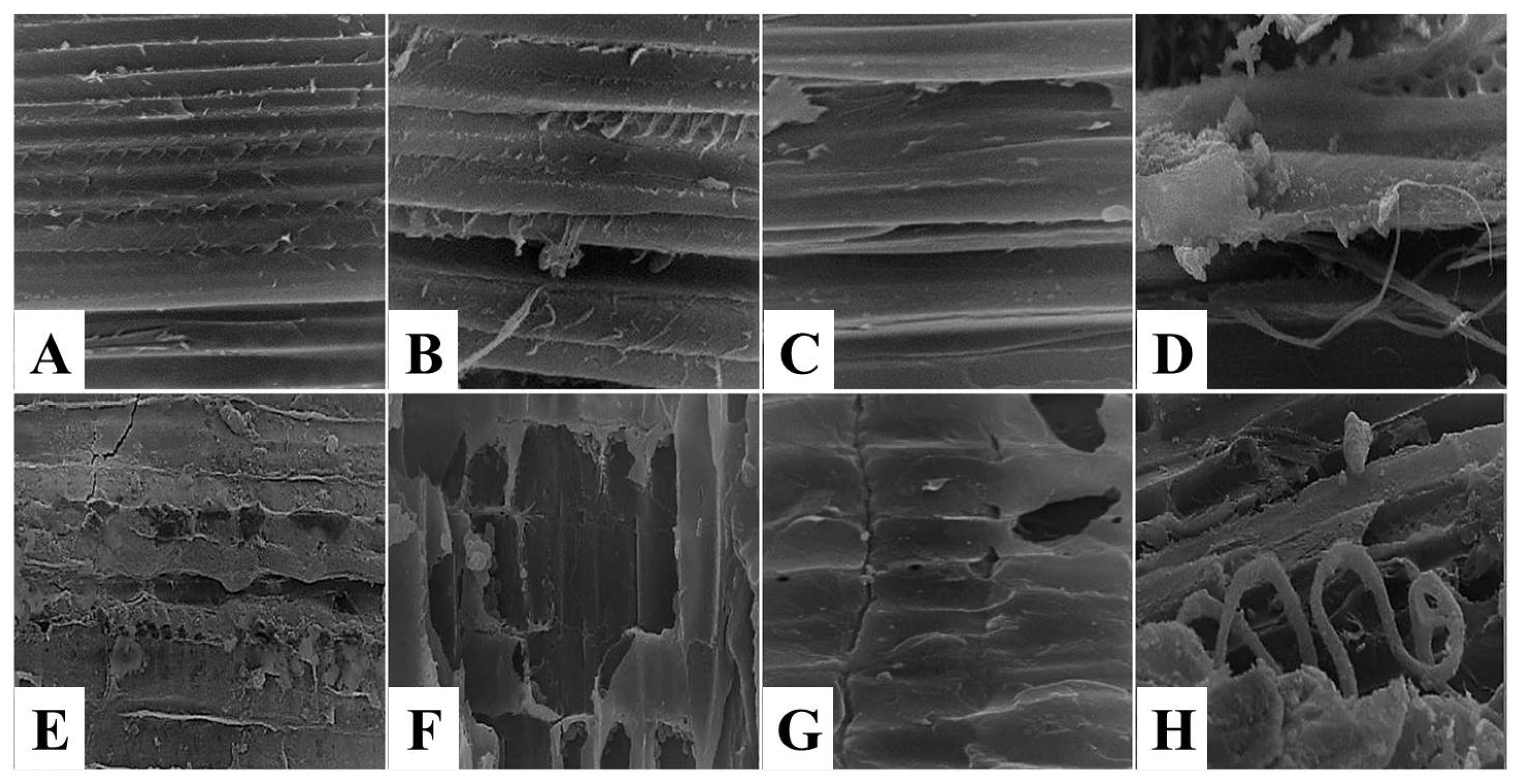
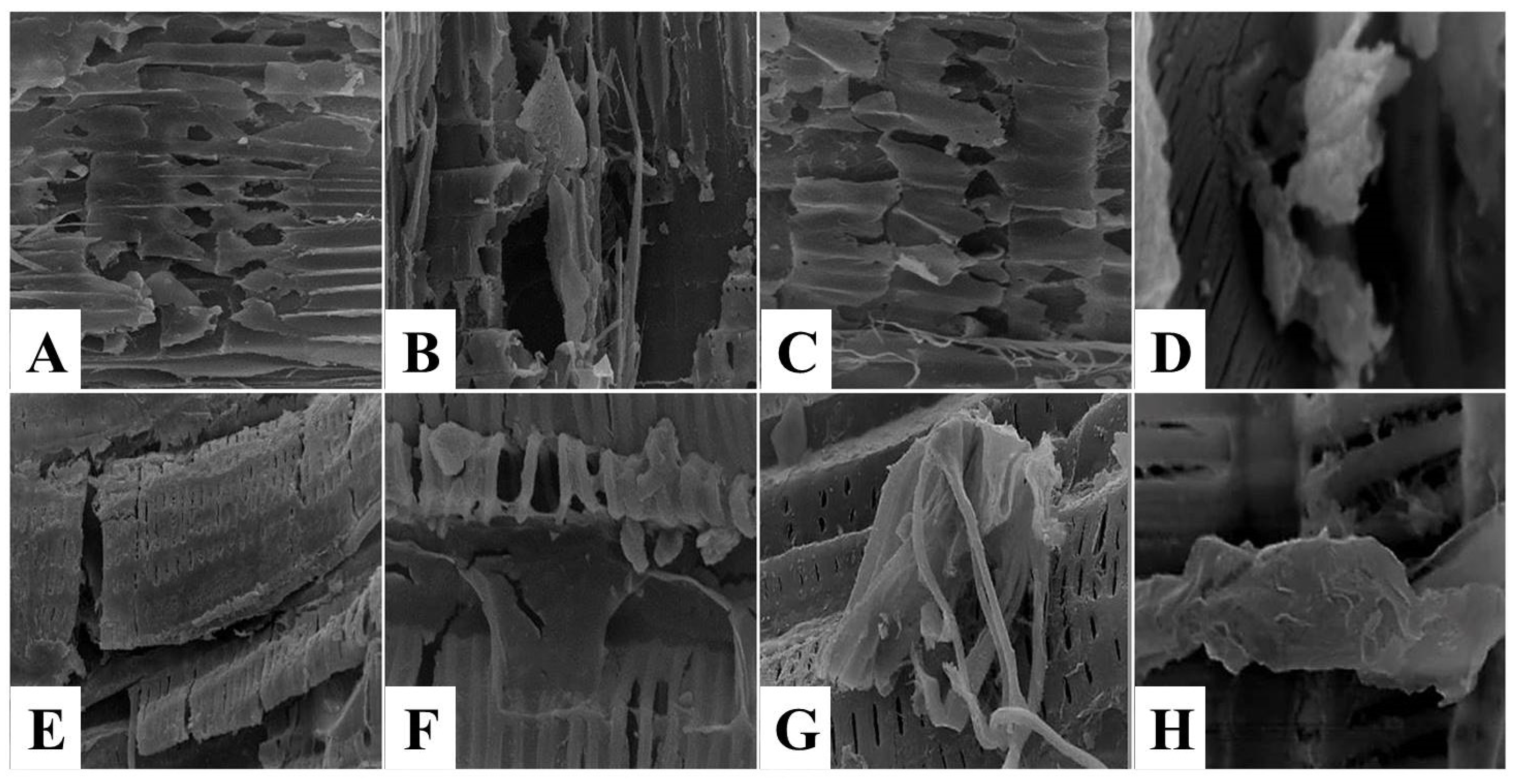
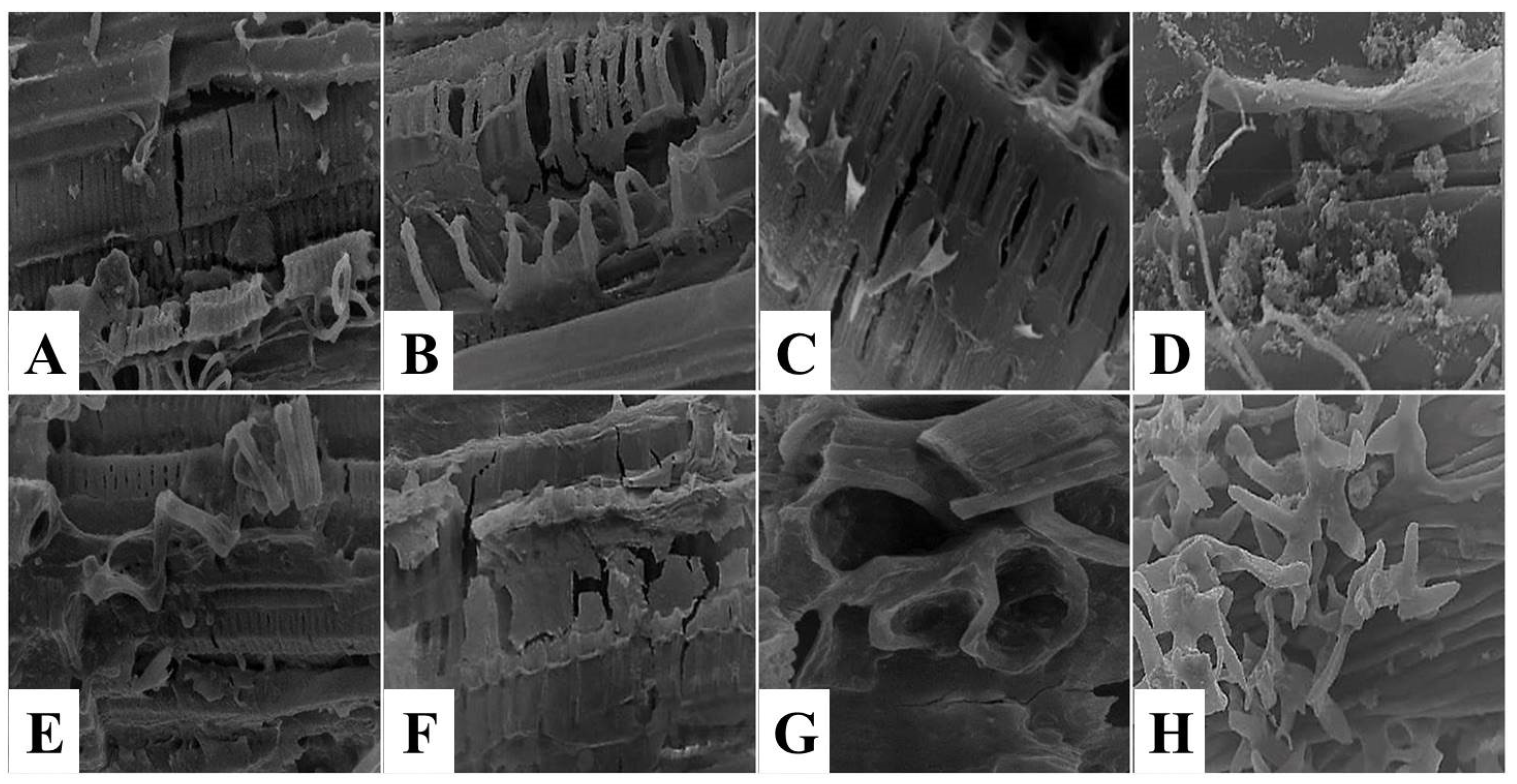
3.2. The Effect of Kiwi and Pineapple Extracts on the Structural Changes of Spent Hen Meat Patties
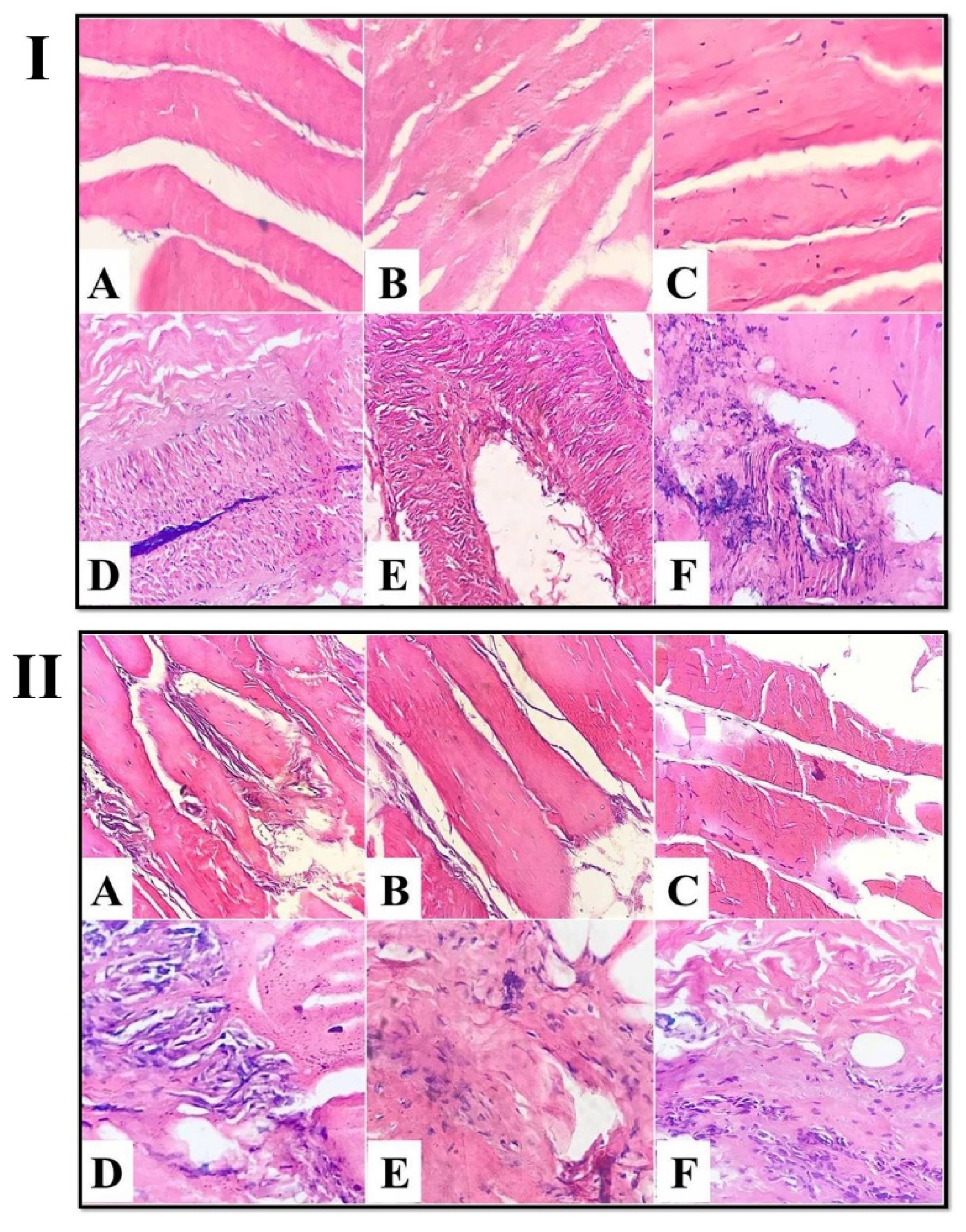
3.2.1. Light Microscope
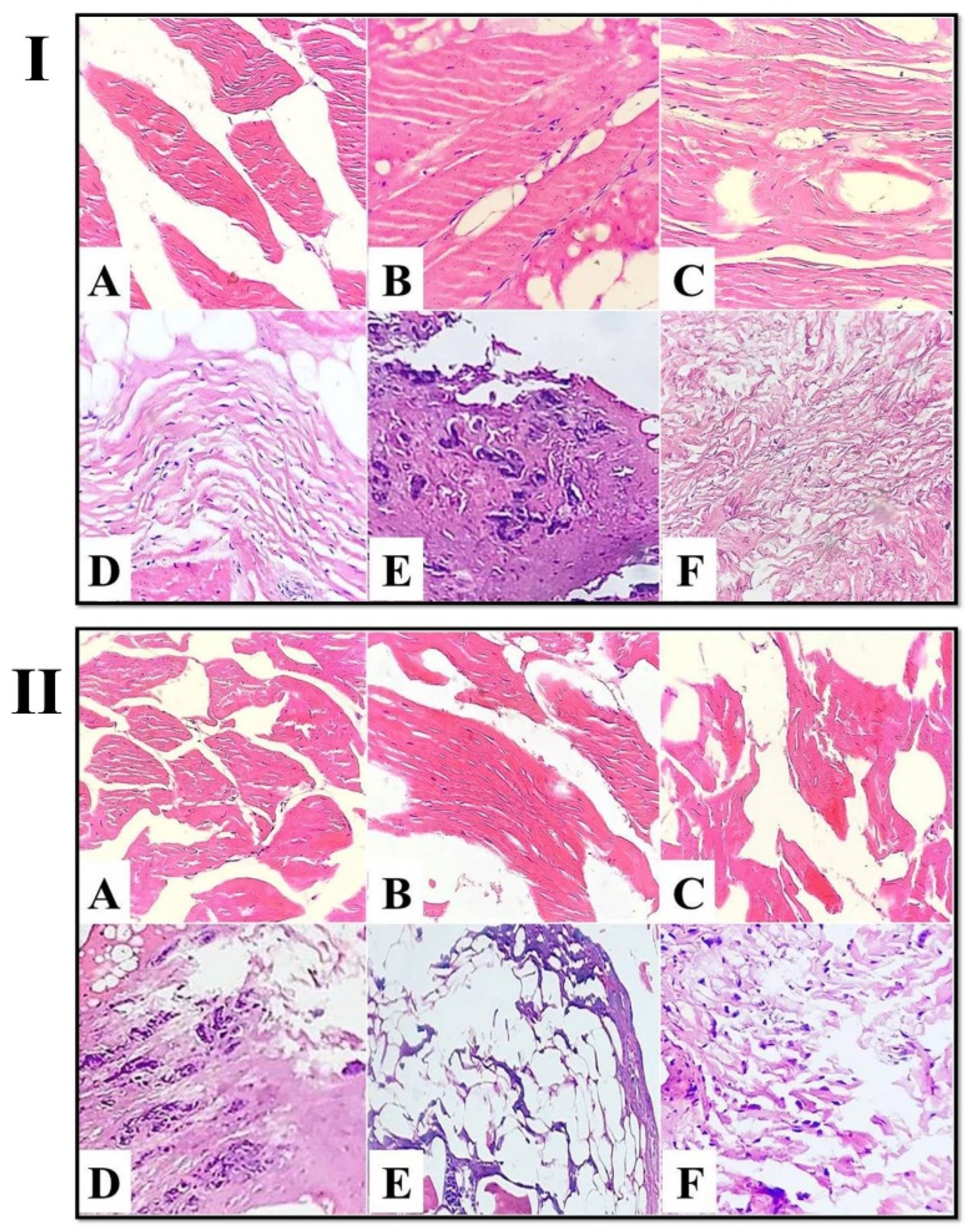
3.2.2. Scanning Electron Microscope
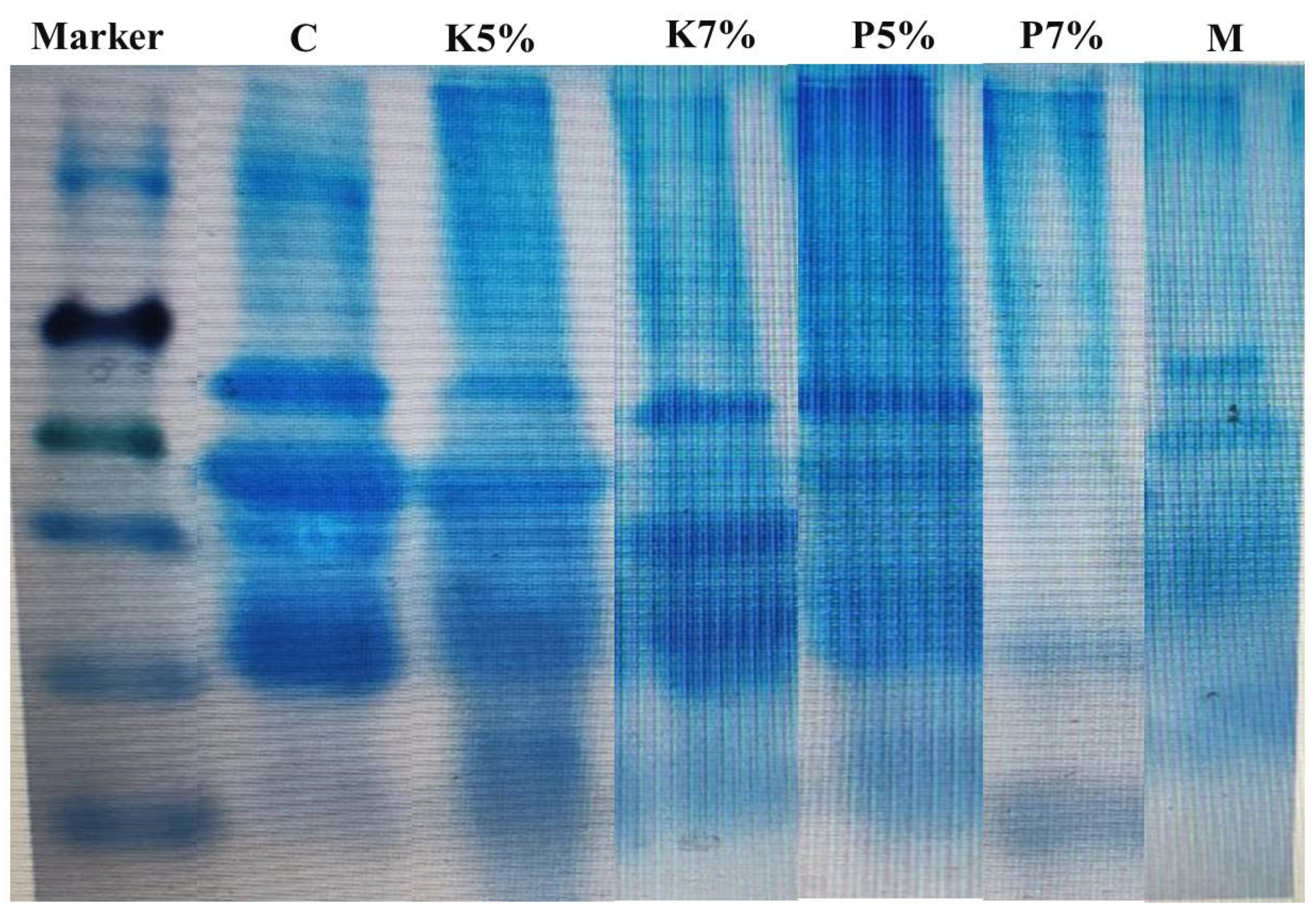
3.3. The Effect of Kiwi and Pineapple Extracts on the Electrophoretic Pattern of Spent Hen Meat Patties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suriani, N.W.; Purnomo, H.; Estiasih, T.; Suwetja, I.K. Physicochemical properties, fatty acids profile and cholesterol content of indigenous Manado chicken, broiler and spent hen meat. Int. J. ChemTech Res. 2014, 6, 3896–3902. [Google Scholar]
- Elshebrawy, H.A.; Abdel-Naeem, H.H.; Mahros, M.A.; Elsayed, H.; Imre, K.; Herman, V.; Morar, A.; Sallam, K.I. Multidrug-resistant Salmonella enterica serovars isolated from frozen chicken carcasses. LWT Food Sci. Technol. 2022, 164, 113647. [Google Scholar] [CrossRef]
- Korhonen, H. Milk-derived bioactive peptides: From science to applications. J. Funct. Foods 2009, 1, 177–187. [Google Scholar] [CrossRef]
- Udenigwe, C.C. Bioinformatics approaches, prospects and challenges of food bioactive peptide research. Trends Food Sci. Technol. 2014, 36, 137–143. [Google Scholar] [CrossRef]
- Li-Chan, E.C. Bioactive peptides and protein hydrolysates: Research trends and challenges for application as nutraceuticals and functional food ingredients. Curr. Opin. Food Sci. 2015, 1, 28–37. [Google Scholar] [CrossRef]
- Fan, H.; Wu, J. Conventional use and sustainable valorization of spent egg-laying hens as functional foods and biomaterials: A review. Bioresour. Bioprocess. 2022, 9, 1–18. [Google Scholar] [CrossRef]
- Singh, R.P.; Verma, S.S. Physico-chemical and sensory quality of chicken patties as influenced by extender and packaging materials. Indian J. Poult. Sci. 2000, 35, 85–88. [Google Scholar]
- Indumathi, J.; Shashikumar, M.; Reddy, G.V.B.; Babu, A.J.; Prakash, M.G. Utilization of spent broiler breeder hen meat to develop value added sausages. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 754–765. [Google Scholar] [CrossRef]
- Abdel-Naeem, H.H.; Talaat, M.M.; Imre, K.; Morar, A.; Herman, V.; El-Nawawi, F.A. Structural changes, electrophoretic pattern, and quality attributes of camel meat treated with fresh ginger extract and papain powder. Foods 2022, 11, 1876. [Google Scholar] [CrossRef]
- Chaurasiya, R.S.; Sakhare, P.Z.; Bhaskar, N.; Hebbar, H.U. Efficacy of reverse micellar extracted fruit bromelain in meat tenderization. J. Food Sci. Technol. 2015, 52, 3870–3880. [Google Scholar] [CrossRef]
- Varilla, C.; Marcone, M.; Paiva, L.; Baptista, J. Bromelain, a group of pineapple proteolytic complex enzymes (Ananas comosus) and their possible therapeutic and clinical effects. A Summary. Foods 2021, 10, 2249. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, F.; Carpena, M.; Fraga-Corral, M.; Echave, J.; Rajoka, M.S.R.; Barba, F.J.; Cao, H.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Valorization of kiwi agricultural waste and industry by-products by recovering bioactive compounds and applications as food additives: A circular economy model. Food Chem. 2022, 370, 131315. [Google Scholar] [CrossRef]
- Kang, G.H.; Kim, S.H.; Kim, J.H.; Kang, H.K.; Kim, D.W.; Seong, P.N.; Cho, S.H.; Park, B.Y.; Kim, D.H. Effect of Flammulina velutipes on spent-hen breast meat tenderization. Poult. Sci. 2012, 91, 232–236. [Google Scholar] [CrossRef]
- Al-Hameed, S.A.H.M.; Al-Jawary, H.A.R.S. Improvement some of chemical and physical properties of the spent hens meat by using natural tenderizers materials. Int. J. Adv. Biol. Res. 2017, 7, 798–803. [Google Scholar]
- Hossain, M.S.; Rokib, M.; Habib, M.; Kabir, M.H.; Hashem, M.A.; Azad, M.A.K.; Rahman, M.M.; Ali, M.S. Quality of spent hen sausages incorporated with fresh ginger extract. Meat Res. 2021, 1, 1–4. [Google Scholar] [CrossRef]
- Abdelrahman, A.G.; Mohamed, H.M.H.; Yassien, N.A. Tenderization of Spent Hen Meat Using Natural Fruits. Master’s Thesis, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt, 2016. [Google Scholar]
- Naewbanij, J.O.; Dorothy, L.H.; Stone, M.B. Roasting vs cooking in a model system: Tenderness of bull adductor muscle conventionally chilled or electrically stimulated-Hot boned. J. Food Sci. 1983, 48, 337–342. [Google Scholar] [CrossRef]
- Shackelford, S.D.; Wheeler, T.L.; Koohmaraie, M. Evaluation of sampling, cookery, and shear force protocols for objective evaluation of lamb longissimus tenderness. J. Anim. Sci. 2004, 82, 802–807. [Google Scholar] [CrossRef]
- Shin, H.; Choi, Y.; Kim, H.; Ryu, Y.; Lee, S.; Kim, B. Tenderization and fragmentation of myofibrillar proteins in bovine Longissimus dorsi muscle using proteolytic extract from Sarcodon aspratus. Food Sci. Technol. 2008, 41, 1389–1395. [Google Scholar] [CrossRef]
- American Meat Science Association (AMSA). Research Guidelines for Cookery, Sensory Evaluation and Instrumental Tenderness Measurements of Fresh Meat; American Meat Science Association: Chicago, IL, USA, 1995. [Google Scholar]
- Banchroft, J.D.; Stevens, A.; Turner, D.R. Theory and Practice of Histological Techniques, 4th ed.; Churchil Livingstone: New York, NY, USA; London, UK; San Francisco, CA, USA; Tokyo, Japan, 1996. [Google Scholar]
- Ketnawa, S.; Rawdkuen, S. Application of bromelain extract for muscle foods tenderization. Food Nutr. Sci. 2011, 2, 5736. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Lengkey, H.A.W.; Garnida, D.; Siwi, J.A.; Edianingsih, P.; Wulandari, E.; Pratama, A. The effect of carrageenan on shelf-life, quality improvement and organoleptic qualities of spent chicken sausages. AgroLife Sci. J. 2016, 5, 115–120. [Google Scholar]
- Kadıoğlu, P.; Karakaya, M.; Unal, K.; Babaoğlu, A.S. Technological and textural properties of spent chicken breast, drumstick and thigh meats as affected by marinating with pineapple fruit juice. Br. Poult. Sci. 2019, 60, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Weng, C.H.; Munawar, N. Effects of different concentrations of pineapple core extract and maceration process on free-range chicken meat quality. Ital. J. Food Sci. 2022, 34, 124–131. [Google Scholar] [CrossRef]
- Bagheri Kakash, S.; Hojjatoleslamy, M.; Babaei, G.; Molavi, H. Kinetic study of the effect of kiwi fruit actinidin on various proteins of chicken meat. Food Sci. Technol. 2019, 39, 980–992. [Google Scholar] [CrossRef]
- Ilayabharathi, D.; Sheriff, F.R.; Raj Manohar, G. Shelf-life of spent chicken sausage and its organoleptic qualities. Tamilnadu J. Vet. Anim. Sci. 2012, 8, 60–67. [Google Scholar]
- Vaithiyanathan, S.; Naveena, B.M.; Muthukumar, M.; Girish, P.S.; Ramakrishna, C.; Sen, A.R.; Babji, Y. Biochemical and physicochemical changes in spent-hen meat during postmortem aging. Poult. Sci. 2008, 87, 180–186. [Google Scholar] [CrossRef]
- Barido, F.H.; Lee, S.K. Changes in proteolytic enzyme activities, tenderness-related traits, and quality properties of spent hen meat affected by adenosine 5′-monophosphate during cold storage. Poult. Sci. 2021, 5, 101056. [Google Scholar] [CrossRef]
- Abdel-Naeem, H.H.; Ebaid, E.M.; Khalel, K.H.; Imre, K.; Morar, A.; Herman, V.; EL-Nawawi, F.A. Decontamination of chicken meat using dielectric barrier discharge cold plasma technology: The effect on microbial quality, physicochemical properties, topographical structure, and sensory attributes. LWT Food Sci. Technol. 2022, 165, 113739. [Google Scholar] [CrossRef]
- Kim, G.D.; Jung, E.Y.; Seo, H.W.; Joo, S.T.; Yang, H.S. Textural and sensory properties of pork jerky adjusted with tenderizers or humectant. Food Sci. Anim. Resour. 2010, 30, 930–937. [Google Scholar] [CrossRef]
- Toohey, E.S.; Kerr, M.J.; Van de Ven, R.; Hopkins, D.L. The effect of a kiwi fruit based solution on meat traits in beef m. semimembranosus (topside). Meat Sci. 2011, 88, 468–471. [Google Scholar] [CrossRef]
- Jiao, Y.; Quek, S.Y.; Gu, M.; Guo, Y.; Liu, Y. Polyphenols from thinned young kiwifruit as natural antioxidant: Protective effects on beef oxidation, physicochemical and sensory properties during storage. Food Control 2020, 108, 106870. [Google Scholar] [CrossRef]
- Singh, B.; Wagh, R.V.; Chatli, M.K.; Mehta, N. Optimization of ethanol-assisted extraction of kiwi peel and antioxidant activity in chicken emulsion. Haryana Vet. 2021, 60, 203–207. [Google Scholar]
- Sharma, S.; Vaidya, D. Application of kiwifruit protease enzyme for tenderization of spent hen chicken. J. Pharmacogn. Phytochem. 2018, 7, 581–584. [Google Scholar]
- Pooona, J.; Singh, P.; Prabhakaran, P. Effect of kiwifruit juice and tumbling on tenderness and lipid oxidation in chicken nuggets. Nutr. Food Sci. 2019, 50, 74–83. [Google Scholar] [CrossRef]
- Żochowska-Kujawska, J.; Lachowicz, K.; Sobczak, M.; Nędzarek, A.; Tórz, A. Effects of natural plant tenderizers on proteolysis and texture of dry sausages produced with wild boar meat addition. Afr. J. Biotechnol. 2013, 12, 5670–5677. [Google Scholar]
- Abdel-Naeem, H.H.; Mohamed, H.M. Improving the physico-chemical and sensory characteristics of camel meat burger patties using ginger extract and papain. Meat Sci. 2016, 118, 52–60. [Google Scholar] [CrossRef]
- Kumar, Y.; Singh, P.; Pandey, A.; Tanwar, V.K.; Kumar, R.R. Augmentation of meat quality attributes of spent hen breast muscle (Pectoralis Major) by marination with lemon juice vis-a-vis ginger extract. J. Anim. Res. 2017, 7, 523–529. [Google Scholar] [CrossRef]
- Unal, K.; Alagöz, E.; Çelik, İ.; Sarıçoban, C. Marination with citric acid, lemon, and grapefruit affects the sensory, textural, and microstructure characteristics of poultry meat. Br. Poult. Sci. 2022, 63, 31–38. Available online: https://www.tandfonline.com/doi/abs/10.1080/00071668.2021.1963674 (accessed on 3 September 2021). [CrossRef] [PubMed]
- Koeipudsa, C.; Malila, Y.; Limpisophon, K. Improving tenderness of breast meat of spent-laying hens using marination in alkaline or acidic solutions. Asia-Pac. J. Sci. Technol. 2019, 24, 1–8. [Google Scholar]
- Rumondor, D.; Tamasoleng, M.; Manangkot, H.; Hadju, R.; Ma’ruf, W. The effect of the storage to the microstructure of angkak sausages of laying chicken meat with the scanning electron microscope (SEM) method. Sci. Pap. Ser. D Anim. Sci. Int. Sess. Sci. Commun. Fac. Anim. Sci. 2021, 64, 380–384. [Google Scholar]
- Ketnawa, S.; Sai-ut, S.; Theppakorn, T.; Chaiwut, P.; Rawdkuen, S. Partitioning of Bromelain from Pineapple peel (Nang Lae cultv.) by Aqueous Two Phase System. Asian J. Food Agro-Ind. 2009, 2, 457–468. [Google Scholar]
- Han, J.; Morton, J.D.; Bekhit, A.E.D.; Sedcole, J.R. Pre-rigor infusion with kiwifruit juice improves lamb tenderness. Meat Sci. 2008, 82, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xiong, Y.L.; Rentfrow, G.K. Kiwifruit protease extract injection reduces toughness of pork loin muscle induced by freeze-thaw abuse. LWT Food Sci. Technol. 2011, 44, 2026–2031. [Google Scholar] [CrossRef]
- Bhaskar, N.; Sachindra, N.M.; Modi, V.K.; Sakhare, P.Z.; Mahendrakar, N.S. Preparation of proteolytic activity rich ginger powder and evaluation of its tenderizing effect on spent-hen muscles. J. Muscle Foods 2006, 17, 174–184. [Google Scholar] [CrossRef]
- Kiran, M.; Naveena, B.M.; Sudhakar, R.K.; Kondal, R.K.; Madhav, R.T. Effect of ammonium hydroxide on textural and ultrastructural properties of spent hen meat. Int. Food Res. J. 2014, 2049–2054. [Google Scholar]

| Collagen Content (g%) | Collagen Solubility (%) | Shear Force (N) | |
|---|---|---|---|
| Control | 1.08 a ± 0.02 | 6.62 f ± 0.72 | 14.42 a ± 0.00 |
| Kiwi 5% | 0.51 b ± 0.02 | 10.44 e ± 0.54 | 11.47 b ± 0.03 |
| Kiwi 7% | 0.34 c ± 0.02 | 15.64 d ± 0.59 | 8.81 c ± 0.01 |
| Pineapple 5% | 0.25 d ± 0.00 | 21.40 c ± 0.20 | 7.94 d ± 0.01 |
| Pineapple 7% | 0.05 f ± 0.01 | 59.11 a ± 2.72 | 3.63 f ± 0.00 |
| Kiwi 5% and Pineapple 5% | 0.12 e ± 0.00 | 39.57 b ± 3.76 | 6.57 e ± 0.00 |
| L* | a* | b* | |
|---|---|---|---|
| Control | 58.28 a ± 0.97 | 6.60 c ± 0.02 | 11.99 a ± 0.10 |
| Kiwi 5% | 57.31 a ± 0.88 | 7.97 b ± 0.02 | 12.37 a ± 0.24 |
| Kiwi 7% | 56.43 a ± 0.92 | 8.67 a ± 0.03 | 12.38 a ± 0.21 |
| Pineapple 5% | 57.64 a ± 0.59 | 7.79 b ± 0.03 | 12.13 a ± 0.14 |
| Pineapple 7% | 57.44 a ± 0.70 | 7.86 b ± 0.09 | 12.14 a ± 0.13 |
| Kiwi 5% and Pineapple 5% | 57.46 a ± 0.77 | 7.89 b ± 0.01 | 12.00 a ± 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Naeem, H.H.S.; Abdelrahman, A.G.; Imre, K.; Morar, A.; Herman, V.; Yassien, N.A. Improving the Structural Changes, Electrophoretic Pattern, and Quality Attributes of Spent Hen Meat Patties by Using Kiwi and Pineapple Extracts. Foods 2022, 11, 3430. https://doi.org/10.3390/foods11213430
Abdel-Naeem HHS, Abdelrahman AG, Imre K, Morar A, Herman V, Yassien NA. Improving the Structural Changes, Electrophoretic Pattern, and Quality Attributes of Spent Hen Meat Patties by Using Kiwi and Pineapple Extracts. Foods. 2022; 11(21):3430. https://doi.org/10.3390/foods11213430
Chicago/Turabian StyleAbdel-Naeem, Heba H. S., Amal G. Abdelrahman, Kálmán Imre, Adriana Morar, Viorel Herman, and Nabil A. Yassien. 2022. "Improving the Structural Changes, Electrophoretic Pattern, and Quality Attributes of Spent Hen Meat Patties by Using Kiwi and Pineapple Extracts" Foods 11, no. 21: 3430. https://doi.org/10.3390/foods11213430
APA StyleAbdel-Naeem, H. H. S., Abdelrahman, A. G., Imre, K., Morar, A., Herman, V., & Yassien, N. A. (2022). Improving the Structural Changes, Electrophoretic Pattern, and Quality Attributes of Spent Hen Meat Patties by Using Kiwi and Pineapple Extracts. Foods, 11(21), 3430. https://doi.org/10.3390/foods11213430








