Androgen Receptor Expression in the Various Regions of Resected Glioblastoma Multiforme Tumors and in an In Vitro Model
Abstract
1. Introduction
2. Results
2.1. Characteristics of The Study Group
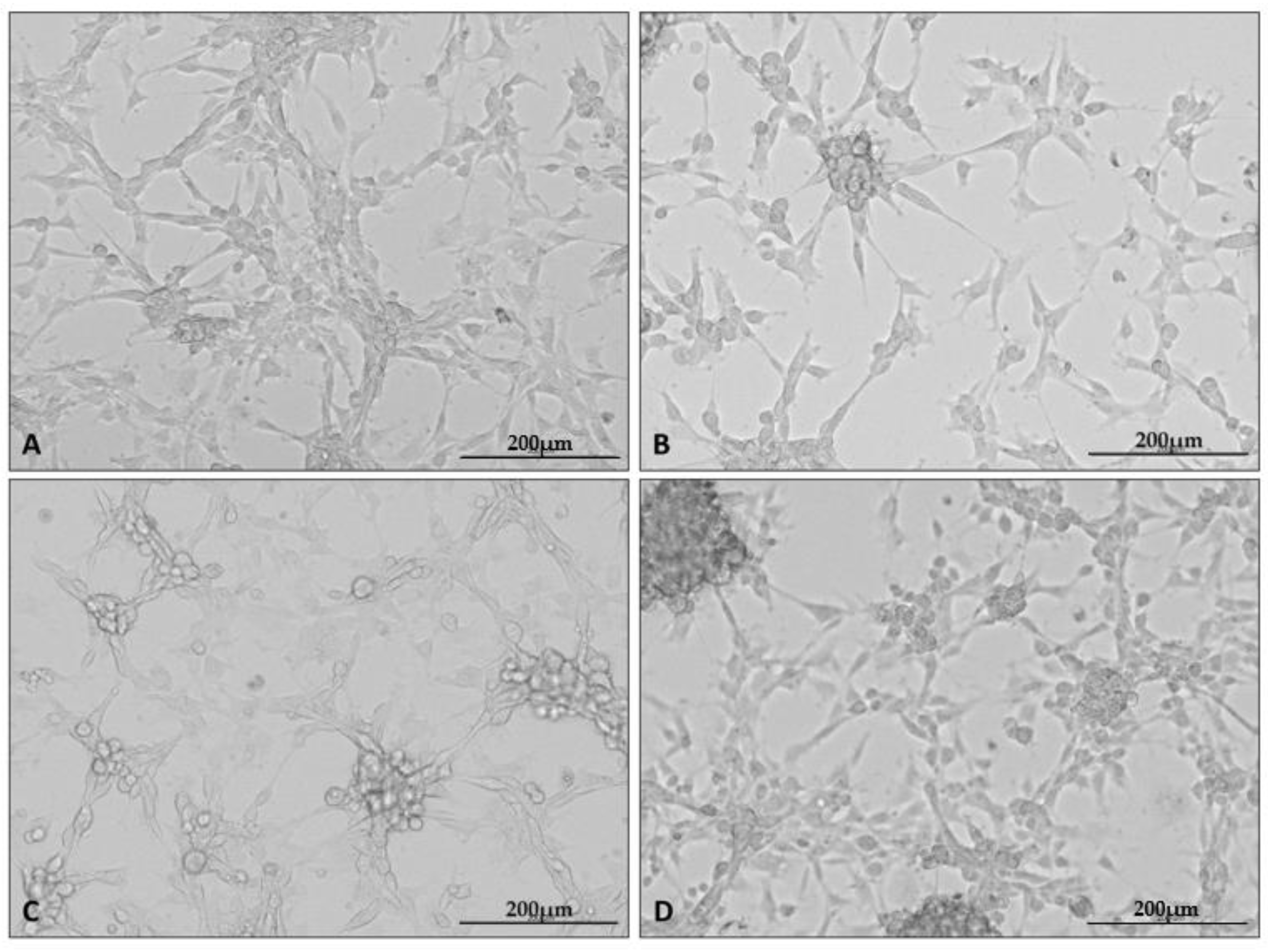
2.2. U87 Cell Line Cultures under Different Conditions
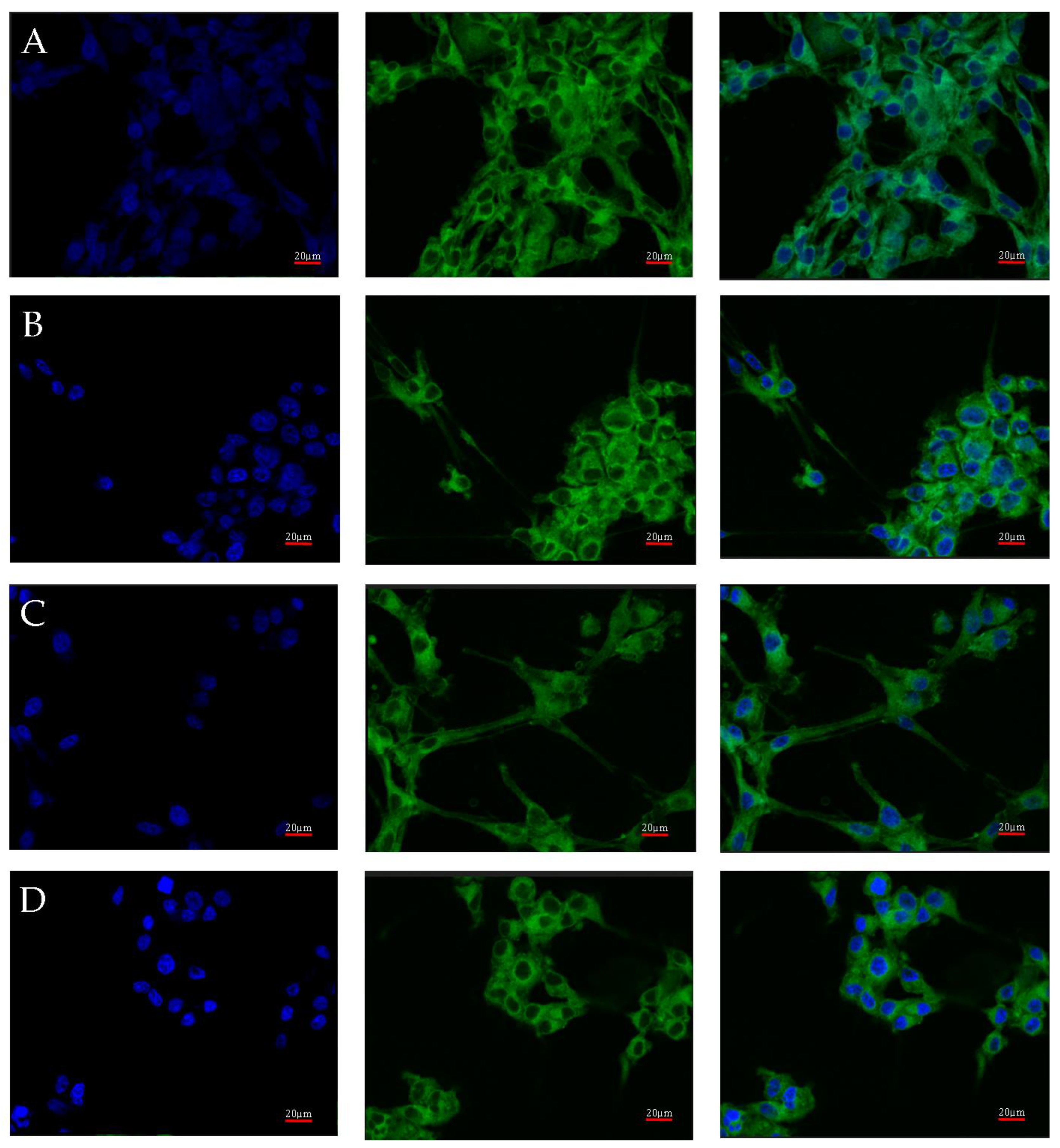
2.3. Changes in AR Gene and Protein Expression in U87 Line Cells Cultured under Different Test Conditions
2.4. Changes in Gene and AR Protein Expression in Individual GBM Structures Obtained from Patients
2.5. Summary of Results
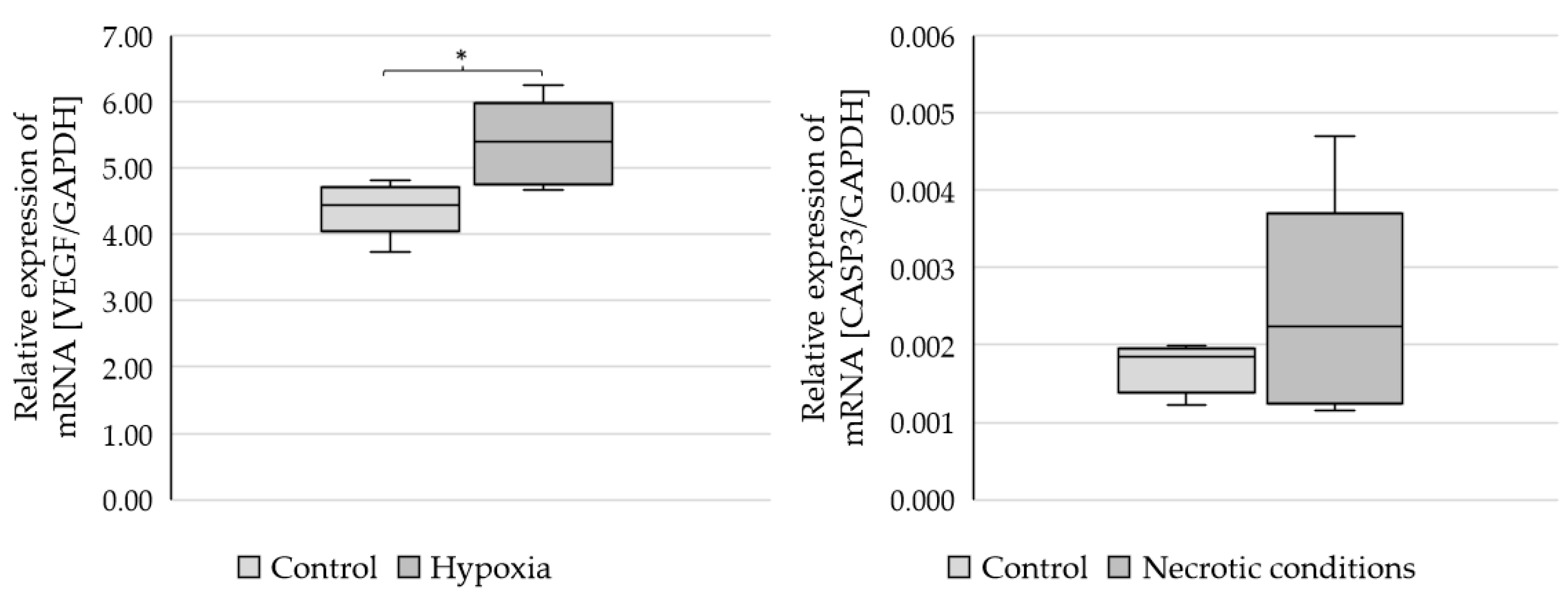
- In vitro AR mRNA expression was higher under nutrient-deficient conditions than in the control and lower under hypoxia than in the control.
- In cell cultures, there were no differences in AR protein expression between the various test conditions.
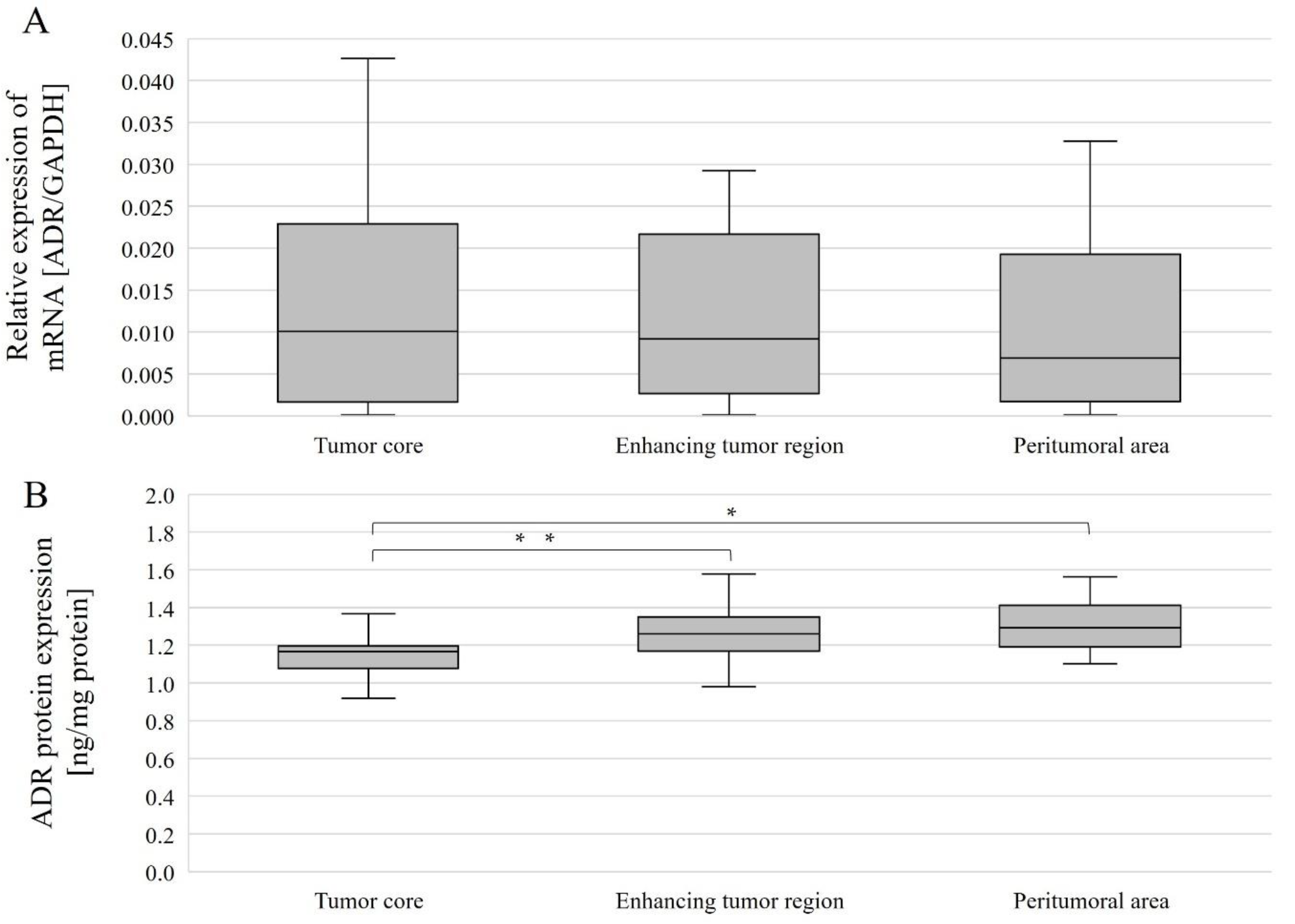
- AR mRNA expression did not differ between different tumor regions taken from patients (analyzed together and by gender).
- In all patients (women and men), AR protein expression was higher in the enhancing tumor region and in the peritumoral area than in the tumor core.
- In women, AR protein expression was higher in the peritumoral area than in the tumor core.
- There were no significant differences between men and women in the expression of the AR gene and AR protein.
3. Discussion
3.1. Model on Patients
3.2. In Vitro Model
4. Materials and Methods
4.1. Patient Samples
4.2. Cell Culture and Treatment
4.3. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Immunohistochemistry
4.6. Confocal Microscopy
4.7. Statistical Analysis
5. Conclusions
Research Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Larjavaara, S.; Mäntylä, R.; Salminen, T.; Haapasalo, H.; Raitanen, J.; Jääskeläinen, J.; Auvinen, A. Incidence of gliomas by anatomic location. Neuro-Oncology 2007, 9, 319–325. [Google Scholar] [CrossRef]
- Lee, C.-H.; Jung, K.-W.; Yoo, H.; Park, S.; Lee, S.H. Epidemiology of Primary Brain and Central Nervous System Tumors in Korea. J. Korean Neurosurg. Soc. 2010, 48, 145–152. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro-Oncology 2020, 22 (Suppl. S2), iv1–iv96. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.V.; Davis, F.G.; Shaw, A.; Louchini, R.; Shack, L.; Woods, R.; Kruchko, C.; Spinelli, J.; Guiot, M.-C.; Perry, J.; et al. Malignant primary brain and other central nervous system tumors diagnosed in Canada from 2009 to 2013. Neuro-Oncology 2019, 21, 360–369. [Google Scholar] [CrossRef]
- Fabbro-Peray, P.; Zouaoui, S.; Darlix, A.; Fabbro, M.; Pallud, J.; Rigau, V.; Mathieu-Daude, H.; Bessaoud, F.; Bauchet, F.; Riondel, A.; et al. Association of patterns of care, prognostic factors, and use of radiotherapy–temozolomide therapy with survival in patients with newly diagnosed glioblastoma: A French national population-based study. J. Neuro-Oncol. 2018, 142, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Mountz, J.; Ahmed, R.; Oborski, M.; Lieberman, F.; Hwang, M. Malignant gliomas: Current perspectives in diagnosis, treatment, and early response assessment using advanced quantitative imaging methods. Cancer Manag. Res. 2014, 6, 149–170. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, T.S.; Bishof, A.M.; Brown, P.D.; Klein, M.; Taphoorn, M.J.; Theodore-Oklota, C. Determining priority signs and symptoms for use as clinical outcomes assessments in trials including patients with malignant gliomas: Panel 1 Report. Neuro-Oncology 2016, 18, ii1–ii12. [Google Scholar] [CrossRef]
- Campen, C.J.; Porter, B.E. Subependymal Giant Cell Astrocytoma (SEGA) Treatment Update. Curr. Treat. Options Neurol. 2011, 13, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Vecchione-Koval, T.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro-Oncology 2017, 19, v1–v88. [Google Scholar] [CrossRef] [PubMed]
- Simińska, D.; Korbecki, J.; Kojder, K.; Kapczuk, P.; Fabiańska, M.; Gutowska, I.; Machoy-Mokrzyńska, A.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Anthropometric Factors in Glioblastoma Multiforme—Literature Review. Brain Sci. 2021, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Dubrow, R.; Darefsky, A.S. Demographic variation in incidence of adult glioma by subtype, United States, 1992-2007. BMC Cancer 2011, 11, 325. [Google Scholar] [CrossRef] [PubMed]
- Gigineishvili, D.; Shengelia, N.; Shalashvili, G.; Rohrmann, S.; Tsiskaridze, A.; Shakarishvili, R. Primary brain tumour epidemiology in Georgia: First-year results of a population-based study. J. Neuro-Oncol. 2013, 112, 241–246. [Google Scholar] [CrossRef]
- Huang, K.; Whelan, E.A.; Ruder, A.M.; Ward, E.M.; Deddens, J.A.; Davis-King, K.E.; Carreoón, T.; Waters, M.A.; Butler, M.A.; Calvert, G.M.; et al. Reproductive Factors and Risk of Glioma in Women. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1583–1588. [Google Scholar] [CrossRef]
- Kabat, G.C.; Park, Y.; Hollenbeck, A.R.; Schatzkin, A.; Rohan, T.E. Reproductive factors and exogenous hormone use and risk of adult glioma in women in the NIH-AARP Diet and Health Study. Int. J. Cancer 2010, 128, 944–950. [Google Scholar] [CrossRef]
- Kabat, G.C.; Etgen, A.M.; Rohan, T.E. Do Steroid Hormones Play a Role in the Etiology of Glioma? Cancer Epidemiol. Biomark. Prev. 2010, 19, 2421–2427. [Google Scholar] [CrossRef]
- Michaud, D.S.; Gallo, V.; Schlehofer, B.; Tjønneland, A.; Olsen, A.; Overvad, K.; Dahm, C.C.; Kaaks, R.; Lukanova, A.; Boeing, H.; et al. Reproductive Factors and Exogenous Hormone Use in Relation to Risk of Glioma and Meningioma in a Large European Cohort Study. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2562–2569. [Google Scholar] [CrossRef] [PubMed]
- Carrano, A.; Juarez, J.; Incontri, D.; Ibarra, A.; Cazares, H.G. Sex-Specific Differences in Glioblastoma. Cells 2021, 10, 1783. [Google Scholar] [CrossRef]
- Tan, M.H.E.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E.-L. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef]
- Chang, C.; Saltzman, A.; Yeh, S.; Young, W.; Keller, E.; Lee, H.-J.; Wang, C.; Mizokami, A. Androgen Receptor: An Overview. Crit. Rev. Eukaryot. Gene Expr. 1995, 5, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, P.; Butti, G.; Zibera, C.; Scerrati, M.; Gibelli, N.; Roselli, R.; Magrassi, L.; Sica, G.; Rossi, G.; della Cuna, G.R. Characteristics and biological role of steroid hormone receptors in neuroepithelial tumors. J. Neurosurg. 1990, 73, 736–742. [Google Scholar] [CrossRef]
- Magrassi, L.; Butti, G.; Silini, E.M.; Bono, F.; Paoletti, P.; Milanesi, G. The expression of genes of the steroid-thyroid hormone receptor superfamily in central nervous system tumors. Anticancer Res. 1993, 13, 859–866. [Google Scholar] [PubMed]
- Zalcman, N.; Canello, T.; Ovadia, H.; Charbit, H.; Zelikovitch, B.; Mordechai, A.; Fellig, Y.; Rabani, S.; Shahar, T.; Lossos, A.; et al. Androgen receptor: A potential therapeutic target for glioblastoma. Oncotarget 2018, 9, 19980–19993. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Dunn, T.A.; Wei, S.; Isharwal, S.; Veltri, R.W.; Humphreys, E.; Han, M.; Partin, A.W.; Vessella, R.L.; Isaacs, W.B.; et al. Ligand-Independent Androgen Receptor Variants Derived from Splicing of Cryptic Exons Signify Hormone-Refractory Prostate Cancer. Cancer Res. 2008, 69, 16–22. [Google Scholar] [CrossRef]
- Li, W.; Graeber, M.B. The molecular profile of microglia under the influence of glioma. Neuro-Oncology 2012, 14, 958–978. [Google Scholar] [CrossRef]
- Kiraga, Ł.; Cheda, Ł.; Taciak, B.; Różańska, K.; Tonecka, K.; Szulc, A.; Kilian, K.; Górka, E.; Rogulski, Z.; Rygiel, T.P.; et al. Changes in hypoxia level of CT26 tumors during various stages of development and comparing different methods of hypoxia determination. PLoS ONE 2018, 13, e0206706. [Google Scholar] [CrossRef]
- Korbecki, J.; Simińska, D.; Gąssowska-Dobrowolska, M.; Listos, J.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Chronic and Cycling Hypoxia: Drivers of Cancer Chronic Inflammation through HIF-1 and NF-κB Activation: A Review of the Molecular Mechanisms. Int. J. Mol. Sci. 2021, 22, 10701. [Google Scholar] [CrossRef]
- Werner, C.K.; Nna, U.J.; Sun, H.; Wilder-Romans, K.; Dresser, J.; Kothari, A.U.; Zhou, W.; Yao, Y.; Rao, A.; Stallard, S.; et al. Expression of the Androgen Receptor Governs Radiation Resistance in a Subset of Glioblastomas Vulnerable to Antiandrogen Therapy. Mol. Cancer Ther. 2020, 19, 2163–2174. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, Y.; Wei, W.; Cong, P.; Ding, Y.; Xiang, L.; Wu, K. Androgen receptor signaling regulates growth of glioblastoma multiforme in men. Tumor Biol. 2014, 36, 967–972. [Google Scholar] [CrossRef]
- Bao, D.; Cheng, C.; Lan, X.; Xing, R.; Chen, Z.; Zhao, H.; Sun, J.; Wang, Y.; Niu, C.; Zhang, B.; et al. Regulation of p53wt glioma cell proliferation by androgen receptor-mediated inhibition of small VCP/p97-interacting protein expression. Oncotarget 2017, 8, 23142–23154. [Google Scholar] [CrossRef]
- Rodríguez-Lozano, D.C.; Velázquez-Vázquez, D.E.; Del Moral-Morales, A.; Camacho-Arroyo, I. Dihydrotestosterone Induces Proliferation, Migration, and Invasion of Human Glioblastoma Cell Lines. OncoTargets Ther. 2020, 13, 8813–8823. [Google Scholar] [CrossRef]
- Orevi, M.; Shamni, O.; Zalcman, N.; Chicheportiche, A.; Mordechai, A.; Moscovici, S.; Shoshan, Y.; Shahar, T.; Charbit, H.; Gutreiman, M.; et al. [18F]-FDHT PET/CT as a tool for imaging androgen receptor expression in high-grade glioma. Neuro-Oncol. Adv. 2021, 3, vdab019. [Google Scholar] [CrossRef]
- Pinacho-Garcia, L.M.; Valdez, R.A.; Navarrete, A.; Cabeza, M.; Segovia, J.; Romano, M.C. The effect of finasteride and dutasteride on the synthesis of neurosteroids by glioblastoma cells. Steroids 2019, 155, 108556. [Google Scholar] [CrossRef] [PubMed]
- Orozco, M.; Valdez, R.; Ramos, L.; Cabeza, M.; Segovia, J.; Romano, M. Dutasteride combined with androgen receptor antagonists inhibit glioblastoma U87 cell metabolism, proliferation, and invasion capacity: Androgen regulation. Steroids 2020, 164, 108733. [Google Scholar] [CrossRef]
- Chuang, J.-Y.; Lo, W.-L.; Ko, C.-Y.; Chou, S.-Y.; Chen, R.-M.; Chang, K.-Y.; Hung, J.-J.; Su, W.-C.; Chang, W.-C.; Hsu, T.-I. Upregulation of CYP17A1 by Sp1-mediated DNA demethylation confers temozolomide resistance through DHEA-mediated protection in glioma. Oncogenesis 2017, 6, e339. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Ko, C.-Y.; Kao, T.-J.; Yang, W.-B.; Tsai, Y.-T.; Chuang, J.-Y.; Hu, S.-L.; Lo, W.-L.; Hsu, T.-I. CYP17A1 Maintains the Survival of Glioblastomas by Regulating SAR1-Mediated Endoplasmic Reticulum Health and Redox Homeostasis. Cancers 2019, 11, 1378. [Google Scholar] [CrossRef]
- Sharpe, M.A.; Baskin, D.S.; Jenson, A.V.; Baskin, A.M. Hijacking Sexual Immuno-Privilege in GBM—An Immuno-Evasion Strategy. Int. J. Mol. Sci. 2021, 22, 10983. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.; Benavides, G.A.; Geng, J.; Koralov, S.; Hu, H.; Darley-Usmar, V.M.; Harrington, L.E. Mitochondrial Oxidative Phosphorylation Regulates the Fate Decision between Pathogenic Th17 and Regulatory T Cells. Cell Rep. 2020, 30, 1898–1909.e4. [Google Scholar] [CrossRef]
- Hou, J.; Liu, Y.; Huang, P.; Wang, Y.; Pei, D.; Tan, R.; Zhang, Y.; Cui, H. RANBP10 promotes glioblastoma progression by regulating the FBXW7/c-Myc pathway. Cell Death Dis. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Muñoz-Sánchez, J.; Chánez-Cárdenas, M.E. The use of cobalt chloride as a chemical hypoxia model. J. Appl. Toxicol. 2018, 39, 556–570. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Hilliard, G.; Ferguson, T.; Millhorn, D.E. Cobalt Inhibits the Interaction between Hypoxia-inducible Factor-α and von Hippel-Lindau Protein by Direct Binding to Hypoxia-inducible Factor-α. J. Biol. Chem. 2003, 278, 15911–15916. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.-L.; Zou, Z.-H.; Tao, T.; Li, J.; Xu, J.; Luo, K.-J.; Liu, Z. Effect of the Recombinant Adenovirus-Mediated HIF-1 Alpha on the Expression of VEGF in the Hypoxic Brain Microvascular Endothelial Cells of Rats. Neuropsychiatr. Dis. Treat. 2020, 16, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Cascio, S.; D’Andrea, A.; Ferla, R.; Surmacz, E.; Gulotta, E.; Amodeo, V.; Bazan, V.; Gebbia, N.; Russo, A. miR-20b modulates VEGF expression by targeting HIF-1α and STAT3 in MCF-7 breast cancer cells. J. Cell. Physiol. 2010, 224, 242–249. [Google Scholar] [CrossRef]
- Nakamura, M.; Yamabe, H.; Osawa, H.; Nakamura, N.; Shimada, M.; Kumasaka, R.; Murakami, R.; Fujita, T.; Osanai, T.; Okumura, K. Hypoxic conditions stimulate the production of angiogenin and vascular endothelial growth factor by human renal proximal tubular epithelial cells in culture. Nephrol. Dial. Transplant. 2006, 21, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Xue, F.; Lan, H.; Li, Z.; Xiao, S.; Yi, T. Multicolor imaging of hydrogen peroxide level in living and apoptotic cells by a single fluorescent probe. Biosens. Bioelectron. 2017, 91, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Parrish, M.; Tay, I.J.J.; Li, N.; Ackerman, S.; He, F.; Kwang, J.; Chow, V.T.; Engelward, B.P. Streptococcus pneumoniae secretes hydrogen peroxide leading to DNA damage and apoptosis in lung cells. Proc. Natl. Acad. Sci. USA 2015, 112, E3421-30. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Yu, Y.; He, S.; Cheng, J.; Gong, Y.; Zhang, Z.; Yang, X.; Xu, B.; Liu, X.; Li, C.-Y.; et al. Dying glioma cells establish a proangiogenic microenvironment through a caspase 3 dependent mechanism. Cancer Lett. 2017, 385, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, H.K. Current Understanding of Hypoxia in Glioblastoma Multiforme and Its Response to Immunotherapy. Cancers 2022, 14, 1176. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Michalek, J.E.; Reardon, D.A.; Wen, P.Y.; Floyd, J.R.; Fox, P.T.; Clarke, G.D.; Jerabek, P.A.; Schmainda, K.M.; Muzi, M.; et al. Assessment of tumor hypoxia and perfusion in recurrent glioblastoma following bevacizumab failure using MRI and 18F-FMISO PET. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, L.; Chen, J.; Sun, Y.; Lin, H.; Jin, R.-A.; Tang, M.; Liang, X.; Cai, X. Increasing AR by HIF-2α inhibitor (PT-2385) overcomes the side-effects of sorafenib by suppressing hepatocellular carcinoma invasion via alteration of pSTAT3, pAKT and pERK signals. Cell Death Dis. 2017, 8, e3095. [Google Scholar] [CrossRef]
- Uo, T.; Sprenger, C.C.; Plymate, S.R. Androgen Receptor Signaling and Metabolic and Cellular Plasticity During Progression to Castration Resistant Prostate Cancer. Front. Oncol. 2020, 10, 580617. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Wang, T.; Zhu, H.; Zhang, P.; Han, R.; Liu, Y.; Ni, P.; Shen, H.; Xu, W.; Xu, H. HMGB1 modulates Lewis cell autophagy and promotes cell survival via RAGE-HMGB1-Erk1/2 positive feedback during nutrient depletion. Immunobiology 2015, 220, 539–544. [Google Scholar] [CrossRef]
- Boonyaratanakornkit, V.; Melvin, V.; Prendergast, P.; Altmann, M.; Ronfani, L.; Bianchi, M.E.; Taraseviciene, L.; Nordeen, S.K.; Allegretto, E.A.; Edwards, D.P. High-Mobility Group Chromatin Proteins 1 and 2 Functionally Interact with Steroid Hormone Receptors to Enhance Their DNA Binding In Vitro and Transcriptional Activity in Mammalian Cells. Mol. Cell. Biol. 1998, 18, 4471–4487. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Korbecki, J.; Kojder, K.; Jeżewski, D.; Simińska, D.; Tarnowski, M.; Kopytko, P.; Safranow, K.; Gutowska, I.; Goschorska, M.; Kolasa-Wołosiuk, A.; et al. Expression of SCD and FADS2 Is Lower in the Necrotic Core and Growing Tumor Area than in the Peritumoral Area of Glioblastoma Multiforme. Biomolecules 2020, 10, 727. [Google Scholar] [CrossRef]
- Baid, U.; Talbar, S.; Rane, S.; Gupta, S.; Thakur, M.H.; Moiyadi, A.; Sable, N.; Akolkar, M.; Mahajan, A. A Novel Approach for Fully Automatic Intra-Tumor Segmentation With 3D U-Net Architecture for Gliomas. Front. Comput. Neurosci. 2020, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Tustison, N.J.; Patel, S.H.; Meyer, C.H. Brain Tumor Segmentation Using an Ensemble of 3D U-Nets and Overall Survival Prediction Using Radiomic Features. Front. Comput. Neurosci. 2020, 14, 25. [Google Scholar] [CrossRef]
- Lemée, J.-M.; Com, E.; Clavreul, A.; Avril, T.; Quillien, V.; de Tayrac, M.; Pineau, C.; Menei, P. Proteomic analysis of glioblastomas: What is the best brain control sample? J. Proteom. 2013, 85, 165–173. [Google Scholar] [CrossRef][Green Version]
- Brat, D.J.; Castellano-Sanchez, A.A.; Hunter, S.B.; Pecot, M.; Cohen, C.; Hammond, E.H.; Devi, S.N.; Kaur, B.; Van Meir, E.G. Pseudopalisades in Glioblastoma Are Hypoxic, Express Extracellular Matrix Proteases, and Are Formed by an Actively Migrating Cell Population. Cancer Res. 2004, 64, 920–927. [Google Scholar] [CrossRef]
- Grube, S.; Göttig, T.; Freitag, D.; Ewald, C.; Kalff, R.; Walter, J. Selection of suitable reference genes for expression analysis in human glioma using RT-qPCR. J. Neuro-Oncol. 2015, 123, 35–42. [Google Scholar] [CrossRef]
- Kreth, S.; Heyn, J.; Grau, S.; Kretzschmar, H.A.; Egensperger, R.; Kreth, F.W. Identification of valid endogenous control genes for determining gene expression in human glioma. Neurooncology 2010, 12, 570–579. [Google Scholar] [CrossRef]





| Gender | Women | Men | No Data Available | ||
| 10 | 14 | - | |||
| Type of work | Physical | Mental | No Data Available | ||
| 13 | 7 | 4 | |||
| Smoking | Yes | No | No Data Available | ||
| 17 | 4 | 3 | |||
| Place of residence | Village | City <10 thousand | City 10–100 thousand | City >100,000 | No Data Available |
| 3 | 4 | 4 | 9 | 4 | |
| Statistical parameters | Average | Minimum | Maximum | Standard Deviation | |
| Age [years] | 61.78 | 41 | 81 | 11.65 | |
| Height [cm] | 171.87 | 147 | 196 | 11.50 | |
| BMI | 28.99 | 21.48 | 38.87 | 4.66 | |
| Standard Medium | Test Medium | ||
|---|---|---|---|
| Necrotic Conditions | Hypoxic Conditions | Nutrient-Deficient Conditions | |
| EMEM (Sigma-Aldrich, Poznań, Poland) | |||
| 10% FBS (inactivated fetal bovine serum) (Gibco Limited, Brigg, UK) | |||
| 100 U/mL penicillin (Gibco Limited) and 100 µg/mL streptomycin (Gibco Limited, Brigg, UK) | |||
| 1% non-essential amino acid (Sigma-Aldrich, Poznań, Poland) | |||
| 2 mM L-glutamine (Sigma-Aldrich, Poznań, Poland) | 0.2 mM L-glutamine (Sigma-Aldrich, Poznań, Poland) | ||
| 1 mM sodium pyruvate (Sigma-Aldrich, Poznań, Poland) | Without sodium pyruvate | ||
| - | 200 µM hydrogen peroxide (Sigma-Aldrich, Poznań, Poland) | 100 µM cobalt chloride (Sigma-Aldrich, Poznań, Poland) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simińska, D.; Korbecki, J.; Kojder, K.; Jeżewski, D.; Tarnowski, M.; Tomasiak, P.; Piotrowska, K.; Masztalewicz, M.; Kolasa, A.; Chlubek, D.; et al. Androgen Receptor Expression in the Various Regions of Resected Glioblastoma Multiforme Tumors and in an In Vitro Model. Int. J. Mol. Sci. 2022, 23, 13004. https://doi.org/10.3390/ijms232113004
Simińska D, Korbecki J, Kojder K, Jeżewski D, Tarnowski M, Tomasiak P, Piotrowska K, Masztalewicz M, Kolasa A, Chlubek D, et al. Androgen Receptor Expression in the Various Regions of Resected Glioblastoma Multiforme Tumors and in an In Vitro Model. International Journal of Molecular Sciences. 2022; 23(21):13004. https://doi.org/10.3390/ijms232113004
Chicago/Turabian StyleSimińska, Donata, Jan Korbecki, Klaudyna Kojder, Dariusz Jeżewski, Maciej Tarnowski, Patrycja Tomasiak, Katarzyna Piotrowska, Marta Masztalewicz, Agnieszka Kolasa, Dariusz Chlubek, and et al. 2022. "Androgen Receptor Expression in the Various Regions of Resected Glioblastoma Multiforme Tumors and in an In Vitro Model" International Journal of Molecular Sciences 23, no. 21: 13004. https://doi.org/10.3390/ijms232113004
APA StyleSimińska, D., Korbecki, J., Kojder, K., Jeżewski, D., Tarnowski, M., Tomasiak, P., Piotrowska, K., Masztalewicz, M., Kolasa, A., Chlubek, D., & Baranowska-Bosiacka, I. (2022). Androgen Receptor Expression in the Various Regions of Resected Glioblastoma Multiforme Tumors and in an In Vitro Model. International Journal of Molecular Sciences, 23(21), 13004. https://doi.org/10.3390/ijms232113004







