Study on Protein Structures of Eight Mung Bean Varieties and Freeze-Thaw Stability of Protein-Stabilized Emulsions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MPIs
2.3. Determination of Albumin and Globulin Content
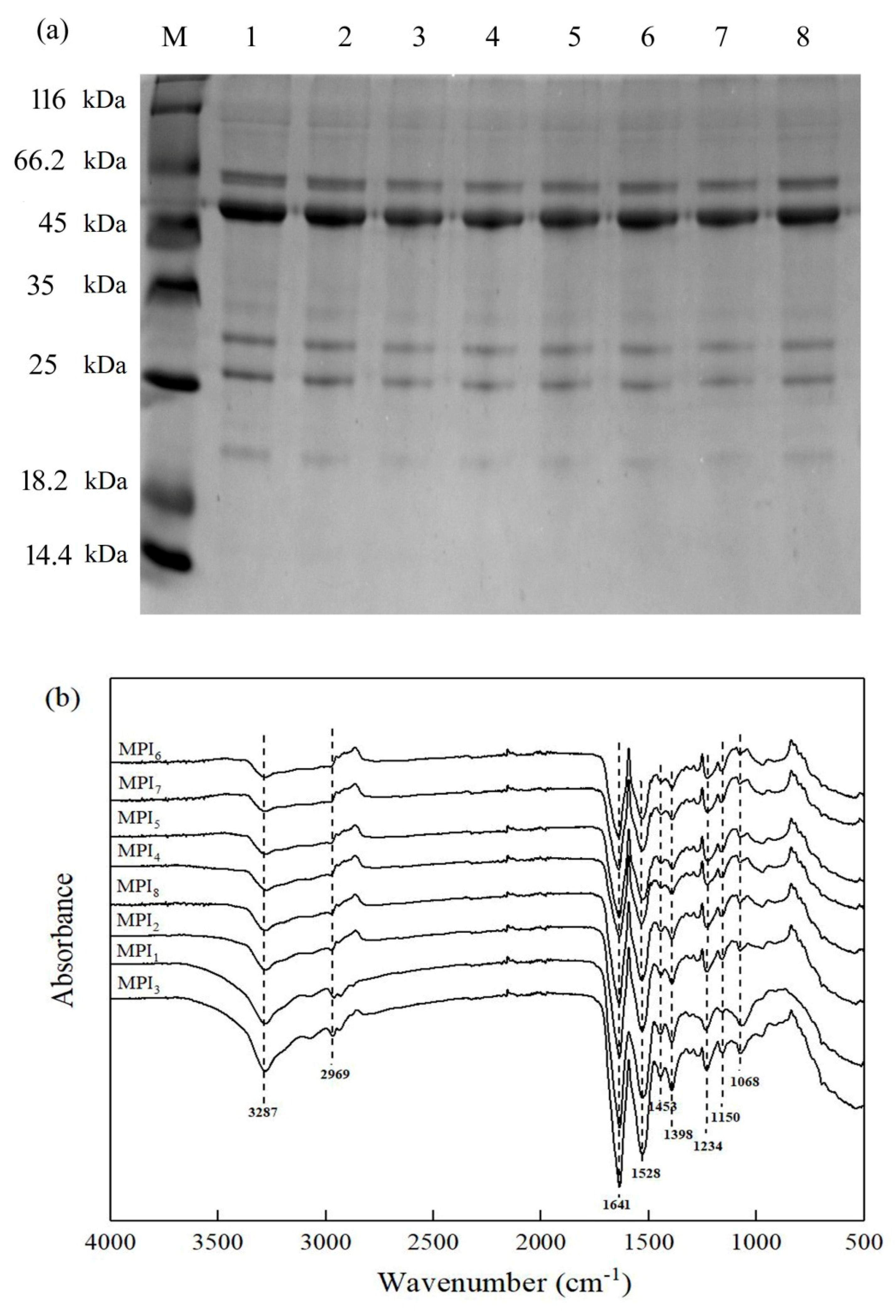
2.4. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.5. Circular Dichroism (CD)
2.6. SDS-PAGE
2.7. Emulsification Determination
2.8. Flexibility Measurement
2.9. Emulsion Preparation
2.10. Freeze-Thaw Cycle
2.11. Creaming Index (CI) Determination
2.12. Oiling-Off Determination
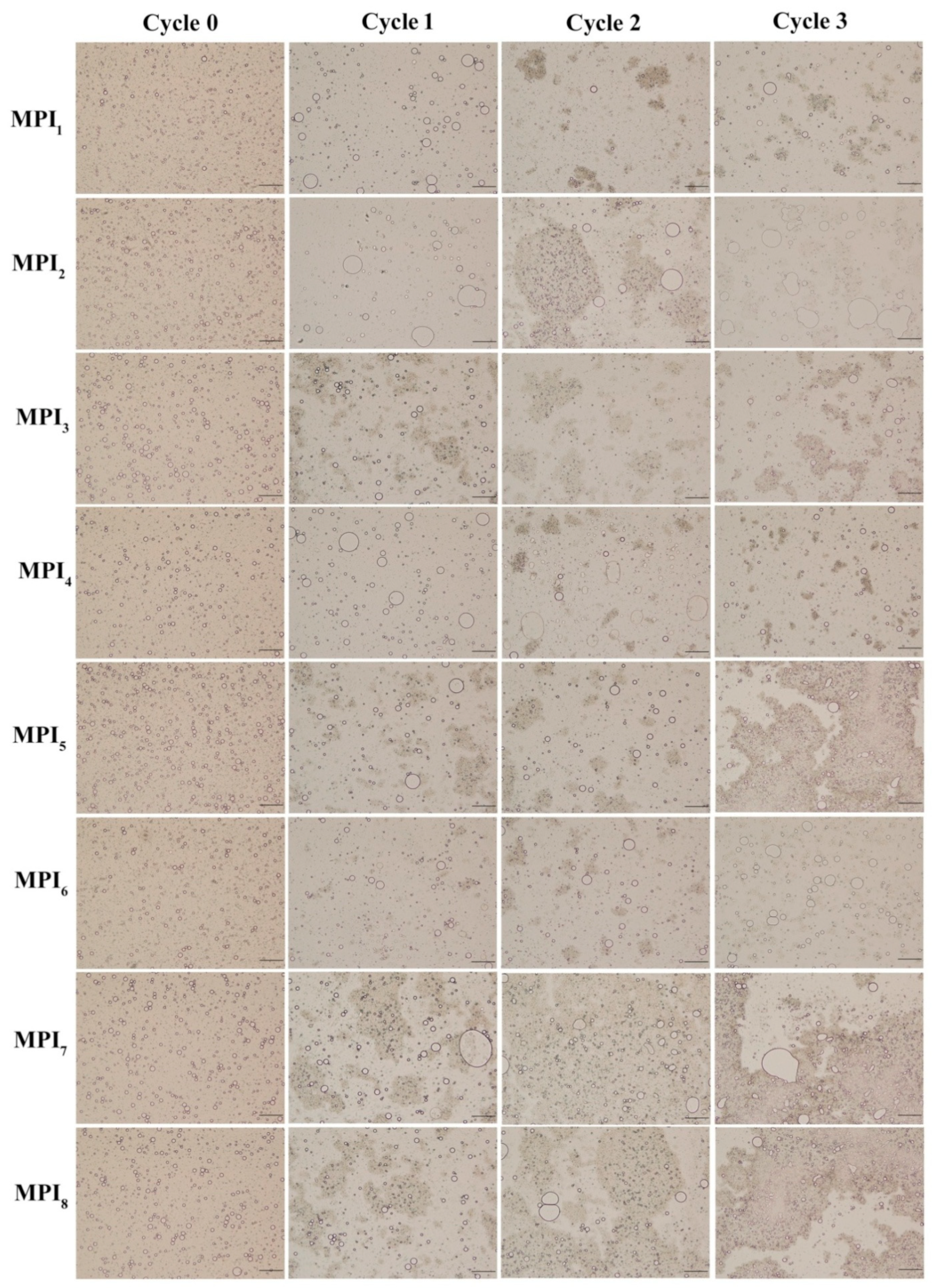
2.13. Optical Microscopy
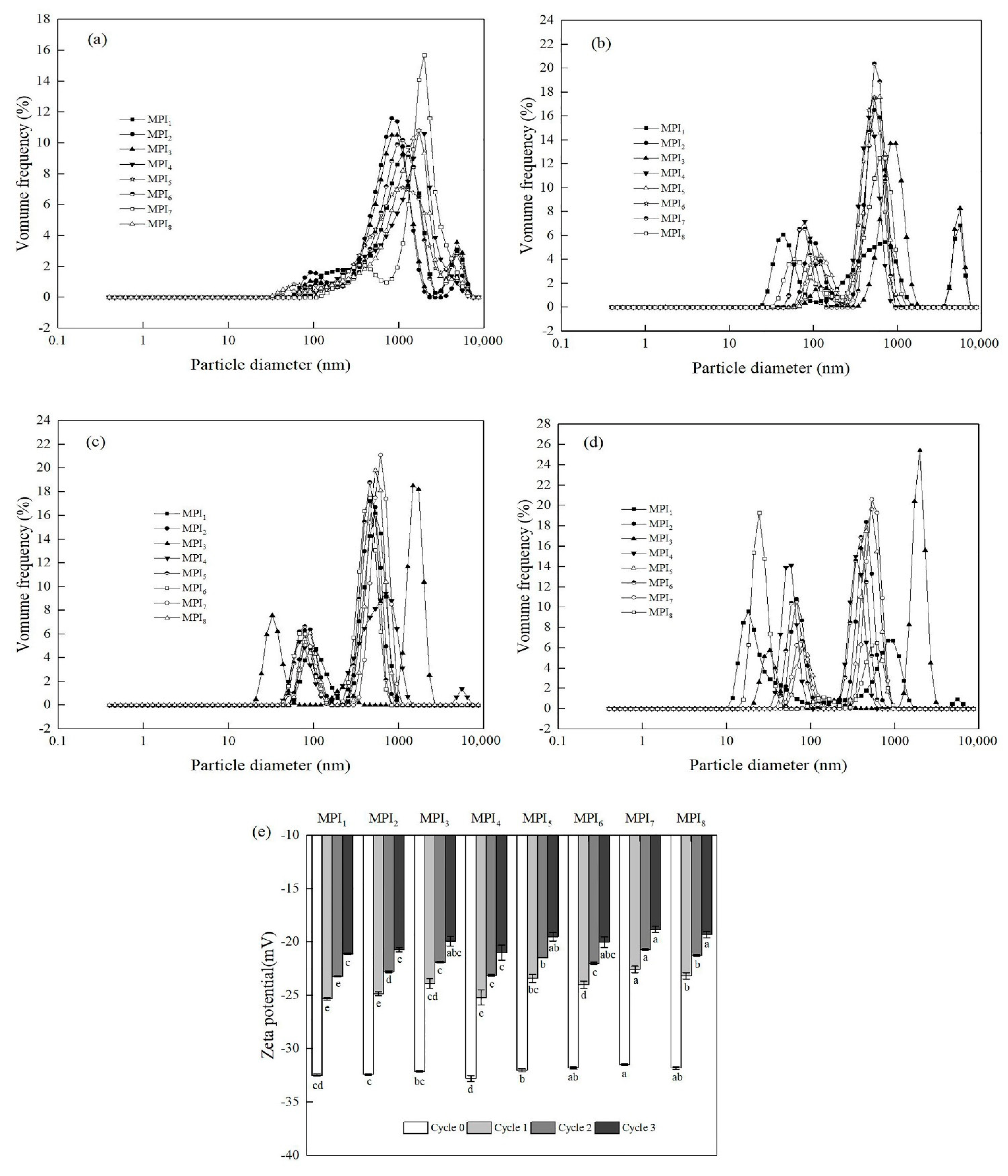
2.14. Determination of Emulsion Particle Size and Zeta Potential
2.15. Data Analysis
3. Results and Discussion
3.1. Analysis of Structural and Emulsifying Properties of Mung Bean Proteins of Eight Mung Bean Varieties
3.1.1. Protein Subunit and Composition Analysis
3.1.2. Secondary Structures of Proteins from Eight Mung Bean Varieties
3.1.3. Emulsification Characteristics of Proteins from Eight Mung Bean Varieties
3.2. Freeze-Thaw Stability of MPI Emulsions
3.2.1. Effects of Freeze-Thaw Cycles on Particle Size and Zeta Potential of MPI Emulsions
3.2.2. Effects of Freeze-Thaw Cycle Number on Creaming Index and Oiling-Off of MPI Emulsions
3.2.3. Effect of Freeze-Thaw Cycles on MPI Emulsion Microstructure
3.3. Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wang, Y.Y.; Zhang, A.Q.; Wang, X.B.; Xu, N.; Jiang, L.Z. The radiation assisted-Maillard reaction comprehensively improves the freeze-thaw stability of soy protein-stabilized oil-in-water emulsions. Food Hydrocoll. 2020, 103, 105684. [Google Scholar] [CrossRef]
- Palazolo, G.G.; Sobral, P.A.; Wagner, J.R. Freeze-thaw stability of oil-in-water emulsions prepared with native and thermally-denatured soybean isolates. Food Hydrocoll. 2011, 25, 398–409. [Google Scholar] [CrossRef]
- Gong, K.J.; Shi, A.M.; Liu, H.Z.; Liu, L.; Hu, H.; Adhikari, B.; Wang, Q. Emulsifying properties and structure changes of spray and freeze-dried peanut protein isolate. J. Food Eng. 2016, 170, 33–40. [Google Scholar] [CrossRef]
- Degner, B.M.; Chung, C.; Schlegel, V.; Hutkins, R.; McClements, D.J. Factors Influencing the Freeze-Thaw Stability of Emulsion-Based Foods. Compr. Rev. Food Sci. Food Saf. 2014, 13, 98–113. [Google Scholar] [CrossRef]
- Zang, X.D.; Liu, P.; Chen, Y.; Wang, J.W.; Yu, G.P.; Xu, H.H. Improved freeze-thaw stability of o/w emulsions prepared with soybean protein isolate modified by papain and transglutaminase. LWT Food Sci. Technol. 2019, 104, 195–201. [Google Scholar] [CrossRef]
- Ogawa, A.; Samoto, M.; Takahashi, K. Soybean allergens and hypoallergenic soybean products. J. Nutr. Sci. Vitaminol. 2000, 46, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Brishti, F.H.; Chay, S.Y.; Muhammad, K.; Ismail-Fitry, M.R.; Zarei, M.; Karthikeyan, S.; Saari, N. Effects of drying techniques on the physicochemical, functional, thermal, structural and rheological properties of mung bean (Vigna radiata) protein isolate powder. Food Res. Int. 2020, 138, 109783. [Google Scholar] [CrossRef] [PubMed]
- Kohno, M.; Sugano, H.; Shigihara, Y.; Shiraishi, Y.; Motoyama, T. Improvement of glucose and lipid metabolism via mung bean protein consumption: Clinical trials of GLUCODIA™ isolated mung bean protein in the USA and Canada. J. Nutr. Sci. 2018, 7, e2. [Google Scholar] [CrossRef] [Green Version]
- Liyanage, R.; Kiramage, C.; Visvanathan, R.; Jayathilake, C.; Weththasinghe, P.; Bangamuwage, R.; Jayawardana, B.C.; Vidanarachchi, J. Hypolipidemic and hypoglycemic potential of raw, boiled, and sprouted mung beans (Vigna radiata L. Wilczek) in rats. J. Food Biochem. 2018, 42, e12457. [Google Scholar] [CrossRef]
- Watanabe, H.; Inaba, Y.; Kimura, K.; Asahara, S.I.; Kido, Y.; Matsumoto, M.; Motoyama, T.; Tachibana, N.; Kaneko, S.; Kohno, M.; et al. Dietary Mung Bean Protein Reduces Hepatic Steatosis, Fibrosis, and Inflammation in Male Mice with Diet-Induced, Nonalcoholic Fatty Liver Disease. J. Nutr. 2017, 147, 52–60. [Google Scholar] [CrossRef]
- Campbell, K.A.; Glatz, C.E.; Johnson, L.A.; Jung, S.; Moura, J.; Kapchie, V.; Murphy, P. Advances in Aqueous Extraction Processing of Soybeans. J. Am. Oil Chem. Soc. 2011, 88, 449–465. [Google Scholar] [CrossRef]
- Keerati-u-rai, M.; Miriani, M.; Iametti, S.; Bonomi, F.; Corredig, M. Structural changes of soy proteins at the oil-water interface studied by fluorescence spectroscopy. Colloids Surf. B 2012, 93, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, S. Protein Stabilization of Emulsions and Foams. J. Food Sci. 2005, 70, R54–R66. [Google Scholar] [CrossRef]
- Du, M.X.; Xie, J.H.; Gong, B.; Xu, X.; Tang, W.; Li, X.; Li, C.; Xie, M. Extraction, physicochemical characteristics and functional properties of Mung bean protein. Food Hydrocoll. 2017, 76, 131–140. [Google Scholar] [CrossRef]
- Feng, H.Y.; Jin, H.; Gao, Y.; Yan, S.Q.; Zhang, Y.; Zhao, Q.S.; Xu, J. Effects of freeze-thaw cycles on the structure and emulsifying properties of peanut protein isolates. Food Chem. 2020, 330, 127215. [Google Scholar] [CrossRef]
- Kato, A.; Ibrahim, H.R.; Watanabe, H.; Honma, K.; Kobayashi, K. Structural and gelling properties of dry-heated egg white proteins. J. Agric. Food Chem. 1990, 38, 32–37. [Google Scholar] [CrossRef]
- Maghamian, N.; Goli, M.; Najarian, A. Ultrasound-assisted preparation of double nano-emulsions loaded with glycyrrhizic acid in the internal aqueous phase and skim milk as the external aqueous phase. LWT Food Sci. Technol. 2021, 141, 110850. [Google Scholar] [CrossRef]
- Koop, J.; Merz, J.; Pietzsch, C.; Schembecker, G. Contribution of Secondary Structure Changes to the Surface Activity of Proteins. J. Biotechnol. 2020, 323, 208–220. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, L.Z.; Li, Y.; Liu, Q.; Wang, S.N.; Sui, X.N. Optimization of Extraction Process of Protein Isolate from Mung Bean. Procedia Eng. 2011, 15, 5250–5258. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.N.; Tang, C.H. Emulsifying and interfacial properties of vicilins: Role of conformational flexibility at quaternary and/or tertiary levels. J. Agric. Food Chem. 2013, 61, 11140–11150. [Google Scholar] [CrossRef]
- Cabezas, D.M.; Pascual, G.N.; Wagner, J.R.; Palazolo, G.G. Nanoparticles assembled from mixtures of whey protein isolate and soluble soybean polysaccharides. Structure, interfacial behavior and application on emulsions subjected to freeze-thawing. Food Hydrocoll. 2019, 95, 445–453. [Google Scholar] [CrossRef]
- Mendoza, E.M.; Adachi, M.; Bernardo, A.E.; Utsumi, S. Mungbean [Vigna radiata (L.) Wilczek] globulins: Purification and characterization. J. Agric. Food Chem. 2001, 49, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Brishti, F.H.; Yea, C.S.; Muhammad, K.; Ismail-Fitry, M.; Zarei, M.; Karthikeyan, S.; Caballero-Briones, F.; Saari, N. Structural and rheological changes of texturized mung bean protein induced by feed moisture during extrusion. Food Chem. 2021, 344, 128643. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.R.; Ding, W.H.; Qu, W.J.; Oladejo, A.O.; Xiong, F.; Zhang, W.W.; He, R.H.; Ma, H.L. Alkali solution extraction of rice residue protein isolates: Influence of alkali concentration on protein functional, structural properties and lysinoalanine formation. Food Chem. 2017, 218, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.X.; Hayakawa, S.; Izumori, K. Modification of ovalbumin with a rare ketohexose through the Maillard reaction: Effect on protein structure and gel properties. J. Agric. Food Chem. 2004, 52, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Sun-Waterhouse, D.X.; Cui, C.; Wang, W.; Dong, K.M. Modification of soy protein isolate by glutaminase for nanocomplexation with curcumin. Food Chem. 2018, 268, 504–512. [Google Scholar] [CrossRef]
- Baier, D.; Purschke, B.; Schmitt, C.; Rawel, H.M.; Knorr, D. Effect of high pressure--low temperature treatments on structural characteristics of whey proteins and micellar caseins. Food Chem. 2015, 187, 354–363. [Google Scholar] [CrossRef]
- Jamdar, S.N.; Rajalakshmi, V.; Pednekar, M.D.; Juan, F.; Yardi, V.; Sharma, A. Influence of degree of hydrolysis on functional properties, antioxidant activity and ACE inhibitory activity of peanut protein hydrolysate. Food Chem. 2010, 121, 178–184. [Google Scholar] [CrossRef]
- Tang, C.H. Globular proteins as soft particles for stabilizing emulsions: Concepts and strategies. Food Hydrocoll. 2020, 103, 105664. [Google Scholar] [CrossRef]
- Tang, C.H.; Sun, X. A comparative study of physicochemical and conformational properties in three vicilins from Phaseolus legumes: Implications for the structure–function relationship. Food Hydrocoll. 2011, 25, 315–324. [Google Scholar] [CrossRef]
- Xu, D.X.; Zhang, J.J.; Cao, Y.P.; Wang, J.; Xiao, J. Influence of microcrystalline cellulose on the microrheological property and freeze-thaw stability of soybean protein hydrolysate stabilized curcumin emulsion. LWT Food Sci. Technol. 2016, 66, 590–597. [Google Scholar] [CrossRef]
- Zang, X.D.; Yue, C.H.; Liu, M.J.; Zheng, H.Y.; Xia, X.F.; Yu, G.P. Improvement of freeze-thaw stability of oil-in-water emulsions prepared with modified soy protein isolates. LWT Food Sci. Technol. 2018, 102, 122–130. [Google Scholar] [CrossRef]
- Noshad, M.; Mohebbi, M.; Shahidi, F.; Koocheki, A. Freeze—Thaw stability of emulsions with soy protein isolate through interfacial engineering. Int. J. Refrig. 2015, 58, 253–260. [Google Scholar] [CrossRef]
- Noh, E.J.; Park, S.Y.; Pak, J.I.; Hong, S.T.; Yun, S.E. Coagulation of soymilk and quality of tofu as affected by freeze treatment of soybeans. Food Chem. 2005, 91, 715–721. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Sun, F.D.; Li, Y.Y.; Liu, Q.; Kong, B.H. Modification of gel properties of soy protein isolate by freeze-thaw cycles are associated with changes of molecular force involved in the gelation. Process. Biochem. 2016, 52, 200–208. [Google Scholar] [CrossRef]
- Markiewicz, M.; Mrozik, W.; Rezwan, K.; Thöming, J.; Hupka, J.; Jungnickel, C. Changes in zeta potential of imidazolium ionic liquids modified minerals--Implications for determining mechanism of adsorption. Chemosphere 2013, 90, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.; Cho, Y.; Decker, E.A.; McClements, D.C. Utilization of polysaccharide coatings to improve freeze–thaw and freeze–dry stability of protein-coated lipid droplets. J. Food Eng. 2008, 86, 508–518. [Google Scholar] [CrossRef]
- Yu, J.; Wang, G.R.; Wang, X.B.; Xu, Y.Y.; Chen, S.; Wang, X.D.; Jiang, L.Z. Improving the freeze-thaw stability of soy protein emulsions via combing limited hydrolysis and Maillard-induced glycation. LWT Food Sci. Technol. 2018, 91, 63–69. [Google Scholar] [CrossRef]
- Adjonu, R.; Doran, G.; Torley, P.; Agboola, S. Whey protein peptides as components of nanoemulsions: A review of emulsifying and biological functionalities. J. Food Eng. 2014, 122, 15–27. [Google Scholar] [CrossRef]
- Tang, C.H.; Shen, L. Role of conformational flexibility in the emulsifying properties of bovine serum albumin. J. Agric. Food Chem. 2013, 61, 3097–3110. [Google Scholar] [CrossRef]
- Kato, A.; Komatsu, K.; Fujimoto, K.; Kobayashi, K. Relationship between surface functional properties and flexibility of proteins detected by the protease susceptibility. J. Agric. Food Chem. 1985, 33, 931–934. [Google Scholar] [CrossRef]
- Poon, S.; Clarke, A.E.; Schultz, C.J. Effect of denaturants on the emulsifying activity of proteins. J. Agric. Food Chem. 2001, 49, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.S.; Decker, E.A.; McClements, D.J. Application of multi-component biopolymer layers to improve the freeze-thaw stability of oil-in-water emulsions: β-lactoglobulin-ι-carrageenan-gelatin. J. Food Eng. 2007, 80, 1246–1254. [Google Scholar] [CrossRef]
- Zhu, Y.; McClements, D.J.; Zhou, W.; Peng, S.; Zhou, L.; Zou, L.; Liu, W. Influence of ionic strength and thermal pretreatment on the freeze-thaw stability of Pickering emulsion gels. Food Chem. 2020, 303, 125401. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Decker, E.A.; Mcclements, D.J. Role of Postadsorption Conformation Changes of β-Lactoglobulin on Its Ability To Stabilize Oil Droplets against Flocculation during Heating at Neutral pH. Langmuir 2002, 18, 7577–7583. [Google Scholar] [CrossRef]
| Parameter | Mung Bean Protein Samples | |||||||
|---|---|---|---|---|---|---|---|---|
| MPI1 | MPI2 | MPI3 | MPI4 | MPI5 | MPI6 | MPI7 | MPI8 | |
| Albumin content (mg/g) | 309.2 ± 3.79 a | 270.1 ± 5.00 b | 236.7 ± 4.37 c | 310.3 ± 5.38 a | 261.6 ± 2.40 b | 212.9 ± 9.42 d | 241.3 ± 6.98 c | 188.4 ± 2.03 e |
| Globulin content (mg/g) | 492.7 ± 5.06 a | 315.0 ± 3.33 e | 356.7 ± 1.07 c | 321.5 ± 2.54 d | 483.4 ± 5.60 b | 301.1 ± 0.09 f | 311.5 ± 0.10 e | 350.25 ± 3.47 c |
| 63.1 kDa (%) | 24.87 ± 0.17 b | 24.93 ± 0.03 b | 23.81 ± 0.11 c | 25.20 ± 0.20 b | 23.57 ± 0.47 c | 23.72 ± 0.52 c | 22.55 ± 0.15 d | 26.18 ± 0.18 a |
| 52.9 kDa (%) | 40.07 ± 0.07 d | 42.82 ± 0.32 b | 45.35 ± 0.25 a | 45.02 ± 0.02 a | 42.58 ± 0.18 b | 40.97 ± 0.20 c | 39.22 ± 0.22 e | 36.69 ± 0.19 f |
| 31.4 kDa (%) | 13.59 ± 0.11 d | 13.02 ± 0.02 e | 13.06 ± 0.06 e | 13.18 ± 0.06 e | 13.28 ± 0.03 e | 13.94 ± 0.18 c | 14.53 ± 0.03 b | 15.31 ± 0.21 a |
| 26.9 kDa (%) | 13.61 ± 0.11 c | 14.02 ± 0.02 b | 13.12 ± 0.12 d | 12.89 ± 0.19 d | 13.86 ± 0.16 bc | 14.04 ± 0.04 b | 15.09 ± 0.09 a | 13.91 ± 0.16 bc |
| 21.4 kDa (%) | 7.86 ± 0.06 b | 5.21 ± 0.11 e | 4.67 ± 0.17 f | 3.70 ± 0.20 g | 6.71 ± 0.21 d | 7.34 ± 0.14 c | 8.61 ± 0.11 a | 7.91 ± 0.06 b |
| α-helix (%) | 17.92 ± 0.12 d | 20.10 ± 0.10 b | 19.33 ± 0.23 c | 20.42 ± 0.22 b | 21.72 ± 0.22 a | 17.92 ± 0.20 d | 19.48 ± 0.08 c | 17.76 ± 0.20 d |
| β-sheet (%) | 32.74 ± 0.08 a | 29.67 ± 0.10 c | 30.59 ± 0.05 b | 30.32 ± 0.03 b | 28.15 ± 0.27 d | 32.74 ± 0.28 a | 30.60 ± 0.12 b | 32.86 ± 0.27 a |
| β-turn (%) | 16.50 ± 0.25 b | 16.71 ± 0.21 ab | 16.58 ± 0.08 ab | 16.91 ± 0.12 a | 16.98 ± 0.08 a | 16.50 ± 0.10 b | 16.64 ± 0.04 ab | 16.35 ± 0.15 b |
| Random coils (%) | 32.83 ± 0.13 d | 33.51 ± 0.11 a | 33.42 ± 0.12 ab | 32.34 ± 0.04 e | 33.15 ± 0.15 bc | 32.83 ± 0.13 d | 33.28 ± 0.08 abc | 33.03 ± 0.03 cd |
| Flexibility | 0.161 ± 0.01 b | 0.149 ± 0.01 c | 0.145 ± 0.01 cd | 0.182 ± 0.01 a | 0.148 ± 0.01 c | 0.137 ± 0.01 d | 0.125 ± 0.01 e | 0.140 ± 0.01 cd |
| EAI (m2/g) | 8.439 ± 0.46 a | 7.687 ± 0.16 b | 7.569 ± 0.20 b | 8.598 ± 0.15 a | 7.564 ± 0.23 b | 6.735 ± 0.29 c | 7.293 ± 0.23 bc | 7.451 ± 0.24 bc |
| ESI (%) | 34.25 ± 0.92 a | 27.36 ± 0.99 b | 25.96 ± 0.48 b | 28.05 ± 1.04 b | 25.49 ± 1.25 bc | 22.94 ± 1.23 cd | 20.13 ± 1.09 d | 22.72 ± 1.15 cd |
| Parameter | Mung Bean Protein Emulsion Samples | |||||||
|---|---|---|---|---|---|---|---|---|
| MPI1 | MPI2 | MPI3 | MPI4 | MPI5 | MPI6 | MPI7 | MPI8 | |
| D4,3-0 (nm) | 343.4 ± 10.40 f | 386.9 ± 14.90 de | 428.9 ± 8.90 abc | 376.5 ± 6.50 e | 406.5 ± 6.50 bcd | 451.7 ± 11.70 a | 432.3 ± 12.30 ab | 403.7 ± 3.70 cde |
| D4,3-1 (nm) | 453.8 ± 7.80 e | 605.8 ± 5.80 d | 707.2 ± 12.20 c | 460.0 ± 15.00 e | 750.8 ± 15.80 bc | 636.7 ± 16.70 d | 1156.0 ± 46.00 a | 769.4 ± 17.40 b |
| D4,3-2 (nm) | 676.7 ± 10.70 e | 640.8 ± 5.80 f | 1982.0 ± 14.00 a | 790.2 ± 12.20 d | 866.2 ± 10.20 c | 779.9 ± 4.90 d | 1196.0 ± 7.00 b | 877.2 ± 7.20 c |
| D4,3-3 (nm) | 722.9 ± 7.90 g | 1054.0 ± 19.00 d | 1997.0 ± 37.00 a | 836.8 ± 6.80 f | 945.7 ± 9.70 e | 960.6 ± 4.60 e | 1317.0 ± 7.00 b | 1227 ± 7.00 c |
| Oiling off-1 (%) | 16.21 ± 0.21 f | 18.14 ± 0.24 e | 21.03 ± 0.23 c | 14.12 ± 0.12 g | 21.38 ± 0.28 bc | 20.02 ± 0.02 d | 26.53 ± 0.33 a | 21.84 ± 0.24 b |
| Oiling off-2 (%) | 16.86 ± 0.09 g | 20.02 ± 0.02 f | 23.50 ± 0.10 c | 15.32 ± 0.15 h | 21.84 ± 0.16 d | 21.38 ± 0.09 e | 26.94 ± 0.12 a | 25.25 ± 0.06 b |
| Oiling off-3 (%) | 16.95 ± 0.07 f | 21.84 ± 0.09 e | 23.69 ± 0.10 c | 16.10 ± 0.08 g | 22.49 ± 0.08 d | 23.50 ± 0.09 c | 31.09 ± 0.09 a | 26.53 ± 0.05 b |
| CI-1 (%) | 15.00 ± 0.60 de | 21.67 ± 0.27 c | 29.52 ± 0.22 b | 10.00 ± 1.00 e | 30.95 ± 0.45 b | 20.58 ± 0.28 c | 57.14 ± 3.14 a | 53.94 ± 1.94 a |
| CI-2 (%) | 16.41 ± 0.16 f | 28.00 ± 0.50 e | 35.00 ± 0.80 c | 12.00 ± 1.00 g | 35.00 ± 0.80 c | 30.95 ± 0.20 d | 59.00 ± 1.00 a | 55.00 ± 1.50 b |
| CI-3 (%) | 20.20 ± 0.20 g | 29.52 ± 0.52 f | 38.10 ± 0.60 d | 12.87 ± 0.87 h | 43.37 ± 0.37 c | 34.76 ± 0.76 e | 59.64 ± 0.49 b | 64.11 ± 0.61 a |
| Albumin Content | Globulin Content | 63.1 (kDa) | 52.9 (kDa) | 31.4 (kDa) | 26.9 (kDa) | 21.4 (kDa) | α-Helix | β-Sheet | β-Turn | Random Coils | Flexibility | EAI | ESI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D4,3 | −0.539 | −0.402 | −0.607 | −0.407 | 0.523 | 0.794 * | 0.532 | 0.083 | 0.049 | −0.099 | 0.515 | −0.820 * | −0.585 | −0.781 * |
| Oiling off | −0.682 | −0.292 | −0.591 | −0.458 | 0.546 | 0.769 * | 0.606 | −0.031 | 0.157 | −0.237 | 0.604 | −0.919 ** | −0.720 * | −0.771 * |
| CI | −0.691 | −0.130 | −0.210 | −0.652 | 0.682 | 0.682 | 0.610 | −0.146 | 0.064 | −0.359 | 0.463 | −0.769 * | −0.507 | −0.715 * |
| Zeta potential | −0.745 * | −0.277 | −0.469 | −0.466 | 0.622 | 0.655 | 0.547 | 0.024 | 0.280 | −0.168 | 0.440 | −0.823 * | −0.698 | −0.848 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Fan, J.; Sun, H.; Jiang, G.; Meng, Y.; Zeng, X.; Yang, Z.; Nan, X.; Kang, L.; Liu, X. Study on Protein Structures of Eight Mung Bean Varieties and Freeze-Thaw Stability of Protein-Stabilized Emulsions. Foods 2022, 11, 3343. https://doi.org/10.3390/foods11213343
Sun H, Fan J, Sun H, Jiang G, Meng Y, Zeng X, Yang Z, Nan X, Kang L, Liu X. Study on Protein Structures of Eight Mung Bean Varieties and Freeze-Thaw Stability of Protein-Stabilized Emulsions. Foods. 2022; 11(21):3343. https://doi.org/10.3390/foods11213343
Chicago/Turabian StyleSun, Hongrui, Jieying Fan, Hongjiao Sun, Guochuan Jiang, Yue Meng, Xianpeng Zeng, Zhiqiang Yang, Xiping Nan, Lining Kang, and Xiangying Liu. 2022. "Study on Protein Structures of Eight Mung Bean Varieties and Freeze-Thaw Stability of Protein-Stabilized Emulsions" Foods 11, no. 21: 3343. https://doi.org/10.3390/foods11213343
APA StyleSun, H., Fan, J., Sun, H., Jiang, G., Meng, Y., Zeng, X., Yang, Z., Nan, X., Kang, L., & Liu, X. (2022). Study on Protein Structures of Eight Mung Bean Varieties and Freeze-Thaw Stability of Protein-Stabilized Emulsions. Foods, 11(21), 3343. https://doi.org/10.3390/foods11213343




