Metabolomic Analysis of Phytochemical Compounds from Agricultural Residues of Eggplant (Solanum melongena L.)
Abstract
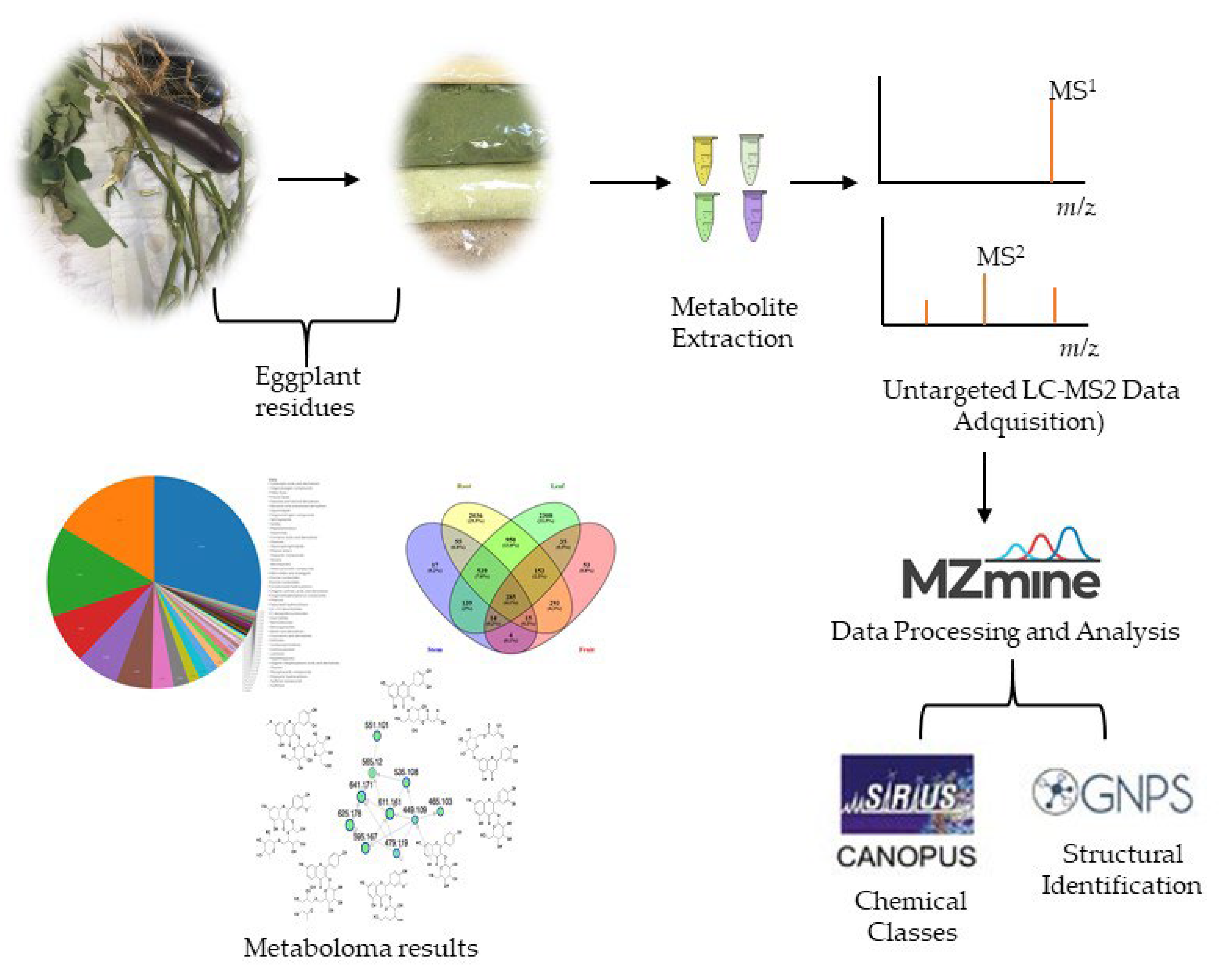
:1. Introduction
2. Results and Discussion
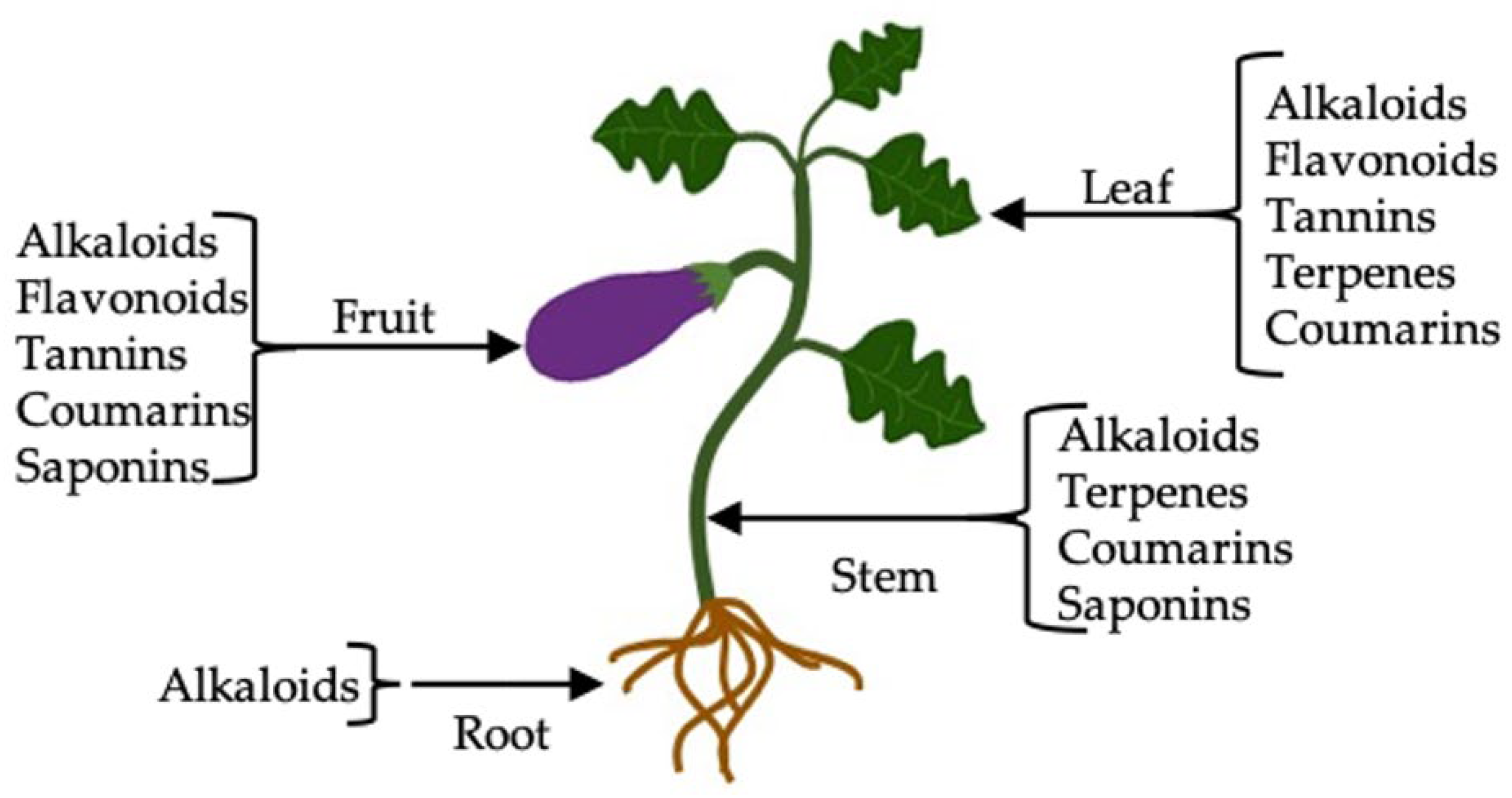
2.1. Phytochemical Screening
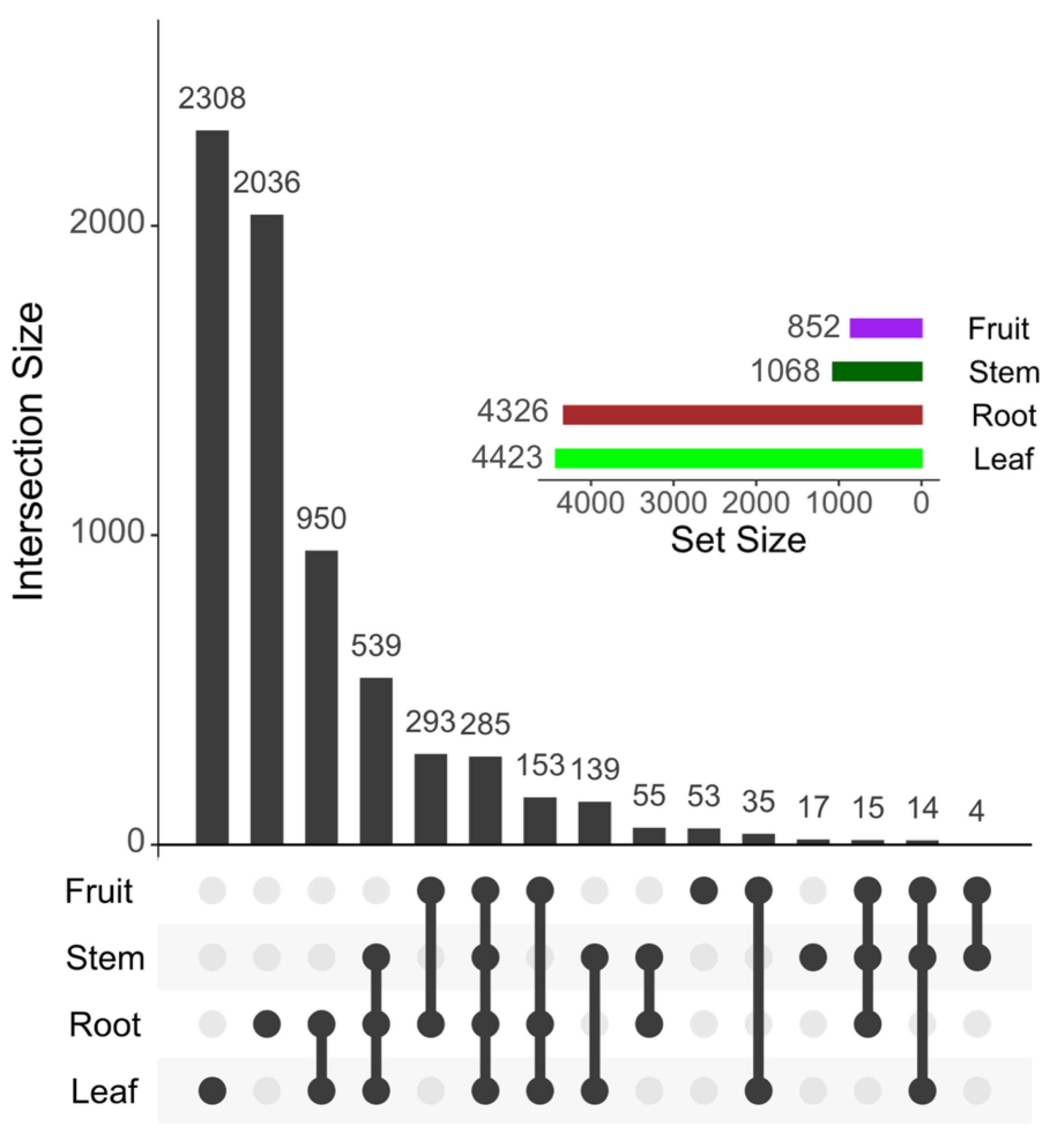
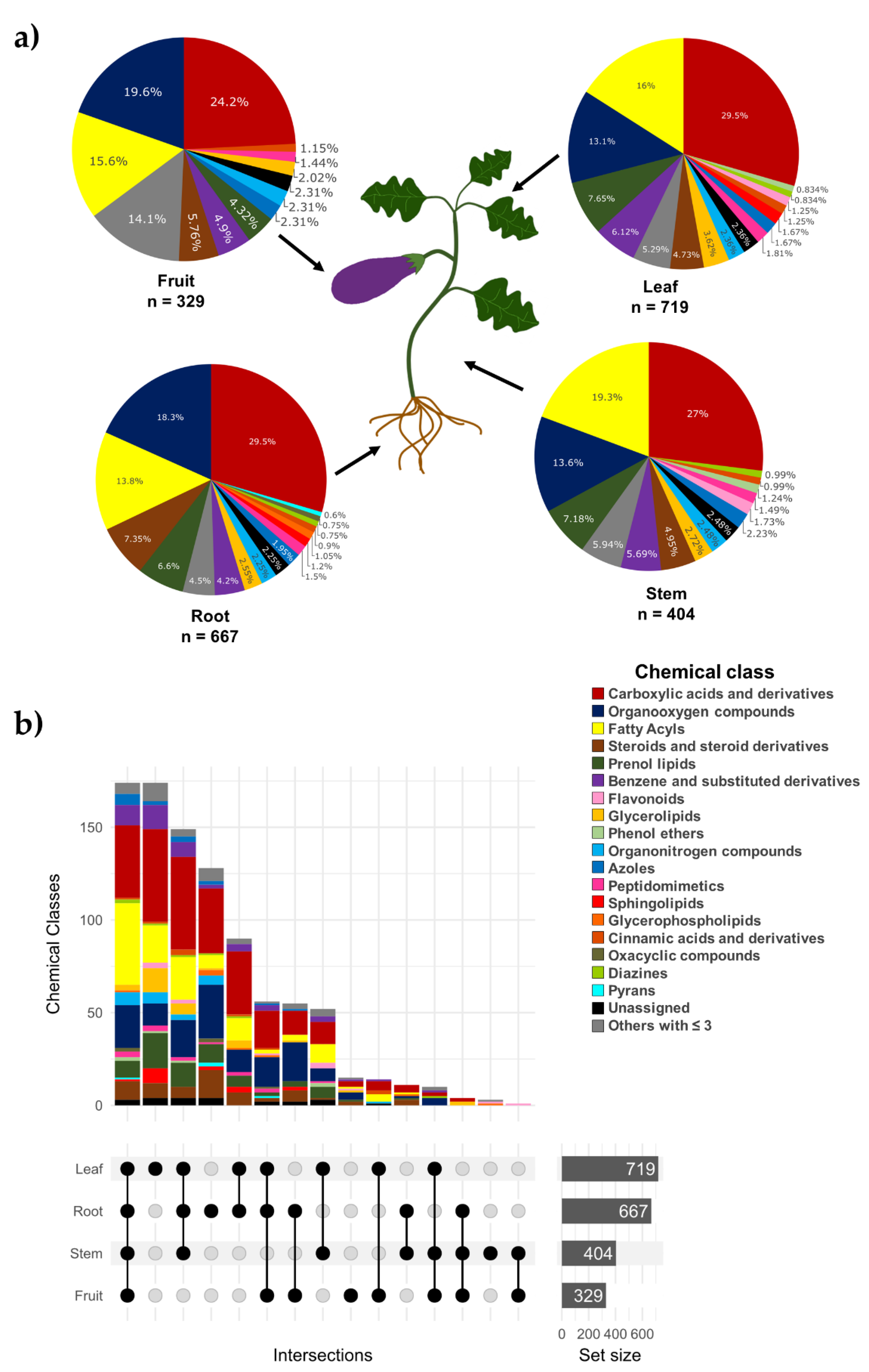
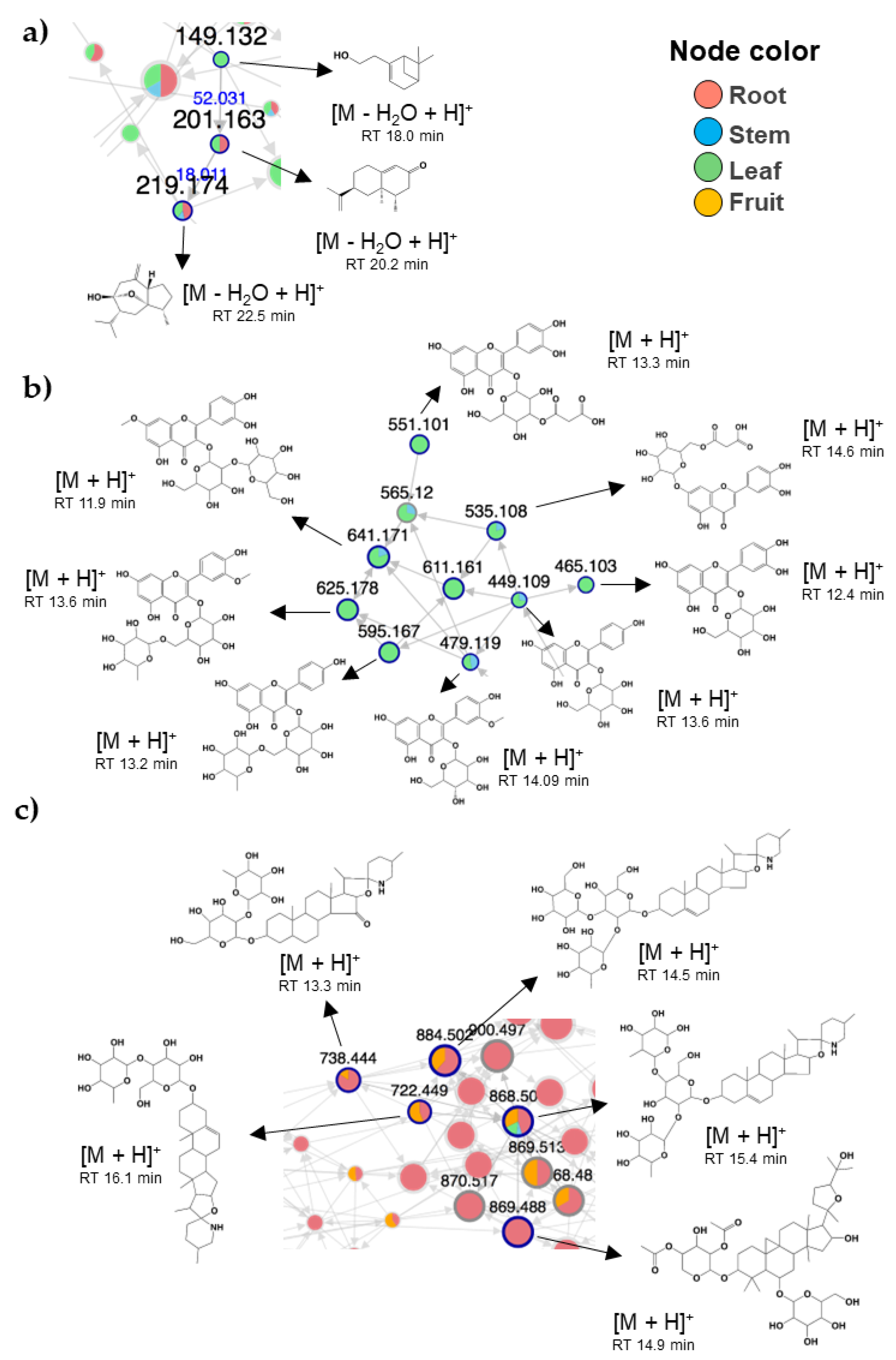
2.2. Metabolomic Analysis
2.3. Phytochemical Content in Eggplant Agricultural Residues
2.3.1. Phenolic Compounds
2.3.2. Flavonoids Content
2.3.3. Anthocyanins
2.3.4. Tannins Total Content
2.3.5. Saponins Total Content
2.3.6. Alkaloids Total Content
2.4. Antioxidant Activity
3. Materials and Methods
3.1. Obtaining and Processing of Agricultural Residues
3.2. Phytochemical Screening
3.3. Mass Spectrometry-Based Untargeted Metabolomic Analysis
3.3.1. Metabolite Extraction
3.3.2. Untargeted LC-MS2 Data Acquisition
3.3.3. LC-MS2 Data Processing and Analysis
3.4. Phytochemical Assays
3.4.1. Methanolic Extraction
3.4.2. Determination of Total Phenolics Compounds
3.4.3. Flavonoids Colorimetric Assay
3.4.4. Quantification of Total Tannins Content
3.4.5. Totals Anthocyanins Content
3.4.6. Determination of Saponins Content
3.4.7. Totals Alkaloids Content
3.5. Antioxidant Activity
3.6. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duque-Acevedo, M.; Belmonte-Ureña, L.J.; Cortés-García, F.J.; Camacho-Ferre, F. Agricultural waste: Review of the evolution, approaches and perspectives on alternative uses. Glob. Ecol. Conserv. 2020, 22, e00902. [Google Scholar] [CrossRef]
- Maurya, R.; Bharti, C.; Singh, T.; Pratap, V. Crop residue management for sustainable agriculture. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 3168–3174. [Google Scholar] [CrossRef]
- Tripathi, N.; Hills, C.D.; Singh, R.S.; Atkinson, C.J. Biomass waste utilisation in low-carbon products: Harnessing a major potential resource. NPJ Clim. Atmos. Sci. 2019, 2, 35. [Google Scholar] [CrossRef] [Green Version]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Rao, U.J.S.P. Sustainable solutions for agro processing waste management: An overview. In Environmental Protection Strategies for Sustainable Development; Malik, A., Grohmann, E., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 65–109. [Google Scholar]
- Malik, A.; Rahman, M.; Ansari, M.I.; Masood, F.; Grohmann, E. Environmental protection strategies: An overview. In Environmental Protection Strategies for Sustainable Development; Malik, A., Grohmann, E., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 1–34. [Google Scholar]
- Sarkar, S.; Skalicky, M.; Hossain, A.; Brestic, M.; Saha, S.; Garai, S.; Brahmachari, K. Management of crop residues for improving input use efficiency and agricultural sustainability. Sustainability 2020, 12, 9808. [Google Scholar] [CrossRef]
- Salim, N.S.M.; Singh, A.; Raghavan, V. Potential utilization of fruit and vegetable wastes for food through drying or extraction techniques. Nov. Tech. Nutri. Food Sci. 2017, 1, 35p. [Google Scholar] [CrossRef] [Green Version]
- Sultana, B.; Hussain, Z.; Hameed, M.; Mushtaq, M. Antioxidant activity among different parts of aubergine (Solanum melongena L.). Pak. J. Bot. 2013, 45, 1443–1448. [Google Scholar]
- Lim, T.K. Solanum melongena. In Edible Medicinal and Non-Medicinal Plants; Lim, T.K., Ed.; Springer: Dordrecht, The Netherlands, 2013; Volume 6, pp. 370–388. [Google Scholar]
- Karimi, A.; Kazemi, M.; Samani, S.A.; Simal-Gandara, J. Bioactive compounds from by-products of eggplant: Functional properties, potential applications and advances in valorization methods. Trends Food Sci. Technol. 2021, 112, 518–531. [Google Scholar] [CrossRef]
- SIAP. Servicio de Información Agroalimentaria y Pesquera. Available online: http://infosiap.siap.gob.mx/gobmx/datosAbiertos_a.php34 (accessed on 16 November 2021).
- Silva, G.F.P.; Pereira, E.; Melgar, B.; Stojković, D.; Sokovic, M.; Calhelha, R.C.; Barros, L. Eggplant fruit (Solanum melongena L.) and bio-residues as a source of nutrients, bioactive compounds, and food colorants, using innovative food technologies. Appl. Sci. 2021, 11, 151. [Google Scholar] [CrossRef]
- SAGARPA. Estadísticas de Producción Agrícola. Available online: www.gob.mx/sagarpa (accessed on 16 December 2020).
- Kazemi, M.; Khodaiyan, F.; Hosseini, S.S.; Najari, Z. An integrated valorization of industrial waste of eggplant: Simultaneous recovery of pectin, phenolics and sequential production of pullulan. Waste Manag. 2019, 100, 101–111. [Google Scholar] [CrossRef]
- Hamzah, R.U.; Agboola, A.R.; Busari, M.B.; Omogu, E.H.; Umar, M.B.; Abubakar, A.N. Evaluation of hepatoprotective effect of methanol extract of Solanum melongena on carbon tetrachloride induced hepatotoxic rats. Eur. J. Med. Plants 2016, 13, 1–12. [Google Scholar] [CrossRef]
- Akanitapichat, P.; Phraibung, K.; Nuchklang, K.; Prompitakkul, S. Antioxidant and hepatoprotective activities of five eggplant varieties. Food Chem. Toxicol. 2010, 48, 3017–3021. [Google Scholar] [CrossRef] [PubMed]
- Mauro, R.P.; Agnello, M.; Rizzo, V.; Graziani, G.; Fogliano, V.; Leonardi, C.; Giuffrida, F. Recovery of eggplant field waste as a source of phytochemicals. Sci. Hortc. 2020, 261, 109023. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant capacity of tea and common vegetables. J. Agric. Food Chem. 1996, 44, 3426–3431. [Google Scholar] [CrossRef]
- Philippi, K.; Tsamandouras, N.; Grigorakis, S.; Makris, D.P. Ultrasound-assisted green extraction of eggplant peel (Solanum melongena) polyphenols using aqueous mixtures of glycerol and ethanol: Optimisation and kinetics. Environ. Process. 2016, 3, 369–386. [Google Scholar] [CrossRef]
- Jung, E.J.; Bae, M.S.; Jo, E.K.; Jo, Y.H.; Lee, S.C. Antioxidant activity of different parts of eggplant. J. Med. Plants Res. 2011, 5, 4610–4615. [Google Scholar]
- Nwanna, E.E.; Ibukun, O.E.; Oboh, G. Inhibitory effects of methanolic extracts of two eggplant species from South-western Nigeria on starch hydrolysing enzymes linked to type-2 diabetes. Afr. J. Pharm. Pharmacol. 2013, 7, 1575–1584. [Google Scholar] [CrossRef] [Green Version]
- Umamageswari, M.S.; Maniyar, Y.A. Evaluation of anti-inflammatory activity of aqueous extract of leaves of Solanum melongena Linn. in experimental animals. J. Clin. Diagn. Res. 2015, 9, FF01–FF03. [Google Scholar] [CrossRef] [PubMed]
- Im, K.; Lee, J.Y.; Byeon, H.; Hwang, K.W.; Kang, W.; Whang, W.K.; Min, H. In vitro antioxidative and anti-inflammatory activities of the ethanol extract of eggplant (Solanum melongena) stalks in macrophage RAW 264.7 cells. Food Agric. Immunol. 2016, 27, 758–771. [Google Scholar] [CrossRef] [Green Version]
- Milner, S.E.; Brunton, N.P.; Jones, P.W.; O’Brien, N.M.; Collins, S.G.; Maguire, A.R. Bioactivities of glycoalkaloids and their aglycones from Solanum species. J. Agric. Food Chem. 2011, 59, 3454–3484. [Google Scholar] [CrossRef] [PubMed]
- Mutalik, S.; Paridhavi, K.; Rao, C.M.; Udupa, N. Antipyretic and analgesic effect of leaves of Solanum melongena Linn. in rodents. Indian J. Pharmacol. 2003, 35, 312–315. [Google Scholar]
- Oliveira, D.M.; Furlong, E.B. Screening of antifungal and antimycotoxigenic activity of plant phenolic extracts. World Mycotoxin J. 2008, 1, 139–146. [Google Scholar] [CrossRef]
- Salamatullah, A.M.; Alkaltham, M.S.; Hayat, K.; Ahmed, M.A.; Arzoo, S.; Husain, F.M.; Alzahrani, A. Bioactive and antimicrobial properties of eggplant (Solanum melongena L.) under microwave cooking. Sustainability 2021, 13, 1519. [Google Scholar] [CrossRef]
- Afshari, F.; Seraj, H.; Sadat, H.Z.; Timajchi, M.; Ensiyeh, O.; Ladan, G.; Ganjibakhsh, M. The cytotoxic effects of eggplant peel extract on human gastric adenocarcinoma cells and normal cells. Mod. Med. Lab. J. 2017, 1, 77–83. [Google Scholar] [CrossRef]
- Fekry, M.I.; Ezzat, S.M.; Salama, M.M.; Alshehri, O.Y.; Al-Abd, A.M. Bioactive glycoalkaloides isolated from Solanum melongena fruit peels with potential anticancer properties against hepatocellular carcinoma cells. Sci. Rep. 2019, 9, 1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabana, M.M.; Salama, M.; Ezzat, S.M.; Ismail, L.R. In Vitro and in vivo anticancer activity of the fruit peels of Solanum melongena L. against hepatocellular carcinoma. J. Carcinog. Mutagen. 2013, 4, 149–154. [Google Scholar]
- Cham, B.E. Intralesion and curaderm BEC5 topical combination therapies of solasodine rhamnosyl glycosides derived from the eggplant or Devil’s apple result in rapid removal of large skin cancers. Methods of treatment compared. Int. J. Clin. Med. 2012, 3, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Cham, B.E.; Daunter, B.; Evans, R.A. Topical treatment of malignant and premalignant skin lesions by very low concentrations of a standard mixture (BEC) of solasodine glycosides. Cancer Lett. 1991, 59, 183–192. [Google Scholar] [CrossRef]
- Horincar, G.; Enachi, E.; Bolea, C.; Râpeanu, G.; Aprodu, I. Value-added lager beer enriched with eggplant (Solanum melongena L.) peel extract. Molecules 2020, 25, 731. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Gu, Y.F.; Su, X.Q.; Li, M.M.; Huo, H.X.; Zhang, J.; Tu, P.F. Anti-inflammatory lignanamides from the roots of Solanum melongena L. Fitoterapia 2014, 98, 110–116. [Google Scholar] [CrossRef]
- Shrivastava, A.; Srivastava, N.; Kumar, N. Phytochemical screening and study of analgesic activity of brinjal leaves. J. Pharma Sci. Monit. 2012, 3, 3028–3033. [Google Scholar]
- Cevallos-Cevallos, J.M.; Reyes-De-Corcuera, J.I.; Etxeberria, E.; Danyluk, M.D.; Rodrick, G.E. Metabolomic analysis in food science: A review. Trends Food Sci. Technol. 2009, 20, 557–566. [Google Scholar] [CrossRef]
- Shulaev, V.; Cortes, D.; Miller, G.; Mittler, R. Metabolomics for plant stress response. Physiol. Plant. 2008, 132, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, A.; Shang, J.; Zhu, Z.; Li, Y.; Wu, X.; Zha, D. Study on browning mechanism of fresh-cut eggplant (Solanum melongena L.) based on metabolomics, enzymatic assays and gene expression. Sci. Rep. 2021, 11, 6937. [Google Scholar] [CrossRef] [PubMed]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.-M.; Chan, T.-F.; Hui, J.H.L. Terpenes and terpenoids in plants: Interactions with environment and insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef]
- Belete, T. Defense mechanisms of plants to insect pests: From morphological to biochemical approach. Trends Tech. Sci. Res. 2018, 2, 30–38. [Google Scholar] [CrossRef]
- Ashrafudoulla, M.; Bellah, S.F.; Alam, F.; Faisal, S.S.; Kafi, M.A.H.; Fuad, F. Phytochemical screening of Solanum nigrum L., S. myriacanthus Dunal, Solanum melongena and Averrhoa bilimbi in Bangladesh. J. Med. Plants Stud. 2016, 4, 35–38. [Google Scholar]
- Tiwari, A.; Jadon, R.S.; Tiwari, P.; Nayak, S. Phytochemical investigations of crown of Solanum melongena fruit. Int. J. Phytomedicine 2009, 1, 9–11. [Google Scholar] [CrossRef] [Green Version]
- Socaciu, C. From phytochemistry to metabolomics: Eight decades of research in plant and food science. Stud. Univ. Babeș Bolyai Chem. 2019, 64, 205–224. [Google Scholar] [CrossRef]
- Dührkop, K.; Nothias, L.F.; Fleischauer, M.; Reher, R.; Ludwig, M.; Hoffmann, M.A.; Böcker, S. Systematic classification of unknown metabolites using high-resolution fragmentation mass spectra. Nat. Biotechnol. 2020, 39, 462–471. [Google Scholar] [CrossRef]
- Djoumbou, F.Y.; Eisner, R.; Knox, C.; Chepelev, L.; Hastings, J.; Owen, G.; Wishart, D.S. ClassyFire: Automated chemical classification with a comprehensive, computable taxonomy. J. Cheminform. 2016, 8, 61. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, M.; Bushehri, A.A.S.; Hassandokht, M.R.; Naghavi, M.R. Evaluation of solasonine content and expression patterns of SGT1 gene in different tissues of two Iranian eggplant (Solanum melongena L.) genotypes. Food Technol. Biotechnol. 2017, 55, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Sulli, M.; Barchi, L.; Toppino, L.; Diretto, G.; Sala, T.; Lanteri, S.; Giuliano, G. An eggplant recombinant inbred population allows the discovery of metabolic QTLs controlling fruit nutritional quality. Front. Plant Sci. 2021, 12, 1–17. [Google Scholar] [CrossRef]
- Hanifah, A.; Maharijaya, A.; Putri, S.P.; Laviña, W.A. Untargeted metabolomics analysis of eggplant (Solanum melongena L.) fruit and its correlation to fruit morphologies. Metabolites 2018, 8, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Ulloa, A.; Delgado-De la Herrán, H.C.; Álvarez-Delgado, C.; Mendoza-Porras, O.; Carballo-Castañeda, R.A.; Donis-Maturano, L.; Villarreal, F. Multi-omics study identifies novel signatures of DNA/RNA, amino acid, peptide, and lipid metabolism by simulated diabetes on coronary endothelial cells. Sci. Rep. 2022, 12, 12027. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ulloa, A.; Diaz, V.S.; Tejeda-Mora, J.A.; Macias-Contreras, M.I.; Castillo, F.D.; Guerrero, A.; Licea-Navarro, A. Chemical profiling provides insights into the metabolic machinery of hydrocarbon-degrading deep-sea microbes. J. Msystems 2020, 5, 1–19. [Google Scholar] [CrossRef]
- Jiménez-Gómez, I.; Valdés-Muñoz, G.; Moreno-Ulloa, A.; Pérez-Llano, Y.; Moreno-Perlín, T.; Silva-Jiménez, H.; Batista-García, R.A. Surviving in the brine: A multi-omics approach for understanding the physiology of the halophile fungus Aspergillus sydowii at saturated NaCl concentration. Front. Microbiol. 2022, 13, 840408. [Google Scholar] [CrossRef]
- Villa-Rodriguez, E.; Moreno-Ulloa, A.; Castro-Longoria, E.; Parra-Cota, F.I.; de Los Santos-Villalobos, S. Integrated omics approaches for deciphering antifungal metabolites produced by a novel Bacillus species, B. cabrialesii TE3(T), against the spot blotch disease of wheat (Triticum turgidum L. subsp. durum). Microbiol. Res. 2021, 251, 126826. [Google Scholar] [CrossRef]
- Al Sinani, S.S.; Eltayeb, E.A.; Kamal, Y.T.; Khan, M.S.; Ahmad, S. Variations in the cytotoxic glycoalkaloids solamargine and solasonine in different parts of the Solanum incanum plant during its growth and development in Oman. J. Taibah Univ. Sci. 2016, 10, 813–822. [Google Scholar] [CrossRef] [Green Version]
- Popova, I.; Sell, B.; Pillai, S.S.; Kuhl, J.; Dandurand, L.M. High-performance liquid chromatography-mass spectrometry analysis of glycoalkaloids from underexploited Solanum species and their acetylcholinesterase inhibition activity. Plants 2022, 11, 269. [Google Scholar] [CrossRef]
- Sanchez-Mata, M.C.; Yokoyama, W.E.; Hong, Y.; Prohens, J. α-Solasonine and α-solamargine contents of gboma (Solanum macrocarpon L.) and scarlet (Solanum aethiopicum L.) eggplants. J. Agric. Food Chem. 2010, 58, 5502–5508. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry and anticarcinogenic mechanisms of glycoalkaloids produced by eggplants, potatoes, and tomatoes. J. Agric. Food Chem. 2015, 63, 3323–3337. [Google Scholar] [CrossRef] [PubMed]
- Plazas, M.; Andujar, I.; Vilanova, S.; Hurtado, M.; Gramazio, P.; Herraiz, F.J.; Prohens, J. Breeding for chlorogenic acid content in eggplant: Interest and prospects. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Haslam, E. Plant polyphenols (syn. vegetable tannins) and chemical defense—A reappraisal. J. Chem. Ecol. 1988, 14, 1789–1805. [Google Scholar] [CrossRef] [PubMed]
- Plazas, M.; López-Gresa, M.P.; Vilanova, S.; Torres, C.; Hurtado, M.; Gramazio, P.; Prohens, J. Diversity and relationships in key traits for functional and apparent quality in a collection of eggplant: Fruit phenolics content, antioxidant activity, polyphenol oxidase activity, and browning. J. Agric. Food Chem. 2013, 61, 8871–8879. [Google Scholar] [CrossRef]
- Lo Scalzo, R.; Fibiani, M.; Francese, G.; D’Alessandro, A.; Rotino, G.L.; Conte, P.; Mennella, G. Cooking influence on physico-chemical fruit characteristics of eggplant (Solanum melongena L.). Food Chem. 2016, 194, 835–842. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Hossain, M.A.; da Silva, J.A.T.; Fujita, M. Plant response and tolerance to abiotic oxidative stress: Antioxidant defense is a key factor. In Crop Stress and its Management: Perspectives and Strategies; Venkateswarlu, B., Shanker, A.K., Shanker, C., Maheswari, M., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 261–315. [Google Scholar]
- Li, K.; Fan, H.; Yin, P.; Yang, L.; Xue, Q.; Li, X.; Liu, Y. Structure-activity relationship of eight high content flavonoids analyzed with a preliminary assign-score method and their contribution to antioxidant ability of flavonoids-rich extract from Scutellaria baicalensis shoots. Arab. J. Chem. 2018, 11, 159–170. [Google Scholar] [CrossRef]
- Di Sotto, A.; Di Giacomo, S.; Amatore, D.; Locatelli, M.; Vitalone, A.; Toniolo, C.; Nencioni, L. A Polyphenol rich extract from Solanum melongena L. DR2 peel exhibits antioxidant properties and anti-herpes simplex virus type 1 activity in vitro. Molecules 2018, 23, 2066. [Google Scholar] [CrossRef] [Green Version]
- Fidrianny, I.; Winarsih, S.; Ruslan, K. Phytochemical content and antioxidant potential of different organs of eggplant (Solanum melongena L.) grown in west Java-Indonesia. Asian J. Pharm. Clin. Res. 2017, 10, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Piao, X.M.; Chung, J.W.; Lee, G.A.; Lee, J.R.; Cho, G.T.; Lee, H.S.; Lee, S.Y. Variation in antioxidant activity and flavonoid aglycones in eggplant (Solanum melongena L.) germplasm. Plant Breed. Biotechnol. Rep. 2014, 2, 396–403. [Google Scholar] [CrossRef] [Green Version]
- Boulekbache-Makhlouf, L.; Medouni, L.; Medouni-Adrar, S.; Arkoub, L.; Madani, K. Effect of solvents extraction on phenolic content and antioxidant activity of the byproduct of eggplant. Ind. Crops Prod. 2013, 49, 668–674. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, Y.; Ren, L.; Lian, H.; Chen, H. Molecular cloning and characterization of anthocyanin biosynthesis genes in eggplant (Solanum melongena L.). Acta Physiol. Plant. 2016, 38, 163. [Google Scholar] [CrossRef]
- Kadhim, N.J.; Al-Rekaby, L.S.; Redha, A.A.; Chappell, J. Chemical composition and antioxidant capacity of eggplant parts during vegetative and flowering stage. J. Phys. Conf. Ser. 2019, 1294, 092013. [Google Scholar] [CrossRef]
- Lee, J.; Wrolstad, R.E. Extraction of anthocyanins and polyphenolics from blueberry processing waste. J. Food Sci. 2004, 69, 564–573. [Google Scholar] [CrossRef]
- Bouhajeb, R.; Selmi, S.; Nakbi, A.; Jlassi, I.; Montevecchi, G.; Flamini, G.; Dabbou, S. Chemical composition analysis, antioxidant, and antibacterial activities of eggplant leaves. Chem. Biodivers. 2020, 17, e2000405. [Google Scholar] [CrossRef] [PubMed]
- Al-Abdulla, B.Y.; Oleiwi, S.D. Estimation of some phytohemicals in extract of eggplant peels and cabbage leaves and using pigment of them in drinks. Mesop. J. Agric. 2019, 47, 128–138. [Google Scholar]
- Ashok, P.K.; Upadhyaya, K. Tannins are astringent. J. Pharma. Phytochem. 2012, 1, 45–50. [Google Scholar]
- Contreras-Angulo, L.; Emus-Medina, A.; Gutierrez-Grijalva, E.; Ambriz-Pérez, D.; Elizalde-Romero, C.; Heredia, J.B. Pharmacological potential of Solanum genus. In Solanum: An Overview; Souza, A.V., Ed.; Nova Science: New York, NY, USA, 2020; Volume 1, p. 199. [Google Scholar]
- Kaunda, J.S.; Zhang, Y.J. The genus Solanum: An ethnopharmacological, phytochemical and biological properties review. Nat. Prod. Bioprospec. 2019, 9, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Carvajal, R.L.; Uribe, Y.H.; Sierra, M.N.; Rueda, N.D. Análisis fitoquímico preliminar de hojas, tallos y semillas de cupatá (Strychnos schultesiana Krukoff). Colomb. For. 2009, 12, 161–170. [Google Scholar] [CrossRef]
- Benjamin, A.; Winner, K.; Constance, N.; Ahamefula, E.; Ljeoma, E.; Chimaraoke, O.; Nchekwube, O. Comparative study of chemical composition of three different eggplant fruit species. Asian Food Sci. J. 2020, 19, 10–16. [Google Scholar] [CrossRef]
- Chowański, S.; Adamski, Z.; Marciniak; Rosiński, G.; Büyükgüzel, E.; Büyükgüzel, K.; Bufo, S.A. A Review of bioinsecticidal activity of Solanaceae alkaloids. Toxins 2016, 8, 60. [Google Scholar] [CrossRef] [Green Version]
- Agoreyo, B.O.; Obansa, E.S.; Obanor, E.O. Comparative nutritional and phytochemical analyses of two varieties of Solanum melongena. Sci. World J. 2012, 7, 5–8. [Google Scholar]
- Edeke, A.; Uchendu, N.; Omeje, K.; Odiba, A.S. Nutritional and pharmacological potentials of Solanum melongena and Solanum aethiopicum fruits. J. Phytopharm. 2021, 10, 61–67. [Google Scholar] [CrossRef]
- Mahmoud, Z.T.; Sadek, F.H. The host plant preference of the leaf miner Liriomyza spp. (diptera: Agromyzidae) on eggplant (Solanum melongena L.) and squash (Cucurbita pepo L.) crops and its relation with the secondary compounds in plant. Plant Arch. 2020, 20, 1034–1038. [Google Scholar]
- Lelario, F.; De Maria, S.; Rivelli, A.R.; Russo, D.; Milella, L.; Bufo, S.A.; Scrano, L.J.T. A complete survey of glycoalkaloids using LC-FTICR-MS and IRMPD in a commercial variety and a local landrace of eggplant (Solanum melongena L.) and their anticholinesterase and antioxidant activities. Toxins 2019, 11, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nenadis, N.; Tsimidou, M.Z. Assessing the activity of natural food antioxidants. In Oxidation in Foods and Beverages and Antioxidant Applications; Decker, E.A., Ed.; Woodhead Publishing: Sawston, UK, 2010; pp. 332–367. [Google Scholar]
- Jo, Y.N.; Jeong, H.R.; Jung, J.-H.; Heo, H.J. The skin protecting effects of ethanolic extracts of eggplant peels. Korean J. Food Sci. Technol. 2012, 44, 94–99. [Google Scholar] [CrossRef] [Green Version]
- Sharma, H.; Chawla, N.; Dhatt, A.S. Nutraceutical content and free radical scavenging capacity of brinjal (Solanum melongena L.) genotypes. Sci. Hortic. 2019, 244, 294–303. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Diatta, K.; Diatta, W.; Fall, A.D.; Dieng, S.I.M.; Mbaye, A.I.; Sarr, A.; Bidjo, C.L. Study of antioxidant activity of stalk and fruit of Solanum melongena L. (Solanaceae). Asian J. Res. Med. Pharm. Sci. 2019, 8, 1–7. [Google Scholar] [CrossRef]
- Boubekri, C.; Lanez, T.; Djouadi, A. A comparative study on antioxidant activities and phenolic contents of five algerian eggplant cultivars. Sci. Study Res. Chem. Chem. Eng. 2015, 16, 29–36. [Google Scholar]
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From chemistry to biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.T.; Gentry, E.C.; McPhail, K.L.; Nothias, L.-F.; Nothias-Esposito, M.; Bouslimani, A.; Dorrestein, P.C. Reproducible molecular networking of untargeted mass spectrometry data using GNPS. Nat. Protoc. 2020, 15, 1954–1991. [Google Scholar] [CrossRef] [PubMed]
- Dührkop, K.; Shen, H.; Meusel, M.; Rousu, J.; Böcker, S. Searching molecular structure databases with tandem mass spectra using CSI:FingerID. Proc. Natl. Acad. Sci. USA 2015, 112, 12580–12585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, L.; Guler, M.; Tagirdzhanov, A.; Lee, Y.Y.; Gurevich, A.; Mohimani, H. MolDiscovery: Learning mass spectrometry fragmentation of small molecules. Nat. Commun. 2021, 12, 3718. [Google Scholar] [CrossRef]
- Holman, J.D.; Tabb, D.L.; Mallick, P. Employing ProteoWizard to Convert Raw Mass Spectrometry Data. Curr. Protoc. Bioinform. 2014, 46, 13.24.1–13.24.9. [Google Scholar] [CrossRef] [Green Version]
- Lex, A.; Gehlenborg, N.; Strobelt, H.; Vuillemot, R.; Pfister, H. UpSet: Visualization of Intersecting Sets. IEEE Trans. Vis. Comput. Graph. 2014, 20, 1983–1992. [Google Scholar] [CrossRef]
- Conway, J.R.; Lex, A.; Gehlenborg, N. UpSetR: An R package for the visualization of intersecting sets and their properties. Bioinformatics 2017, 33, 2938–2940. [Google Scholar] [CrossRef] [Green Version]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Böcker, S. SIRIUS 4: A rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, M.; Nothias, L.F.; Dührkop, K.; Koester, I.; Fleischauer, M.; Hoffmann, M.A.; Böcker, S. Database-independent molecular formula annotation using Gibbs sampling through ZODIAC. Nat. Mach. Intell. 2020, 2, 629–641. [Google Scholar] [CrossRef]
- Hoffmann, M.A.; Nothias, L.F.; Ludwig, M.; Fleischauer, M.; Gentry, E.C.; Witting, M.; Böcker, S. High-confidence structural annotation of metabolites absent from spectral libraries. Nat. Biotechnol. 2022, 40, 411–421. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W.E. The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Ghasemi, K.; Ghasemi, Y.; Ebrahimzadeh, M.A. Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. J. Pak. Pharm. Sci. 2009, 22, 277–281. [Google Scholar]
- Barman, K.; Rai, S.N. In vitro nutrient digestibility, gas production and tannin metabolites of Acacia nilotica pods in goats. Asian-Aust. J Anim. Sci. 2008, 21, 59–65. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Blümmel, M.; Borowy, N.K.; Becker, K. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J. Sci. Food Agric. 1993, 61, 161–165. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Hucl, P. A rapid method for quantifying total anthocyanins in blue aleurone and purple pericarp wheats. Cereal Chem. 1999, 76, 350–354. [Google Scholar] [CrossRef]
- Hiai, S.; Oura, H.; Nakajima, T. Color reaction of some sapogenins and saponins with vanillin and sulfuric acid. J. Planta Med. 1976, 29, 116–122. [Google Scholar] [CrossRef]
- Shamsa, F.; Monsef, H.; Ghamooshi, R.; Verdian-Rizi, M. Spectrophotometric determination of total alkaloids in some Iranian medicinal plants. J. Appl. Hortic. 2010, 12, 69–70. [Google Scholar]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of methods to determine antioxidant capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Bandeira, N. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]

| Metabolite | Assay | Solvent | Eggplant Residue | |||
|---|---|---|---|---|---|---|
| Root | Leaf | Stem | Fruit | |||
| Alkaloids | Dragendorff | Hexane | + | + | + | + |
| Methanol | + | + | + | + | ||
| Water | + | + | + | + | ||
| Mayer | Hexane | − | + | + | − | |
| Methanol | − | + | + | − | ||
| Water | − | + | + | − | ||
| Wagner | Hexane | + | + | + | + | |
| Methanol | + | + | + | + | ||
| Water | + | + | + | + | ||
| Terpenes | Libermann-Buchard | Hexane | − | + | − | − |
| Methanol | − | + | + | − | ||
| Water | − | − | − | − | ||
| Salkowsky | Hexane | − | + | − | − | |
| Methanol | − | + | + | − | ||
| Water | − | − | − | − | ||
| Flavonoids | Shinoda | Hexane | − | + | − | − |
| Methanol | − | + | − | + | ||
| Water | + | − | − | |||
| Tannins | Ferric Chloride | Hexane | − | + | − | + |
| Methanol | − | + | − | + | ||
| Water | − | + | − | + | ||
| Coumarins | Hexane | − | + | + | + | |
| Methanol | − | + | + | + | ||
| Water | − | + | − | + | ||
| Saponins | Foam | Hexane | − | − | − | − |
| Methanol | − | − | − | + | ||
| Water | − | − | + | + | ||
| Eggplant | Phenolic Compounds | Flavonoids | Anthocyanins | Tannins | Saponins | Alkaloids |
|---|---|---|---|---|---|---|
| Residue | (mg CAE/100g) | (mg QE/100g) | (mg C3GE/100g) | (mg CE/100g) | (mg DE/100g) | (mg AE/100g) |
| Root | 354.9 ±2 3 d | 23.1 ± 3 c | 0.0 ± 0 d | 56.4 ± 12 d | 9.8 ± 2 b | 653.4 ± 15 c |
| Leaf | 2454.9 ± 135 a | 39.3 ± 7 b | 7.3 ± 0.5 b | 650.9 ± 42 a | 40.0 ± 0.6 a | 3935.0 ± 173 a |
| Stem | 521.4 ± 37 c | 5.4 ± 2 d | 3.9 ± 0.8 c | 146.0 ± 4 c | 8.8 ± 0.6 b | 788.6 ± 67 c |
| Fruit | 2056.8 ± 193 b | 207.0 ± 10 a | 36.7 ± 1 a | 303.7 ± 5 b | 43.5 ± 5 a | 2689.5 ± 220 b |
| Eggplant Residue | ABTS Radical Scavenging IC50 (µg/mL) | DPPH Radical Scavenging IC50 (µg/mL) |
|---|---|---|
| Root | 2086.0 | 4249.9 |
| Leaf | 689.5 | 455.9 |
| Stem | 2093.3 | 2412.3 |
| Fruit | 2626.4 | 2236.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras-Angulo, L.A.; Moreno-Ulloa, A.; Carballo-Castañeda, R.A.; León-Felix, J.; Romero-Quintana, J.G.; Aguilar-Medina, M.; Ramos-Payán, R.; Heredia, J.B. Metabolomic Analysis of Phytochemical Compounds from Agricultural Residues of Eggplant (Solanum melongena L.). Molecules 2022, 27, 7013. https://doi.org/10.3390/molecules27207013
Contreras-Angulo LA, Moreno-Ulloa A, Carballo-Castañeda RA, León-Felix J, Romero-Quintana JG, Aguilar-Medina M, Ramos-Payán R, Heredia JB. Metabolomic Analysis of Phytochemical Compounds from Agricultural Residues of Eggplant (Solanum melongena L.). Molecules. 2022; 27(20):7013. https://doi.org/10.3390/molecules27207013
Chicago/Turabian StyleContreras-Angulo, Laura Aracely, Aldo Moreno-Ulloa, Rommel A. Carballo-Castañeda, Josefina León-Felix, José Geovanni Romero-Quintana, Maribel Aguilar-Medina, Rosalío Ramos-Payán, and J. Basilio Heredia. 2022. "Metabolomic Analysis of Phytochemical Compounds from Agricultural Residues of Eggplant (Solanum melongena L.)" Molecules 27, no. 20: 7013. https://doi.org/10.3390/molecules27207013







