Bio-Based Adhesives for Orthopedic Applications: Sources, Preparation, Characterization, Challenges, and Future Perspectives
Abstract
1. Introduction
1.1. Bone Fracture Healing
1.2. Bio-Adhesion and Bio-Based Adhesives
2. Sources and Types of Bio-Based Adhesives
3. Preparation of Bio-Based Adhesives
4. Characterization of Bio-Adhesives
4.1. In Vitro Methods
4.1.1. Shear Strength Measurement
4.1.2. Peel Strength Evaluation
4.1.3. Flow through Experiment and Plate Method
4.2. Ex Vitro Methods
4.2.1. Adhesion Weight Method
4.2.2. Fluorescent Probe Methods
5. Challenges
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsiridis, E.; Upadhyay, N.; Giannoudis, P. Molecular Aspects of Fracture Healing: Which Are the Important Molecules? Int. J. Care Inj. 2007, 38S1, S11–S25. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Tsiridis, E.; Giannoudis, P.V. Current Concepts of Molecular Aspects of Bone Healing. Injury 2005, 36, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Marsell, R.; Einhorn, T.A. The Biology of Fracture Healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Barnes, G.L.; Kostenuik, P.J.; Gerstenfeld, L.C.; Einhorn, T.A. Growth Factor Regulation of Fracture Repair. J. Bone Miner. Res. 1999, 14, 1805–1815. [Google Scholar] [CrossRef]
- Al-Aql, Z.S.; Alagl, A.S.; Graves, D.T.; Gerstenfeld, L.C.; Einhorn, T.A. Molecular Mechanisms Controlling Bone Formation During Fracture Healing and Distraction Osteogenesis. J. Dent. Res. 2008, 87, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Brió Pérez, M.; Cao, C.; de Beer, S. Switching (Bio-) Adhesion and Friction in Liquid by Stimulus Responsive Polymer Coatings. Eur. Polym. J. 2021, 147, 110298. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Punia, S.; Dhakane-Lad, J.; Dhumal, S.; Changan, S.; Senapathy, M.; Berwal, M.K.; Sampathrajan, V.; Sayed, A.A.S.; et al. Plant-Based Proteins and Their Multifaceted Industrial Applications. Lwt 2022, 154, 112620. [Google Scholar] [CrossRef]
- Sardella, E.; Gristina, R.; Fanelli, F.; Veronico, V.; Da Ponte, G.; Kroth, J.; Fracassi, F.; Favia, P. How to Confer a Permanent Bio-Repelling and Bio-Adhesive Character to Biomedical Materials through Cold Plasmas. Appl. Sci. 2020, 10, 9101. [Google Scholar] [CrossRef]
- Majeed, H.; Rehman, K.; Ali, A.; Khalid, M.F.; Akash, M.S.H. Wound Healing Adhesives. In Green Adhesives; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 181–204. [Google Scholar]
- Xiang, L. Molecular Interaction and Adhesion Mechanisms of Mussel-Inspired Adhesive Coatings; University of Alberta: Alberta, Canada, 2020. [Google Scholar]
- Ramesh, M.; Kumar, L.R. Bioadhesives. In Green Adhesives: Preparation, Properties and Applications; John Wiley & Sons: Inc Hoboken, NJ, USA, 2020; pp. 145–163. [Google Scholar]
- Rathi, S.; Saka, R.; Domb, A.J.; Khan, W. Protein-Based Bioadhesives and Bioglues. Polym. Adv. Technol. 2019, 30, 217–234. [Google Scholar] [CrossRef]
- Bolghari, N.; Shahsavarani, H.; Anvari, M.; Habibollahi, H. A Novel Recombinant Chimeric Bio-Adhesive Protein Consisting of Mussel Foot Protein 3, 5, Gas Vesicle Protein A, and CsgA Curli Protein Expressed in Pichia Pastoris. AMB Express 2022, 12, 1–19. [Google Scholar] [CrossRef]
- Medeiros Borsagli, F.G.L.; Carvalho, I.C.; Mansur, H.S. Amino Acid-Grafted and N-Acylated Chitosan Thiomers: Construction of 3D Bio-Scaffolds for Potential Cartilage Repair Applications. Int. J. Biol. Macromol. 2018, 114, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Janarthanan, G.; Tran, H.N.; Cha, E.; Lee, C.; Das, D.; Noh, I. 3D Printable and Injectable Lactoferrin-Loaded Carboxymethyl Cellulose-Glycol Chitosan Hydrogels for Tissue Engineering Applications. Mater. Sci. Eng. C 2020, 113, 111008. [Google Scholar] [CrossRef] [PubMed]
- Shahryarimorad, K.; Alipour, A.; Honar, Y.S.; Abtahi, B. In Silico Prediction and in Vitro Validation of the Effect of PH on Adhesive Behaviour of the Fused CsgA - MFP3 Protein. AMB Express 2022, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, S.; Pourmadadi, M.; Yazdian, F.; Ghadami, A. The Synthesis and Characterization of Targeted Delivery Curcumin Using Chitosan-Magnetite-Reduced Graphene Oxide as Nano-Carrier. Int. J. Biol. Macromol. 2021, 186, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Alqosaibi, A.I. Nanocarriers for Anticancer Drugs: Challenges and Perspectives. Saudi J. Biol. Sci. 2022, 29, 103298. [Google Scholar] [CrossRef] [PubMed]
- Chopra, H.; Kumar, S.; Singh, I. Bioadhesive Hydrogels and Their Applications. In Bioadhesives in Drug Delivery; John Wiley & Sons: Inc Hoboken, NJ, USA, 2020; pp. 147–170. ISBN 9781119640240. [Google Scholar]
- Lyu, Q.; Peng, L.; Hong, X.; Fan, T.; Li, J.; Cui, Y.; Zhang, H.; Zhao, J. Smart Nano-Micro Platforms for Ophthalmological Applications: The State-of-the-Art and Future Perspectives. Biomaterials 2021, 270, 120682. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, J.; Wang, X.; Zhang, M.; Li, C.; Zhou, J. Recent Advances on Synthetic and Polysaccharide Adhesives for Biological Hemostatic Applications. Front. Bioeng. Biotechnol. 2020, 8, 926. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Chen, J.; Wang, J.; Zeng, H.; Yu, J. Recent Progress in Synthesis and Application of Mussel-Inspired Adhesives. Nanoscale 2020, 12, 1307–1324. [Google Scholar] [CrossRef]
- Bu, Y.; Pandit, A. Cohesion Mechanisms for Bioadhesives. Bioact. Mater. 2022, 13, 105–118. [Google Scholar] [CrossRef]
- Patil, N.A.; Kandasubramanian, B. Functionalized Polylysine Biomaterials for Advanced Medical Applications: A Review. Eur. Polym. J. 2021, 146, 110248. [Google Scholar] [CrossRef]
- Korde, J.M.; Kandasubramanian, B. Biocompatible Alkyl Cyanoacrylates and Their Derivatives as Bio-Adhesives. Biomater. Sci. 2018, 6, 1691–1711. [Google Scholar] [CrossRef] [PubMed]
- Al-Abassi, A.; Papini, M.; Towler, M. Review of Biomechanical Studies and Finite Element Modeling of Sternal Closure Using Bio-Active Adhesives. Bioengineering 2022, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.M. A Review of Calcium Phosphate Cements and Acrylic Bone Cements as Injectable Materials for Bone Repair and Implant Fixation. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019872594. [Google Scholar] [CrossRef]
- Chen, S.; Gil, C.J.; Ning, L.; Jin, L.; Perez, L.; Kabboul, G.; Tomov, M.L.; Serpooshan, V. Adhesive Tissue Engineered Scaffolds: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 683079. [Google Scholar] [CrossRef] [PubMed]
- Venturella, F. Development of Nontoxic Bio–Adhesives for Wet Environments; Università degli Studi di Palermo: Palermo, Italy, 2021. [Google Scholar]
- Gillman, N.; Lloyd, D.; Bindra, R.; Ruan, R.; Zheng, M. Surgical Applications of Intracorporal Tissue Adhesive Agents: Current Evidence and Future Development. Expert Rev. Med. Devices 2020, 17, 443–460. [Google Scholar] [CrossRef]
- Park, K.; Kim, S.; Jo, Y.; Park, J.; Kim, I.; Hwang, S.; Lee, Y.; Kim, S.Y.; Seo, J. Lubricant Skin on Diverse Biomaterials with Complex Shapes via Polydopamine-Mediated Surface Functionalization for Biomedical Applications. Bioact. Mater. 2022. [Google Scholar] [CrossRef]
- Zheng, D.; Ruan, H.; Chen, W.; Zhang, Y.; Cui, W.; Chen, H.; Shen, H. Advances in Extracellular Vesicle Functionalization Strategies for Tissue Regeneration. Bioact. Mater. 2022. [Google Scholar] [CrossRef]
- Liu, C.; Yu, Q.; Yuan, Z.; Guo, Q.; Liao, X.; Han, F.; Feng, T.; Liu, G.; Zhao, R.; Zhu, Z.; et al. Engineering the Viscoelasticity of Gelatin Methacryloyl (GelMA) Hydrogels via Small “Dynamic Bridges” to Regulate BMSC Behaviors for Osteochondral Regeneration. Bioact. Mater. 2022. [Google Scholar] [CrossRef]
- Shokri, M.; Dalili, F.; Kharaziha, M.; Baghaban Eslaminejad, M.; Ahmadi Tafti, H. Strong and Bioactive Bioinspired Biomaterials, next Generation of Bone Adhesives. Adv. Colloid Interface Sci. 2022, 305, 102706. [Google Scholar] [CrossRef]
- Wang, X.; Fang, X.; Gao, X.; Wang, H.; Li, S.; Li, C.; Qing, Y.; Qin, Y. Strong Adhesive and Drug-Loaded Hydrogels for Enhancing Bone–Implant Interface Fixation and Anti-Infection Properties. Colloids Surf. B Biointerfaces 2022, 219, 112817. [Google Scholar] [CrossRef]
- Du, J.; Zhou, Y.; Bao, X.; Kang, Z.; Huang, J.; Xu, G.; Yi, C.; Li, D. Surface Polydopamine Modification of Bone Defect Repair Materials: Characteristics and Applications. Front. Bioeng. Biotechnol. 2022, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Abourehab, M.A.S.; Rajendran, R.R.; Singh, A.; Pramanik, S.; Shrivastav, P.; Ansari, M.J.; Manne, R.; Amaral, L.S.; Deepak, A. Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art. Int. J. Mol. Sci. 2022, 23, 9035. [Google Scholar] [CrossRef] [PubMed]
- Bohara, S.; Suthakorn, J. Surface Coating of Orthopedic Implant to Enhance the Osseointegration and Reduction of Bacterial Colonization: A Review. Biomater. Res. 2022, 26, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Wang, J.; Cong, Y.; Fu, J. Recent Progress in Polymer Hydrogel Bioadhesives. J. Polym. Sci. 2021, 59, 1312–1337. [Google Scholar] [CrossRef]
- Espinoza-Ramirez, A.; Fuentes-Rodriguez, H.; Hernandez-Herrera, E.; Mora-Sandi, A.; Vega-Baudrit, J.R. Nanobiodiversity and Biomimetic Adhesives Development: From Nature to Production and Application. J. Biomater. Nanobiotechnol. 2019, 10, 78–101. [Google Scholar] [CrossRef]
- Zheng, G.; Cui, Y.; Lu, L.; Guo, M.; Hu, X.; Wang, L.; Yu, S.; Sun, S.; Li, Y.; Zhang, X.; et al. Microfluidic Chemostatic Bioreactor for High-Throughput Screening and Sustainable Co-Harvesting of Biomass and Biodiesel in Microalgae. Bioact. Mater. 2022, 1–11. [Google Scholar] [CrossRef]
- Samyn, P. A Platform for Functionalization of Cellulose, Chitin/Chitosan, Alginate with Polydopamine: A Review on Fundamentals and Technical Applications. Int. J. Biol. Macromol. 2021, 178, 71–93. [Google Scholar] [CrossRef]
- Dongre, R.S. Marine Polysaccharides in Pharmaceutical Uses; Springer: Berlin/Heidelberg, Germany, 2022; ISBN 9783030357344. [Google Scholar]
- Bashir, S.M.; Rather, G.A.; Patrício, A.; Haq, Z.; Sheikh, A.A.; Zahoor, M.; Singh, H.; Khan, A.A.; Imtiyaz, S.; Ahmad, S.B. Chitosan Nanoparticles: A Versatile Platform for Biomedical Applications. Materials 2022, 15, 6521. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Pramanik, S.; Abdelgawad, M.A.; Abualsoud, B.M.; Kadi, A.; Ansari, M.J.; Deepak, A. Recent Advances of Chitosan Formulations in Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 10975. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cui, G.; Dan, N.; Huang, Y.; Bai, Z.; Yang, C.; Dan, W. Preparation and Characterization of Dopamine–Sodium Carboxymethyl Cellulose Hydrogel. SN Appl. Sci. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Jaramillo, J.; Rodriguez-Oliva, I.; Abian, O.; Palomo, J.M. Specific Chemical Incorporation of L-DOPA and Functionalized l-DOPA-Hyaluronic Acid in Candida Antarctica Lipase: Creating Potential Mussel-Inspired Bioadhesives. SN Appl. Sci. 2020, 2, 1–12. [Google Scholar] [CrossRef]
- Lutz, T.M.; Kimna, C.; Casini, A.; Lieleg, O. Bio-Based and Bio-Inspired Adhesives from Animals and Plants for Biomedical Applications. Mater. Today Bio. 2022, 13, 100203. [Google Scholar] [CrossRef] [PubMed]
- Sun, J. Fabrication and Mechanical Properties of Supercharged Polypeptides Based Biomaterials: From Adhesives to Fibers; University of Groningen: The Netherlands, 2020. [Google Scholar]
- Pandey, N.; Soto-Garcia, L.F.; Liao, J.; Zimmern, P.; Nguyen, K.T.; Hong, Y. Mussel-Inspired Bioadhesives in Healthcare: Design Parameters, Current Trends, and Future Perspectives. Biomater. Sci. 2020, 8, 1240–1255. [Google Scholar] [CrossRef] [PubMed]
- Kang, V.; Lengerer, B.; Wattiez, R.; Flammang, P. Molecular Insights into the Powerful Mucus-Based Adhesion of Limpets (Patella Vulgata L.): Molecular Insights into Limpets Adhesion. Open Biol. 2020, 10, 200019. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Zhang, S.; Zhang, W.; Li, J.; Han, Y. Mussel-Inspired Polymer Materials Derived from Nonphytogenic and Phytogenic Catechol Derivatives and Their Applications. Polym. Int. 2021, 70, 1209–1224. [Google Scholar] [CrossRef]
- Shi, C.; Chen, X.; Zhang, Z.; Chen, Q.; Shi, D.; Kaneko, D. Mussel Inspired Bio-Adhesive with Multi-Interactions for Tissue Repair. J. Biomater. Sci. Polym. Ed. 2020, 31, 491–503. [Google Scholar] [CrossRef]
- Park, M.K.; Li, M.X.; Yeo, I.; Jung, J.; Yoon, B.I.L.; Joung, Y.K. Balanced Adhesion and Cohesion of Chitosan Matrices by Conjugation and Oxidation of Catechol for High-Performance Surgical Adhesives. Carbohydr. Polym. 2020, 248, 116760. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Li, S.; Pei, M.; Yang, H.; Gu, S.; Tao, Y.; Ye, D.; Zhou, Y.; Xu, W.; Xiao, P. Dopamine-Modified Hyaluronic Acid Hydrogel Adhesives with Fast-Forming and High Tissue Adhesion. ACS Appl. Mater. Interfaces 2020, 12, 18225–18234. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Zhang, J.; Wang, W.; Gong, L.; Zhang, L.; Yan, B.; Zeng, H. Nanomechanics of π-Cation-π Interaction with Implications for Bio-Inspired Wet Adhesion. Acta Biomater. 2020, 117, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Ventura, I.V.P. Characterization of Glycoproteins Involved in Sea Urchin Adhesion; University of Coimbra: Portugal, 2020. [Google Scholar]
- Capitain, C.; Wagner, S.; Hummel, J.; Tippkötter, N. Investigation of C–N Formation Between Catechols and Chitosan for the Formation of a Strong, Novel Adhesive Mimicking Mussel Adhesion. Waste Biomass Valorization 2021, 12, 1761–1779. [Google Scholar] [CrossRef]
- Kwon, Y.; Yang, D.H.; Lee, D. A Titanium Surface-Modified with Nano-Sized Hydroxyapatite and Simvastatin Enhances Bone Formation and Osseointegration. J. Biomed. Nanotechnol. 2015, 11, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Guo, C.; Dong, W.; He, F.; Zhao, S.; Yang, G. Effect of Thin Nano-Hydroxyapatite Coating on Implant Osseointegration in Ovariectomized Rats. Oral Maxillofac. Surg. 2012, 113, 48–53. [Google Scholar] [CrossRef]
- Pang, K.-M.; Lee, J.-K.; Seo, Y.-K.; Kim, S.-M.; Kim, M.-J.; Lee, J.-H. Biologic Properties of Nano-Hydroxyapatite: An in Vivo Study of Calvarial Defects, Ectopic Bone Formation and Bone Implantation. Biomed. Mater. Eng. 2015, 25, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Juliadmi, D.; Nuswantoro, N.F.; Fajri, H.; Indriyani, I.Y.; Affi, J.; Manjas, M.; Tjong, D.H.; Gunawarman. The Coating of Bovine-Source Hydroxyapatite on Titanium Alloy (Ti-6Al-4V ELI) Using Electrophoretic Deposition for Biomedical Application. Mater. Sci. Forum 2020, 1000, 97–106. [Google Scholar] [CrossRef]
- Kusrini, E.; Sontang, M. Characterization of X-Ray Diffraction and Electron Spin Resonance: Effects of Sintering Time and Temperature on Bovine Hydroxyapatite. Radiat. Phys. Chem. 2012, 81, 118–125. [Google Scholar] [CrossRef]
- Khandan, A.; Abdellahi, M.; Ozada, N.; Ghayour, H. Study of the Bioactivity, Wettability and Hardness Behaviour of the Bovine Hydroxyapatite-Diopside Bio-Nanocomposite Coating. J. Taiwan Inst. Chem. Eng. 2016, 60, 538–546. [Google Scholar] [CrossRef]
- Mihailescu, N.; Stan, G.E.; Duta, L.; Chifiriuc, C.M.; Bleotu, C.; Sopronyi, M.; Luculescu, C.; Oktar, F.N.; Mihailescu, I.N. Structural, Compositional, Mechanical Characterization and Biological Assessment of Bovine-Derived Hydroxyapatite Coatings Reinforced with MgF 2 or MgO for Implants Functionalization. Mater. Sci. Eng. C 2016, 59, 863–874. [Google Scholar] [CrossRef]
- Gunawarman; Mulyadi, I.H.; Arif, Z.; Nuswantoro, N.F.; Affi, J.; Niinomi, M. Effect of Particle Size on Adhesion Strength of Bovine Hydroxyapatite Layer on Ti-12Cr Coated by Using Electrophoretic Deposition (EPD) Method. In Proceedings of the 2nd Conference on Innovation in Technology (CITES 2020), Padang, Indonesia, 4–5 November 2020; pp. 1–8. [Google Scholar]
- Fajri, H.; Ramadhan, F.; Nuswantoro, N.F.; Juliadmi, D.; Tjong, D.H.; Manjas, M.; Affi, J.; Yetri, Y.; Gunawarman. Electrophoretic Deposition (EPD) of Natural Hydroxyapatite Coatings on Titanium Ti-29Nb-13Ta-4. 6Zr Substrates for Implant Material. Mater. Sci. Forum 2020, 1000, 123–131. [Google Scholar] [CrossRef]
- Gunawarman; Affi, J.; Yetri, Y.; Ilhamdi; Juliadmi, D.; Nuswantoro, N.F.; Fajri, H.; Ahli, A.; Gundini, R.; Nur, H. Synthesis and Characterization of Calcium Precursor for Hydroxyapatite Synthesis from Blood Clam Shell (Anadara Antiquata) Using Planetary Ball Mill Process. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Padang, Indonesia, 8–9 November 2018; pp. 1–6. [Google Scholar]
- Khalili, V.; Khalil-allafi, J.; Xia, W.; Parsa, A.B.; Frenzel, J.; Somsen, C.; Eggeler, G. Preparing Hydroxyapatite-Silicon Composite Suspensions with Homogeneous Distribution of Multi-Walled Carbon Nano-Tubes for Electrophoretic Coating of NiTi Bone Implant and Their Effect on the Surface Morphology. Appl. Surf. Sci. 2016, 366, 158–165. [Google Scholar] [CrossRef]
- Gunawarman; Nuswantoro, N.F.; Juliadmi, D.; Fajri, H.; Budiman, A.; Djong, H.T.; Manjas, M. Hydroxyapatite Coatings on Titanium Alloy TNTZ Using Electrophoretic Deposition. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Padang, Indonesia, 8–9 November 2018; pp. 1–11. [Google Scholar]
- Juliadmi, D.; Fauzi, V.R.; Gunawarman; Nur, H.; Idris, M.H. Hydroxyapatite Coating on Titanium Alloy Ti-6Al-4V with Electrophoretic Deposition (EPD) for Dental Root Application. Int. J. Adv. Sci. Eng. Informational Technol. 2017, 7, 2152–2158. [Google Scholar] [CrossRef]
- Łukaszewska-Kuska, M.; Krawczyk, P.; Martyla, A.; Hędzelek, W.; Dorocka-bobkowska, B.; Dorocka-bobkowska, B. Hydroxyapatite Coating on Titanium Endosseous Implants for Improved Osseointegration: Physical and Chemical Considerations Address for Correspondence. Adv. Clin. Exper. Med. 2018, 27, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Xu, X.; Feng, X.; Deng, X.; Wu, S.; Liu, X.; Yang, C. Gold Nanoparticles-Loaded Hydroxyapatite Composites Guide Osteogenic Differentiation of Human Mesenchymal Stem Cells through Wnt/β–Catenin Signaling Pathway. Int. J. Nanomed. 2019, 14, 6151–6163. [Google Scholar] [CrossRef] [PubMed]
- Nuswantoro, N.F.; Juliadmi, D.; Fajri, H.; Manjas, M.; Suharti, N.; Tjong, D.H.; Affi, J.; Gunawarman. Electrophoretic Deposition Performance of Hydroxyapatite Coating on Titanium Alloys for Orthopedic Implant Application. Mater. Sci. Forum 2020, 1000, 69–81. [Google Scholar] [CrossRef]
- Nuswantoro, N.F.; Manjas, M.; Suharti, N.; Juliadmi, D.; Fajri, H.; Tjong, D.H.; Affi, J.; Niinomi, M.; Gunawarman. Hydroxyapatite Coating on Titanium Alloy TNTZ for Increasing Osseointegration and Reducing Inflammatory Response in Vivo on Rattus Norvegicus Wistar Rats. Ceram. Int. 2021, 47, 16094–16100. [Google Scholar] [CrossRef]
- Nuswantoro, N.F.; Gunawarman; Saputra, M.R.; Nanda, I.P.; Idris, M.H.; Arafat, A. Microstructure Analysis of Hydroxyapatite Coating on Stainless Steel 316L Using Investment Casting Technique for Implant Application. Int. J. Adv. Sci. Eng. Inform. Technol. 2018, 8, 2168–2174. [Google Scholar] [CrossRef]
- Beig, B.; Liaqat, U.; Niazi, M.F.K.; Douna, I.; Zahoor, M.; Niazi, M.B.K. Current Challenges and Innovative Developments in Hydroxyapatite-Based Coatings on Metallic Materials for Bone Implantation: A Review. Coatings 2020, 10, 1249. [Google Scholar] [CrossRef]
- Bose, S.; Tarafder, S.; Bandyopadhyay, A. Hydroxyapatite Coatings for Metallic Implants. In Hydroxyapatite (HAP) for Biomedical Applications. Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 7, pp. 143–157. ISBN 9781782420330. [Google Scholar]
- Nuswantoro, N.F.; Maulana, I.; Djong, H.T.; Manjas, M.; Gunawarman. Hydroxyapatite Coating on New Type Titanium, TNTZ, Using Electrophoretic Deposition. J. Ocean. Mech. Aerosp. 2018, 56, 1–4. [Google Scholar]
- Gunawarman; Affi, J.; Sutanto, A.; Putri, D.M.; Juliadmi, D.; Nuswantoro, N.F.; Fajri, H.; Tjong, D.H.; Manjas, M. Adhesion Strength of Hydroxyapatite Coating on Titanium Alloy (Ti-6Al- 4V ELI) for Biomedical Application. In Proceedings of the International Colloquium on Computational and Experimental Mechanics (ICCEM 2020), Selangor, Malaysia, 25–26 June 2020; pp. 1–9. [Google Scholar]
- Araghi, A.; Hadianfard, M.J. Fabrication and Characterization of Functionally Graded Hydroxyapatite/TiO2 Multilayer Coating on Ti-6Al-4V Titanium Alloy for Biomedical Applications. Ceram. Int. 2015, 41, 12668–12679. [Google Scholar] [CrossRef]
- Nuswantoro, N.F.; Budiman, I.; Septiawarman, A.; Djong, H.T.; Manjas, M.; Gunawarman. Effect of Applied Voltage and Coating Time on Nano Hydroxyapatite Coating on Titanium Alloy Ti6Al4V Using Electrophoretic Deposition for Orthopaedic Implant Application. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Bandung, Indonesia, 6–7 September 2018; Volume 547, pp. 1–11. [Google Scholar]
- Bovand, D.; Allazadeh, M.R.; Rasouli, S.; Khodadad, E.; Borhani, E. Studying the Effect of Hydroxyapatite Particles in Osteoconductivity of Ti-HA Bioceramic. J. Aust. Ceram. Soc. 2019, 55, 395–403. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, W.; Zheng, Y.; Wang, H.; Sun, Y.; Zhang, Y.; Luo, J.; Zhang, H. Self-Adhesive Lubricated Coating for Enhanced Bacterial Resistance. Bioact. Mater. 2021, 6, 2535–2545. [Google Scholar] [CrossRef] [PubMed]
- Alinavaz, S.; Mahdavinia, G.R.; Jafari, H.; Hazrati, M.; Akbari, A. Hydroxyapatite (HA)-Based Hybrid Bionanocomposite Hydrogels: Ciprofloxacin Delivery, Release Kinetics and Antibacterial Activity. J. Mol. Struct. 2021, 1225, 129095. [Google Scholar] [CrossRef]
- Albu, A.M.; Draghicescu, W.; Munteanu, T.; Ion, R.; Mitran, V.; Cimpean, A.; Popescu, S.; Pirvu, C. Nitrodopamine vs Dopamine as an Intermediate Layer for Bone Regeneration Applications. Mater. Sci. Eng. C 2019, 98, 461–471. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, J.; Li, B.; Li, D.; Meng, Z.; Sun, S.K. Biocompatible Therapeutic Albumin/Genipin Bioglue for Postoperative Wound Adhesion and Residual Tumor Ablation. Biomaterials 2021, 279, 121179. [Google Scholar] [CrossRef] [PubMed]
- Vargas Villanueva, J.G.; Sarmiento Huertas, P.A.; Galan, F.S.; Esteban Rueda, R.J.; Briceño Triana, J.C.; Casas Rodriguez, J.P. Bio-Adhesion Evaluation of a Chitosan-Based Bone Bio-Adhesive. Int. J. Adhes. Adhes. 2019, 92, 80–88. [Google Scholar] [CrossRef]
- Lu, D.; Wang, H.; Wang, X.; Li, Y.; Guo, H.; Sun, S.; Zhao, X.; Yang, Z.; Lei, Z. Biomimetic Chitosan-Graft-Polypeptides for Improved Adhesion in Tissue and Metal. Carbohydr. Polym. 2019, 215, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Nayal, A.; Malhotra, S.; Koul, V. Dual Functionalized Chitosan Based Composite Hydrogel for Haemostatic Efficacy and Adhesive Property. Carbohydr. Polym. 2020, 247, 116757. [Google Scholar] [CrossRef]
- Lei, H.; Xiong, M.; Xiao, J.; Zheng, L.; Zhuang, Q. Fluorine-Free Coating with Low Surface Energy and Anti-Biofouling Properties. Prog. Org. Coatings 2018, 124, 158–164. [Google Scholar] [CrossRef]
- Ma, L.; Cheng, S.; Ji, X.; Zhou, Y.; Zhang, Y.; Li, Q.; Tan, C.; Peng, F.; Zhang, Y.; Huang, W. Immobilizing Magnesium Ions on 3D Printed Porous Tantalum Scaffolds with Polydopamine for Improved Vascularization and Osteogenesis. Mater. Sci. Eng. C 2020, 117, 111303. [Google Scholar] [CrossRef]
- Smith, J.A.; Mele, E.; Rimington, R.P.; Capel, A.J.; Lewis, M.P.; Silberschmidt, V.V.; Li, S. Polydimethylsiloxane and Poly(Ether) Ether Ketone Functionally Graded Composites for Biomedical Applications. J. Mech. Behav. Biomed. Mater. 2019, 93, 130–142. [Google Scholar] [CrossRef]
- Kazi, G.A.S.; Yamamoto, O. Effectiveness of the Sodium Alginate as Surgical Sealant Materials. Wound Med. 2019, 24, 18–23. [Google Scholar] [CrossRef]
- Shirzaei Sani, E.; Portillo Lara, R.; Aldawood, Z.; Bassir, S.H.; Nguyen, D.; Kantarci, A.; Intini, G.; Annabi, N. An Antimicrobial Dental Light Curable Bioadhesive Hydrogel for Treatment of Peri-Implant Diseases. Matter 2019, 1, 926–944. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.J.S. Development of Poly (2-Oxazoline) -Based Bone-Adhesive Biomaterials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021. [Google Scholar]
- Wales, D.J.; Keshavarz, M.; Howe, C.; Yeatman, E. 3D Printability Assessment of Poly(Octamethylene Maleate (Anhydride) Citrate) and Poly(Ethylene Glycol) Diacrylate Copolymers for Biomedical Applications. Appl. Polym. Mater. 2022. [Google Scholar] [CrossRef]
- Feng, C.; Wang, F.; Xu, Z.; Sui, H.; Fang, Y.; Tang, X.; Shen, X. Characterization of Soybean Protein Adhesives Modified by Xanthan Gum. Coatings 2018, 8, 342. [Google Scholar] [CrossRef]
- Xue, B.; Gu, J.; Li, L.; Yu, W.; Yin, S.; Qin, M.; Jiang, Q.; Wang, W.; Cao, Y. Hydrogel Tapes for Fault-Tolerant Strong Wet Adhesion. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.J.; Yoon, S.J.; Koo, J.H.; Yoon, Y.; Byun, H.J.; Kim, H.S.; Khang, G.; Chun, H.J.; Yang, D.H. β-Cyclodextrin/Triclosan Complex-Grafted Methacrylated Glycol Chitosan Hydorgel by Photocrosslinking via Visible Light Irradiation for a Tissue Bio-Adhesive. Int. J. Mol. Sci. 2021, 22, 700. [Google Scholar] [CrossRef]
- Negrescu, A.M.; Mitran, V.; Draghicescu, W.; Popescu, S.; Pirvu, C.; Ionascu, I.; Soare, T.; Uzun, S.; Croitoru, S.M.; Cimpean, A. TiO2 Nanotubes Functionalized with Icariin for an Attenuated In Vitro Immune Response and Improved In Vivo Osseointegration. J. Funct. Biomater. 2022, 13, 43. [Google Scholar] [CrossRef]
- Scalzone, A.; Bonifacio, M.A.; Cometa, S.; Cucinotta, F.; De Giglio, E.; Ferreira, A.M.; Gentile, P. PH-Triggered Adhesiveness and Cohesiveness of Chondroitin Sulfate-Catechol Biopolymer for Biomedical Applications. Front. Bioeng. Biotechnol. 2020, 8, 1–14. [Google Scholar] [CrossRef]
- Zou, C.Y.; Lei, X.X.; Hu, J.J.; Jiang, Y.L.; Li, Q.J.; Song, Y.T.; Zhang, Q.Y.; Li-Ling, J.; Xie, H.Q. Multi-Crosslinking Hydrogels with Robust Bio-Adhesion and pro-Coagulant Activity for First-Aid Hemostasis and Infected Wound Healing. Bioact. Mater. 2022, 16, 388–402. [Google Scholar] [CrossRef]
- Yu, J.; Qin, Y.; Yang, Y.; Zhao, X.; Zhang, Z.; Zhang, Q.; Su, Y.; Zhang, Y.; Cheng, Y. Robust Hydrogel Adhesives for Emergency Rescue and Gastric Perforation Repair. Bioact. Mater. 2022, 19, 703–716. [Google Scholar] [CrossRef]
- Tabatabaei, F.; Rasoulianboroujeni, M.; Yadegari, A.; Tajik, S.; Moharamzadeh, K.; Tayebi, L. Osteo-Mucosal Engineered Construct: In Situ Adhesion of Hard-Soft Tissues. Mater. Sci. Eng. C 2021, 128, 112255. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Wang, X.; Yue, O.; Zheng, M.; Zhang, H.; Liu, X. Development of a Multifunctional Injectable Temperature-Sensitive Gelatin-Based Adhesive Double-Network Hydrogel. Mater. Sci. Eng. C 2021, 134, 112556. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liang, C.; Wei, Z.; Bai, Y.; Bhaduri, S.B.; Webster, T.J.; Bian, L.; Yang, L. Injectable Biomaterials for Translational Medicine. Mater. Today 2019, 28, 81–97. [Google Scholar] [CrossRef]
- Xu, R.; Yu, F.; Huang, L.; Zhou, W.; Wang, Y.; Wang, F.; Sun, X.; Chang, G.; Fang, M.; Zhang, L.; et al. Isocyanate-Terminated Urethane-Based Dental Adhesive Bridges Dentinal Matrix Collagen with Adhesive Resin. Acta Biomater. 2019, 83, 140–152. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, J.N.; Lee, J.; Lee, H.; Park, W.H. Enzymatically Cross-Linked Poly(γ-Glutamic Acid) Hydrogel with Enhanced Tissue Adhesive Property. ACS Biomater. Sci. Eng. 2020, 6, 3103–3113. [Google Scholar] [CrossRef]
- Tao, C.; Jin, M.; Yao, H.; Wang, D.A. Dopamine Based Adhesive Nano-Coatings on Extracellular Matrix (ECM) Based Grafts for Enhanced Host-Graft Interfacing Affinity. Nanoscale 2021, 13, 18148–18159. [Google Scholar] [CrossRef]
- Zhang, Y.; Debenedictis, E.P.; Keten, S. Cohesive and Adhesive Properties of Crosslinked Semiflexible Biopolymer Networks. Soft Matter 2019, 15, 3807–3816. [Google Scholar] [CrossRef]
- Wang, A. Molecular Mechanisms Governing the Mechanics of Polymeric and Protein-Based Materials. Adv. Drug Del. Rev. 2021. [Google Scholar]
- Liu, H.; Yuan, M.; Sonamuthu, J.; Yan, S.; Huang, W.; Cai, Y.; Yao, J. A Dopamine-Functionalized Aqueous-Based Silk Protein Hydrogel Bioadhesive for Biomedical Wound Closure. New J. Chem. 2020, 44, 884–891. [Google Scholar] [CrossRef]
- Wang, H.; Su, X.; Chai, Z.; Tian, Z.; Xie, W.; Wang, Y.; Wan, Z.; Deng, M.; Yuan, Z.; Huang, J. A Hydra Tentacle-Inspired Hydrogel with Underwater Ultra-Stretchability for Adhering Adipose Surfaces. Chem. Eng. J. 2022, 428, 131049. [Google Scholar] [CrossRef]
- Zussma, M.; Giladi, S.; Zilberman, M. In Vitro Characterization of Injectable Chlorhexidine-Eluting Gelatin Hydrogels for Local Treatment of Periodontal Infections. Polym. Adv. Technol. 2022, 1–12. [Google Scholar]
- Eshkol-Yogev, I.; Tobias, T.; Keren, A.; Gilhar, A.; Gilboa, E.; Furer, A.; Ullman, Y.; Zilberman, M. Dual Composite Bioadhesives for Wound Closure Applications: An in Vitro and in Vivo Study. Polym. Adv. Technol. 2022, 1–16. [Google Scholar] [CrossRef]
- Karami, P. Design and Development of Injectable, Tough and Intrinsically-Adhesive Hydrogels for Biomedical Applications. EPFL 2021. [Google Scholar]
- Lu, X.; Shi, S.; Li, H.; Gerhard, E.; Lu, Z.; Tan, X.; Li, W.; Rahn, K.M.; Xie, D.; Xu, G.; et al. Magnesium Oxide-Crosslinked Low-Swelling Citrate-Based Mussel-Inspired Tissue Adhesives. Biomaterials 2020, 232, 119719. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, P.; Yang, C.; Duan, H.; Hong, W. Switchable Adhesion between Hydrogels by Wrinkling. Extrem. Mech. Lett. 2021, 43, 101193. [Google Scholar] [CrossRef]
- Li, A.; Xu, Z.; Sun, N.; Si, Z.; Xu, Y.; Guo, X. Cellulose-Reinforced Catechol-Modified Polyacrylic Acid-Zn2+ Coacervate as Strong Composite Adhesive. J. Appl. Polym. Sci. 2019, 136, 3–9. [Google Scholar] [CrossRef]
- Moini, N.; Khaghanipour, M.; Kabiri, K.; Salimi, A.; Zohuriaan-Mehr, M.J.; Jahandideh, A. Engineered Green Adhesives Based on Demands: Star-Shaped Glycerol-Lactic Acid Oligomers in Anaerobic Adhesives. ACS Sustain. Chem. Eng. 2019, 7, 16247–16256. [Google Scholar] [CrossRef]
- Xu, X.; Sui, B.; Liu, X.; Sun, J. A Bioinspired and High-Strengthed Hydrogel for Regeneration of Perforated Temporomandibular Joint Disc: Construction and Pleiotropic Immunomodulatory Effects. Bioact. Mater. 2022. [Google Scholar] [CrossRef]
- Yi, B.; Zhou, B.; Song, Z.; Yu, L.; Wang, W.; Liu, W. Step-Wise CAG@PLys@PDA-Cu2+ Modification on Micropatterned Nanofibers for Programmed Endothelial Healing. Bioact. Mater. 2022. [Google Scholar] [CrossRef]
- Chávez-Villarreal, A.; de los Ángeles Andrea Carvajal-Montes de Oca, M.; Garza-Enríquez, M.; Elizondo-Cantú, O. The Use of Cyanoacrylate in Surgical Procedure in Periodontics: A Literature Review. Int. J. Appl. Dent. Sci. 2019, 5, 330–332. [Google Scholar]
- Anupama Devi, V.K.; Shyam, R.; Palaniappan, A.; Jaiswal, A.K.; Oh, T.H.; Nathanael, A.J. Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications. Polymers 2021, 13, 3782. [Google Scholar] [CrossRef] [PubMed]
- Khadem, E.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Das, O.; Berto, F. Cutting-Edge Progress in Stimuli-Responsive Bioadhesives: From Synthesis to Clinical Applications . Polymers 2022, 14, 1709. [Google Scholar] [CrossRef] [PubMed]
- Hyldbakk, A.; Mørch, Y.; Snipstad, S.; Åslund, A.K.O.; Klinkenberg, G.; Nakstad, V.T.; Wågbø, A.M.; Schmid, R.; Molesworth, P.P. Identification of Novel Cyanoacrylate Monomers for Use in Nanoparticle Drug Delivery Systems Prepared by Miniemulsion Polymerisation—A Multistep Screening Approach. Int. J. Pharm. X 2022, 4, 100124. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhuang, B.; Wang, X.; Wu, Z.; Wei, W.; Aladejana, J.T.; Hou, X.; Yves, K.G.; Xie, Y.; Liu, J. Chitosan Used as a Specific Coupling Agent to Modify Starch in Preparation of Adhesive Film. J. Clean. Prod. 2020, 277, 123210. [Google Scholar] [CrossRef]
- Salama, A.H.; Abdelkhalek, A.A.; Elkasabgy, N.A. Etoricoxib-Loaded Bio-Adhesive Hybridized Polylactic Acid-Based Nanoparticles as an Intra-Articular Injection for the Treatment of Osteoarthritis. Int. J. Pharm. 2020, 578, 119081. [Google Scholar] [CrossRef] [PubMed]
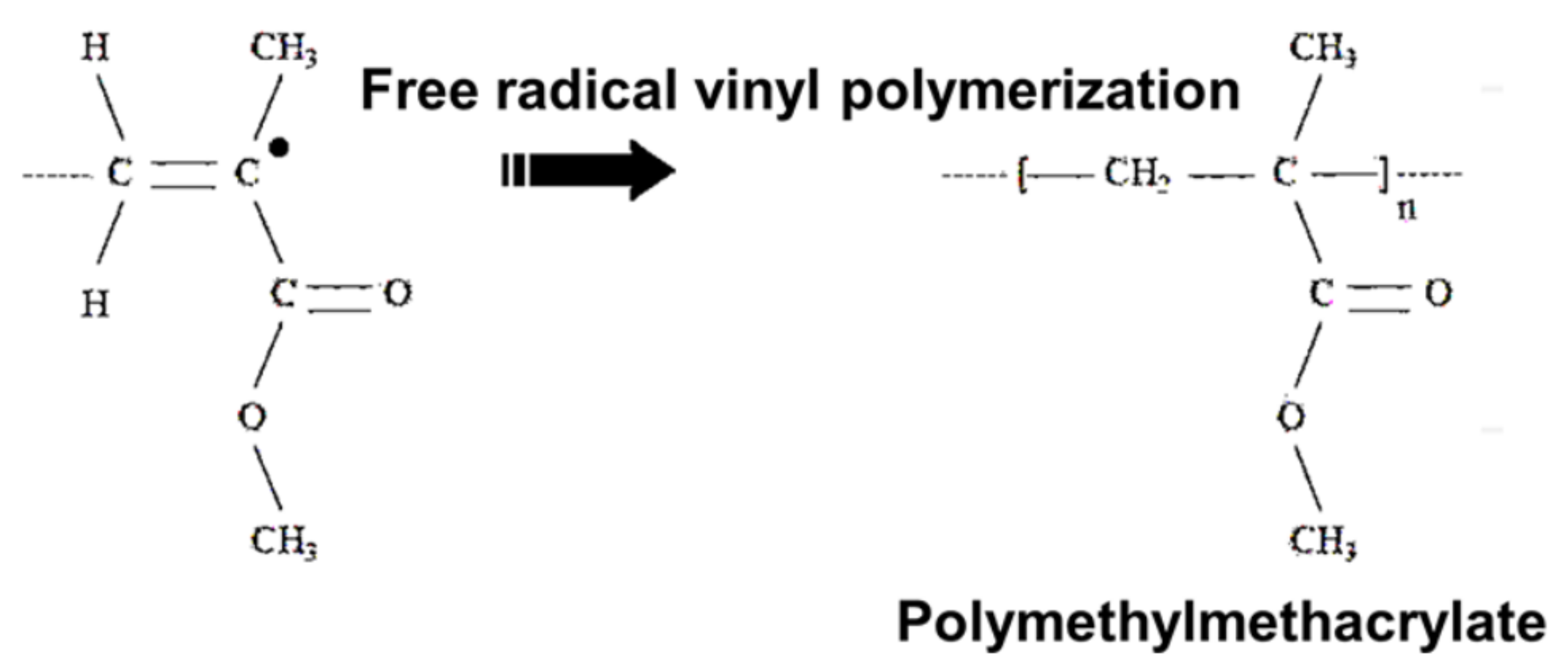
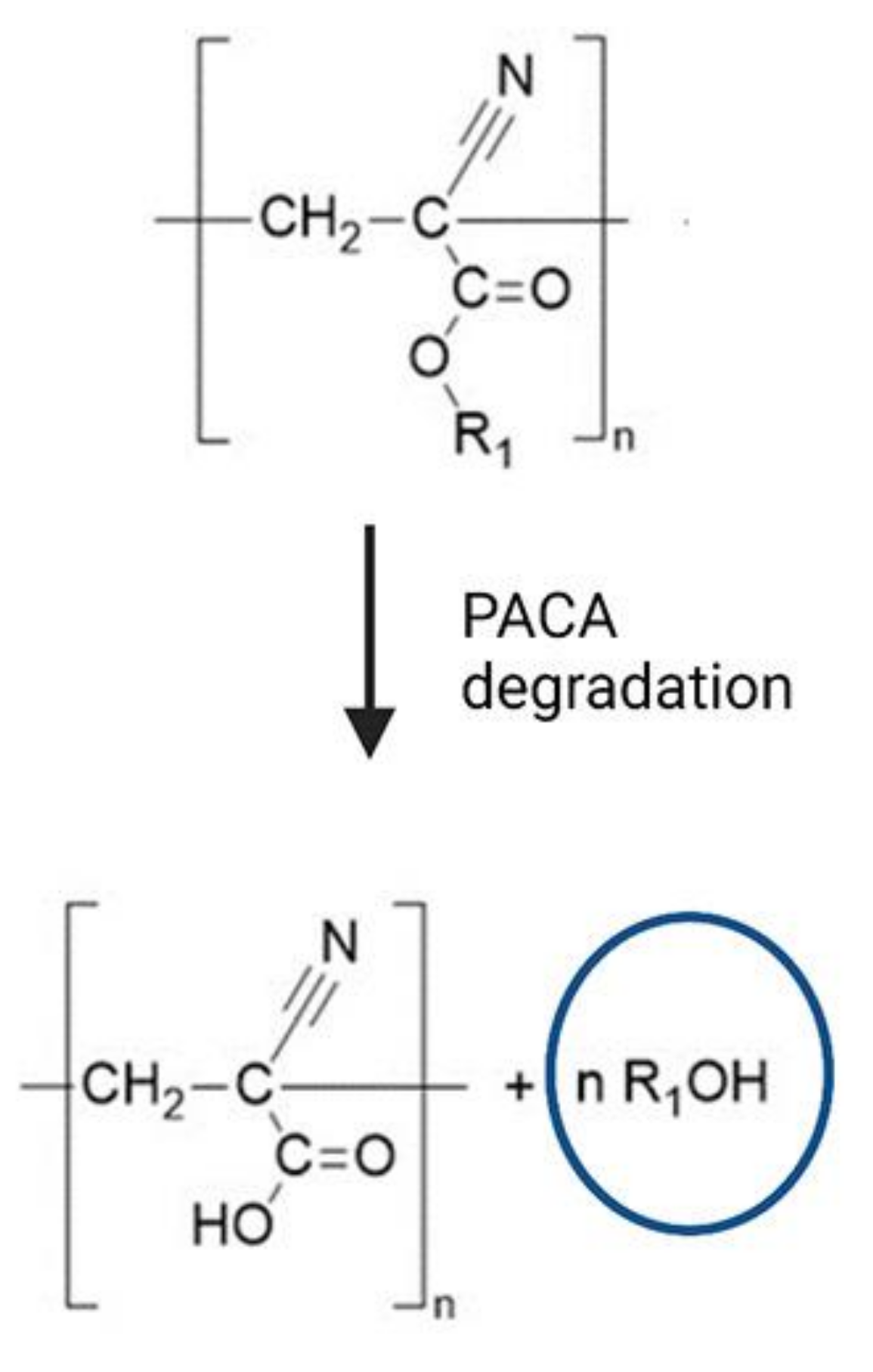
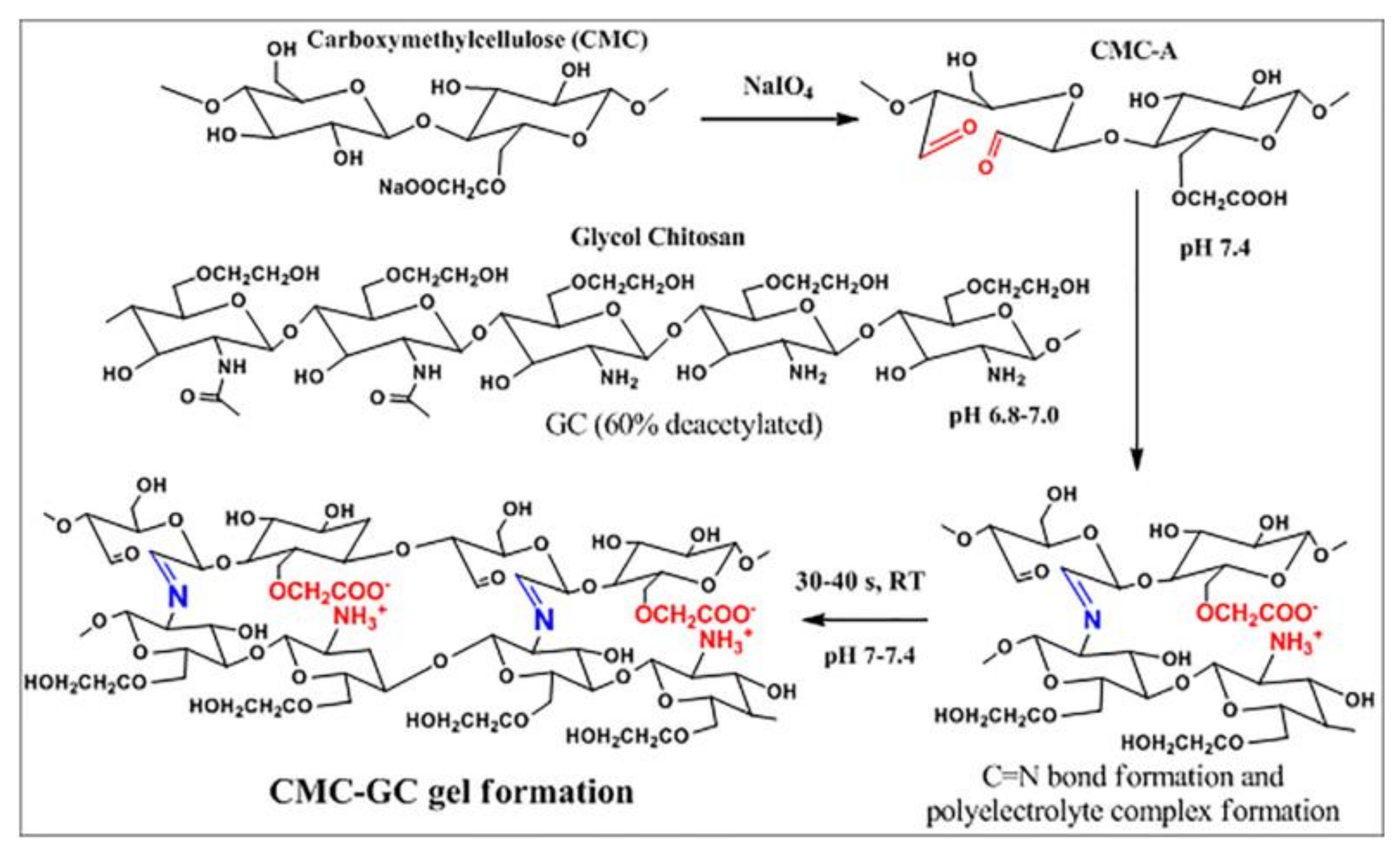
| No. | Materials | Mechanical Properties | Preparation | Applications | Reference |
|---|---|---|---|---|---|
| 1. |
|
| Polymerization |
| [84] |
| 2. |
|
| Polymerization | Bone Scaffold | [85] |
| 3. |
|
| Polymerization | Implant Coating | [86] |
| 4. |
|
| Polymerization | Tissue glue | [87] |
| 5. |
|
| Cross-linking | Bone glue | [88] |
| 6. |
|
| Ring opening polymerization | Bone glue | [89] |
| 7. |
|
| Cross-linking | Bone Glue | [90] |
| 8. |
|
| Polymerization | Anti-bacterial implant coating | [91] |
| 9. |
|
| 3D Printing | Scaffold and drug release | [92] |
| 10. |
|
| Polymerization |
| [93] |
| 11. |
|
| Cross-linking | Tissue engineering | [94] |
| 12. |
|
| Cross-linking | Scaffold for teeth | [95] |
| 13. |
|
| Polymerization | Bone implant | [96] |
| 14. |
|
|
| Scaffold | [97] |
| 15. |
|
| Polymerization | Bone adhesive | [98] |
| 16. |
|
| Cross-linking |
| [99] |
| 17. |
|
| Photo-cross-linking via Visible Light Irradiation | Tissue bio-adhesive and anti-bacterial | [100] |
| 18. |
|
| Electrochemical Anodization | Bone implant osseointegration | [101] |
| 19. |
|
| Carbodiimide coupling reaction | Soft tissue engineering | [102] |
| 20. |
|
|
|
| [103] |
| 21. |
|
| Polymerization |
| [104] |
| 22. |
|
| Melt grafting | Implant Coating | [86] |
| 23. |
|
| Cross-linking | Bone scaffold | [105] |
| 24. |
|
|
| Wound healing | [106] |
| 25. |
|
|
|
| [107] |
| 26. |
|
| Polymerization | Orthodontic | [108] |
| 27. |
|
| Polymerization |
| [56] |
| 28. |
|
| Cross-linking | Tissue adhesive | [109] |
| 29. |
|
| Coupling reaction |
| [110] |
| 30. |
|
|
|
| [111] |
| 31. |
|
| Polymerization | Tissue engineering | [112] |
| 32. |
|
| Cross-linking | Wound healing | [55] |
| 33. |
|
| Cross-linking | Wound healing | [113] |
| 34. |
|
|
| Tissue adhesive | [52] |
| 35. |
|
| Genetic engineering | Tissue adhesive | [51] |
| 36. |
|
| Cross-linking |
| [114] |
| 37. |
|
| Cross-linking |
| [115] |
| 38. |
|
| Cross-linking | Bone Bio-Adhesive | [88] |
| 39. |
|
|
| Surgical Adhesive | [54] |
| 40. |
|
| Cross-linking |
| [53] |
| 41. |
|
|
| Tissue adhesive | [47] |
| 42. |
|
|
|
| [49] |
| 43. |
|
| Cross-linking | Wound healing | [116] |
| 44. |
|
| Cross-linking | Tissue engineering | [117] |
| 45. |
|
| Cross-linking | Tissue engineering | [118] |
| 46. |
|
| Cross-linking | Tissue engineering | [119] |
| 47. |
|
| 3D printing |
| [105] |
| 48. |
|
| Amidation reaction | Implant coating | [120] |
| 49. |
|
| Condensation reaction of glycerin and LA | Biomedical application | [121] |
| 50. |
|
| Cross-linking | Spinal sealant | [30] |
| 51. |
|
|
| Wound healing | [46] |
| 52. |
|
| Michael-type addition | Bio-glue | [58] |
| 53. |
|
| Cross-linking |
| [50] |
| 54. |
|
| Cross-linking | temporomandibular joint disc | [122] |
| 55. |
|
| step-wise modifi- cation of parallel-microgroove-patterned | Endothelial healing | [123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuswantoro, N.F.; Lubis, M.A.R.; Juliadmi, D.; Mardawati, E.; Antov, P.; Kristak, L.; Hua, L.S. Bio-Based Adhesives for Orthopedic Applications: Sources, Preparation, Characterization, Challenges, and Future Perspectives. Designs 2022, 6, 96. https://doi.org/10.3390/designs6050096
Nuswantoro NF, Lubis MAR, Juliadmi D, Mardawati E, Antov P, Kristak L, Hua LS. Bio-Based Adhesives for Orthopedic Applications: Sources, Preparation, Characterization, Challenges, and Future Perspectives. Designs. 2022; 6(5):96. https://doi.org/10.3390/designs6050096
Chicago/Turabian StyleNuswantoro, Nuzul Ficky, Muhammad Adly Rahandi Lubis, Dian Juliadmi, Efri Mardawati, Petar Antov, Lubos Kristak, and Lee Seng Hua. 2022. "Bio-Based Adhesives for Orthopedic Applications: Sources, Preparation, Characterization, Challenges, and Future Perspectives" Designs 6, no. 5: 96. https://doi.org/10.3390/designs6050096
APA StyleNuswantoro, N. F., Lubis, M. A. R., Juliadmi, D., Mardawati, E., Antov, P., Kristak, L., & Hua, L. S. (2022). Bio-Based Adhesives for Orthopedic Applications: Sources, Preparation, Characterization, Challenges, and Future Perspectives. Designs, 6(5), 96. https://doi.org/10.3390/designs6050096










