Pathways to Alzheimer’s Disease: The Intersecting Roles of Clusterin and Apolipoprotein E in Amyloid-β Regulation and Neuronal Health
Abstract
1. Introduction
2. Clusterin and Alzheimer’s Disease
2.1. Interactions of CLU with Aβ
2.2. Role of CLU in Aβ Clearance from the Brain
2.3. Cellular Risk Factors Associated with CLU Protein in Alzheimer’s Disease: Implications for Lipid Metabolism, Homeostasis, and Neuronal Apoptosis
3. APOE and Alzheimer’s Disease
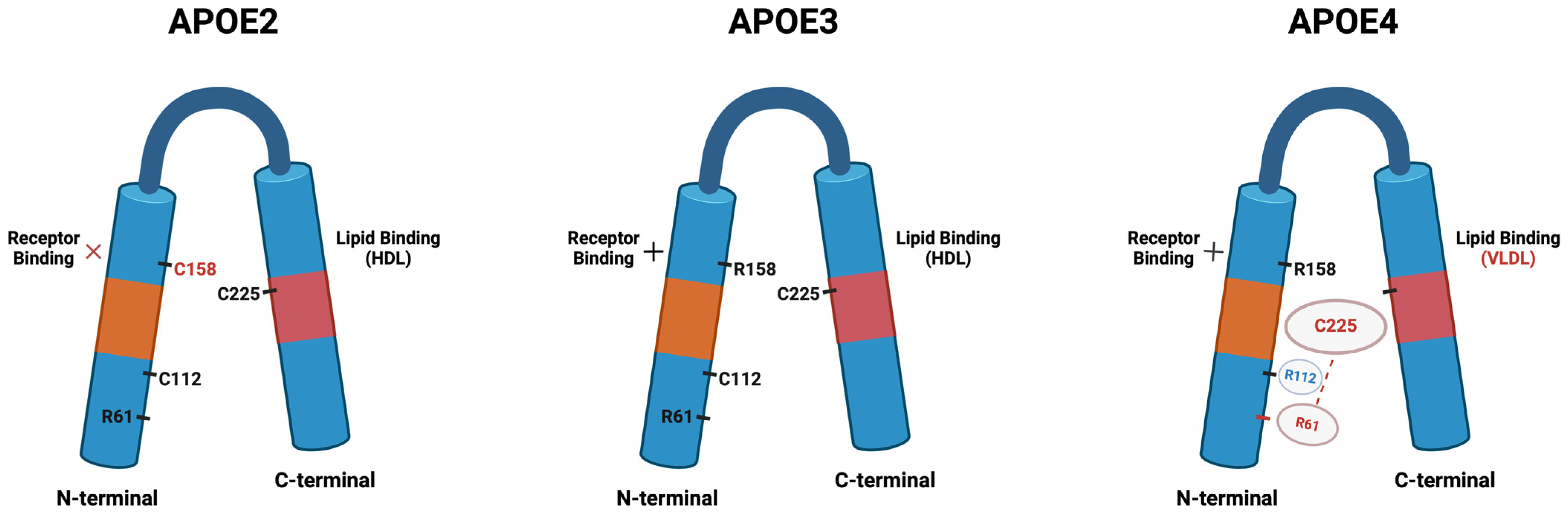
3.1. APOE–Isoforms and Structure
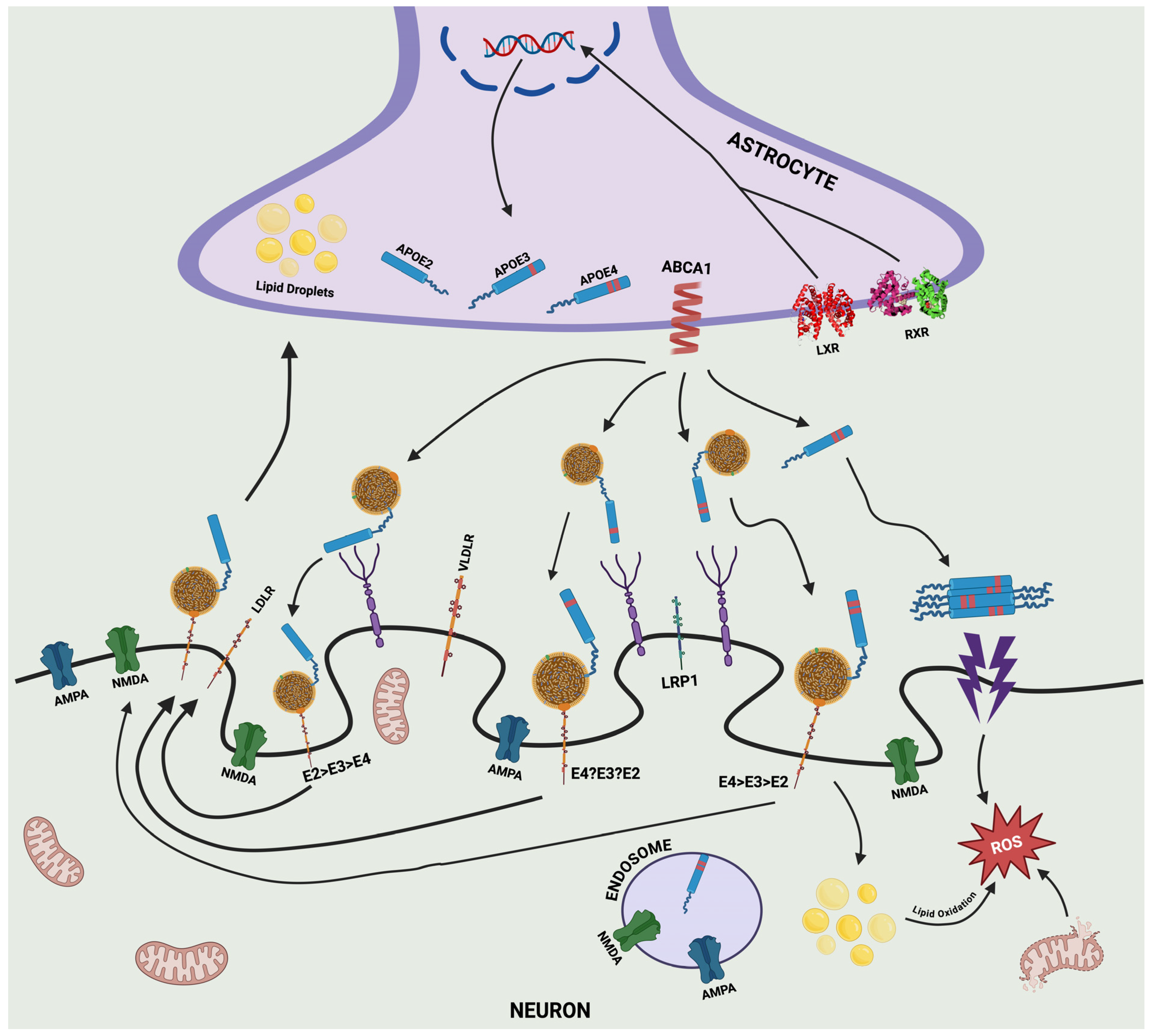
3.2. APOE–Secretion and Production
3.3. Distribution of APOE in the Blood, Brain, and Cerebrospinal Fluid
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Association. 2014 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. J. Alzheimers Assoc. 2014, 10, e47–e92. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Miners, J.S.; Clarke, P.; Love, S. Clusterin Levels Are Increased in Alzheimer’s Disease and Influence the Regional Distribution of Aβ. Brain Pathol. Zurich Switz. 2017, 27, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Barage, S.H.; Sonawane, K.D. Amyloid Cascade Hypothesis: Pathogenesis and Therapeutic Strategies in Alzheimer’s Disease. Neuropeptides 2015, 52, 1–18. [Google Scholar] [CrossRef]
- Vinters, H.V. Cerebral Amyloid Angiopathy. A Critical Review. Stroke 1987, 18, 311–324. [Google Scholar] [CrossRef]
- Jellinger, K.A. Alzheimer Disease and Cerebrovascular Pathology: An Update. J. Neural Transm. Vienna Austria 1996 2002, 109, 813–836. [Google Scholar] [CrossRef]
- Roher, A.E.; Kuo, Y.-M.; Esh, C.; Knebel, C.; Weiss, N.; Kalback, W.; Luehrs, D.C.; Childress, J.L.; Beach, T.G.; Weller, R.O.; et al. Cortical and Leptomeningeal Cerebrovascular Amyloid and White Matter Pathology in Alzheimer’s Disease. Mol. Med. Camb. Mass 2003, 9, 112–122. [Google Scholar] [CrossRef]
- Revesz, T.; Holton, J.L.; Lashley, T.; Plant, G.; Rostagno, A.; Ghiso, J.; Frangione, B. Sporadic and Familial Cerebral Amyloid Angiopathies. Brain Pathol. Zurich Switz. 2002, 12, 343–357. [Google Scholar] [CrossRef]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene Dose of Apolipoprotein E Type 4 Allele and the Risk of Alzheimer’s Disease in Late Onset Families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Briggs, M.E.; Hyman, B.T.; Kokoris, G.J.; Takis, C.; Kanter, D.S.; Kase, C.S.; Pessin, M.S. Apolipoprotein E Epsilon 4 Is Associated with the Presence and Earlier Onset of Hemorrhage in Cerebral Amyloid Angiopathy. Stroke 1996, 27, 1333–1337. [Google Scholar] [CrossRef]
- Tarasoff-Conway, J.M.; Carare, R.O.; Osorio, R.S.; Glodzik, L.; Butler, T.; Fieremans, E.; Axel, L.; Rusinek, H.; Nicholson, C.; Zlokovic, B.V.; et al. Clearance Systems in the Brain-Implications for Alzheimer Disease. Nat. Rev. Neurol. 2015, 11, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.O.; Subash, M.; Preston, S.D.; Mazanti, I.; Carare, R.O. Perivascular Drainage of Amyloid-Beta Peptides from the Brain and Its Failure in Cerebral Amyloid Angiopathy and Alzheimer’s Disease. Brain Pathol. Zurich Switz. 2008, 18, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.A.; Härtig, W.; Kacza, J.; Schliebs, R.; Weller, R.O.; Nicoll, J.A.; Carare, R.O. Perivascular Drainage of Solutes Is Impaired in the Ageing Mouse Brain and in the Presence of Cerebral Amyloid Angiopathy. Acta Neuropathol. 2011, 121, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.A.; Sullivan, P.M.; Hands, S.; Weller, R.O.; Nicoll, J.A.R.; Carare, R.O. Disruption of Arterial Perivascular Drainage of Amyloid-β from the Brains of Mice Expressing the Human APOE Ε4 Allele. PLoS ONE 2012, 7, e41636. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.A.; Gentleman, S.M.; Nicoll, J.A.; Carare, R.O. Prenatal High-Fat Diet Alters the Cerebrovasculature and Clearance of β-Amyloid in Adult Offspring. J. Pathol. 2015, 235, 619–631. [Google Scholar] [CrossRef]
- Carare, R.O.; Teeling, J.L.; Hawkes, C.A.; Püntener, U.; Weller, R.O.; Nicoll, J.A.; Perry, V.H. Immune Complex Formation Impairs the Elimination of Solutes from the Brain: Implications for Immunotherapy in Alzheimer’s Disease. Acta Neuropathol. Commun. 2013, 1, 48. [Google Scholar] [CrossRef]
- Zekonyte, J.; Sakai, K.; Nicoll, J.A.R.; Weller, R.O.; Carare, R.O. Quantification of Molecular Interactions between ApoE, Amyloid-Beta (Aβ) and Laminin: Relevance to Accumulation of Aβ in Alzheimer’s Disease. Biochim. Biophys. Acta 2016, 1862, 1047–1053. [Google Scholar] [CrossRef]
- Foster, E.M.; Dangla-Valls, A.; Lovestone, S.; Ribe, E.M.; Buckley, N.J. Clusterin in Alzheimer’s Disease: Mechanisms, Genetics, and Lessons From Other Pathologies. Front. Neurosci. 2019, 13, 164. [Google Scholar] [CrossRef]
- Nuutinen, T.; Suuronen, T.; Kauppinen, A.; Salminen, A. Clusterin: A Forgotten Player in Alzheimer’s Disease. Brain Res. Rev. 2009, 61, 89–104. [Google Scholar] [CrossRef]
- Yuste-Checa, P.; Bracher, A.; Hartl, F.U. The Chaperone Clusterin in Neurodegeneration-Friend or Foe? BioEssays News Rev. Mol. Cell. Dev. Biol. 2022, 44, e2100287. [Google Scholar] [CrossRef]
- Lambert, J.-C.; Heath, S.; Even, G.; Campion, D.; Sleegers, K.; Hiltunen, M.; Combarros, O.; Zelenika, D.; Bullido, M.J.; Tavernier, B.; et al. Genome-Wide Association Study Identifies Variants at CLU and CR1 Associated with Alzheimer’s Disease. Nat. Genet. 2009, 41, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Jansen, I.E.; Savage, J.E.; Watanabe, K.; Bryois, J.; Williams, D.M.; Steinberg, S.; Sealock, J.; Karlsson, I.K.; Hägg, S.; Athanasiu, L.; et al. Genome-Wide Meta-Analysis Identifies New Loci and Functional Pathways Influencing Alzheimer’s Disease Risk. Nat. Genet. 2019, 51, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, M.; Wang, R.; Bassett, S.S.; Avramopoulos, D. Alzheimer’s Risk Variants in the Clusterin Gene Are Associated with Alternative Splicing. Transl. Psychiatry 2011, 1, e18. [Google Scholar] [CrossRef] [PubMed]
- Padhy, B.; Nanda, G.G.; Chowdhury, M.; Padhi, D.; Rao, A.; Alone, D.P. Role of an Extracellular Chaperone, Clusterin in the Pathogenesis of Pseudoexfoliation Syndrome and Pseudoexfoliation Glaucoma. Exp. Eye Res. 2014, 127, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.-Y.; Yu, J.-T.; Cui, W.-Z.; Zhong, X.-L.; Wu, Z.-C.; Zhang, Q.; Tan, L. Blood Clusterin Levels, Rs9331888 Polymorphism, and the Risk of Alzheimer’s Disease. J. Alzheimers Dis. JAD 2012, 29, 515–519. [Google Scholar] [CrossRef]
- Allen, M.; Zou, F.; Chai, H.S.; Younkin, C.S.; Crook, J.; Pankratz, V.S.; Carrasquillo, M.M.; Rowley, C.N.; Nair, A.A.; Middha, S.; et al. Novel Late-Onset Alzheimer Disease Loci Variants Associate with Brain Gene Expression. Neurology 2012, 79, 221–228. [Google Scholar] [CrossRef]
- Karch, C.M.; Jeng, A.T.; Nowotny, P.; Cady, J.; Cruchaga, C.; Goate, A.M. Expression of Novel Alzheimer’s Disease Risk Genes in Control and Alzheimer’s Disease Brains. PLoS ONE 2012, 7, e50976. [Google Scholar] [CrossRef]
- Deming, Y.; Xia, J.; Cai, Y.; Lord, J.; Holmans, P.; Bertelsen, S.; Holtzman, D.; Morris, J.C.; Bales, K.; Pickering, E.H.; et al. A Potential Endophenotype for Alzheimer’s Disease: Cerebrospinal Fluid Clusterin. Neurobiol. Aging 2016, 37, 208.e1–208.e9. [Google Scholar] [CrossRef]
- Tan, L.; Wang, H.-F.; Tan, M.-S.; Tan, C.-C.; Zhu, X.-C.; Miao, D.; Yu, W.-J.; Jiang, T.; Tan, L.; Yu, J.-T.; et al. Effect of CLU Genetic Variants on Cerebrospinal Fluid and Neuroimaging Markers in Healthy, Mild Cognitive Impairment and Alzheimer’s Disease Cohorts. Sci. Rep. 2016, 6, 26027. [Google Scholar] [CrossRef]
- DeMattos, R.B.; Cirrito, J.R.; Parsadanian, M.; May, P.C.; O’Dell, M.A.; Taylor, J.W.; Harmony, J.A.K.; Aronow, B.J.; Bales, K.R.; Paul, S.M.; et al. ApoE and Clusterin Cooperatively Suppress Abeta Levels and Deposition: Evidence That ApoE Regulates Extracellular Abeta Metabolism in Vivo. Neuron 2004, 41, 193–202. [Google Scholar] [CrossRef]
- Lidström, A.M.; Bogdanovic, N.; Hesse, C.; Volkman, I.; Davidsson, P.; Blennow, K. Clusterin (Apolipoprotein J) Protein Levels Are Increased in Hippocampus and in Frontal Cortex in Alzheimer’s Disease. Exp. Neurol. 1998, 154, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.S.; Thompson, W.K.; Holland, D.; Hess, C.P.; Brewer, J.B.; Zetterberg, H.; Blennow, K.; Andreassen, O.A.; McEvoy, L.K.; Hyman, B.T.; et al. The Role of Clusterin in Amyloid-β Associated Neurodegeneration. JAMA Neurol. 2014, 71, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Thambisetty, M.; Simmons, A.; Velayudhan, L.; Hye, A.; Campbell, J.; Zhang, Y.; Wahlund, L.-O.; Westman, E.; Kinsey, A.; Güntert, A.; et al. Association of Plasma Clusterin Concentration with Severity, Pathology, and Progression in Alzheimer Disease. Arch. Gen. Psychiatry 2010, 67, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Schrijvers, E.M.C.; Koudstaal, P.J.; Hofman, A.; Breteler, M.M.B. Plasma Clusterin and the Risk of Alzheimer Disease. JAMA 2011, 305, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Fandos, N.; Pérez-Grijalba, V.; Pesini, P.; Olmos, S.; Bossa, M.; Villemagne, V.L.; Doecke, J.; Fowler, C.; Masters, C.L.; Sarasa, M.; et al. Plasma amyloid β 42/40 ratios as biomarkers for amyloid β cerebral deposition in cognitively normal individuals. Alzheimers Dement. 2017, 8, 179–187. [Google Scholar] [CrossRef]
- Giannakopoulos, P.; Kövari, E.; French, L.E.; Viard, I.; Hof, P.R.; Bouras, C. Possible Neuroprotective Role of Clusterin in Alzheimer’s Disease: A Quantitative Immunocytochemical Study. Acta Neuropathol. 1998, 95, 387–394. [Google Scholar] [CrossRef]
- Narayan, P.; Holmström, K.M.; Kim, D.-H.; Whitcomb, D.J.; Wilson, M.R.; St George-Hyslop, P.; Wood, N.W.; Dobson, C.M.; Cho, K.; Abramov, A.Y.; et al. Rare Individual Amyloid-β Oligomers Act on Astrocytes to Initiate Neuronal Damage. Biochemistry 2014, 53, 2442–2453. [Google Scholar] [CrossRef]
- DeMattos, R.B.; O’dell, M.A.; Parsadanian, M.; Taylor, J.W.; Harmony, J.A.K.; Bales, K.R.; Paul, S.M.; Aronow, B.J.; Holtzman, D.M. Clusterin Promotes Amyloid Plaque Formation and Is Critical for Neuritic Toxicity in a Mouse Model of Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2002, 99, 10843–10848. [Google Scholar] [CrossRef]
- Narayan, P.; Orte, A.; Clarke, R.W.; Bolognesi, B.; Hook, S.; Ganzinger, K.A.; Meehan, S.; Wilson, M.R.; Dobson, C.M.; Klenerman, D. The Extracellular Chaperone Clusterin Sequesters Oligomeric Forms of the Amyloid-β(1-40) Peptide. Nat. Struct. Mol. Biol. 2011, 19, 79–83. [Google Scholar] [CrossRef]
- Lakins, J.N.; Poon, S.; Easterbrook-Smith, S.B.; Carver, J.A.; Tenniswood, M.P.R.; Wilson, M.R. Evidence That Clusterin Has Discrete Chaperone and Ligand Binding Sites. Biochemistry 2002, 41, 282–291. [Google Scholar] [CrossRef]
- Poon, S.; Treweek, T.M.; Wilson, M.R.; Easterbrook-Smith, S.B.; Carver, J.A. Clusterin Is an Extracellular Chaperone That Specifically Interacts with Slowly Aggregating Proteins on Their Off-Folding Pathway. FEBS Lett. 2002, 513, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Dabbs, R.A.; Wilson, M.R. Expression and Purification of Chaperone-Active Recombinant Clusterin. PLoS ONE 2014, 9, e86989. [Google Scholar] [CrossRef] [PubMed]
- Cascella, R.; Conti, S.; Tatini, F.; Evangelisti, E.; Scartabelli, T.; Casamenti, F.; Wilson, M.R.; Chiti, F.; Cecchi, C. Extracellular Chaperones Prevent Aβ42-Induced Toxicity in Rat Brains. Biochim. Biophys. Acta 2013, 1832, 1217–1226. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oda, T.; Wals, P.; Osterburg, H.H.; Johnson, S.A.; Pasinetti, G.M.; Morgan, T.E.; Rozovsky, I.; Stine, W.B.; Snyder, S.W.; Holzman, T.F. Clusterin (apoJ) Alters the Aggregation of Amyloid Beta-Peptide (A Beta 1-42) and Forms Slowly Sedimenting A Beta Complexes That Cause Oxidative Stress. Exp. Neurol. 1995, 136, 22–31. [Google Scholar] [CrossRef]
- Lambert, M.P.; Barlow, A.K.; Chromy, B.A.; Edwards, C.; Freed, R.; Liosatos, M.; Morgan, T.E.; Rozovsky, I.; Trommer, B.; Viola, K.L.; et al. Diffusible, Nonfibrillar Ligands Derived from Abeta1-42 Are Potent Central Nervous System Neurotoxins. Proc. Natl. Acad. Sci. USA 1998, 95, 6448–6453. [Google Scholar] [CrossRef]
- Jha, N.K.; Jha, S.K.; Kumar, D.; Kejriwal, N.; Sharma, R.; Ambasta, R.K.; Kumar, P. Impact of Insulin Degrading Enzyme and Neprilysin in Alzheimer’s Disease Biology: Characterization of Putative Cognates for Therapeutic Applications. J. Alzheimers Dis. JAD 2015, 48, 891–917. [Google Scholar] [CrossRef]
- Deane, R.; Bell, R.D.; Sagare, A.; Zlokovic, B.V. Clearance of Amyloid-Beta Peptide across the Blood-Brain Barrier: Implication for Therapies in Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2009, 8, 16–30. [Google Scholar] [CrossRef]
- Matsubara, E.; Soto, C.; Governale, S.; Frangione, B.; Ghiso, J. Apolipoprotein J and Alzheimer’s Amyloid Beta Solubility. Biochem. J. 1996, 316, 671–679. [Google Scholar] [CrossRef]
- Bell, R.D.; Sagare, A.P.; Friedman, A.E.; Bedi, G.S.; Holtzman, D.M.; Deane, R.; Zlokovic, B.V. Transport Pathways for Clearance of Human Alzheimer’s Amyloid Beta-Peptide and Apolipoproteins E and J in the Mouse Central Nervous System. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2007, 27, 909–918. [Google Scholar] [CrossRef]
- Calero, M.; Rostagno, A.; Matsubara, E.; Zlokovic, B.; Frangione, B.; Ghiso, J. Apolipoprotein J (Clusterin) and Alzheimer’s Disease. Microsc. Res. Tech. 2000, 50, 305–315. [Google Scholar] [CrossRef]
- Howlett, D.R.; Hortobágyi, T.; Francis, P.T. Clusterin Associates Specifically with Aβ40 in Alzheimer’s Disease Brain Tissue. Brain Pathol. 2013, 23, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Craggs, L.; Taylor, J.; Slade, J.Y.; Chen, A.; Hagel, C.; Kuhlenbaeumer, G.; Borjesson-Hanson, A.; Viitanen, M.; Kalimo, H.; Deramecourt, V.; et al. Clusterin/Apolipoprotein J Immunoreactivity Is Associated with White Matter Damage in Cerebral Small Vessel Diseases. Neuropathol. Appl. Neurobiol. 2016, 42, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Manousopoulou, A.; Gatherer, M.; Smith, C.; Nicoll, J.A.R.; Woelk, C.H.; Johnson, M.; Kalaria, R.; Attems, J.; Garbis, S.D.; Carare, R.O. Systems Proteomic Analysis Reveals That Clusterin and Tissue Inhibitor of Metalloproteinases 3 Increase in Leptomeningeal Arteries Affected by Cerebral Amyloid Angiopathy. Neuropathol. Appl. Neurobiol. 2017, 43, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Raulin, A.C.; Doss, S.V.; Trottier, Z.A.; Ikezu, T.C.; Bu, G.; Liu, C.C. ApoE in Alzheimer’s disease: Pathophysiology and therapeutic strategies. Mol. Neurodegener. 2022, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Akasaka, Y.; Ishii, T.; Komiyama, K.; Masuda, S.; Asuwa, N.; Choi-Miura, N.H.; Tomita, M. Distribution and Synthesis of Apolipoprotein J in the Atherosclerotic Aorta. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 665–672. [Google Scholar] [CrossRef]
- Squitti, R.; Polimanti, R.; Bucossi, S.; Ventriglia, M.; Mariani, S.; Manfellotto, D.; Vernieri, F.; Cassetta, E.; Ursini, F.; Rossini, P.M. Linkage Disequilibrium and Haplotype Analysis of the ATP7B Gene in Alzheimer’s Disease. Rejuvenation Res. 2013, 16, 3–10. [Google Scholar] [CrossRef]
- Elliott, D.A.; Weickert, C.S.; Garner, B. Apolipoproteins in the Brain: Implications for Neurological and Psychiatric Disorders. Clin. Lipidol. 2010, 51, 555–573. [Google Scholar] [CrossRef]
- Lee, K.-B.; Jeon, J.-H.; Choi, I.; Kwon, O.-Y.; Yu, K.; You, K.-H. Clusterin, a Novel Modulator of TGF-Beta Signaling, Is Involved in Smad2/3 Stability. Biochem. Biophys. Res. Commun. 2008, 366, 905–909. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Tischfield, M.; Williams, J.; Smallwood, P.M.; Rattner, A.; Taketo, M.M.; Nathans, J. Canonical WNT Signaling Components in Vascular Development and Barrier Formation. J. Clin. Investig. 2014, 124, 3825–3846. [Google Scholar] [CrossRef]
- Materia, S.; Cater, M.A.; Klomp, L.W.J.; Mercer, J.F.B.; La Fontaine, S. Clusterin (Apolipoprotein J), a Molecular Chaperone That Facilitates Degradation of the Copper-ATPases ATP7A and ATP7B. J. Biol. Chem. 2011, 286, 10073–10083. [Google Scholar] [CrossRef]
- Janelidze, S.; Mattsson, N.; Stomrud, E.; Lindberg, O.; Palmqvist, S.; Zetterberg, H.; Blennow, K.; Hansson, O. CSF Biomarkers of Neuroinflammation and Cerebrovascular Dysfunction in Early Alzheimer Disease. Neurology 2018, 91, e867–e877. [Google Scholar] [CrossRef] [PubMed]
- Ewers, M.; Franzmeier, N.; Suárez-Calvet, M.; Morenas-Rodriguez, E.; Caballero, M.A.A.; Kleinberger, G.; Piccio, L.; Cruchaga, C.; Deming, Y.; Dichgans, M.; et al. Increased Soluble TREM2 in Cerebrospinal Fluid Is Associated with Reduced Cognitive and Clinical Decline in Alzheimer’s Disease. Sci. Transl. Med. 2019, 11, eaav6221. [Google Scholar] [CrossRef] [PubMed]
- Bellaver, B.; Ferrari-Souza, J.P.; Uglione da Ros, L.; Carter, S.F.; Rodriguez-Vieitez, E.; Nordberg, A.; Pellerin, L.; Rosa-Neto, P.; Leffa, D.T.; Zimmer, E.R. Astrocyte Biomarkers in Alzheimer Disease: A Systematic Review and Meta-Analysis. Neurology 2021, 96, e2944–e2955. [Google Scholar] [CrossRef] [PubMed]
- Moretti, R.M.; Montagnani Marelli, M.; Mai, S.; Cariboni, A.; Scaltriti, M.; Bettuzzi, S.; Limonta, P. Clusterin Isoforms Differentially Affect Growth and Motility of Prostate Cells: Possible Implications in Prostate Tumorigenesis. Cancer Res. 2007, 67, 10325–10333. [Google Scholar] [CrossRef]
- Rizzi, F.; Coletta, M.; Bettuzzi, S. Chapter 2: Clusterin (CLU): From One Gene and Two Transcripts to Many Proteins. Adv. Cancer Res. 2009, 104, 9–23. [Google Scholar] [CrossRef]
- Xie, Z.; Harris-White, M.E.; Wals, P.A.; Frautschy, S.A.; Finch, C.E.; Morgan, T.E. Apolipoprotein J (Clusterin) Activates Rodent Microglia in Vivo and in Vitro. J. Neurochem. 2005, 93, 1038–1046. [Google Scholar] [CrossRef]
- Cunin, P.; Beauvillain, C.; Miot, C.; Augusto, J.-F.; Preisser, L.; Blanchard, S.; Pignon, P.; Scotet, M.; Garo, E.; Fremaux, I.; et al. Clusterin Facilitates Apoptotic Cell Clearance and Prevents Apoptotic Cell-Induced Autoimmune Responses. Cell Death Dis. 2016, 7, e2215. [Google Scholar] [CrossRef]
- Yang, C.R.; Leskov, K.; Hosley-Eberlein, K.; Criswell, T.; Pink, J.J.; Kinsella, T.J.; Boothman, D.A. Nuclear Clusterin/XIP8, an x-Ray-Induced Ku70-Binding Protein That Signals Cell Death. Proc. Natl. Acad. Sci. USA 2000, 97, 5907–5912. [Google Scholar] [CrossRef]
- Zellweger, T.; Kiyama, S.; Chi, K.; Miyake, H.; Adomat, H.; Skov, K.; Gleave, M.E. Overexpression of the Cytoprotective Protein Clusterin Decreases Radiosensitivity in the Human LNCaP Prostate Tumour Model. BJU Int. 2003, 92, 463–469. [Google Scholar] [CrossRef]
- Zellweger, T.; Miyake, H.; Cooper, S.; Chi, K.; Conklin, B.S.; Monia, B.P.; Gleave, M.E. Antitumor Activity of Antisense Clusterin Oligonucleotides Is Improved in Vitro and in Vivo by Incorporation of 2’-O-(2-Methoxy)Ethyl Chemistry. J. Pharmacol. Exp. Ther. 2001, 298, 934–940. [Google Scholar]
- Chen, Y.; Strickland, M.R.; Soranno, A.; Holtzman, D.M. Apolipoprotein E: Structural Insights and Links to Alzheimer Disease Pathogenesis. Neuron 2021, 109, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.G.; Hamby, M.E.; McReynolds, M.L.; Ray, W.J. The Role of APOE4 in Disrupting the Homeostatic Functions of Astrocytes and Microglia in Aging and Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Weisgraber, K.H.; Innerarity, T.L.; Mahley, R.W. Abnormal Lipoprotein Receptor-Binding Activity of the Human E Apoprotein Due to Cysteine-Arginine Interchange at a Single Site. J. Biol. Chem. 1982, 257, 2518–2521. [Google Scholar] [CrossRef] [PubMed]
- Fagan, A.M.; Watson, M.; Parsadanian, M.; Bales, K.R.; Paul, S.M.; Holtzman, D.M. Human and Murine ApoE Markedly Alters A Beta Metabolism before and after Plaque Formation in a Mouse Model of Alzheimer’s Disease. Neurobiol. Dis. 2002, 9, 305–318. [Google Scholar] [CrossRef]
- Liao, F.; Zhang, T.J.; Jiang, H.; Lefton, K.B.; Robinson, G.O.; Vassar, R.; Sullivan, P.M.; Holtzman, D.M. Murine versus Human Apolipoprotein E4: Differential Facilitation of and Co-Localization in Cerebral Amyloid Angiopathy and Amyloid Plaques in APP Transgenic Mouse Models. Acta Neuropathol. Commun. 2015, 3, 70. [Google Scholar] [CrossRef]
- Tai, L.M.; Balu, D.; Avila-Munoz, E.; Abdullah, L.; Thomas, R.; Collins, N.; Valencia-Olvera, A.C.; LaDu, M.J. EFAD Transgenic Mice as a Human APOE Relevant Preclinical Model of Alzheimer’s Disease. J. Lipid Res. 2017, 58, 1733–1755. [Google Scholar] [CrossRef]
- Eisenberg, D.T.A.; Kuzawa, C.W.; Hayes, M.G. Worldwide Allele Frequencies of the Human Apolipoprotein E Gene: Climate, Local Adaptations, and Evolutionary History. Am. J. Phys. Anthropol. 2010, 143, 100–111. [Google Scholar] [CrossRef]
- Bekris, L.M.; Millard, S.P.; Galloway, N.M.; Vuletic, S.; Albers, J.J.; Li, G.; Galasko, D.R.; DeCarli, C.; Farlow, M.R.; Clark, C.M.; et al. Multiple SNPs Within and Surrounding the Apolipoprotein E Gene Influence Cerebrospinal Fluid Apolipoprotein E Protein Levels. J. Alzheimers Dis. JAD 2008, 13, 255–266. [Google Scholar] [CrossRef]
- Husain, M.A.; Laurent, B.; Plourde, M. APOE and Alzheimer’s Disease: From Lipid Transport to Physiopathology and Therapeutics. Front. Neurosci. 2021, 15, 630502. [Google Scholar] [CrossRef]
- Abondio, P.; Sazzini, M.; Garagnani, P.; Boattini, A.; Monti, D.; Franceschi, C.; Luiselli, D.; Giuliani, C. The Genetic Variability of APOE in Different Human Populations and Its Implications for Longevity. Genes 2019, 10, 222. [Google Scholar] [CrossRef]
- Kern, S.; Mehlig, K.; Kern, J.; Zetterberg, H.; Thelle, D.; Skoog, I.; Lissner, L.; Blennow, K.; Börjesson-Hanson, A. The Distribution of Apolipoprotein E Genotype over the Adult Lifespan and in Relation to Country of Birth. Am. J. Epidemiol. 2015, 181, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Kockx, M.; Traini, M.; Kritharides, L. Cell-Specific Production, Secretion, and Function of Apolipoprotein E. J. Mol. Med. Berl. Ger. 2018, 96, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M.; Fryer, J.D.; Sullivan, P.M.; Christopher, E.A.; Wahrle, S.E.; DeMattos, R.B.; O’Dell, M.A.; Fagan, A.M.; Lashuel, H.A.; Walz, T.; et al. Production and Characterization of Astrocyte-Derived Human Apolipoprotein E Isoforms from Immortalized Astrocytes and Their Interactions with Amyloid-Beta. Neurobiol. Dis. 2005, 19, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, I.B.; Wilhelmus, M.M.M.; Kox, M.; Veerhuis, R.; de Waal, R.M.W.; Verbeek, M.M. Apolipoprotein E Protects Cultured Pericytes and Astrocytes from D-Abeta(1-40)-Mediated Cell Death. Brain Res. 2010, 1315, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Bernardo, A.; Walker, D.; Kanegawa, T.; Mahley, R.W.; Huang, Y. Profile and Regulation of Apolipoprotein E (ApoE) Expression in the CNS in Mice with Targeting of Green Fluorescent Protein Gene to the ApoE Locus. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 4985–4994. [Google Scholar] [CrossRef]
- Buttini, M.; Masliah, E.; Yu, G.-Q.; Palop, J.J.; Chang, S.; Bernardo, A.; Lin, C.; Wyss-Coray, T.; Huang, Y.; Mucke, L. Cellular Source of Apolipoprotein E4 Determines Neuronal Susceptibility to Excitotoxic Injury in Transgenic Mice. Am. J. Pathol. 2010, 177, 563–569. [Google Scholar] [CrossRef]
- Kockx, M.; Guo, D.L.; Huby, T.; Lesnik, P.; Kay, J.; Sabaretnam, T.; Jary, E.; Hill, M.; Gaus, K.; Chapman, J.; et al. Secretion of Apolipoprotein E From Macrophages Occurs via a Protein Kinase A– and Calcium-Dependent Pathway Along the Microtubule Network. Circ. Res. 2007, 101, 607–616. [Google Scholar] [CrossRef]
- Frenkel-Pinter, M.; Shmueli, M.D.; Raz, C.; Yanku, M.; Zilberzwige, S.; Gazit, E.; Segal, D. Interplay between Protein Glycosylation Pathways in Alzheimer’s Disease. Sci. Adv. 2017, 3, e1601576. [Google Scholar] [CrossRef]
- Boix, C.P.; Lopez-Font, I.; Cuchillo-Ibañez, I.; Sáez-Valero, J. Amyloid Precursor Protein Glycosylation Is Altered in the Brain of Patients with Alzheimer’s Disease. Alzheimers Res. Ther. 2020, 12, 96. [Google Scholar] [CrossRef]
- Haukedal, H.; Freude, K.K. Implications of Glycosylation in Alzheimer’s Disease. Front. Neurosci. 2020, 14, 625348. [Google Scholar] [CrossRef]
- Flowers, S.A.; Grant, O.C.; Woods, R.J.; Rebeck, G.W. O-Glycosylation on Cerebrospinal Fluid and Plasma Apolipoprotein E Differs in the Lipid-Binding Domain. Glycobiology 2020, 30, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Schulman, I.G. Liver X Receptors Link Lipid Metabolism and Inflammation. FEBS Lett. 2017, 591, 2978–2991. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, M.S.; Donizetti, A.; Iannotta, L.; Aliperti, V.; Cupidi, C.; Bruni, A.C.; Cigliano, L. Brain-Derived Neurotrophic Factor Modulates Cholesterol Homeostasis and Apolipoprotein E Synthesis in Human Cell Models of Astrocytes and Neurons. J. Cell. Physiol. 2018, 233, 6925–6943. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Fan, J.; Kulic, I.; Koh, C.; Clark, A.; Meuller, J.; Engkvist, O.; Barichievy, S.; Raynoschek, C.; Hicks, R.; et al. Axl Receptor Tyrosine Kinase Is a Regulator of Apolipoprotein E. Mol. Brain 2020, 13, 66. [Google Scholar] [CrossRef]
- Hong, C.; Tontonoz, P. Liver X Receptors in Lipid Metabolism: Opportunities for Drug Discovery. Nat. Rev. Drug Discov. 2014, 13, 433–444. [Google Scholar] [CrossRef]
- Wood, H. Retinoid X Receptor Mediates Brain Clean-up after Stroke. Nat. Rev. Neurol. 2020, 16, 128–129. [Google Scholar] [CrossRef]
- Schierle, S.; Merk, D. Therapeutic Modulation of Retinoid X Receptors—SAR and Therapeutic Potential of RXR Ligands and Recent Patents. Expert Opin. Ther. Pat. 2019, 29, 605–621. [Google Scholar] [CrossRef]
- Fitz, N.F.; Nam, K.N.; Koldamova, R.; Lefterov, I. Therapeutic Targeting of Nuclear Receptors, Liver X and Retinoid X Receptors, for Alzheimer’s Disease. Br. J. Pharmacol. 2019, 176, 3599–3610. [Google Scholar] [CrossRef]
- Koldamova, R.; Lefterov, I. Role of LXR and ABCA1 in the Pathogenesis of Alzheimer’s Disease—Implications for a New Therapeutic Approach. Curr. Alzheimer Res. 2007, 4, 171–178. [Google Scholar] [CrossRef]
- Jacobo-Albavera, L.; Domínguez-Pérez, M.; Medina-Leyte, D.J.; González-Garrido, A.; Villarreal-Molina, T. The Role of the ATP-Binding Cassette A1 (ABCA1) in Human Disease. Int. J. Mol. Sci. 2021, 22, 1593. [Google Scholar] [CrossRef]
- Fitz, N.F.; Cronican, A.A.; Saleem, M.; Fauq, A.H.; Chapman, R.; Lefterov, I.; Koldamova, R. Abca1 Deficiency Affects Alzheimer’s Disease-Like Phenotype in Human ApoE4 But Not in ApoE3-Targeted Replacement Mice. J. Neurosci. 2012, 32, 13125–13136. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Calle, R.; Konings, S.C.; Frontiñán-Rubio, J.; García-Revilla, J.; Camprubí-Ferrer, L.; Svensson, M.; Martinson, I.; Boza-Serrano, A.; Venero, J.L.; Nielsen, H.M.; et al. APOE in the Bullseye of Neurodegenerative Diseases: Impact of the APOE Genotype in Alzheimer’s Disease Pathology and Brain Diseases. Mol. Neurodegener. 2022, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Mahley, R.W. Central Nervous System Lipoproteins: ApoE and Regulation of Cholesterol Metabolism. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Nieweg, K.; Schaller, H.; Pfrieger, F.W. Marked Differences in Cholesterol Synthesis between Neurons and Glial Cells from Postnatal Rats. J. Neurochem. 2009, 109, 125–134. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, H.; Basak, J.; Kim, J. Apolipoprotein E in Synaptic Plasticity and Alzheimer’s Disease: Potential Cellular and Molecular Mechanisms. Mol. Cells 2014, 37, 767–776. [Google Scholar] [CrossRef]
- Lane-Donovan, C.; Wong, W.M.; Durakoglugil, M.S.; Wasser, C.R.; Jiang, S.; Xian, X.; Herz, J. Genetic Restoration of Plasma ApoE Improves Cognition and Partially Restores Synaptic Defects in ApoE-Deficient Mice. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 10141–10150. [Google Scholar] [CrossRef]
- Tensaouti, Y.; Yu, T.-S.; Kernie, S.G. Apolipoprotein E Regulates the Maturation of Injury-Induced Adult-Born Hippocampal Neurons Following Traumatic Brain Injury. PLoS ONE 2020, 15, e0229240. [Google Scholar] [CrossRef]
- Vitali, C.; Wellington, C.L.; Calabresi, L. HDL and Cholesterol Handling in the Brain. Cardiovasc. Res. 2014, 103, 405–413. [Google Scholar] [CrossRef]
- Hottman, D.A.; Chernick, D.; Cheng, S.; Wang, Z.; Li, L. HDL and Cognition in Neurodegenerative Disorders. Neurobiol. Dis. 2014, 72 Pt A, 22–36. [Google Scholar] [CrossRef]
- Mahley, R.W. Apolipoprotein E: From Cardiovascular Disease to Neurodegenerative Disorders. J. Mol. Med. Berl. Ger. 2016, 94, 739–746. [Google Scholar] [CrossRef]
- Riddell, D.R.; Zhou, H.; Atchison, K.; Warwick, H.K.; Atkinson, P.J.; Jefferson, J.; Xu, L.; Aschmies, S.; Kirksey, Y.; Hu, Y.; et al. Impact of Apolipoprotein E (ApoE) Polymorphism on Brain ApoE Levels. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 11445–11453. [Google Scholar] [CrossRef]
- Sullivan, P.M.; Han, B.; Liu, F.; Mace, B.E.; Ervin, J.F.; Wu, S.; Koger, D.; Paul, S.; Bales, K.R. Reduced Levels of Human apoE4 Protein in an Animal Model of Cognitive Impairment. Neurobiol. Aging 2011, 32, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, G.; Xu, Q.; Huang, Y.; Weisgraber, K.H. Effect of Domain Interaction on Apolipoprotein E Levels in Mouse Brain. J. Neurosci. 2005, 25, 10658–10663. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sullivan, P.M.; Mace, B.E.; Maeda, N.; Schmechel, D.E. Marked Regional Differences of Brain Human Apolipoprotein e Expression in Targeted Replacement Mice. Neuroscience 2004, 124, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Giannisis, A.; Patra, K.; Edlund, A.K.; Nieto, L.A.; Benedicto-Gras, J.; Moussaud, S.; de la Rosa, A.; Twohig, D.; Bengtsson, T.; Fu, Y.; et al. Brain Integrity Is Altered by Hepatic APOE Ε4 in Humanized-Liver Mice. Mol. Psychiatry 2022, 27, 3533–3543. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, J.D.; Burchett, J.M.; Restivo, J.L.; Schuler, D.R.; Verghese, P.B.; Mahan, T.E.; Landreth, G.E.; Castellano, J.M.; Jiang, H.; Cirrito, J.R.; et al. In Vivo Measurement of Apolipoprotein E from the Brain Interstitial Fluid Using Microdialysis. Mol. Neurodegener. 2013, 8, 13. [Google Scholar] [CrossRef]
- Linton, M.F.; Gish, R.; Hubl, S.T.; Bütler, E.; Esquivel, C.; Bry, W.I.; Boyles, J.K.; Wardell, M.R.; Young, S.G. Phenotypes of Apolipoprotein B and Apolipoprotein E after Liver Transplantation. J. Clin. Investig. 1991, 88, 270–281. [Google Scholar] [CrossRef]
- Getz, G.S.; Reardon, C.A. Apoprotein E as a Lipid Transport and Signaling Protein in the Blood, Liver, and Artery Wall. J. Lipid Res. 2009, 50, S156–S161. [Google Scholar] [CrossRef]
- Huynh, T.-P.V.; Wang, C.; Tran, A.C.; Tabor, G.T.; Mahan, T.E.; Francis, C.M.; Finn, M.B.; Spellman, R.; Manis, M.; Tanzi, R.E.; et al. Lack of Hepatic apoE Does Not Influence Early Aβ Deposition: Observations from a New APOE Knock-in Model. Mol. Neurodegener. 2019, 14, 37. [Google Scholar] [CrossRef]
- Wahrle, S.E.; Shah, A.R.; Fagan, A.M.; Smemo, S.; Kauwe, J.S.K.; Grupe, A.; Hinrichs, A.; Mayo, K.; Jiang, H.; Thal, L.J.; et al. Apolipoprotein E Levels in Cerebrospinal Fluid and the Effects of ABCA1 Polymorphisms. Mol. Neurodegener. 2007, 2, 7. [Google Scholar] [CrossRef]
- Martínez-Morillo, E.; Hansson, O.; Atagi, Y.; Bu, G.; Minthon, L.; Diamandis, E.P.; Nielsen, H.M. Total Apolipoprotein E Levels and Specific Isoform Composition in Cerebrospinal Fluid and Plasma from Alzheimer’s Disease Patients and Controls. Acta Neuropathol. 2014, 127, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Girod, M.; Fonbonne, C.; Salvador, A.; Clément, Y.; Lantéri, P.; Amouyel, P.; Lambert, J.C.; Lemoine, J. Total ApoE and ApoE4 Isoform Assays in an Alzheimer’s Disease Case-Control Study by Targeted Mass Spectrometry (N = 669): A Pilot Assay for Methionine-Containing Proteotypic Peptides. Mol. Cell. Proteomics MCP 2012, 11, 1389–1403. [Google Scholar] [CrossRef] [PubMed]
- Zokaei, N.; Giehl, K.; Sillence, A.; Neville, M.J.; Karpe, F.; Nobre, A.C.; Husain, M. Sex and APOE: A Memory Advantage in Male APOE Ε4 Carriers in Midlife. Cortex J. Devoted Study Nerv. Syst. Behav. 2017, 88, 98–105. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laslo, A.; Laslo, L.; Arbănași, E.-M.; Ujlaki-Nagi, A.-A.; Chinezu, L.; Ivănescu, A.D.; Arbănași, E.-M.; Cărare, R.O.; Cordoș, B.A.; Popa, I.A.; et al. Pathways to Alzheimer’s Disease: The Intersecting Roles of Clusterin and Apolipoprotein E in Amyloid-β Regulation and Neuronal Health. Pathophysiology 2024, 31, 545-558. https://doi.org/10.3390/pathophysiology31040040
Laslo A, Laslo L, Arbănași E-M, Ujlaki-Nagi A-A, Chinezu L, Ivănescu AD, Arbănași E-M, Cărare RO, Cordoș BA, Popa IA, et al. Pathways to Alzheimer’s Disease: The Intersecting Roles of Clusterin and Apolipoprotein E in Amyloid-β Regulation and Neuronal Health. Pathophysiology. 2024; 31(4):545-558. https://doi.org/10.3390/pathophysiology31040040
Chicago/Turabian StyleLaslo, Alexandru, Laura Laslo, Eliza-Mihaela Arbănași, Alexandru-Andrei Ujlaki-Nagi, Laura Chinezu, Adrian Dumitru Ivănescu, Emil-Marian Arbănași, Roxana Octavia Cărare, Bogdan Andrei Cordoș, Ioana Adriana Popa, and et al. 2024. "Pathways to Alzheimer’s Disease: The Intersecting Roles of Clusterin and Apolipoprotein E in Amyloid-β Regulation and Neuronal Health" Pathophysiology 31, no. 4: 545-558. https://doi.org/10.3390/pathophysiology31040040
APA StyleLaslo, A., Laslo, L., Arbănași, E.-M., Ujlaki-Nagi, A.-A., Chinezu, L., Ivănescu, A. D., Arbănași, E.-M., Cărare, R. O., Cordoș, B. A., Popa, I. A., & Brînzaniuc, K. (2024). Pathways to Alzheimer’s Disease: The Intersecting Roles of Clusterin and Apolipoprotein E in Amyloid-β Regulation and Neuronal Health. Pathophysiology, 31(4), 545-558. https://doi.org/10.3390/pathophysiology31040040







