Abstract
The use of catheter-based irreversible electroporation in clinical cardiac laboratories, termed pulsed-field ablation (PFA), is gaining international momentum among cardiac electrophysiology proceduralists for the non-thermal management of both atrial and ventricular tachyrhythmogenic substrates. One area of potential application for PFA is in the mitigation of ventricular tachycardia (VT) risk in the setting of ischemia-mediated myocardial fibrosis, as evidenced by recently published clinical case reports. The efficacy of tissue electroporation has been documented in other branches of science and medicine; however, ventricular PFA’s potential advantages and pitfalls are less understood. This comprehensive review will briefly summarize the pathophysiological mechanisms underlying VT and then summarize the pre-clinical and adult clinical data published to date on PFA’s effectiveness in treating monomorphic VT. These data will be contrasted with the effectiveness ascribed to thermal cardiac ablation modalities to treat VT, namely radiofrequency energy and liquid nitrogen-based cryoablation.
1. Introduction
Ventricular tachycardia (VT) is a wide-complex tachyrhythm (QRS duration on surface electrocardiogram > 120 milliseconds) that disrupts normal sinus rhythm and may lead to hemodynamic instability. This arrhythmia originates from within the ventricles and can be clinically defined as ≥3 consecutive ventricular beats occurring at ≥100 beats per minute. VT is categorized by the duration of the episode and the QRS morphology. Based on duration, VT is divided into non-sustained VT and sustained VT. Non-sustained VT terminates spontaneously within 30 s, whereas sustained VT lasts longer than 30 s or requires termination due to hemodynamic instability in <30 s [1]. Based on QRS morphology, VT can be classified as monomorphic (mmVT) or polymorphic. On electrocardiographic assessment, mmVT consists of a singular, consistent QRS morphology with minimal beat-to-beat variation (Figure 1), while polymorphic VT depicts varying QRS morphologies beat-to-beat (Figure 2) [1,2]. The most common cause of mmVT is structural sequelae from ischemic heart disease, but it may also arise from non-ischemic cardiomyopathies, iatrogenic etiologies, Purkinje system defects, or idiopathic etiologies [3]. Several causes of polymorphic VT exist, including R-on-T phenomenon, acute myocardial ischemia, congenital short QT syndrome, acquired long QT syndrome, catecholaminergic, torsade de pointes, bidirectional VT [4], and Brugada syndrome.
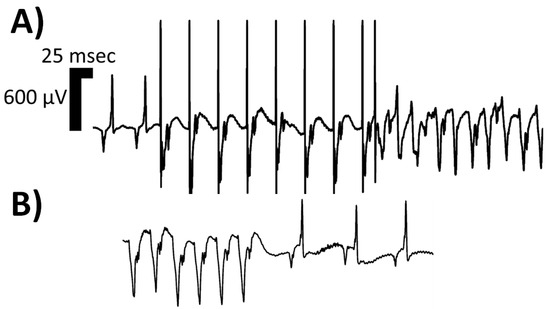
Figure 1.
Isolated limb lead of a single rodent’s surface electrocardiogram depicting one morphologic classification of sustained ventricular tachycardia (VT), namely monomorphic VT. In Panel (A), two sinus complexes begin the rhythm strip, followed by an eight (8) S1–S2 programmed electrical stimulation (PES) drivetrain protocol, executed at twice diastolic-threshold on the epicardial surface using microelectrodes; the resulting monomorphic VT can be appreciated with P-waves continuing at the intrinsic sinoatrial node rate. In Panel (B), the spontaneous termination of the monomorphic VT is depicted, with a sinus pause and resumption of intrinsic conduction system activity at a slower rate than pre-PES. The rat suffered from heart failure with reduced ejection fraction induced via permanent left coronary artery ligation. With the onset of the induced monomorphic VT, the rodent’s invasive blood pressure readings no longer exhibited pulsatility, and the mean arterial pressure dropped. Abbreviations: msec—milliseconds; μV—microvolts.
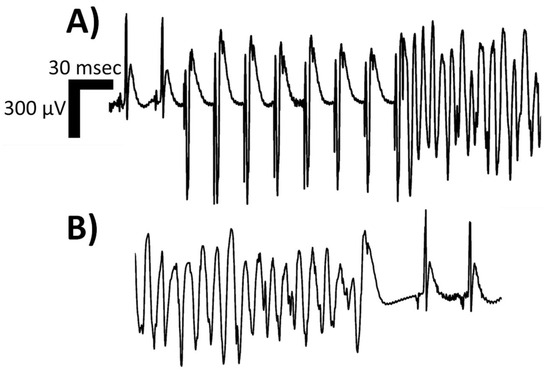
Figure 2.
Isolated limb lead of a single rodent’s surface electrocardiogram depicting one morphologic classification of sustained ventricular tachycardia (VT), namely polymorphic VT. In Panel (A), two sinus complexes begin the rhythm strip, followed by an eight (8) S1–S2 programmed electrical stimulation (PES) drivetrain protocol, executed at twice diastolic-threshold on the epicardial surface using microelectrodes; the resulting polymorphic VT can be appreciated with P-waves continuing at the intrinsic sinoatrial node rate. In Panel (B), the spontaneous termination of the polymorphic VT is depicted, with a sinus pause and resumption of intrinsic conduction system activity at a slower rate than pre-PES. The rat suffered from heart failure with reduced ejection fraction induced via permanent left coronary artery ligation. With the onset of the induced polymorphic VT, the rodent’s invasive blood pressure readings no longer exhibited pulsatility, and the mean arterial pressure dropped. Abbreviations: msec—milliseconds; μV—microvolts.
Pulsed-field ablation (PFA) is a tunable non-thermal ablative technique that, with the appropriate settings, is capable of inducing irreversible cell death via phospholipid bilayer electroporation in selective cardiac tissue populations with minimal damage to anatomically adjacent structures. This technique has recently been adapted to catheter-based technology to allow for clinical use in cardiac electrophysiology laboratories, though its use for research applications such as transmembrane transportation of relatively large macromolecules (ex.: plasmids) dates back to the early 1980s [5]. Furthermore, its use for clinical applications, such as selectively destroying malignant cells in oncology patients, dates back to the early 1990s [6]. PFA has demonstrated in pre-clinical [7] and non-randomized clinical [8] studies an acceptable degree of efficacy with respect to preventing atrial fibrillation recurrence over a short interval (approximately 1 year) while also maintaining an exceptionally favorable safety profile through the avoidance of collateral damage typically observed with post-thermal catheter ablation techniques.
Recently published case reports have illuminated the potential for PFA utilization for substrate suppression in the setting of recurrent ventricular tachycardia secondary to multiple underlying etiologies [9,10,11,12,13]. These preliminary reports likely signal the beginning of an expansion of the clinical electrophysiologists’ armamentarium to include a non-thermal catheter-based therapy in addition to the well-described catheter-based radiofrequency, cryoballoon, and laser options. As such, a careful review of the literature published to date is warranted in order to expedite regulatory approval in the United States of America [14], maximize the proportion of clinical decisions backed by evidence, and support the eventual generation of evidence- and consensus-based clinical guidelines for the use of PFA for ventricular tachyrhythms. In the present review, we aim to comprehensively review the pathophysiological mechanisms underlying VT, summarize the pre-clinical and adult clinical data published to date on PFA’s effectiveness in treating mmVT, and contrast these data with the effectiveness ascribed to thermal cardiac ablation modalities in the treatment of mmVT.
2. Pathophysiology of Ventricular Tachycardia
2.1. Etiologies Leading to Ventricular Tachycardia
2.1.1. Myocardial Infarction, Adverse Remodeling, and Re-Entry
Sustained mmVT is nearly exclusively found secondary to the adverse ventricular remodeling associated with acute- or chronic ischemic heart disease. Adverse ventricular remodeling is frequently observed months to years post-acute myocardial infarction without appropriate pharmacologic suppression, where previously healthy contractile myocytes become irreparably damaged and are eventually replaced by myofibroblast-mediated fibrous tissue [15,16]. Within this myocardial scar are bundles of stunned cardiomyocytes with poor intercellular coupling and subsequently exhibit delayed electrical conduction.
The presence of non-conductive tissue with spatially distributed pockets of conductive myocardium that have impaired repolarization creates a substrate for re-entry [17,18,19]. The criteria for anatomic re-entry are satisfied, namely, a fixed anatomic obstacle mediated by the focus of scar tissue, a circuit-like excitation wavefront pathway through impaired bundles, and unidirectional conduction block facilitated by locally prolonged repolarization in the setting of globally heterogenous repolarization [20] (Figure 3). A myocardial scar provides a fixed arrhythmogenic substrate and a single ventricular focus that consequentially favors mmVT pathophysiology [3].
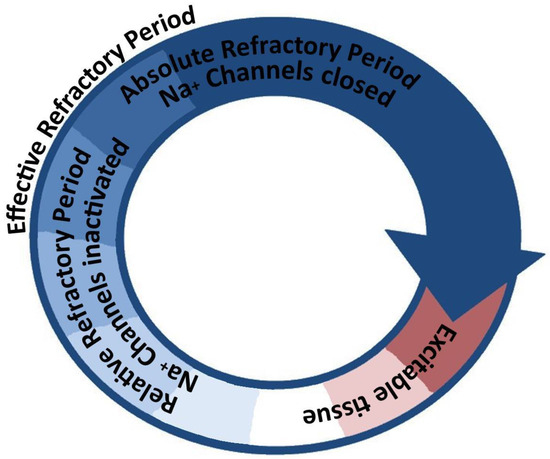
Figure 3.
A stylized diagram illustrating the concept of arrhythmogenic re-entry. A circuit (not necessarily circular in shape, illustrated as such for simplicity) that contains a continuous path of conductive tissue, spatially separated in any of the three physical dimensions, that exhibits a heterogenous distribution of conduction velocities and/or repolarization rates can facilitate re-entry. A sufficiently large circuit, enabled either by anatomic (fixed) tissue separation or by functional (slowed conduction velocity) tissue separation, can allow a unified depolarization wavefront (arrowhead) to continuously meet excitable tissue and thus the circuit to activate with a certain cycle frequency. Abbreviations: Na+—sodium.
In the acute phase of myocardial ischemia, transient sub-clinical ischemia and/or therapeutic reperfusion in acute coronary syndrome (ACS) can cause regional variations in myocyte membrane voltage stability. This instability can lead to ectopic depolarizations or increased automaticity described as R-on-T, Ashman phenomenon, or long-short coupling. These irregular, unregulated focal depolarizations can act as a nidus for triggered activity and initiate hemodynamically significant ventricular arrhythmias [21]. Although VT from ACS is predominately polymorphic, early studies have described an increased risk of mmVT with superimposed acute ischemia on a healed myocardial scar [22,23].
2.1.2. Congenital and Acquired Cardiomyopathies
The incidence of mmVT is certainly higher in ischemic cardiomyopathy relative to non-ischemic; however, cases have been reported [24]. Akin to scar formation from myocardial infarction, inflammatory and degenerative processes can also lead to fibrotic tissue replacement of previously healthy myocytes, thus predisposing to the re-entrant form of VT. Causes of non-ischemic cardiomyopathy are vast and include familial cardiomyopathies such as arrhythmogenic right ventricular cardiomyopathy and non-compaction, autoimmune conditions such as cardiac sarcoidosis and cardiac amyloidosis, or infectious etiologies such as untreated Chagas disease and chronic viral myocarditis. Patients with established non-ischemic cardiomyopathies exhibit a surprisingly high incidence of mmVT via scar-related re-entry mechanisms, as evidenced by the seminal study from Marchlinski et al. [25].
2.1.3. Iatrogenic Causes
Congenital heart disease (CHD) that is incompatible with life and consequently requires surgical repair increases the risk for life-threatening arrhythmias. The majority of VT in CHD occurs predominantly by re-entry mechanisms and is quintessentially illustrated in early-to-middle-aged adults with repaired tetralogy of Fallot (rTOF). Well-defined anatomic isthmuses bordered by regions of unexcitable tissue are created by the numerous suture lines created by the congenital heart surgeons to the anatomical barriers and slowed conduction necessary for re-entry circuits [26]. The arrhythmogenic substrates in rTOF include postsurgical scars following right ventricular outflow tract incisions, valve annuli, and patches [27]. In addition to the expected post-operative surgical scarring, patients with CHD can also develop VT due to adverse ventricular remodeling from impaired function, increased workload, or subsequent conduction system destruction.
2.1.4. Purkinje System Disease
Bundle-branch re-entrant VT (BBR-VT) accounts for approximately 8% of sustained mmVTs [28] and can involve the right/left bundle, their branches, or the His bundle. Bundle-branch diseases, notably left-bundle branch blocks (LBBBs), facilitate interventricular conduction delays, which lead to ventricular contraction desynchrony and, if sufficiently severe, chronic, or hemodynamically significant, can lead to myocardial fibrosis via activation of the renin–angiotensin–aldosterone axis due to impaired perfusion of the periphery. The diffuse scar tissue deposition from this systemic process can provide an arrhythmogenic substrate for the formation of re-entrant circuits. These abnormalities disrupt the intrinsic cardiac conduction system, further increasing the likelihood of ventricular arrhythmias, including mmVT.
2.1.5. Idiopathic Ventricular Tachycardia
Idiopathic VT is a small subset of tachyarrhythmias that occurs in patients without structural heart disease. The VT focus can be located anywhere in the heart but predominantly arises from the right ventricular outflow tract (RVOT) and, less commonly, from the left ventricular outflow tract [29]. RVOT-VT is frequently precipitated by high adrenergic states such as exercise, intense emotions, and illness [30]. Individuals affected by idiopathic VT are typically female, young, and healthy and thus require a thorough diagnostic workup for other causes and then for underlying heart disease. Unlike other causes of VT, this type is relatively benign given its transient nature in otherwise unremarkable cardiac systems and thus is associated with a low risk of sudden cardiac death [31].
2.2. Molecular Mechanisms of Monomorphic Ventricular Re-Entry
2.2.1. Calcium Handling
Under optimal cardiomyocyte conditions, a sodium influx-mediated membrane action potential initiates the opening of voltage-gated L-type Ca2+ channels (LTCCs), leading to Ca2+ release from the sarcoplasmic reticulum (SR), facilitating allosteric manipulation of thin filament regulatory proteins and actin–myosin cross-bridge cycling. LTCCs play a vital role in maintaining membrane depolarization throughout the plateau phase of the action potential. For this reason, the L-type calcium current (ICa,L) is crucial for preserving optimal action potential duration (APD) and illustrates why any alternations in ICa,L kinetics carry a high arrhythmogenic potential.
2.2.2. Action Potential Prolongation and Repolarization Heterogeneity
In structural heart disease, a myriad of compensatory and pathophysiologic electrophysiological alterations ensue, precipitating a persistent proarrhythmic state. Ischemia-mediated calcium dysregulation (via sarco-endoplasmic reticulum adenosine triphosphate-ase expression downregulation and allosteric inhibition in addition to uncontrolled calcium sparks from failing ryanodine receptor clusters) and impaired potassium efflux [18] can directly lead to apoptosis via caspase activation or can lead to an impaired resting membrane potential and subsequent cell death. These two electrolyte abnormalities increase APD and ultimately exacerbate the repolarization reserve to depletion. Under conditions of acute metabolic stress, this low reserve state can lead to electrical alternans and, subsequently, mechanical alternans [32] as well as increase the likelihood of re-entrant arrhythmia via furthering the global cardiac repolarization heterogeneity.
This dispersion of repolarization can allow early afterdepolarizations, occurring during phases two or three of the cardiac action potential near the absolute refractory period, or the higher risk delayed afterdepolarizations occurring during phase four in the relative refractory period, to perturb the systems sufficiently to activate a dormant re-entrant system.
2.2.3. Dysregulated Na+ Handling
The rapid upstroke of phase one in the cardiomyocyte action potential is primarily attributed to the influx of sodium ions (Na+) through the sodium current (INa), making it predominantly responsible for tissue conduction velocity. In myocytes battered by local hypoxia and impaired membrane voltage regulation, the properties of the INa fail to completely inactivate and/or close throughout the action potential, resulting in late INa. This late depolarizing current has been demonstrated to be induced by the Ca2+/calmodulin-dependent protein kinase II pathways, which are activated in the presence of structural heart disease to facilitate salvaging cardiac function via calcium desensitization [33]. The heightened intracellular Na+ concentration subsequently triggers an additional increase in cytosolic Ca2+ levels via membrane-bound Na+-Ca2+ exchangers. These mechanisms collectively contribute to APD dispersion and elevated arrhythmogenic risk [18].
3. Pulsed-Field Ablation for Arrhythmogenic Substrate Suppression
3.1. Regulatory Status of Clinical Pulsed-Field Ablation
PFA achieved regulatory approval for clinical use in the European Medicines Agency when Farapulse Inc., a subsidiary of Boston Scientific, achieved “Conformité Européene” in January of 2021 for the treatment of paroxysmal atrial fibrillation in adults [34] and shortly thereafter in March of 2021, Medtronic attained the same approval for their Affera™ Mapping and Ablation System. Though the same systems are also being utilized in the United States of America for various clinical conditions, including persistent left superior vena cava [35], neither has yet to achieve regulatory approval for clinical use by the Food and Drug Administration nor the Chinese National Medical Products Administration, though investigations are pending [36]. A third system, the Galaxy Medical/Galvanize Therapeutics CENTAURI™ System, attained European regulatory approval in August of 2022 [37], making it the most recent approval to date. As many as thirteen additional PFA systems are still in the development pipeline; however, no information regarding regulatory pursuits for pediatric indications can be found, though clinical case reports are arising [38].
3.2. Foundational Findings Supporting Pulsed-Field Ablation
3.2.1. Mechanism of Action
Irreversible electroporation is the process of exposing a cell’s phospholipid bilayer membrane to nanosecond strong “non-thermal” [39] electric fields (voltage) to overwhelm the membrane’s inherent electrical capacitance threshold and creating sufficiently large membrane pores that cell death via necrosis or apoptosis. This process is tunable in that the voltage, pulse duration, pulse frequency, drivetrain, and application site can be adjusted independently; the optimization of these settings of cardiac catheter-based ablation is referred to as pulsed-field ablation. Electroporation requires cell membranes and thus creates specificity for biologically active membranes within the relatively precise electric field, thus sparing non-membranous connective tissue and support matrices. In addition, the small field of action minimizes damage to biologically active tissues outside of the electric field, such as nervous tissue, though variable non-fatal damage and regeneration have been described [40]. In addition, the non-thermal mechanism of action does not denature tissue or activate any damage-sensitive innate receptors for non-specific inflammation [41]. A minimized inflammatory response yields less fibrosis and off-target tissue damage. This non-thermal technique was adapted to force-sensing catheter-based systems and ultimately made uniform with a “single shot” operation, which avoids any theoretical cancellation effects from rapid serial dosing [42].
3.2.2. Preclinical Proof-of-Concept
Published data evaluating the molecular effects of PFA on cell cultures have revealed a differential response and toxicity threshold from both murine and human cardiomyocytes, neurons, and cardiac adipocytes based on electric field strengths [43,44,45,46,47]. Human esophageal smooth muscle cells have also been assessed and exhibited a greater resistance to FPA [48]. Dose-response assessments have been generated for isolated rodent ventricles [49]. In addition to differences in population thresholds, in silico models are optimized to maximize myocytes’ toxicity based on cell orientation relative to the electric field [50] and tissue fiber orientations [51].
Langendorff and whole-animal studies have been completed in swine [52,53,54,55,56,57], canine [58], ovine [59], and even rabbit [60] models using various PFA catheter types and methodologies. Consensus findings include the creation of transmural lesions via concentrated apoptosis with no evidence of thermal-based tissue damage. Though most articles do not document any adverse effects from PFA, coronary artery spasms and chronic stenosis via neointimal hyperplasia have been appreciated.
3.3. Pulsed-Field Ablation for Monomorphic Ventricular Tachycardia
3.3.1. Pre-Clinical Efficacy for Monomorphic Ventricular Tachycardia
Unfortunately, no pre-clinical data assessing the safety, efficacy, or reproducibility of PFA for VT exist in animal models of human cardiac disease. However, pre-clinical reports on the efficacy of ventricular PFA [53,56,57,61], as well as the safety of ventricular PFA [62], are increasing. All five studies were conducted in swine [53,56,57,61,62] and had short-term endpoints (ranging from 2 to 7 days [57] to 6 to 8 weeks [53]). Sample sizes were small (ranging from 4 to 10 swine), though appropriate for preliminary de novo safety study designs. Translational studies in models that closely recapitulate the pathophysiological processes encountered in the clinical cardiac electrophysiology lab (namely rodent [17] and swine models of ischemic cardiomyopathy assessed with clinically relevant programmed electrical stimulation techniques) are needed to carefully detail the immediate-, short-, and long-term efficacy of PFA for mmVT.
3.3.2. Adult Clinical Efficacy for Monomorphic Ventricular Tachycardia
Clinical data for the management of patients suffering from VT are presently limited to the case report and case series stage of development [9,10,11,12,13]. Though the etiologies varied from scar-mediated [9,10,11,13] to ventricular aneurysm [11], and arrhythmogenic cardiomyopathy [11], atrioventricular malformations [12], non-ischemic cardiomyopathy [13,63], and even ectopy [11,64], no adverse events were reported in any of these observational studies (Table 1). Of note, the longest time period that a patient was monitored for potential arrhythmia recurrence post-PFA was 6 months [9]. Though concerns for coronary artery vasospasm and/or stenosis [65,66] secondary to neointimal hyperplasia, as well as nerve damage [67], have been raised, the volume-adjusted incidence suggests a lower risk of these neurovascular complications compared to the thermal catheter-based ablation modalities used for comparable substrates.

Table 1.
A table highlighting the pertinent qualities of the clinical case reports on the use of pulsed-field ablation for ventricular tachycardia. Case reports/series are listed in order of reference number and include the PFA technology parameters, if available. Abbreviations: Ref—reference, M—male, F—female, PFA—pulsed-field ablation, MI—myocardial infarction, ICD—implantable cardioverter defibrillator, defibs—defibrillations, VT—ventricular tachycardia, mmVT—monomorphic ventricular tachycardia, ATP—anti-tachycardia pacing, mm—millimeters, kV—kiloVolts, HFrEF—heart failure with reduced ejection fraction, LVEF—left ventricular ejection fraction, RFA—radiofrequency ablation, CRT-D—cardiac resynchronization therapy-defibrillator, aRV—atrialized right ventricle, RV—right ventricle, LAD—left anterior descending coronary artery, W—watts, C—Celsius, RVOT—right ventricular outflow tract, PVCs—premature ventricular contractions, sec—seconds, LV—left ventricle.
3.3.3. Outcome Comparison with Thermal Cardiac Ablation
Due to the preliminary stage of PFA for mmVT management and likely also due to the publication bias for positive-result studies, all (6/6, 100%) case reports and case studies published to date describe success with regard to the primary outcome of arrhythmia cessation and/or failed VT induction. The success rate for managing mmVT secondary to coronary disease over a six-month time interval has been approximated to 62% [68]. Laser energy continues to be investigated at the pre-clinical level for ventricular rhythm applications [69]; nonetheless, its use remains focused on lead extractions due to operator familiarity with radiofrequency energy. Similarly, cryoballoons have been utilized for ventricular substrates at the case series level [70] but are rarely used for monomorphic ventricular tachycardia, and thus, no success rates nor complication rates can be confidently assessed.
4. Conclusions
Chronic compensatory changes post-ischemic insult produce ischemia-mediated calcium dysregulation, impaired potassium efflux, global action potential duration dispersion, and increased arrhythmogenic risk. These electrophysiologic changes paired with structural scar tissue can propagate the initiation of ventricular tachyrhthms, including monomorphic ventricular tachycardia (mmVT). Though current management of pharmacologically resistant mmVT includes catheter ablation with a thermal mechanism of action, recurrence occurs, and anatomical challenges and safety risks must be carefully considered. Irreversible electroporation provides a non-thermal option for the invasive cardiac electrophysiologist and may increase the overall efficacy of catheter ablation for difficult-to-treat or anatomically limiting cardiac tachyrhythms. At the present time, pre-clinical data supporting pulsed-field ablation (PFA) for mmVT are lackluster; however, clinical case reports are mounting. Additional work regarding the safety, efficacy, and long-term durability of PFA for mmVT is needed.
Author Contributions
M.L.R.—manuscript and table drafting. I.R.C.—manuscript and table drafting, review, and revision; creation of figures. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data utilized in this review article are publicly available.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2018, 72, e91–e220, Erratum in J. Am. Coll. Cardiol. 2018, 72, 1760. [Google Scholar] [CrossRef] [PubMed]
- Buxton, A.E.; Calkins, H.; Callans, D.J.; DiMarco, J.P.; Fisher, J.D.; Greene, H.L.; Haines, D.E.; Hayes, D.L.; Heidenreich, P.A.; Miller, J.M.; et al. ACC/AHA/HRS 2006 key data elements and definitions for electrophysiological studies and procedures: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (ACC/AHA/HRS Writing Committee to Develop Data Standards on Electrophysiology). J. Am. Coll. Cardiol. 2006, 48, 2360–2396. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, W.G.; Soejima, K. Catheter ablation for ventricular tachycardia. Circulation 2007, 115, 2750–2760. [Google Scholar] [CrossRef]
- Ransom, J.L.; Wong, K.C.; Kircher, J.; Usry, C.; Larson, C. Bidirectional Ventricular Tachycardia in a Young Female: A Case of Andersen-Tawil Syndrome. Mil. Med. 2023, 188, e412–e416. [Google Scholar] [CrossRef] [PubMed]
- Neumann, E.; Schaefer-Ridder, M.; Wang, Y.; Hofschneider, P.H. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982, 1, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Dev, S.B.; Hofmann, G.A. Electrochemotherapy--a novel method of cancer treatment. Cancer Treat. Rev. 1994, 20, 105–115. [Google Scholar] [CrossRef]
- Yavin, H.D.; Higuchi, K.; Younis, A.; Anter, E. Lattice-tip catheter for single-shot pulmonary vein isolation with pulsed field ablation. J. Interv. Card. Electrophysiol. 2023, 66, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Haines, D.E.; Boersma, L.V.; Sood, N.; Natale, A.; Marchlinski, F.E.; Calkins, H.; Sanders, P.; Packer, D.L.; Kuck, K.H.; et al. Pulsed Field Ablation for the Treatment of Atrial Fibrillation: PULSED AF Pivotal Trial. Circulation 2023, 147, 1422–1432. [Google Scholar] [CrossRef]
- Ouss, A.; van Stratum, L.; van der Voort, P.; Dekker, L. First in human pulsed field ablation to treat scar-related ventricular tachycardia in ischemic heart disease: A case report. J. Interv. Card. Electrophysiol. 2022, 66, 509–510. [Google Scholar] [CrossRef]
- Martin, C.A.; Zaw, M.T.; Jackson, N.; Morris, D.; Costanzo, P. First worldwide use of pulsed-field ablation for ventricular tachycardia ablation via a retrograde approach. J. Cardiovasc. Electrophysiol. 2023, 34, 1772–1775. [Google Scholar] [CrossRef]
- Lozano-Granero, C.; Hirokami, J.; Franco, E.; Tohoku, S.; Matía-Francés, R.; Schmidt, B.; Hernández-Madrid, A.; Zamorano Gómez, J.L.; Moreno, J.; Chun, J. Case Series of Ventricular Tachycardia Ablation With Pulsed-Field Ablation: Pushing Technology Further (Into the Ventricle). JACC Clin. Electrophysiol. 2023, 9, 1990–1994. [Google Scholar] [CrossRef] [PubMed]
- Krause, U.; Bergau, L.; Zabel, M.; Müller, M.J.; Paul, T. Flowerpower: Pulsed field ablation of ventricular tachycardia in a patient with Ebstein’s anomaly. Eur. Heart J. Case Rep. 2023, 7, ytad093. [Google Scholar] [CrossRef] [PubMed]
- Adragão, P.; Matos, D.; Carmo, P.; Costa, F.M.; Ramos, S. Pulsed-field ablation vs radiofrequency ablation for ventricular tachycardia: First in-human case of histologic lesion analysis. Heart Rhythm. 2023, 20, 1395–1398. [Google Scholar] [CrossRef] [PubMed]
- Ekanem, E.; Reddy, V.Y.; Schmidt, B.; Reichlin, T.; Neven, K.; Metzner, A.; Hansen, J.; Blaauw, Y.; Maury, P.; Arentz, T.; et al. Multi-national survey on the methods, efficacy, and safety on the post-approval clinical use of pulsed field ablation (MANIFEST-PF). Europace 2022, 24, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Dhanjal, T.S.; Lellouche, N.; von Ruhland, C.J.; Abehsira, G.; Edwards, D.H.; Dubois-Randé, J.L.; Moschonas, K.; Teiger, E.; Williams, A.J.; George, C.H. Massive Accumulation of Myofibroblasts in the Critical Isthmus Is Associated With Ventricular Tachycardia Inducibility in Post-Infarct Swine Heart. JACC Clin. Electrophysiol. 2017, 3, 703–714. [Google Scholar] [CrossRef]
- D’Souza, K.M.; Biwer, L.A.; Madhavpeddi, L.; Ramaiah, P.; Shahid, W.; Hale, T.M. Persistent change in cardiac fibroblast physiology after transient ACE inhibition. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1346–H1353. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chinyere, I.R.; Moukabary, T.; Hutchinson, M.D.; Lancaster, J.J.; Juneman, E.; Goldman, S. Progression of infarct-mediated arrhythmogenesis in a rodent model of heart failure. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H108–H116. [Google Scholar] [CrossRef]
- Chinyere, I.R.; Hutchinson, M.; Moukabary, T.; Lancaster, J.; Goldman, S.; Juneman, E. Monophasic action potential amplitude for substrate mapping. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H667–H673. [Google Scholar] [CrossRef]
- Chinyere, I.R.; Hutchinson, M.; Moukabary, T.; Koevary, J.W.; Juneman, E.; Goldman, S.; Lancaster, J.J. Modulating the Infarcted Ventricle’s Refractoriness with an Epicardial Biomaterial. J. Investig. Med. 2021, 69, 364–370. [Google Scholar] [CrossRef]
- Ciaccio, E.J.; Coromilas, J.; Ashikaga, H.; Cervantes, D.O.; Wit, A.L.; Peters, N.S.; McVeigh, E.R.; Garan, H. Reprint of ‘Model of unidirectional block formation leading to reentrant ventricular tachycardia in the infarct border zone of postinfarction canine hearts’. Comput. Biol. Med. 2015, 65, 256–266. [Google Scholar] [CrossRef]
- Morton, M.B.; Morton, J.B.; Mond, H.G. Aberrant Ventricular Conduction: Revisiting an Old Concept. Heart Lung Circ. 2023, 32, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Moroe, K.; Mayrovitz, H.N.; Sampsell, R.; Furukawa, N.; Myerburg, R.J. Arrhythmogenic effects of graded coronary blood flow reductions superimposed on prior myocardial infarction in dogs. Circulation 1991, 84, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Stambler, B.S.; Akosah, K.O.; Mohanty, P.K.; Wood, M.A.; Ellenbogen, K.A. Myocardial ischemia and induction of sustained ventricular tachyarrhythmias: Evaluation using dobutamine stress echocardiography-electrophysiologic testing. J. Cardiovasc. Electrophysiol. 2004, 15, 901–907. [Google Scholar] [CrossRef]
- De Potter, T.; Balt, J.C.; Boersma, L.; Sacher, F.; Neuzil, P.; Reddy, V.; Grigorov, I.; Verma, A. First-in-Human Experience With Ultra-Low Temperature Cryoablation for Monomorphic Ventricular Tachycardia. JACC Clin. Electrophysiol. 2023, 9, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Hsia, H.H.; Callans, D.J.; Marchlinski, F.E. Characterization of endocardial electrophysiological substrate in patients with nonischemic cardiomyopathy and monomorphic ventricular tachycardia. Circulation 2003, 108, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Caldaroni, F.; Lo Rito, M.; Chessa, M.; Varrica, A.; Micheletti, A.; Pappone, C.; Giamberti, A. Surgical ablation of ventricular tachycardia in patients with repaired tetralogy of Fallot†. Eur. J. Cardiothorac. Surg. 2019, 55, 845–850. [Google Scholar] [CrossRef]
- Kapel, G.F.; Sacher, F.; Dekkers, O.M.; Watanabe, M.; Blom, N.A.; Thambo, J.B.; Derval, N.; Schalij, M.J.; Jalal, Z.; Wijnmaalen, A.P.; et al. Arrhythmogenic anatomical isthmuses identified by electroanatomical mapping are the substrate for ventricular tachycardia in repaired Tetralogy of Fallot. Eur. Heart J. 2017, 38, 268–276. [Google Scholar] [CrossRef]
- Mizusawa, Y.; Sakurada, H.; Nishizaki, M.; Ueda-Tatsumoto, A.; Fukamizu, S.; Hiraoka, M. Characteristics of bundle branch reentrant ventricular tachycardia with a right bundle branch block configuration: Feasibility of atrial pacing. Europace 2009, 11, 1208–1213. [Google Scholar] [CrossRef]
- Badhwar, N.; Scheinman, M.M. Idiopathic ventricular tachycardia: Diagnosis and management. Curr. Probl. Cardiol. 2007, 32, 7–43. [Google Scholar] [CrossRef]
- Ward, R.C.; van Zyl, M.; DeSimone, C.V. Idiopathic Ventricular Tachycardia. J. Clin. Med. 2023, 12, 930. [Google Scholar] [CrossRef]
- Buxton, A.E.; Waxman, H.L.; Marchlinski, F.E.; Simson, M.B.; Cassidy, D.; Josephson, M.E. Right ventricular tachycardia: Clinical and electrophysiologic characteristics. Circulation 1983, 68, 917–927. [Google Scholar] [CrossRef]
- Chinyere, I.R.; Moukabary, T.; Goldman, S.; Juneman, E. Electrical and mechanical alternans during ventricular tachycardia with moderate chronic heart failure. J. Electrocardiol. 2018, 51, 33–37. [Google Scholar] [CrossRef]
- Liu, X.; Ren, L.; Yu, S.; Li, G.; He, P.; Yang, Q.; Wei, X.; Thai, P.N.; Wu, L.; Huo, Y. Late sodium current in synergism with Ca2+/calmodulin-dependent protein kinase II contributes to β-adrenergic activation-induced atrial fibrillation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2023, 378, 20220163. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Bordignon, S.; Neven, K.; Reichlin, T.; Blaauw, Y.; Hansen, J.; Adelino, R.; Ouss, A.; Füting, A.; Roten, L.; et al. EUropean real-world outcomes with Pulsed field ablatiOn in patients with symptomatic atRIAl fibrillation: Lessons from the multi-centre EU-PORIA registry. Europace 2023, 25, euad185. [Google Scholar] [CrossRef]
- Tohoku, S.; Schmidt, B.; Bordignon, S.; Chen, S.; Bologna, F.; Urbanek, L.; Pansera, F.; Chun, K.R.J. Pulsed Field Ablation for Persistent Superior Vena Cava: New Solution for an Old Problem. JACC Case Rep. 2022, 4, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Lehmann, J.W.; Gerstenfeld, E.P.; Mugglin, A.S.; Schneider, C.W.; Achyutha, A.B.; Mansour, M. A randomized controlled trial of pulsed field ablation versus standard-of-care ablation for paroxysmal atrial fibrillation: The ADVENT trial rationale and design. Heart Rhythm O2 2023, 4, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Anić, A.; Phlips, T.; Brešković, T.; Koopman, P.; Girouard, S.; Mediratta, V.; Jurišić, Z.; Sikirić, I.; Lisica, L.; Vijgen, J. Pulsed field ablation using focal contact force-sensing catheters for treatment of atrial fibrillation: Acute and 90-day invasive remapping results. Europace 2023, 25, euad147. [Google Scholar] [CrossRef]
- Rossillo, A.; Borio, G.; Vittadello, S.; Spadaro, G.L.; Bonanno, C.; Raviele, A.; Caprioglio, F. Focal atrial tachycardia arising from left superior pulmonary vein in a pediatric patient, safely treated by pulsed-field ablation. J. Cardiovasc. Electrophysiol. 2023, 34, 1764–1767. [Google Scholar] [CrossRef]
- Aycock, K.N.; Campelo, S.N.; Davalos, R.V. A Comparative Modeling Study of Thermal Mitigation Strategies in Irreversible Electroporation Treatments. J. Heat. Transfer. 2022, 144, 031206. [Google Scholar] [CrossRef]
- Kim, M.Y.; Stavrakis, S. For Better or Worse, Pulse Field Ablation Is Kinder to Some Nerves. JACC Clin. Electrophysiol. 2022, 8, 905–907. [Google Scholar] [CrossRef]
- Teng, P.; Wu, Y.; Chen, R.; Hong, L.; Wu, B.; Liu, L.; Ma, L.; Zhao, H.; Wu, S. Pulsed field ablation as a precise approach for cardiac arrhythmia treatment via cardiac microenvironment remodeling. Bioelectrochemistry 2023, 154, 108502. [Google Scholar] [CrossRef] [PubMed]
- Polajžer, T.; Dermol-Černe, J.; Reberšek, M.; O’Connor, R.; Miklavčič, D. Cancellation effect is present in high-frequency reversible and irreversible electroporation. Bioelectrochemistry 2020, 132, 107442. [Google Scholar] [CrossRef] [PubMed]
- Avazzadeh, S.; O’Brien, B.; Coffey, K.; O’Halloran, M.; Keane, D.; Quinlan, L.R. Establishing Irreversible Electroporation Electric Field Potential Threshold in A Suspension In Vitro Model for Cardiac and Neuronal Cells. J. Clin. Med. 2021, 10, 5443. [Google Scholar] [CrossRef]
- Avazzadeh, S.; Dehkordi, M.H.; Owens, P.; Jalali, A.; O’Brien, B.; Coffey, K.; O’Halloran, M.; Fernhead, H.O.; Keane, D.; Quinlan, L.R. Establishing electroporation thresholds for targeted cell specific cardiac ablation in a 2D culture model. J. Cardiovasc. Electrophysiol. 2022, 33, 2050–2061. [Google Scholar] [CrossRef]
- Baena-Montes, J.M.; O’Halloran, T.; Clarke, C.; Donaghey, K.; Dunne, E.; O’Halloran, M.; Quinlan, L.R. Electroporation Parameters for Human Cardiomyocyte Ablation In Vitro. J. Cardiovasc. Dev. Dis. 2022, 9, 240. [Google Scholar] [CrossRef] [PubMed]
- Casciola, M.; Feaster, T.K.; Caiola, M.J.; Keck, D.; Blinova, K. Human in vitro assay for irreversible electroporation cardiac ablation. Front. Physiol. 2023, 13, 1064168. [Google Scholar] [CrossRef]
- Masuyama, K.; Ajijola, O.A. Irreversible electroporation for cardiac ablation: Optimizing cell type-specific effects. J. Cardiovasc. Electrophysiol. 2022, 33, 2062–2063. [Google Scholar] [CrossRef]
- Casciola, M.; Keck, D.; Feaster, T.K.; Blinova, K. Human cardiomyocytes are more susceptible to irreversible electroporation by pulsed electric field than human esophageal cells. Physiol. Rep. 2022, 10, e15493. [Google Scholar] [CrossRef]
- Chaigne, S.; Sigg, D.C.; Stewart, M.T.; Hocini, M.; Batista Napotnik, T.; Miklavčič, D.; Bernus, O.; Benoist, D. Reversible and Irreversible Effects of Electroporation on Contractility and Calcium Homeostasis in Isolated Cardiac Ventricular Myocytes. Circ. Arrhythm. Electrophysiol. 2022, 15, e011131. [Google Scholar] [CrossRef]
- Scuderi, M.; Dermol-Černe, J.; Batista Napotnik, T.; Chaigne, S.; Bernus, O.; Benoist, D.; Sigg, D.C.; Rems, L.; Miklavčič, D. Characterization of Experimentally Observed Complex Interplay between Pulse Duration, Electrical Field Strength, and Cell Orientation on Electroporation Outcome Using a Time-Dependent Nonlinear Numerical Model. Biomolecules 2023, 13, 727. [Google Scholar] [CrossRef]
- Xie, F.; Zemlin, C.W. Effect of Twisted Fiber Anisotropy in Cardiac Tissue on Ablation with Pulsed Electric Fields. PLoS ONE 2016, 11, e0152262. [Google Scholar] [CrossRef] [PubMed]
- Varghese, F.; Philpott, J.M.; Neuber, J.U.; Hargrave, B.; Zemlin, C.W. Surgical Ablation of Cardiac Tissue with Nanosecond Pulsed Electric Fields in Swine. Cardiovasc. Eng. Technol. 2023, 14, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Im, S.I.; Higuchi, S.; Lee, A.; Stillson, C.; Buck, E.; Morrow, B.; Schenider, K.; Speltz, M.; Gerstenfeld, E.P. Pulsed Field Ablation of Left Ventricular Myocardium in a Swine Infarct Model. JACC Clin. Electrophysiol. 2022, 8, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.; Zilberman, I.; Krywanczyk, A.; Higuchi, K.; Yavin, H.D.; Sroubek, J.; Anter, E. Effect of Pulsed-Field and Radiofrequency Ablation on Heterogeneous Ventricular Scar in a Swine Model of Healed Myocardial Infarction. Circ. Arrhythm. Electrophysiol. 2022, 15, e011209. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, I.; Reddy, V.Y.; Wang, B.J.; Dukkipati, S.R.; Chaudhry, H.W.; Santos-Gallego, C.G.; Koruth, J.S. Pulsed Field Ablation of the Porcine Ventricle Using a Focal Lattice-Tip Catheter. Circ. Arrhythm. Electrophysiol. 2022, 15, e011120. [Google Scholar] [CrossRef]
- Sandhu, U.; Alkukhun, L.; Kheiri, B.; Hodovan, J.; Chiang, K.; Splanger, T.; Castellvi, Q.; Zhao, Y.; Nazer, B. In vivo pulsed-field ablation in healthy vs. chronically infarcted ventricular myocardium: Biophysical and histologic characterization. Europace 2023, 25, 1503–1509. [Google Scholar] [CrossRef]
- Kawamura, I.; Reddy, V.Y.; Santos-Gallego, C.G.; Wang, B.J.; Chaudhry, H.W.; Buck, E.D.; Mavroudis, G.; Jerrell, S.; Schneider, C.W.; Speltz, M.; et al. Electrophysiology, Pathology, and Imaging of Pulsed Field Ablation of Scarred and Healthy Ventricles in Swine. Circ. Arrhythm. Electrophysiol. 2023, 16, e011369. [Google Scholar] [CrossRef]
- Fan, S.; Jia, F.; Cui, Y.; Wu, D.; He, L.; Zhang, F.; Xue, Z.; Xu, X.; Lu, F.; Ma, W.; et al. Study on the process of cardiomyocyte apoptosis after pulsed field ablation. Front. Cardiovasc. Med. 2023, 10, 1112131. [Google Scholar] [CrossRef]
- Aryana, A.; Ji, S.Y.; Hata, C.; de la Rama, A.; Nguyen, K.; Panescu, D. Preclinical evaluation of a novel single-shot pulsed field ablation system for pulmonary vein and atrial ablation. J. Cardiovasc. Electrophysiol. 2023, 34, 2203–2212. [Google Scholar] [CrossRef]
- Xie, F.; Varghese, F.; Pakhomov, A.G.; Semenov, I.; Xiao, S.; Philpott, J.; Zemlin, C. Ablation of Myocardial Tissue With Nanosecond Pulsed Electric Fields. PLoS ONE 2015, 10, e0144833. [Google Scholar] [CrossRef]
- Koruth, J.S.; Kuroki, K.; Iwasawa, J.; Viswanathan, R.; Brose, R.; Buck, E.D.; Donskoy, E.; Dukkipati, S.R.; Reddy, V.Y. Endocardial ventricular pulsed field ablation: A proof-of-concept preclinical evaluation. Europace 2020, 22, 434–439. [Google Scholar] [CrossRef]
- Alkukhun, L.; Sandhu, U.; Hodovan, J.; Zhao, Y.; Chiang, K.; Castellvi, Q.; Stenzel, P.; Woltjer, R.; Li, X.; Barajas, R.F.; et al. Multi-modality imaging assessment of microbubbles and cerebral emboli in left ventricular pulsed field ablation. J. Interv. Card. Electrophysiol. 2023; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Weyand, S.; Löbig, S.; Seizer, P. First in human focal pulsed field ablation to treat an epicardial VT focus with an endocardial approach in non-ischemic cardiomyopathy. J. Interv. Card. Electrophysiol. 2023, 66, 1057–1058. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Chen, S.; Tohoku, S.; Bordignon, S.; Bologna, F.; Chun, K.R.J. Single shot electroporation of premature ventricular contractions from the right ventricular outflow tract. Europace 2022, 24, 597. [Google Scholar] [CrossRef]
- Ladejobi, A.; Christopoulos, G.; Tan, N.; Ladas, T.P.; Tri, J.; van Zyl, M.; Yasin, O.; Sugrue, A.; Khabsa, M.; Uecker, D.R.; et al. Effects of Pulsed Electric Fields on the Coronary Arteries in Swine. Circ. Arrhythm. Electrophysiol. 2022, 15, e010668. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, S.; Im, S.I.; Stillson, C.; Buck, E.D.; Jerrell, S.; Schneider, C.W.; Speltz, M.; Gerstenfeld, E.P. Effect of Epicardial Pulsed Field Ablation Directly on Coronary Arteries. JACC Clin. Electrophysiol. 2022, 8, 1486–1496. [Google Scholar] [CrossRef]
- Pansera, F.; Bordignon, S.; Bologna, F.; Tohoku, S.; Chen, S.; Urbanek, L.; Schmidt, B.; Chun, K.J. Catheter ablation induced phrenic nerve palsy by pulsed field ablation-completely impossible? A case series. Eur. Heart J. Case Rep. 2022, 6, ytac361. [Google Scholar] [CrossRef] [PubMed]
- Marchlinski, F.E.; Haffajee, C.I.; Beshai, J.F.; Dickfeld, T.L.; Gonzalez, M.D.; Hsia, H.H.; Schuger, C.D.; Beckman, K.J.; Bogun, F.M.; Pollak, S.J.; et al. Long-Term Success of Irrigated Radiofrequency Catheter Ablation of Sustained Ventricular Tachycardia: Post-Approval THERMOCOOL VT Trial. J. Am. Coll. Cardiol. 2016, 67, 674–683. [Google Scholar] [CrossRef]
- Krist, D.; Linz, D.; Schotten, U.; Zeemering, S.; Leenen, D. A Novel Laser Energy Ablation Catheter for Endocardial Cavo-Tricuspid Isthmus Ablation and Epicardial Ventricular Lesion Formation: An in vivo Proof-of-Concept Study. Front. Med. Technol. 2022, 4, 834856. [Google Scholar] [CrossRef]
- Cook, C.; Welter-Frost, A.; Wilson, D.R.; Herweg, B. Cryoballoon ablation for refractory ventricular tachycardia. HeartRhythm Case Rep. 2022, 9, 203–207. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).