A Refractory Electrical Storm after Acute Myocardial Infarction: The Role of Temporary Ventricular Overdrive Pacing as a Bridge to ICD Implantation
Abstract
1. Introduction
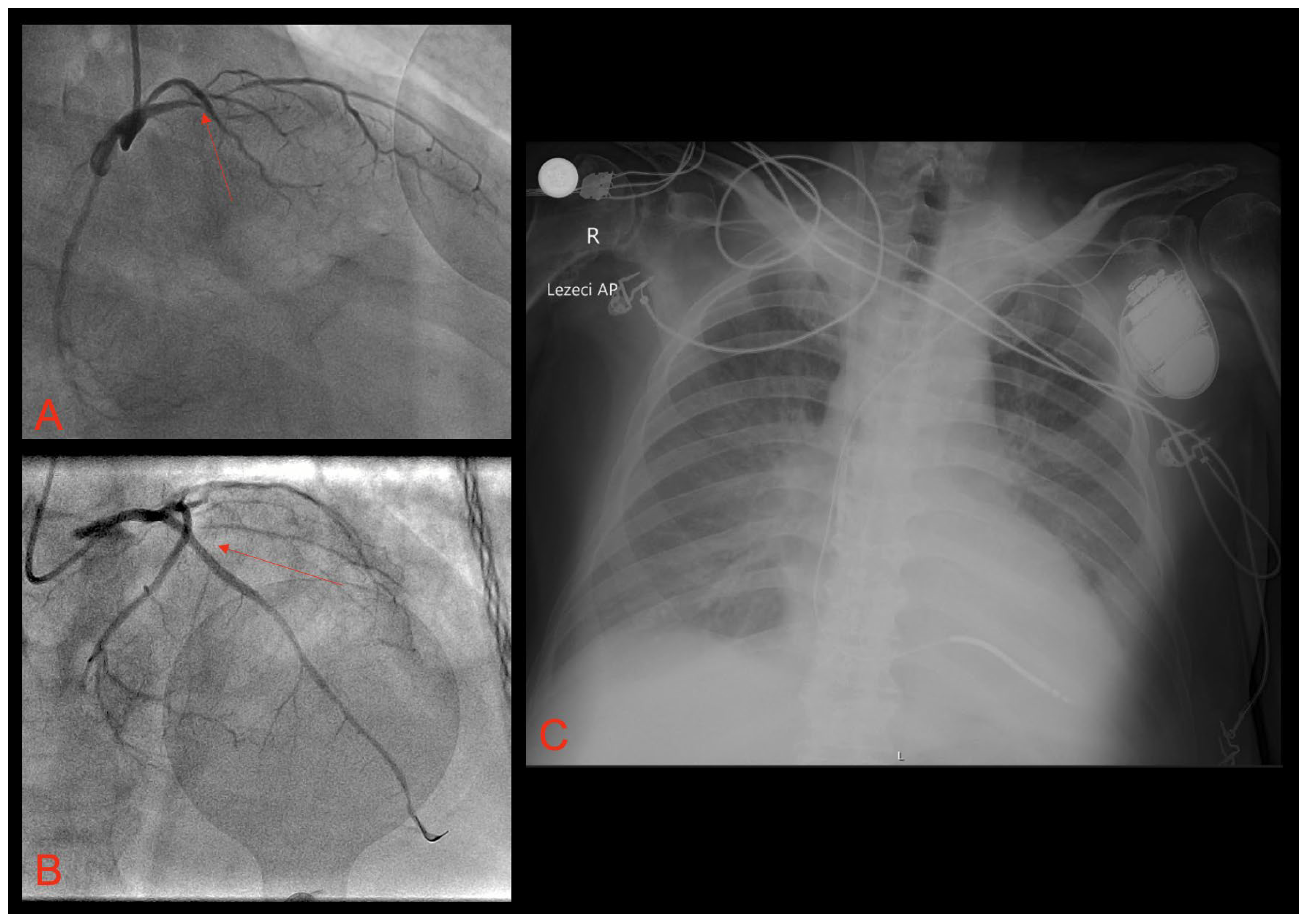
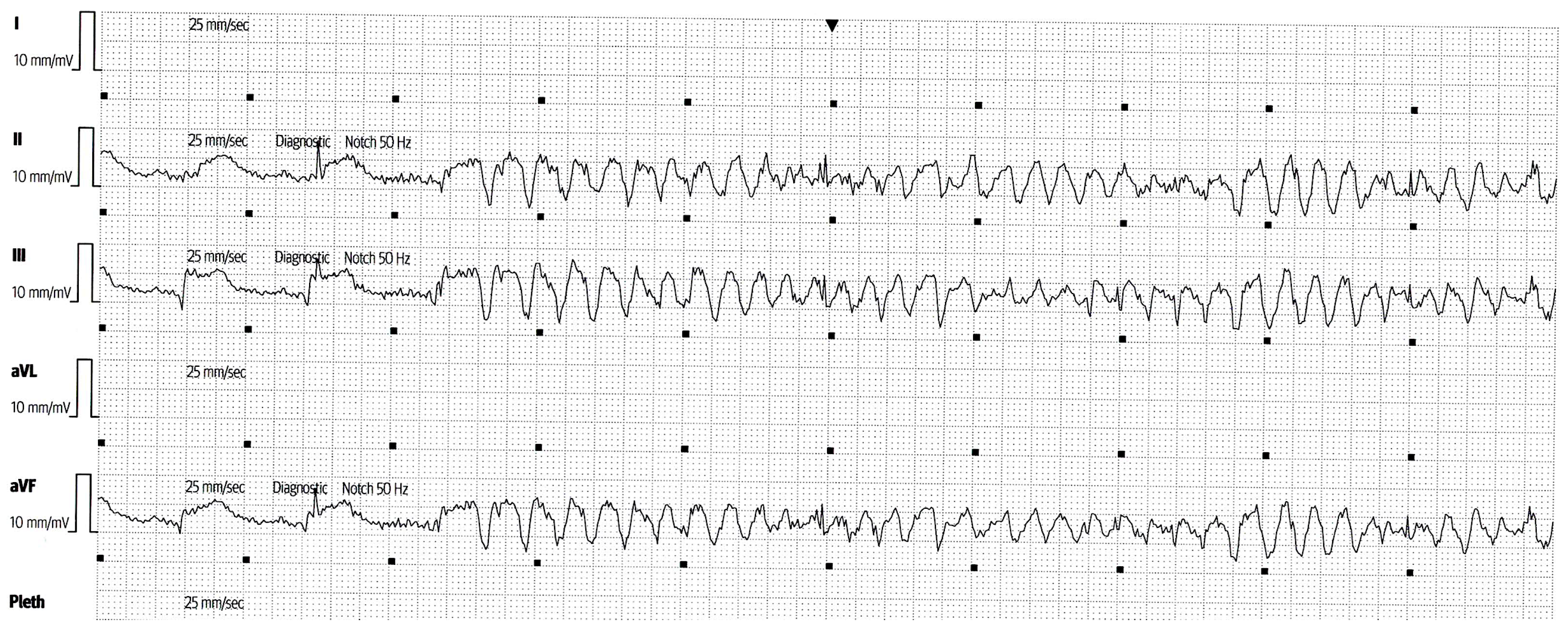
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hohnloser, S.H.; Al-Khalidi, H.R.; Pratt, C.M.; Brum, J.M.; Tatla, D.S.; Tchou, P.; Dorian, P. Shock Inhibition Evaluation with AzimiLiDe (SHIELD) Investigators. Electrical storm in patients with an implantable defibrillator: Incidence, features, and preventive therapy: Insights from a randomized trial. Eur. Heart J. 2006, 27, 3027–3032. [Google Scholar] [CrossRef] [PubMed]
- Piccini, J.P.; Berger, J.S.; Brown, D.L. Early sustained ventricular arrhythmias complicating acute myocardial infarction. Am. J. Med. 2008, 121, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Gorenek, B.; Blomström Lundqvist, C.; Brugada Terradellas, J.; Camm, A.J.; Hindricks, G.; Huber, K.; Kirchhof, P.; Kuck, K.H.; Kudaiberdieva, G.; Lin, T.; et al. Cardiac arrhythmias in acute coronary syndromes: Position paper from the joint EHRA, ACCA, and EAPCI task force. Europace 2014, 16, 1655–1673. [Google Scholar] [CrossRef]
- Pedersen, C.T.; Kay, G.N.; Kalman, J.; Borggrefe, M.; Della-Bella, P.; Dickfeld, T.; Dorian, P.; Huikuri, H.; Kim, Y.H.; Knight, B.; et al. EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Europace 2014, 16, 1257–1283. [Google Scholar] [CrossRef] [PubMed]
- Proietti, R.; Sagone, A. Electrical Storm: Incidence, Prognosis and Therapy. Indian Pacing Electrophysiol. J. 2011, 11, 34–42. [Google Scholar] [PubMed]
- Greene, M.; Newman, D.; Geist, M.; Paquette, M.; Heng, D.; Dorian, P. Is electrical storm in ICD patients the sign of a dying heart? Outcome of patients with clusters of ventricular tachyarrhythmias. Europace 2000, 2, 263–269. [Google Scholar] [CrossRef]
- Elsokkari, I.; Sapp, J.L. Electrical Storm: Prognosis and management. Prog. Cardiovasc Dis. 2021, 66, 70–79. [Google Scholar] [CrossRef]
- Arya, A.; Haghjoo, M.; Dehghani, M.R.; Fazelifar, A.F.; Nikoo, M.H.; Bagherzadeh, A.; Sadr-Ameli, M.A. Prevalence and predictors of electrical storm in patients with implantable cardioverter-defibrillator. Am. J. Cardiol. 2006, 97, 389–392. [Google Scholar] [CrossRef]
- Schwartz, P.J.; Spazzolini, C.; Priori, S.G.; Crotti, L.; Vicentini, A.; Landolina, M.; Gasparini, M.; Wilde, A.A.; Knops, R.E.; Denjoy, I.; et al. Who are the long-QT syndrome patients who receive an implantable cardioverter-defibrillator and what happens to them?: Data from the European Long-QT syndrome implantable cardioverter-defibrillator (LQTS ICD) registry. Circulation 2010, 122, 1272–1282. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Magdi, M.; Mubasher, M.; Alzaeem, H.; Hamid, T. Resistant Ventricular Arrhythmia and the Role of Overdrive Pacing in the Suppression of the Electrical Storm. Case Rep. Cardiol. 2019, 2019, 6592927. [Google Scholar] [CrossRef] [PubMed]
- Kurisu, S.; Inoue, I.; Kawagoe, T.; Ishihara, M.; Shimatani, Y.; Mitsuba, N.; Hata, T.; Nakama, Y.; Kisaka, T.; Kijima, Y. Temporary overdriving pacing as an adjunct to antiarrhythmic drug therapy for electrical storm in acute myocardial infarction. Circ. J. 2005, 69, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.C.; Bottcher, M.; Nielsen, T.T.; Pedersen, A.K.; Andersen, H.R. Regional myocardial blood flow in patients with sick sinus syndrome randomized to long-term single chamber atrial or dual chamber pacing: Effect pacing mode and rate. J. Am. Coll. Cardiol. 2000, 35, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- European Heart Rhythm Association; Heart Rhythm Society; Zipes, D.P.; Camm, A.J.; Borggrefe, M.; Buxton, A.E.; Chaitman, B.; Fromer, M.; Gregoratos, G.; Klein, G.; et al. ACC/AHA/ESC 2006 Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). J. Am. Coll. Cardiol. 2006, 48, e247–e346. [Google Scholar] [CrossRef]
- Priori, S.G.; Wilde, A.A.; Horie, M.; Cho, Y.; Behr, E.R.; Berul, C.; Blom, N.; Brugada, J.; Chiang, C.E.; Huikuri, H.; et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Europace 2013, 15, 1389–1406. [Google Scholar] [CrossRef]
- Chalupová, M.; Suter, P.; Graf, D.; Cook, S. Temporary atrial overdrive pacing during a drug-refractory electrical storm in acute myocardial infarction. BMJ Case Rep. 2021, 14, e242100. [Google Scholar] [CrossRef]
- Kamakura, T.; Kamoshida, J.; Toda, K.; Wayama, K.; Wada, M.; Kusano, K. Ventricular fibril-lation initiated by reentry involving the Purkinje network in a patient with after myo-cardial infarction. HeartRhythm. Case Rep. 2023, 9, 618–623. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2018, 138, e210–e271. [Google Scholar] [CrossRef]
- Kowlgi, G.N.; Cha, Y.M. Management of ventricular electrical storm: A contemporary appraisal. Europace 2020, 22, 1768–1780. [Google Scholar] [CrossRef]
- Meng, L.; Tseng, C.H.; Shivkumar, K.; Ajijola, O. Efficacy of Stellate Ganglion Blockade in Managing Electrical Storm: A Systematic Review. JACC Clin. Electrophysiol. 2017, 3, 942–949. [Google Scholar] [CrossRef]
- Nayyar, S.; Ganesan, A.N.; Brooks, A.G.; Sullivan, T.; Roberts-Thomson, K.C.; Sanders, P. Venturing into ventricular arrhythmia storm: A systematic review and meta-analysis. Eur. Heart J. 2013, 34, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, Y.; Hocini, M.; Nogami, A.; Maury, P.; Peichl, P.; Iwasaki, Y.; Masuda, K.; Denis, A.; Voglimacci-Stephanopoli, Q.; Wichterle, D.; et al. Catheter ablation of refractory ventricular fibrillation storm after myocardial infarction: A multicenter study. Circulation 2019, 139, 2315–2325. [Google Scholar] [CrossRef] [PubMed]
- Dinov, B.; Arya, A.; Bertagnolli, L.; Schirripa, V.; Schoene, K.; Sommer, P.; Bollmann, A.; Rolf, S.; Hindricks, G. Early referral for ablation of scar-related ventricular tachycardia is associated with improved acute and long-term outcomes: Results from the Heart Center of Leipzig ventricular tachycardia registry. Circ. Arrhythm. Electrophysiol. 2014, 7, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Luo, H.; Mao, T.; Xiang, C.; Hu, H.; Zhao, J.; Wang, X.; Wang, J.; Liu, H.; Yu, L.; et al. Stereotactic arrhythmia radioablation: A novel therapy for cardiac arrhythmia. Heart Rhythm. 2023, 20, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Le Pennec-Prigent, S.; Flecher, E.; Auffret, V.; Leurent, G.; Daubert, J.C.; Leclercq, C.; Mabo, P.; Verhoye, J.P.; Martins, R.P. Effectiveness of Extracorporeal Life Support for Patients with Cardiogenic Shock Due to Intractable Arrhythmic Storm. Crit. Care Med. 2017, 45, e281–e289. [Google Scholar] [CrossRef]
- Vergara, P.; Tung, R.; Vaseghi, M.; Brombin, C.; Frankel, D.S.; Di Biase, L.; Nagashima, K.; Tedrow, U.; Tzou, W.S.; Sauer, W.H.; et al. Successful ventricular tachycardia ablation in patients with electrical storm reduces recurrences and improves survival. Heart Rhythm. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Do, D.H.; Bradfield, J.; Ajijola, O.A.; Vaseghi, M.; Le, J.; Rahman, S.; Mahajan, A.; Nogami, A.; Boyle, N.G.; Shivkumar, K. Thoracic Epidural Anesthesia Can Be Effective for the Short-Term Management of Ventricular Tachycardia Storm. J. Am. Heart Assoc. 2017, 6, e007080. [Google Scholar] [CrossRef]
- Bourke, T.; Vaseghi, M.; Michowitz, Y.; Sankhla, V.; Shah, M.; Swapna, N.; Boyle, N.G.; Mahajan, A.; Narasimhan, C.; Lokhandwala, Y.; et al. Neuraxial Modulation for Re-fractory Ventricular Arrhythmias: Value of Thoracic Epidural Anesthesia and Surgical Left Cardiac Sympathetic Denervation. Circulation 2010, 121, 2255–2262. [Google Scholar] [CrossRef]
- Vaseghi, M.; Barwad, P.; Malavassi Corrales, F.J.; Tandri, H.; Mathuria, N.; Shah, R.; Sorg, J.M.; Gima, J.; Mandal, K.; Sàenz Morales, L.C.; et al. Cardiac Sympathetic De-nervation for Refractory Ventricular Arrhythmias. J. Am. Coll. Cardiol. 2017, 69, 3070–3080. [Google Scholar] [CrossRef]

| Drug | Dose | Administration |
|---|---|---|
| Amiodarone | 150 mg bolus + 1200 mg continuous infusion during 24 h | intravenous |
| Lidocaine | Bolus dose of 1 mg/kg, with repeat bolus dose of 0.5 mg/kg, followed by the continuous infusion of 20 mcg/kg/min during 24 h | intravenous |
| Metoprolol | 5 mg intravenously every 5 min; up to 3 doses | intravenous |
| Magnesium-sulphate | 2 g bolus dose | intravenous |
| Propofol | 50 mg bolus dose + continuous infusion of 100 mcg/kg/min | intravenous |
| Propranolol | 40 mg twice a day after acute stabilization | oral |
| Drug | Dose |
|---|---|
| Amiodarone | IV: bolus of 150 mg for stable VT; maintenance: 1 mg/min × 6 h, then 0.5 mg/min × 18 h; PO: 400 mg × q 8–12 h for 7–14 days, then 200–400 mg daily. |
| Lidocaine | IV: bolus of 1–1.5 mg/kg, can repeat up to total of 3 mg/kg, maintenance: 1–4 mg/min. |
| Propranolol | IV: 1–3 mg q5 min to a maximum of 5 mg; PO: 10–40 mg q6 h, immediate release; 60–160 mg q12 h, extended release. |
| Mexiletin | 150–300 mg; PO: q8–12 h. |
| Procainamide | IV: bolus of 10 mg/kg over 20 min, maintenance: 2–3 g/24 h; oral: 500–1250 mg q6 h. |
| Quinidine | Quinidine sulfate: 200–600 mg; PO: q6–12 h; quinidine gluconate at 324–648 mg; PO: q8 h; IV loading dose: 800 mg/50 mL, maintenance: 50 mg/min. |
| Sotalol | IV: 7 5 mg q12 h; PO: 80–160 mg q12 h. |
| Metoprolol | IV: 5 mg q5 min up to 3 doses; PO: metoprolol tartarate at 25–100 mg q12 h. |
| Esmolol | IV: bolus of 0.5 mg/kg, maintenance: 0.05 mg/kg/min. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meter, M.; Borovac, J.A. A Refractory Electrical Storm after Acute Myocardial Infarction: The Role of Temporary Ventricular Overdrive Pacing as a Bridge to ICD Implantation. Pathophysiology 2024, 31, 44-51. https://doi.org/10.3390/pathophysiology31010004
Meter M, Borovac JA. A Refractory Electrical Storm after Acute Myocardial Infarction: The Role of Temporary Ventricular Overdrive Pacing as a Bridge to ICD Implantation. Pathophysiology. 2024; 31(1):44-51. https://doi.org/10.3390/pathophysiology31010004
Chicago/Turabian StyleMeter, Mijo, and Josip Andelo Borovac. 2024. "A Refractory Electrical Storm after Acute Myocardial Infarction: The Role of Temporary Ventricular Overdrive Pacing as a Bridge to ICD Implantation" Pathophysiology 31, no. 1: 44-51. https://doi.org/10.3390/pathophysiology31010004
APA StyleMeter, M., & Borovac, J. A. (2024). A Refractory Electrical Storm after Acute Myocardial Infarction: The Role of Temporary Ventricular Overdrive Pacing as a Bridge to ICD Implantation. Pathophysiology, 31(1), 44-51. https://doi.org/10.3390/pathophysiology31010004






