Current Views about the Inflammatory Damage Triggered by Bacterial Superantigens and Experimental Attempts to Neutralize Superantigen-Mediated Toxic Effects with Natural and Biological Products
Abstract
1. Introduction
2. T Cell Receptor and T Cell Activation
3. Modalities of Superantigen Binding to TCR and Induction of the Inflammatory Pathway
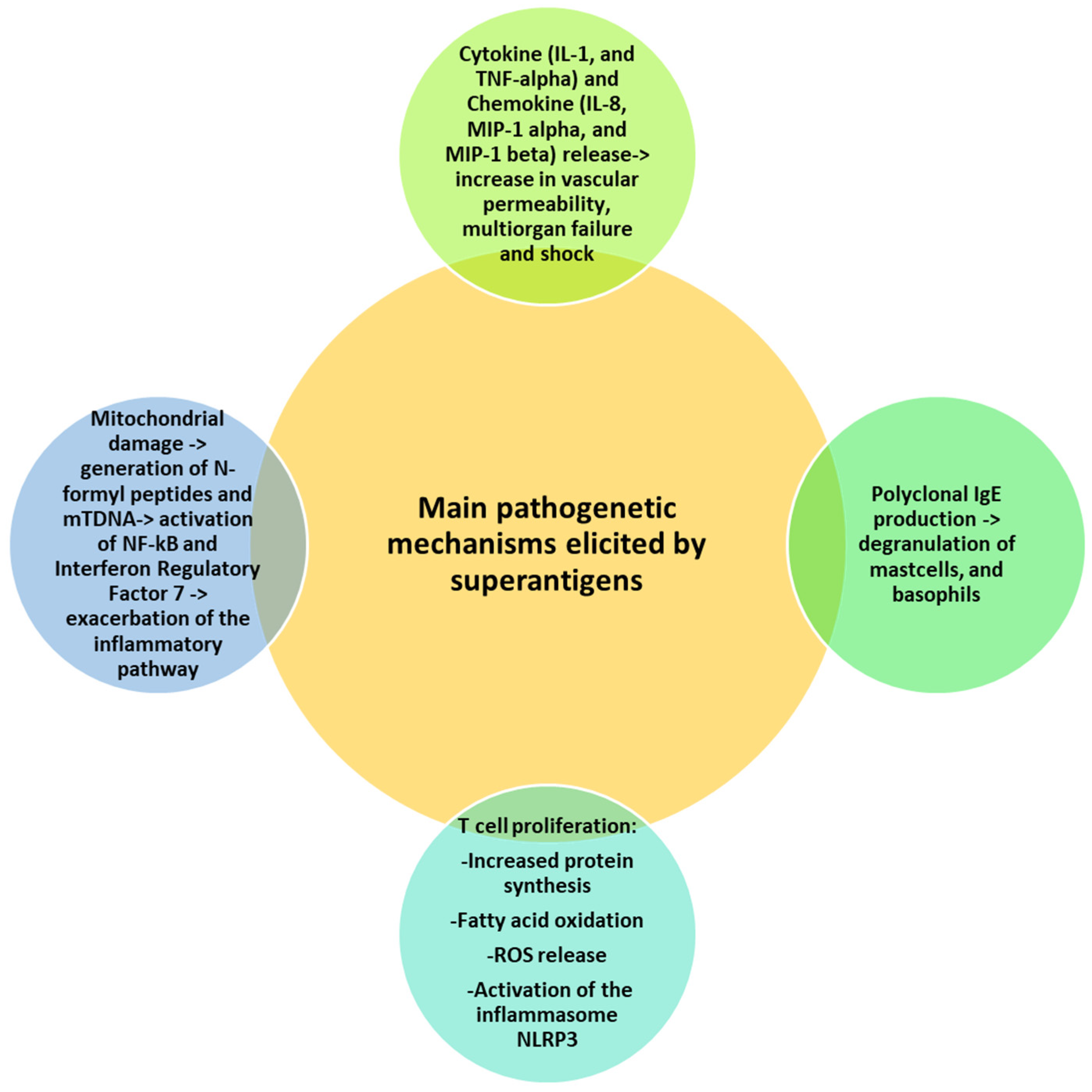
4. Superantigen-Mediated Disease
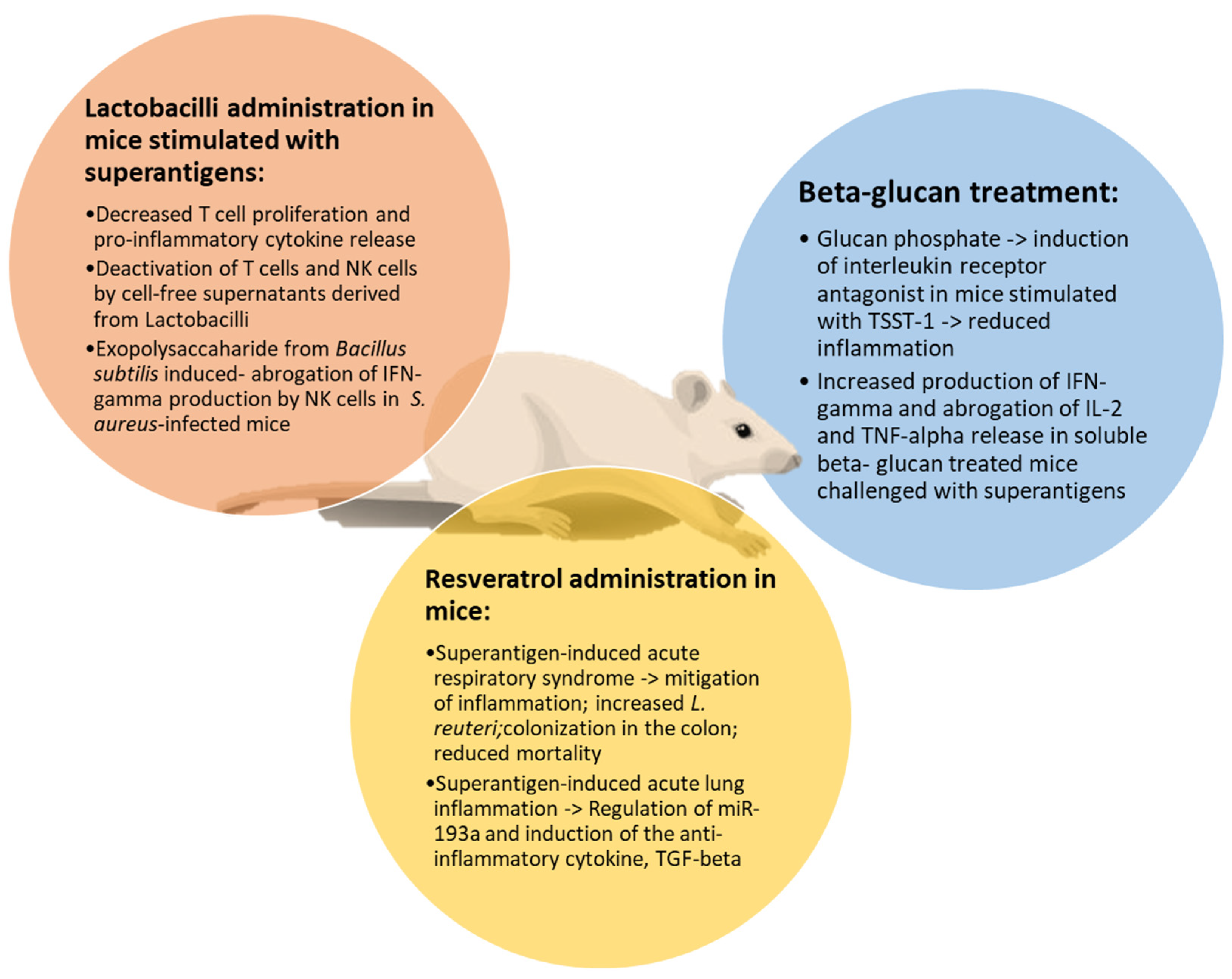
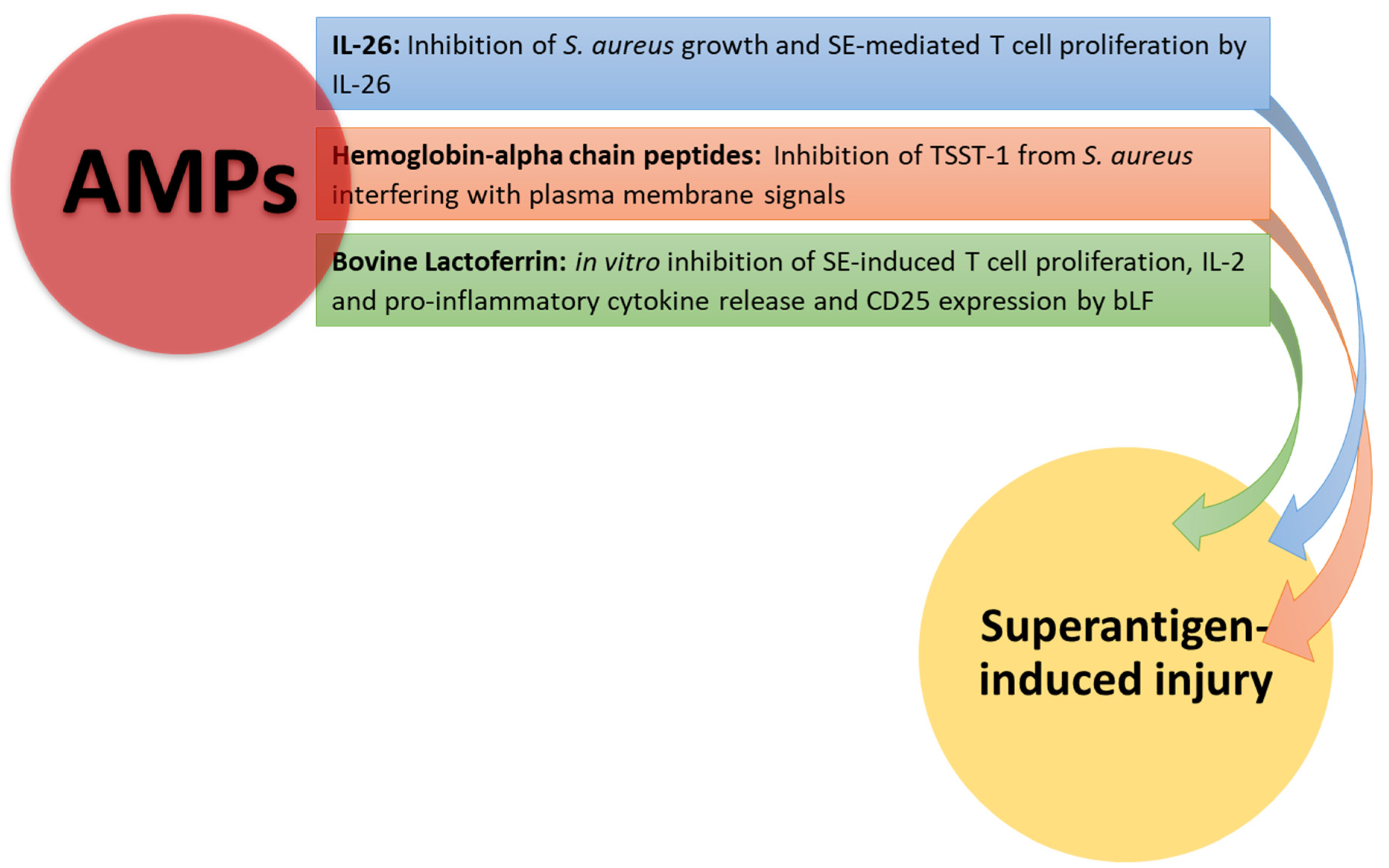
5. Natural and Biological Products as an Alternative Treatment of Superantigen-Mediated Damage
5.1. Polyphenols
5.2. Beta-Glucans
5.3. Lactobacilli
5.4. Antimicrobial Peptides
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALI | Acute lung injury |
| AMPs | Antimicrobial peptides |
| APCs | Antigen-presenting cells |
| b-LF | Bovine lactoferrin |
| CDR | Complementary determining regions |
| CFs | Cell-free supernatants |
| DCs | Dendritic cells |
| EPs | Exopolysaccharides |
| ER | Endoplasmic reticular |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IFN | Interferon |
| IL | Interleukin |
| LF | Lactoferrin |
| MHC | Major histocompatibility complex |
| MIP-1 | Macrophage inflammatory protein-1 |
| NFPs | N-formyl peptides |
| NLRs | Nod-like receptors |
| PAMPS | Pathogen-associated molecular patterns |
| PTKs | Protein tyrosine kinases |
| RES | Resveratrol |
| ROS | Reactive oxygen species |
| SEs | Staphylococcal enterotoxins |
| SPEs | Streptococcal pyrogenic exotoxins |
| Th | T helper |
| TGF-beta | Transforming growth factor-beta |
| TLRs | Toll-like receptors |
| TNF-alpha | Tumor necrosis factor-alpha |
| TREG | T regulatory |
| TRM | T resident memory |
| TSS | Toxic shock syndrome |
| TSST-1 | Toxic shock syndrome toxin-1 |
References
- Le, J.; Kulatheepan, Y.; Jeyaseelan, S. Role of toll-like receptors and nod-like receptors in acute lung infection. Front. Immunol. 2023, 14, 1249098. [Google Scholar] [CrossRef] [PubMed]
- Gül, E.; Fattinger, S.A.; Sellin, M.E.; Hardt, W.D. Epithelial inflammasomes, gasdermins, and mucosal inflammation—Lessons from Salmonella and Shigella infected mice. Semin. Immunol. 2023, 70, 101812. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.S.; Oh, S.F. Unconventional immune cells in the gut mucosal barrier: Regulation by symbiotic microbiota. Exp. Mol. Med. 2023, 55, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Imbimbo, C.; Ballini, A.; Crocetto, F.; Scacco, S.; Cantore, S.; Di Zazzo, E.; Colella, M.; Jirillo, E. Testicular Immunity and Its Connection with the Microbiota. Physiological and Clinical Implications in the Light of Personalized Medicine. J. Pers. Med. 2022, 12, 1335. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.I.; Farber, D.L. Tissue-Resident Immune Cells in Humans. Annu. Rev. Immunol. 2022, 40, 195–220. [Google Scholar] [CrossRef] [PubMed]
- Al, B.; Suen, T.K.; Placek, K.; Netea, M.G. Innate (learned) memory. J. Allergy Clin. Immunol. 2023, 152, 551–566. [Google Scholar] [CrossRef]
- Nofi, C.P.; Wang, P.; Aziz, M. Chromatin-Associated Molecular Patterns (CAMPs) in sepsis. Cell Death Dis. 2022, 13, 700. [Google Scholar] [CrossRef]
- Kaplan, M.J. Casting a Wide NET. J. Immunol. 2022, 209, 843–844. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef]
- Janssen, E.M.; Lemmens, E.E.; Wolfe, T.; Christen, U.; von Herrath, M.G.; Schoenberger, S.P. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 2003, 421, 852–856. [Google Scholar] [CrossRef]
- Shedlock, D.J.; Shen, H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 2003, 300, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Jiao, A.; Liu, H.; Ding, R.; Zheng, H.; Zhang, C.; Feng, Z.; Lei, L.; Wang, X.; Su, Y.; Yang, X.; et al. Med1 Controls Effector CD8+ T Cell Differentiation and Survival through C/EBPβ-Mediated Transcriptional Control of T-bet. J. Immunol. 2022, 209, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. A brief history of T cell help to B cells. Nat. Rev. Immunol. 2015, 15, 185–189. [Google Scholar] [CrossRef]
- La Gruta, N.L.; Gras, S.; Daley, S.R.; Thomas, P.G.; Rossjohn, J. Understanding the drivers of MHC restriction of T cell receptors. Nat. Rev. Immunol. 2018, 18, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Kaech, S.M.; Cui, W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 2012, 12, 749–761. [Google Scholar] [CrossRef]
- Laidlaw, B.J.; Craft, J.E.; Kaech, S.M. The multifaceted role of CD4(+) T cells in CD8(+) T cell memory. Nat. Rev. Immunol. 2016, 16, 102–111. [Google Scholar] [CrossRef]
- Surh, C.D.; Sprent, J. Homeostasis of naive and memory T cells. Immunity. 2008, 29, 848–862. [Google Scholar] [CrossRef]
- Noli Truant, S.; Redolfi, D.M.; Sarratea, M.B.; Malchiodi, E.L.; Fernández, M.M. Superantigens, a Paradox of the Immune Response. Toxins 2022, 14, 800. [Google Scholar] [CrossRef]
- Jensen, I.J.; Farber, D.L. Gutsy memory T cells stand their ground against pathogens. Sci. Immunol. 2022, 7, eade7168. [Google Scholar] [CrossRef]
- Li, H.; Llera, A.; Malchiodi, E.L.; Mariuzza, R.A. The structural basis of T cell activation by superantigens. Annu. Rev. Immunol. 1999, 17, 435–466. [Google Scholar] [CrossRef]
- Kolla, H.B.; Tirumalasetty, C.; Sreerama, K.; Ayyagari, V.S. An immunoinformatics approach for the design of a multi-epitope vaccine targeting super antigen TSST-1 of Staphylococcus aureus. J. Genet. Eng. Biotechnol. 2021, 19, 69. [Google Scholar] [CrossRef] [PubMed]
- Balzanelli, M.G.; Distratis, P.; Aityan, S.K.; Amatulli, F.; Catucci, O.; Cefalo, A.; De Michele, A.; Dipalma, G.; Inchingolo, F.; Lazzaro, R.; et al. An Alternative “Trojan Horse” Hypothesis for COVID-19: Immune Deficiency of IL-10 and SARS-CoV-2 Biology. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jupin, C.; Anderson, S.; Damais, C.; Alouf, J.E.; Parant, M. Toxic shock syndrome toxin 1 as an inducer of human tumor necrosis factors and gamma interferon. J. Exp. Med. 1988, 167, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Tessier, P.A.; Naccache, P.H.; Diener, K.R.; Gladue, R.P.; Neote, K.S.; Clark-Lewis, I.; McColl, S.R. Induction of acute inflammation in vivo by staphylococcal superantigens. II. Critical role for chemokines, ICAM-1, and TNF-alpha. J. Immunol. 1998, 161, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, T. Costimulatory receptors for the superantigen staphylococcal enterotoxin B on human vascular endothelial cells and T cells. J. Leukoc. Biol. 1994, 56, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Recent Advances on the Anti-Inflammatory and Antioxidant Properties of Red Grape Polyphenols: In Vitro and In Vivo Studies. Antioxidants 2019, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Kazemifard, N.; Dehkohneh, A.; Baradaran Ghavami, S. Probiotics and probiotic-based vaccines: A novel approach for improving vaccine efficacy. Front. Med. 2022, 9, 940454. [Google Scholar] [CrossRef]
- Han, X.; Luo, R.; Ye, N.; Hu, Y.; Fu, C.; Gao, R.; Fu, S.; Gao, F. Research progress on natural β-glucan in intestinal diseases. Int. J. Biol. Macromol. 2022, 219, 1244–1260. [Google Scholar] [CrossRef]
- Reddy, K.V.; Yedery, R.D.; Aranha, C. Antimicrobial peptides: Premises and promises. Int. J. Antimicrob. Agents 2004, 24, 536–547. [Google Scholar] [CrossRef]
- Magrone, T.; Russo, M.A.; Jirillo, E. Antimicrobial peptides in human disease: Therapeutic approaches. Second of two parts. Curr. Pharm. Des. 2018, 24, 1148–1156. [Google Scholar] [CrossRef]
- Galperin, M.; Farenc, C.; Mukhopadhyay, M.; Jayasinghe, D.; Decroos, A.; Benati, D.; Tan, L.L.; Ciacchi, L.; Reid, H.H.; Rossjohn, J.; et al. CD4+ T cell-mediated HLA class II cross-restriction in HIV controllers. Sci. Immunol. 2018, 3, eaat0687. [Google Scholar] [CrossRef] [PubMed]
- Willcox, B.E.; Willcox, C.R. γδ TCR ligands: The quest to solve a 500-million-year-old mystery [published correction appears. Nat. Immunol. 2019, 20, 516. [Google Scholar] [CrossRef] [PubMed]
- Burn, T.N.; Miot, C.; Gordon, S.M.; Culberson, E.J.; Diamond, T.; Kreiger, P.A.; Hayer, K.E.; Bhattacharyya, A.; Jones, J.M.; Bassing, C.H.; et al. The RAG1 Ubiquitin Ligase Domain Stimulates Recombination of TCRβ and TCRα Genes and Influences Development of αβ T Cell Lineages. J. Immunol. 2022, 209, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, S.; Lamothe, P.A.; Soghoian, D.Z.; Kazer, S.W.; Cole, M.B.; Shalek, A.K.; Yosef, N.; Jones, R.B.; Donaghey, F.; Nwonu, C.; et al. Antiviral CD8+ T Cells Restricted by Human Leukocyte Antigen Class II Exist during Natural HIV Infection and Exhibit Clonal Expansion. Immunity 2016, 45, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Calabi, F.; Jarvis, J.M.; Martin, L.; Milstein, C. Two classes of CD1 genes. Eur. J. Immunol. 1989, 19, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Arstila, T.P.; Casrouge, A.; Baron, V.; Even, J.; Kanellopoulos, J.; Kourilsky, P. A direct estimate of the human alphabeta T cell receptor diversity. Science 1999, 286, 958–961. [Google Scholar] [CrossRef]
- Tian, L.; Zhou, W.; Wu, X.; Hu, Z.; Qiu, L.; Zhang, H.; Chen, X.; Zhang, S.; Lu, Z. CTLs: Killers of intracellular bacteria. Front. Cell Infect. Microbiol. 2022, 12, 967679. [Google Scholar] [CrossRef]
- Jenkins, M.K.; Moon, J.J. The role of naive T cell precursor frequency and recruitment in dictating immune response magnitude. J. Immunol. 2012, 188, 4135–4140. [Google Scholar] [CrossRef]
- Thakur, A.; Mikkelsen, H.; Jungersen, G. Intracellular Pathogens: Host Immunity and Microbial Persistence Strategies. J. Immunol. Res. 2019, 2019, 1356540. [Google Scholar] [CrossRef]
- Cruz-Adalia, A.; Ramirez-Santiago, G.; Calabia-Linares, C.; Torres-Torresano, M.; Feo, L.; Galán-Díez, M.; Fernandez-Ruiz, E.; Pereiro, E.; Guttmann, P.; Chiappi, M.; et al. T cells kill bacteria captured by transinfection from dendritic cells and confer protection in mice. Cell Host Microbe 2014, 15, 611–622. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Ding, R.; Jiao, A.; Zheng, H.; Zhang, C.; Feng, Z.; Su, Y.; Yang, X.; Lei, L.; et al. The Transcription Factor Zfp335 Promotes Differentiation and Persistence of Memory CD8+ T Cells by Regulating TCF-1. J. Immunol. 2022, 209, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Q.; Xia, J.; Zeng, W.; Luo, W.; Liu, L.; Zeng, X.; Cao, D. The intestinal γδ T cells: Functions in the gut and in the distant organs. Front. Immunol. 2023, 14, 1206299. [Google Scholar] [CrossRef] [PubMed]
- Park, S.L.; Zaid, A.; Hor, J.L.; Christo, S.N.; Prier, J.E.; Davies, B.; Alexandre, Y.O.; Gregory, J.L.; Russell, T.A.; Gebhardt, T.; et al. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat. Immunol. 2018, 19, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.J.; Gu, S.; Luoma, A.M. Human gamma delta T cells: Evolution and ligand recognition. Cell Immunol. 2015, 296, 31–40. [Google Scholar] [CrossRef]
- Khairallah, C.; Chu, T.H.; Sheridan, B.S. Tissue Adaptations of Memory and Tissue-Resident Gamma Delta T Cells. Front. Immunol. 2018, 9, 2636. [Google Scholar] [CrossRef]
- Abrahmsén, L.; Dohlsten, M.; Segrén, S.; Björk, P.; Jonsson, E.; Kalland, T. Characterization of two distinct MHC class II binding sites in the superantigen staphylococcal enterotoxin A. EMBO J. 1995, 14, 2978–2986. [Google Scholar] [CrossRef]
- Hudson, K.R.; Tiedemann, R.E.; Urban, R.G.; Lowe, S.C.; Strominger, J.L.; Fraser, J.D. Staphylococcal enterotoxin A has two cooperative binding sites on major histocompatibility complex class II. J. Exp. Med. 1995, 182, 711–720. [Google Scholar] [CrossRef]
- Ulrich, R.G.; Bavari, S.; Olson, M.A. Staphylococcal enterotoxins A and B share a common structural motif for binding class II major histocompatibility complex molecules. Nat. Struct. Biol. 1995, 2, 554–560. [Google Scholar] [CrossRef]
- Hopkins, P.A.; Fraser, J.D.; Pridmore, A.C.; Russell, H.H.; Read, R.C.; Sriskandan, S. Superantigen recognition by HLA class II on monocytes up-regulates toll-like receptor 4 and enhances proinflammatory responses to endotoxin. Blood 2005, 105, 3655–3662. [Google Scholar] [CrossRef]
- Grundström, S.; Cederbom, L.; Sundstedt, A.; Scheipers, P.; Ivars, F. Superantigen-induced regulatory T cells display different suppressive functions in the presence or absence of natural CD4+CD25+ regulatory T cells in vivo. J. Immunol. 2003, 170, 5008–5017. [Google Scholar] [CrossRef]
- Li, S.J.; Hu, D.L.; Maina, E.K.; Shinagawa, K.; Omoe, K.; Nakane, A. Superantigenic activity of toxic shock syndrome toxin-1 is resistant to heating and digestive enzymes. J. Appl. Microbiol. 2011, 110, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Smith-Garvin, J.E.; Koretzky, G.A.; Jordan, M.S. T cell activation. Annu. Rev. Immunol. 2009, 27, 591–619. [Google Scholar] [CrossRef] [PubMed]
- Linsley, P.S.; Ledbetter, J.A. The role of the CD28 receptor during T cell responses to antigen. Annu. Rev. Immunol. 1993, 11, 191–212. [Google Scholar] [CrossRef]
- Isakov, N.; Altman, A. PKC-theta-mediated signal delivery from the TCR/CD28 surface receptors. Front. Immunol. 2012, 3, 273. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, T.; Stiles, B.G. The staphylococcal enterotoxin (SE) family: SEB and siblings. Virulence 2013, 4, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Isakov, N.; Altman, A. Regulation of immune system cell functions by protein kinase C. Front. Immunol. 2013, 4, 384. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Colella, M.; Charitos, I.A.; Di Domenico, M.; Palmirotta, R.; Jirillo, E. Microbial and Host Metabolites at the Backstage of Fever: Current Knowledge about the Co-Ordinate Action of Receptors and Molecules Underlying Pathophysiology and Clinical Implications. Metabolites 2023, 13, 461. [Google Scholar] [CrossRef]
- Peng, L.; Jiang, J.; Chen, T.; Xu, D.; Hou, F.; Huang, Q.; Peng, Y.; Ye, C.; Hu, D.L.; Fang, R. Toxic Shock Syndrome Toxin 1 Induces Immune Response via the Activation of NLRP3 Inflammasome. Toxins 2021, 13, 68. [Google Scholar] [CrossRef]
- Krakauer, T. Staphylococcal Superantigens: Pyrogenic Toxins Induce Toxic Shock. Toxins 2019, 11, 178. [Google Scholar] [CrossRef]
- Huzella, L.M.; Buckley, M.J.; Alves, D.A.; Stiles, B.G.; Krakauer, T. Central roles for IL-2 and MCP-1 following intranasal exposure to SEB: A new mouse model. Res. Vet. Sci. 2009, 86, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.X.; Gilmore, K.J.; Szabo, P.A.; Zeppa, J.J.; Baroja, M.L.; Haeryfar, S.M.; McCormick, J.K. Superantigens subvert the neutrophil response to promote abscess formation and enhance Staphylococcus aureus survival in vivo. Infect. Immun. 2014, 82, 3588–3598. [Google Scholar] [CrossRef] [PubMed]
- Neumann, B.; Engelhardt, B.; Wagner, H.; Holzmann, B. Induction of acute inflammatory lung injury by staphylococcal enterotoxin B. J. Immunol. 1997, 158, 1862–1871. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Muzio, E.L.; Bottalico, L.; Spirito, F.; Charitos, I.A.; Passarelli, P.C.; Jirillo, E. Subversion of the Oral Microbiota and Induction of Immune-Mediated Systemic Inflammation with Special Reference to Periodontitis. Current Knowledge and Perspectives. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, J.P.; Marone, G. Protein Fv: An endogenous immunoglobulin superantigen and superallergen. Chem. Immunol. Allergy 2007, 93, 58–76. [Google Scholar] [CrossRef]
- Marone, G.; Rossi, F.W.; Detoraki, A.; Granata, F.; Marone, G.; Genovese, A.; Spadaro, G. Role of superallergens in allergic disorders. Chem. Immunol. Allergy 2007, 93, 195–213. [Google Scholar] [CrossRef]
- Sørensen, M.; Klingenberg, C.; Wickman, M.; Sollid, J.U.; Furberg, A.S.; Bachert, C.; Bousquet, J. Staphylococcus aureus enterotoxin sensitization is associated with allergic poly-sensitization and allergic multimorbidity in adolescents. Allergy 2017, 72, 1548–1555. [Google Scholar] [CrossRef]
- Solinas, G.; Karin, M. JNK1 and IKKbeta: Molecular links between obesity and metabolic dysfunction. FASEB J. 2010, 24, 2596–2611. [Google Scholar] [CrossRef]
- Shenderov, K.; Riteau, N.; Yip, R.; Mayer-Barber, K.D.; Oland, S.; Hieny, S.; Fitzgerald, P.; Oberst, A.; Dillon, C.P.; Green, D.R.; et al. Cutting edge: Endoplasmic reticulum stress licenses macrophages to produce mature IL-1β in response to TLR4 stimulation through a caspase-8- and TRIF-dependent pathway. J. Immunol. 2014, 192, 2029–2033. [Google Scholar] [CrossRef]
- Santacroce, L.; Palmirotta, R.; Bottalico, L.; Charitos, I.A.; Colella, M.; Topi, S.; Jirillo, E. Crosstalk between the Resident Microbiota and the Immune Cells Regulates Female Genital Tract Health. Life 2023, 13, 1531. [Google Scholar] [CrossRef]
- Nakahira, K.; Hisata, S.; Choi, A.M. The Roles of Mitochondrial Damage-Associated Molecular Patterns in Diseases. Antioxid. Redox Signal. 2015, 23, 1329–1350. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.L.; Nakane, A. Mechanisms of staphylococcal enterotoxin-induced emesis. Eur. J. Pharmacol. 2014, 722, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Di Serio, F.; Lovero, R.; D’Agostino, D.; Nisi, L.; Miragliotta, G.; Contino, R.; Man, A.; Ciccone, M.M.; Santacroce, L. Evaluation of procalcitonin, Vitamin D and C-reactive protein levels in septic patients with positive emocoltures. Our preliminary experience. Acta Med. Mediterr. 2016, 32, 1911–1914. [Google Scholar] [CrossRef]
- Grumann, D.; Ruotsalainen, E.; Kolata, J.; Kuusela, P.; Järvinen, A.; Kontinen, V.P.; Bröker, B.M.; Holtfreter, S. Characterization of infecting strains and superantigen-neutralizing antibodies in Staphylococcus aureus bacteremia. Clin. Vaccine Immunol. 2011, 18, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.C. New perspectives on Kawasaki disease. Arch. Dis. Child. 2019, 104, 616–617. [Google Scholar] [CrossRef] [PubMed]
- Topi, S.; Bottalico, L.; Charitos, I.A.; Colella, M.; Di Domenico, M.; Palmirotta, R.; Santacroce, L. Biomolecular Mechanisms of Autoimmune Diseases and Their Relationship with the Resident Microbiota: Friend or Foe? Pathophysiology 2022, 29, 507–536. [Google Scholar] [CrossRef]
- Leung, D.Y.M.; Schlievert, P.M. Kawasaki syndrome: Role of superantigens revisited. FEBS J. 2021, 288, 1771–1777. [Google Scholar] [CrossRef]
- Carretta, D.M.; Silva, A.M.; D’Agostino, D.; Topi, S.; Lovero, R.; Charitos, I.A.; Wegierska, A.E.; Montagnani, M.; Santacroce, L. Cardiac Involvement in COVID-19 Patients: A Contemporary Review. Infect. Dis. Rep. 2021, 13, 494–517. [Google Scholar] [CrossRef]
- Halloran, P.F. Immunosuppressive drugs for kidney transplantation [published correction appears. N. Engl. J. Med. 2005, 352, 1056. [Google Scholar] [CrossRef]
- Komisar, J.L.; Weng, C.F.; Oyejide, A.; Hunt, R.E.; Briscoe, C.; Tseng, J. Cellular and cytokine responses in the circulation and tissue reactions in the lung of rhesus monkeys (Macaca mulatta) pretreated with cyclosporin A and challenged with staphylococcal enterotoxin B. Toxicol. Pathol. 2001, 29, 369–378. [Google Scholar] [CrossRef]
- Tilahun, A.Y.; Karau, M.J.; Clark, C.R.; Patel, R.; Rajagopalan, G. The impact of tacrolimus on the immunopathogenesis of staphylococcal enterotoxin-induced systemic inflammatory response syndrome and pneumonia. Microbes Infect. 2012, 14, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.; Stabilini, A.; Roncarolo, M.G. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 2005, 105, 4743–4748. [Google Scholar] [CrossRef] [PubMed]
- Darenberg, J.; Söderquist, B.; Normark, B.H.; Norrby-Teglund, A. Differences in potency of intravenous polyspecific immunoglobulin G against streptococcal and staphylococcal superantigens: Implications for therapy of toxic shock syndrome. Clin. Infect. Dis. 2004, 38, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Tilahun, M.E.; Rajagopalan, G.; Shah-Mahoney, N.; Lawlor, R.G.; Tilahun, A.Y.; Xie, C.; Natarajan, K.; Margulies, D.H.; Ratner, D.I.; Osborne, B.A.; et al. Potent neutralization of staphylococcal enterotoxin B by synergistic action of chimeric antibodies. Infect. Immun. 2010, 78, 2801–2811. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Hatswell, A.J.; Nathan, P.; Lebmeier, M.; Lee, D. The Predicted Impact of Ipilimumab Usage on Survival in Previously Treated Advanced or Metastatic Melanoma in the UK. PLoS ONE 2015, 10, e0145524. [Google Scholar] [CrossRef] [PubMed]
- Varshney, A.K.; Wang, X.; Cook, E.; Dutta, K.; Scharff, M.D.; Goger, M.J.; Fries, B.C. Generation, characterization, and epitope mapping of neutralizing and protective monoclonal antibodies against staphylococcal enterotoxin B-induced lethal shock. J. Biol. Chem. 2014, 289, 13706. [Google Scholar] [CrossRef]
- Whitfield, S.J.C.; Taylor, C.; Risdall, J.E.; Griffiths, G.D.; Jones, J.T.A.; Williamson, E.D.; Rijpkema, S.; Saraiva, L.; Vessillier, S.; Green, A.C.; et al. Interference of the T Cell and Antigen-Presenting Cell Costimulatory Pathway Using CTLA4-Ig (Abatacept) Prevents Staphylococcal Enterotoxin B Pathology. J. Immunol. 2017, 198, 3989–3998. [Google Scholar] [CrossRef]
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Taking Advantage of Plant Defense Mechanisms to Promote Human Health. The Plant Immune System. First of Two Parts. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 1183–1195. [Google Scholar] [CrossRef]
- Magrone, T.; Russo, M.A.; Jirillo, E. Antimicrobial Peptides: Phylogenic Sources and Biological Activities. First of Two Parts. Curr. Pharm. Des. 2018, 24, 1043–1053. [Google Scholar] [CrossRef]
- Nagy-Bota, M.C.; Man, A.; Santacroce, L.; Brinzaniuc, K.; Pap, Z.; Pacurar, M.; Pribac, M.; Ciurea, C.N.; Pintea-Simon, I.A.; Kovacs, M. Essential Oils as Alternatives for Root-Canal Treatment and Infection Control against Enterococcus faecalis—A Preliminary Study. Appl. Sci. 2021, 11, 1422. [Google Scholar] [CrossRef]
- Marzulli, G.; Magrone, T.; Kawaguchi, K.; Kumazawa, Y.; Jirillo, E. Fermented grape marc (FGM): Immunomodulating properties and its potential exploitation in the treatment of neurodegenerative diseases. Curr. Pharm. Des. 2012, 18, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, T.; Shinozaki, R.; Udono, M.; Katakura, Y. Identification and Functional Evaluation of Polyphenols That Induce Regulatory T Cells. Nutrients 2022, 14, 2862. [Google Scholar] [CrossRef] [PubMed]
- Rasooly, R.; Do, P.M.; Friedman, M. Inhibition of biological activity of staphylococcal enterotoxin A (SEA) by apple juice and apple polyphenols. J. Agric. Food Chem. 2010, 58, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.L.; Vicario, M.; Wang, A.; Moreto, M.; McKay, D.M. Immune cell activation and subsequent epithelial dysfunction by Staphylococcus enterotoxin B is attenuated by the green tea polyphenol (-)-epigallocatechin gallate. Cell Immunol. 2005, 237, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Alghetaa, H.; Mohammed, A.; Zhou, J.; Singh, N.; Nagarkatti, M.; Nagarkatti, P. Resveratrol-mediated attenuation of superantigen-driven acute respiratory distress syndrome is mediated by microbiota in the lungs and gut. Pharmacol. Res. 2021, 167, 105548. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Zhao, L.; Sun, Y.; Wang, X.; Shi, X. HMGB1 and Toll-like receptors: Potential therapeutic targets in autoimmune diseases. Mol. Med. 2023, 29, 117. [Google Scholar] [CrossRef]
- Lyu, Y.; Wang, T.; Huang, S.; Zhang, Z. Mitochondrial Damage-Associated Molecular Patterns and Metabolism in the Regulation of Innate Immunity. J. Innate Immun. 2023, 15, 665–679. [Google Scholar] [CrossRef]
- Tirunavalli, S.K.; Pramatha, S.; Eedara, A.C.; Walvekar, K.P.; Immanuel, C.; Potdar, P.; Nayak, P.G.; Chamallamudi, M.R.; Sistla, R.; Chilaka, S.; et al. Protective effect of β-glucan on Poly(I:C)-induced acute lung injury/inflammation: Therapeutic implications of viral infections in the respiratory system. Life Sci. 2023, 330, 122027. [Google Scholar] [CrossRef]
- Gordon, S. Pattern recognition receptors: Doubling up for the innate immune response. Cell 2002, 111, 927–930. [Google Scholar] [CrossRef]
- Battle, J.; Ha, T.; Li, C.; Della Beffa, V.; Rice, P.; Kalbfleisch, J.; Browder, W.; Williams, D. Ligand binding to the (1 --> 3)-beta-D-glucan receptor stimulates NFkappaB activation, but not apoptosis in U937 cells. Biochem. Biophys. Res. Commun. 1998, 249, 499–504. [Google Scholar] [CrossRef]
- Altstaedt, J.; Kirchner, H.; Rink, L. Cytokine production of neutrophils is limited to interleukin-8. Immunology 1996, 89, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Ha, T.; Li, C.; Kalbfleisch, J.H.; Laffan, J.J.; Ferguson, D.A. Inhibiting early activation of tissue nuclear factor-kappa B and nuclear factor interleukin 6 with (1-->3)-beta-D-glucan increases long-term survival in polymicrobial sepsis. Surgery 1999, 126, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Li, C.; Ha, T.; Ozment-Skelton, T.; Kalbfleisch, J.H.; Preiszner, J.; Brooks, L.; Breuel, K.; Schweitzer, J.B. Modulation of the phosphoinositide 3-kinase pathway alters innate resistance to polymicrobial sepsis. J. Immunol. 2004, 172, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Luhm, J.; Langenkamp, U.; Hensel, J.; Frohn, C.; Brand, J.M.; Hennig, H.; Rink, L.; Koritke, P.; Wittkopf, N.; Williams, D.L.; et al. Beta-(1-->3)-D-glucan modulates DNA binding of nuclear factors kappaB, AT and IL-6 leading to an anti-inflammatory shift of the IL-1beta/IL-1 receptor antagonist ratio. BMC Immunol. 2006, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef]
- Soltys, J.; Quinn, M.T. Modulation of endotoxin- and enterotoxin-induced cytokine release by in vivo treatment with beta-(1,6)-branched beta-(1,3)-glucan. Infect. Immun. 1999, 67, 244–252. [Google Scholar] [CrossRef]
- Yang, X.; Chi, H.; Wu, M.; Wang, Z.; Lang, Q.; Han, Q.; Wang, X.; Liu, X.; Li, Y.; Wang, X.; et al. Discovery and characterization of SARS-CoV-2 reactive and neutralizing antibodies from humanized CAMouseHG mice through rapid hybridoma screening and high-throughput single-cell V(D)J sequencing. Front. Immunol. 2022, 13, 992787. [Google Scholar] [CrossRef]
- Arrigoni, R.; Ballini, A.; Topi, S.; Bottalico, L.; Jirillo, E.; Santacroce, L. Antibiotic Resistance to Mycobacterium tuberculosis and Potential Use of Natural and Biological Products as Alternative Anti-Mycobacterial Agents. Antibiotics 2022, 11, 1431. [Google Scholar] [CrossRef]
- de Moreno de Leblanc, A.; Del Carmen, S.; Zurita-Turk, M.; Santos Rocha, C.; Van De Guchte, M.; Azevedo, V.; Miyoshi, A.; LeBlanc, J.G. Importance of IL-10 modulation by probiotic microorganisms in gastrointestinal inflammatory diseases. ISRN Gastroenterol. 2011, 2011, 892971. [Google Scholar] [CrossRef]
- Ashraf, R.; Vasiljevic, T.; Smith, S.C.; Donkor, O.N. Effect of cell-surface components and metabolites of lactic acid bacteria and probiotic organisms on cytokine production and induction of CD25 expression in human peripheral mononuclear cells. J. Dairy Sci. 2014, 97, 2542–2558. [Google Scholar] [CrossRef]
- Colella, M.; Charitos, I.A.; Ballini, A.; Cafiero, C.; Topi, S.; Palmirotta, R.; Santacroce, L. Microbiota revolution: How gut microbes regulate our lives. World J. Gastroenterol. 2023, 29, 4368–4383. [Google Scholar] [CrossRef] [PubMed]
- Haileselassie, Y.; Johansson, M.A.; Zimmer, C.L.; Björkander, S.; Petursdottir, D.H.; Dicksved, J.; Petersson, M.; Persson, J.O.; Fernandez, C.; Roos, S.; et al. Lactobacilli regulate Staphylococcus aureus 161:2-induced pro-inflammatory T-cell responses in vitro. PLoS ONE 2013, 8, e77893. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.A.; Björkander, S.; Mata Forsberg, M.; Qazi, K.R.; Salvany Celades, M.; Bittmann, J.; Eberl, M.; Sverremark-Ekström, E. Probiotic Lactobacilli Modulate Staphylococcus aureus-Induced Activation of Conventional and Unconventional T cells and NK Cells. Front. Immunol. 2016, 7, 273. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Charitos, I.A.; Cantore, S.; Topi, S.; Bottalico, L.; Santacroce, L. About Functional Foods: The Probiotics and Prebiotics State of Art. Antibiotics 2023, 12, 635. [Google Scholar] [CrossRef]
- Santacroce, L.; Cagiano, R.; Del Prete, R.; Bottalico, L.; Sabatini, R.; Carlaio, R.G.; Prejbeanu, R.; Vermesan, H.; Dragulescu, S.I.; Vermesan, D.; et al. Helicobacter pylori infection and gastric MALTomas: An up-to-date and therapy highlight. Clin. Ter. 2008, 159, 457–462. [Google Scholar] [PubMed]
- Merriman, J.A.; Nemeth, K.A.; Schlievert, P.M. Novel antimicrobial peptides that inhibit gram positive bacterial exotoxin synthesis. PLoS ONE 2014, 9, e95661. [Google Scholar] [CrossRef] [PubMed]
- Woetmann, A.; Alhede, M.; Dabelsteen, S.; Bjarnsholt, T.; Rybtke, M.; Nastasi, C.; Krejsgaard, T.; Andersen, M.H.; Bonefeld, C.M.; Geisler, C.; et al. Interleukin-26 (IL-26) is a novel anti-microbial peptide produced by T cells in response to staphylococcal enterotoxin. Oncotarget 2018, 9, 19481–19489. [Google Scholar] [CrossRef]
- Yami, H.A.; Tahmoorespur, M.; Javadmanesh, A.; Tazarghi, A.; Sekhavati, M.H. The immunomodulatory effects of lactoferrin and its derived peptides on NF-κB signaling pathway: A systematic review and meta-analysis. Immun. Inflamm. Dis. 2023, 11, e972. [Google Scholar] [CrossRef]
- Ianiro, G.; Rosa, L.; Bonaccorsi di Patti, M.C.; Valenti, P.; Musci, G.; Cutone, A. Lactoferrin: From the structure to the functional orchestration of iron homeostasis. Biometals 2023, 36, 391–416. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santacroce, L.; Topi, S.; Charitos, I.A.; Lovero, R.; Luperto, P.; Palmirotta, R.; Jirillo, E. Current Views about the Inflammatory Damage Triggered by Bacterial Superantigens and Experimental Attempts to Neutralize Superantigen-Mediated Toxic Effects with Natural and Biological Products. Pathophysiology 2024, 31, 18-31. https://doi.org/10.3390/pathophysiology31010002
Santacroce L, Topi S, Charitos IA, Lovero R, Luperto P, Palmirotta R, Jirillo E. Current Views about the Inflammatory Damage Triggered by Bacterial Superantigens and Experimental Attempts to Neutralize Superantigen-Mediated Toxic Effects with Natural and Biological Products. Pathophysiology. 2024; 31(1):18-31. https://doi.org/10.3390/pathophysiology31010002
Chicago/Turabian StyleSantacroce, Luigi, Skender Topi, Ioannis Alexandros Charitos, Roberto Lovero, Paolo Luperto, Raffaele Palmirotta, and Emilio Jirillo. 2024. "Current Views about the Inflammatory Damage Triggered by Bacterial Superantigens and Experimental Attempts to Neutralize Superantigen-Mediated Toxic Effects with Natural and Biological Products" Pathophysiology 31, no. 1: 18-31. https://doi.org/10.3390/pathophysiology31010002
APA StyleSantacroce, L., Topi, S., Charitos, I. A., Lovero, R., Luperto, P., Palmirotta, R., & Jirillo, E. (2024). Current Views about the Inflammatory Damage Triggered by Bacterial Superantigens and Experimental Attempts to Neutralize Superantigen-Mediated Toxic Effects with Natural and Biological Products. Pathophysiology, 31(1), 18-31. https://doi.org/10.3390/pathophysiology31010002










