The Endothelial Glycocalyx and Retinal Hemodynamics
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Enzyme Treatments
2.3. Preparative Surgery for Intravital Microscopy
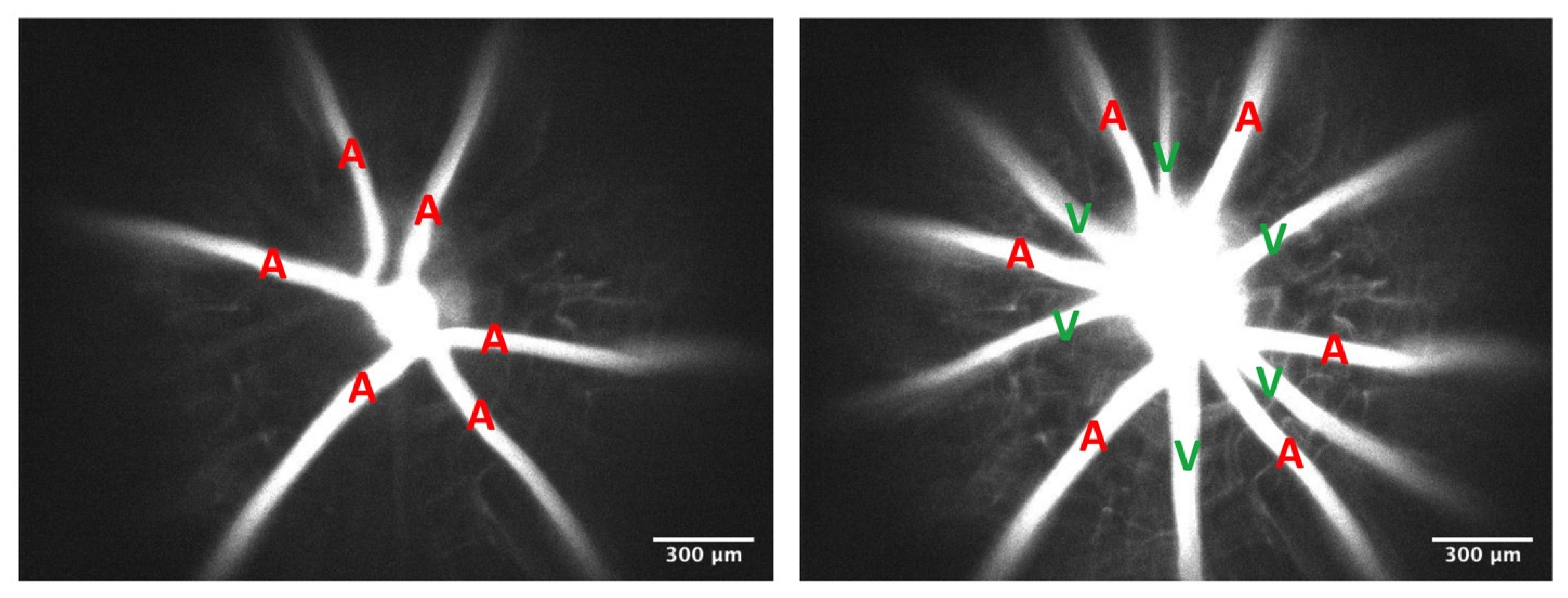
2.4. Measurements of Retinal Velocity, Diameter, and Hematocrit
2.5. Measurements of Retinal Flow Rate and Shear Rate
2.6. Measurements of Mean Circulation Time
2.7. In Vivo Thrombosis Induction
2.8. Statistics
3. Results
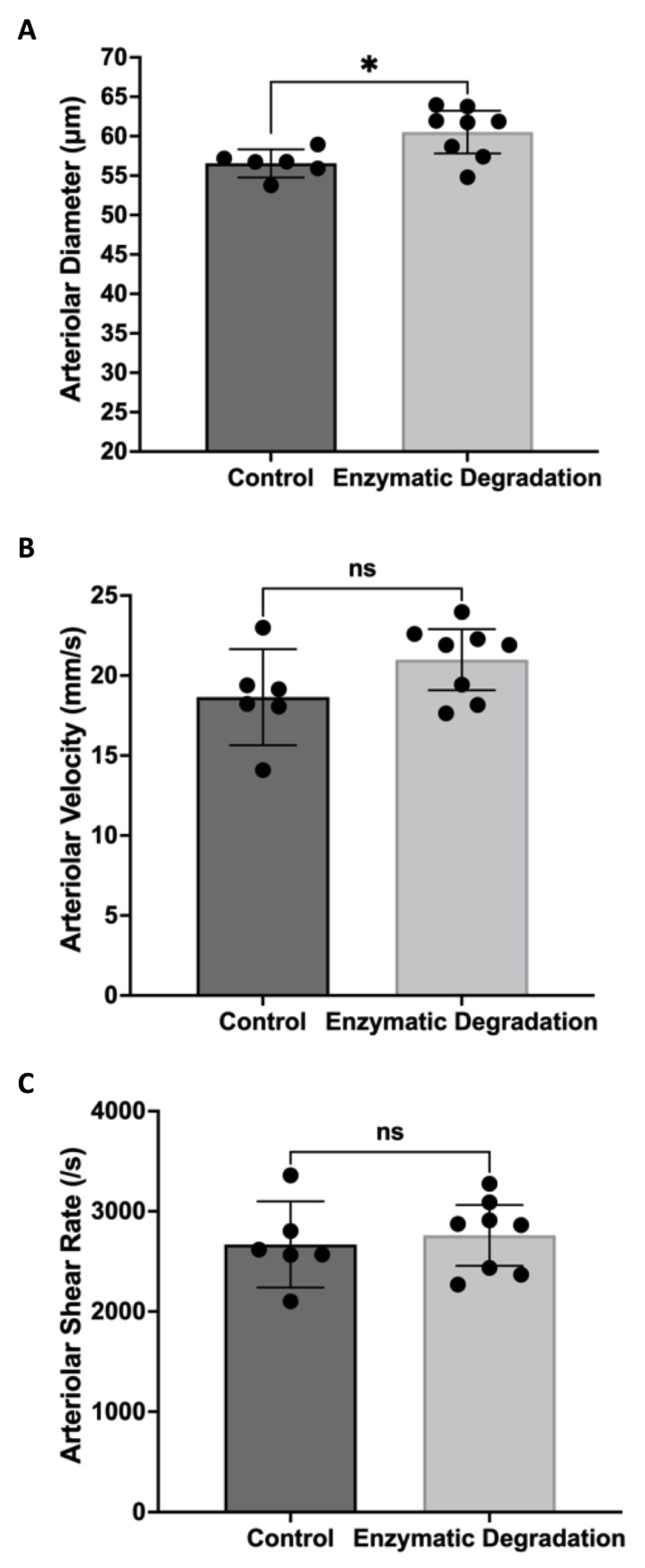
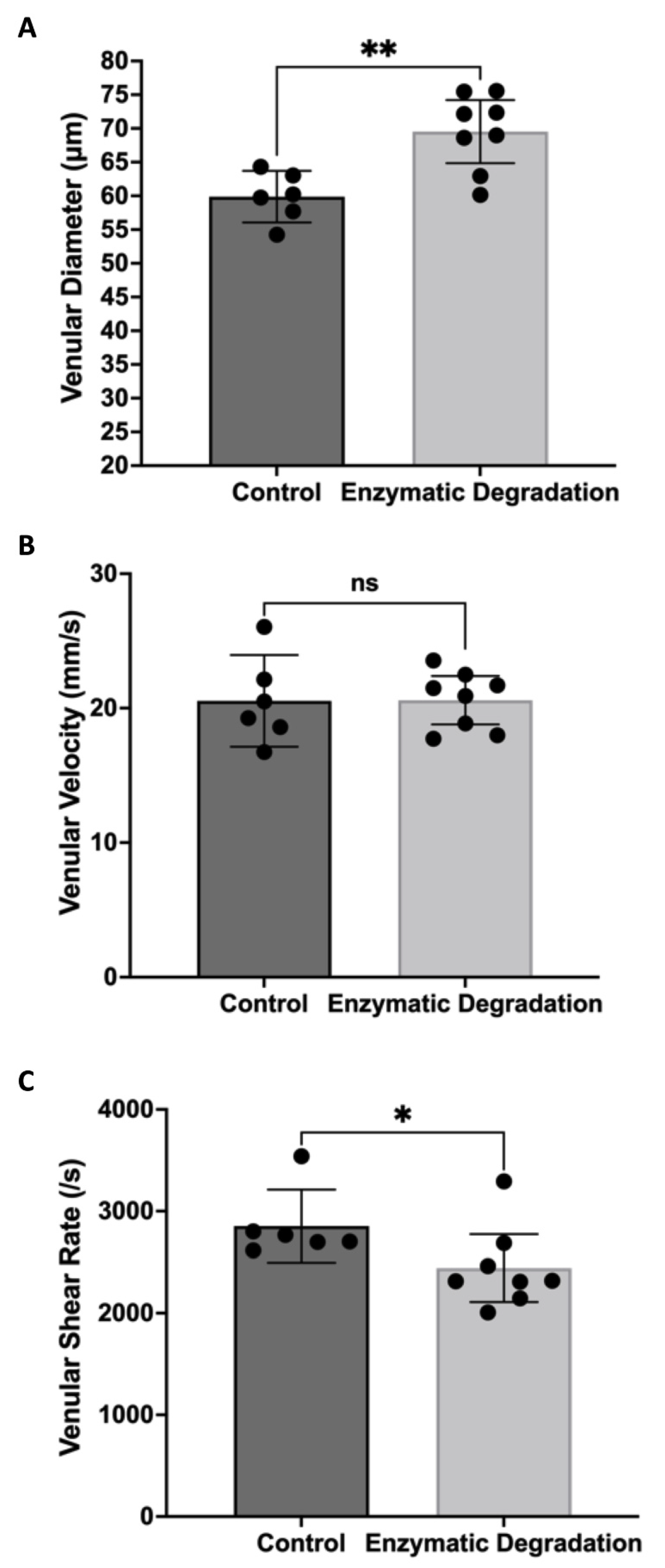
3.1. Effect of the Endothelial Glycocalyx on Retinal Hemodynamics
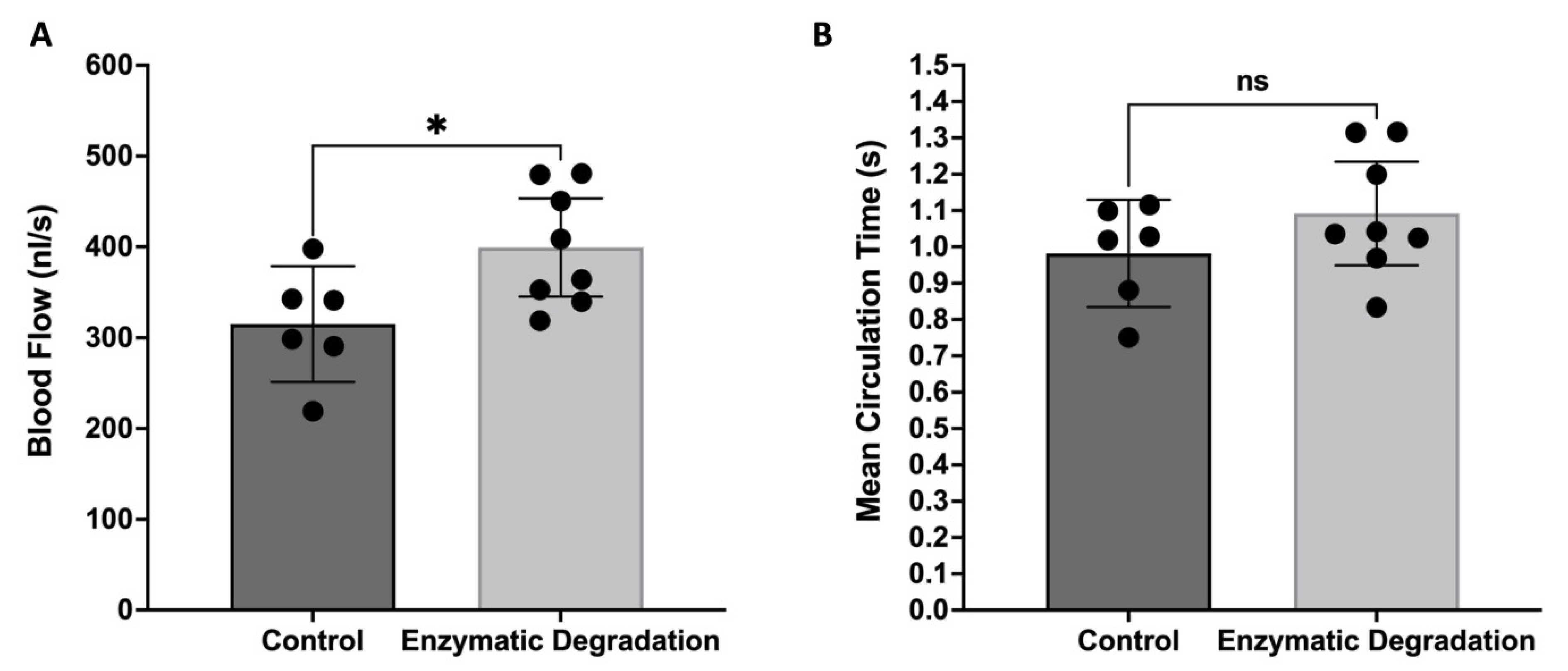
3.2. Effect of the Endothelial Glycocalyx on the Retinal Blood Flow and MCT
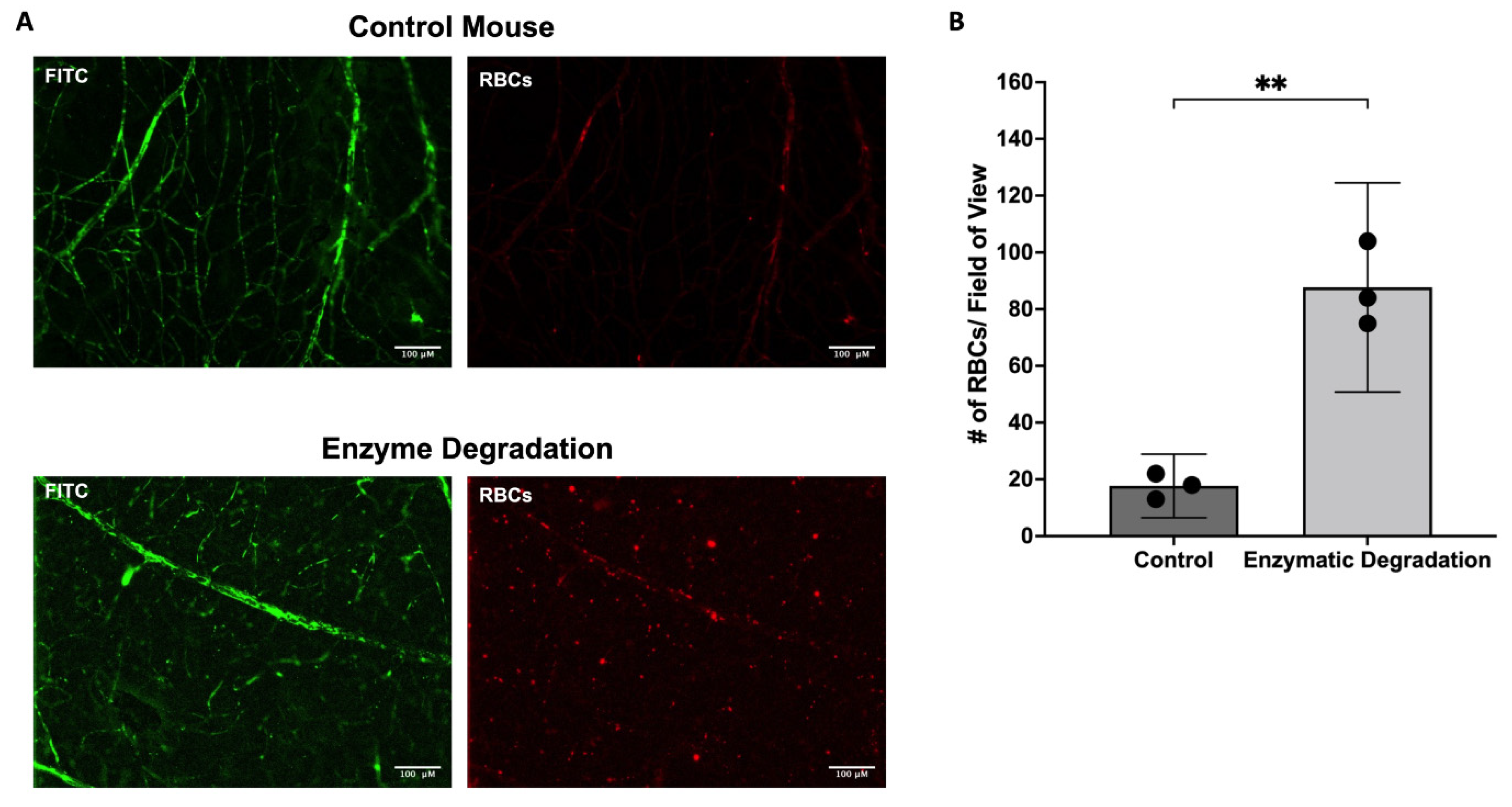
3.3. Effect of the Endothelial Glycocalyx on Retinal RBC Distribution
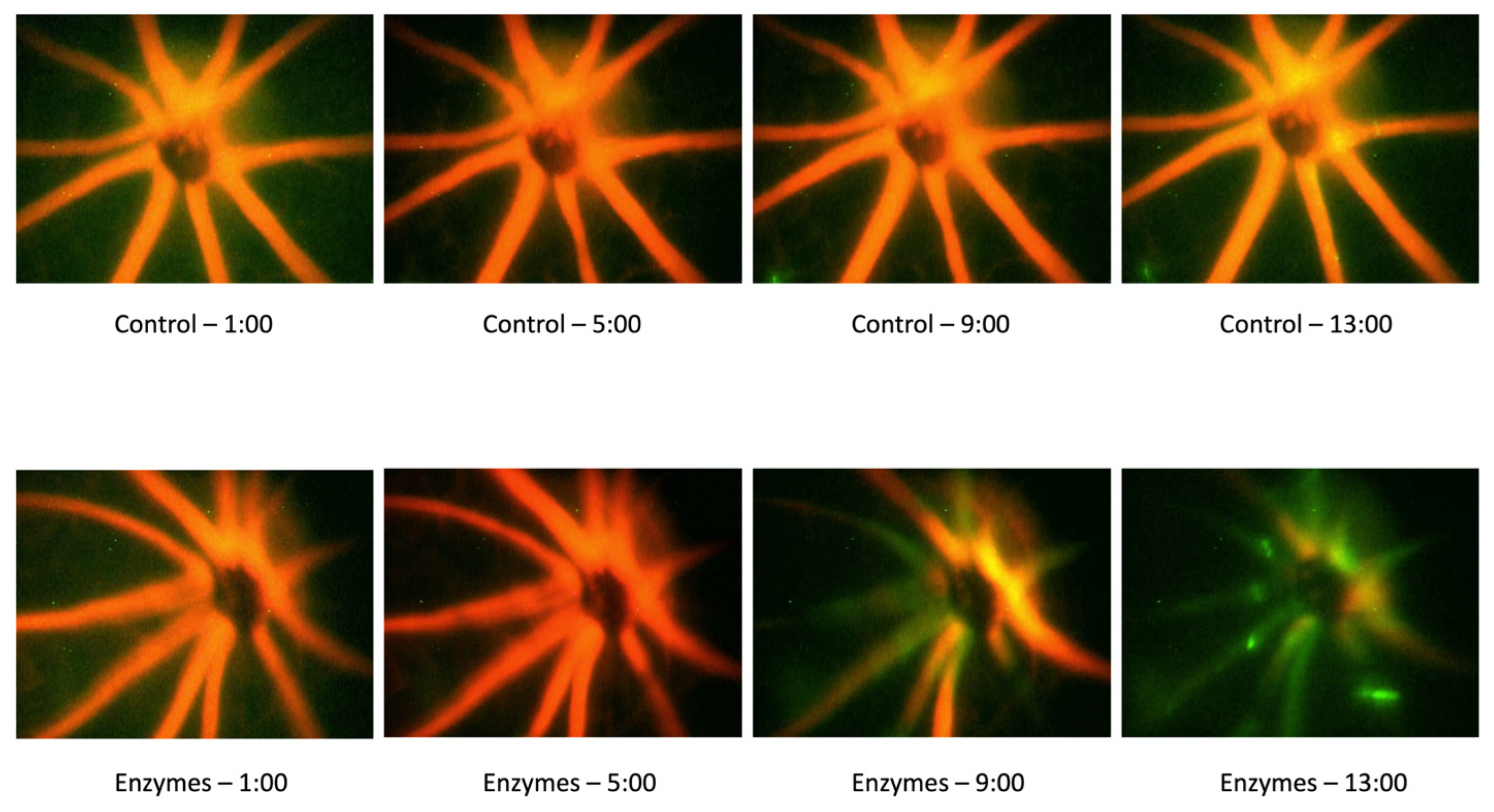
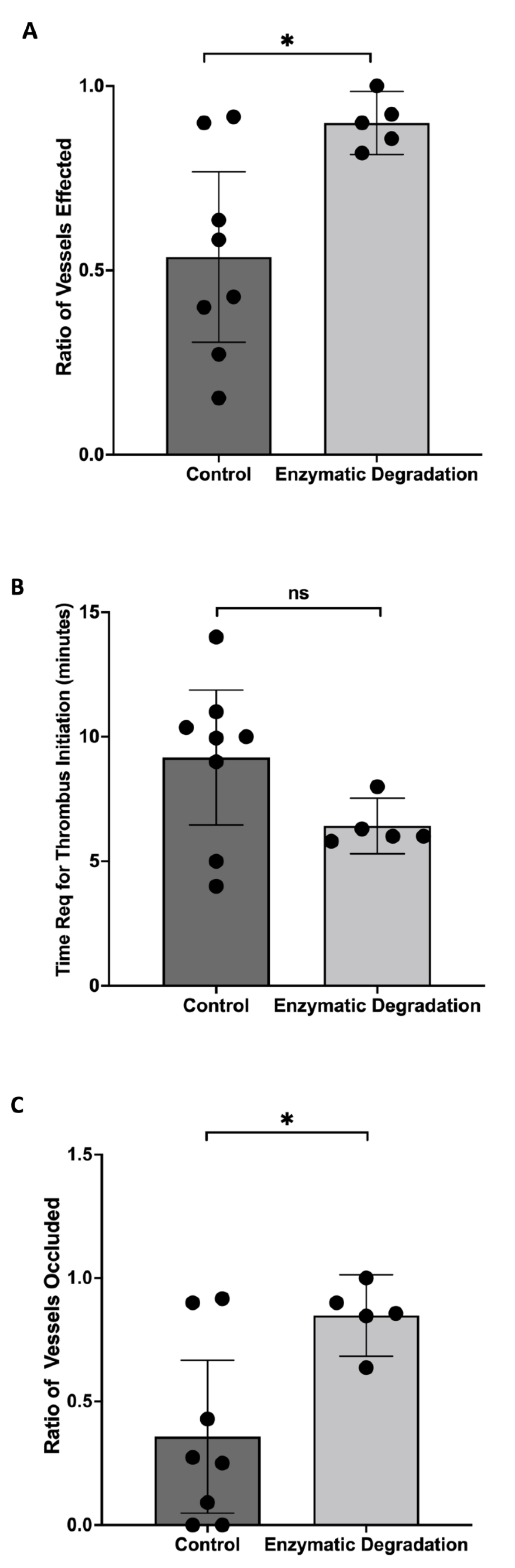
3.4. Effect of the Endothelial Glycocalyx Degradation on Thrombosis Induction
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.M.J.; Oude Egbrink, M.G. The endothelial glycocalyx: Composition, functions, and visualization. Pflug. Arch. 2007, 454, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Weinbaum, S.; Cancel, L.M.; Fu, B.M.; Tarbell, J.M. The Glycocalyx and Its Role in Vascular Physiology and Vascular Related Diseases. Cardiovasc. Eng. Technol. 2020, 12, 37–71. [Google Scholar] [CrossRef] [PubMed]
- Kundra, P.; Goswami, S. Endothelial glycocalyx: Role in body fluid homeostasis and fluid management. Indian J. Anaesth. 2019, 63, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Rabodzey, A.; Dewey, J.C.F. Glycocalyx modulates the motility and proliferative response of vascular endothelium to fluid shear stress. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1023–H1030. [Google Scholar] [CrossRef]
- Baeyens, N.; Mulligan-Kehoe, M.J.; Corti, F.; Simon, D.D.; Ross, T.D.; Rhodes, J.M.; Wang, T.Z.; Mejean, C.O.; Simons, M.; Humphrey, J.; et al. Syndecan 4 is required for endothelial alignment in flow and atheroprotective signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 17308–17313. [Google Scholar] [CrossRef]
- Weinbaum, S.; Zhang, X.; Han, Y.; Vink, H.; Cowin, S.C. Mechanotransduction and flow across the endothelial glycocalyx. Proc. Natl. Acad. Sci. USA 2003, 100, 7988–7995. [Google Scholar] [CrossRef]
- Bartosch, A.M.W.; Mathews, R.; Mahmoud, M.M.; Cancel, L.M.; Haq, Z.S.; Tarbell, J.M. Heparan sulfate proteoglycan glypican-1 and PECAM-1 cooperate in shear-induced endothelial nitric oxide production. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Pries, A.R.; Secomb, T.W.; Jacobs, H.; Sperandio, M.; Osterloh, K.; Gaehtgens, P. Microvascular blood flow resistance: Role of endothelial surface layer. Am. J. Physiol. Circ. Physiol. 1997, 273, H2272–H2279. [Google Scholar] [CrossRef]
- Ruane-O’Hora, T.; Markos, F. The Arteriolar Glycocalyx Plays a Role in the Regulation of Blood Flow in the Iliac of the Anaesthetised Pig. Physiol. Res. 2018, 67, 41–44. [Google Scholar] [CrossRef]
- Harris, N.R.; Leskova, W.; Kaur, G.; Eshaq, R.S.; Carter, P.R. Blood flow distribution and the endothelial surface layer in the diabetic retina. Biorheology 2019, 56, 181–189. [Google Scholar] [CrossRef]
- Desjardins, C.; Duling, B.R. Heparinase treatment suggests a role for the endothelial cell glycocalyx in regulation of capillary hematocrit. Am. J. Physiol. Circ. Physiol. 1990, 258, H647–H654. [Google Scholar] [CrossRef] [PubMed]
- Vink, H.; Duling, B.R. Identification of Distinct Luminal Domains for Macromolecules, Erythrocytes, and Leukocytes Within Mammalian Capillaries. Circ. Res. 1996, 79, 581–589. [Google Scholar] [CrossRef] [PubMed]
- McClatchey, P.M.; Schafer, M.; Hunter, K.S.; Reusch, J.E.B. The endothelial glycocalyx promotes homogenous blood flow distribution within the microvasculature. Am. J. Physiol. Circ. Physiol. 2016, 311, H168–H176. [Google Scholar] [CrossRef] [PubMed]
- Cabrales, P.; Vázquez, B.Y.S.; Tsai, A.G.; Intaglietta, M. Microvascular and capillary perfusion following glycocalyx degradation. J. Appl. Physiol. 2007, 102, 2251–2259. [Google Scholar] [CrossRef]
- Broekhuizen, L.N.; Lemkes, B.A.; Mooij, H.L.; Meuwese, M.C.; Verberne, H.; Holleman, F.; Schlingemann, R.O.; Nieuwdorp, M.; Stroes, E.S.G.; Vink, H. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia 2010, 53, 2646–2655. [Google Scholar] [CrossRef]
- Kumase, F.; Morizane, Y.; Mohri, S.; Takasu, I.; Ohtsuka, A.; Ohtsuki, H. Glycocalyx degradation in retinal and choroidal capillary endothelium in rats with diabetes and hypertension. Acta Med. Okayama 2010, 64, 277–283. [Google Scholar]
- Leskova, W.; Pickett, H.; Eshaq, R.S.; Shrestha, B.; Pattillo, C.B.; Harris, N.R. Effect of diabetes and hyaluronidase on the retinal endothelial glycocalyx in mice. Exp. Eye Res. 2018, 179, 125–131. [Google Scholar] [CrossRef]
- Kaur, G.; Song, Y.; Xia, K.; McCarthy, K.; Zhang, F.; Linhardt, R.J.; Harris, N.R. Effect of high glucose on glycosaminoglycans in cultured retinal endothelial cells and rat retina. Glycobiology 2022, 32, 720–734. [Google Scholar] [CrossRef]
- Kaur, G.; Rogers, J.; Rashdan, N.A.; Cruz-Topete, D.; Pattillo, C.B.; Hartson, S.D.; Harris, N.R. Hyperglycemia-induced effects on glycocalyx components in the retina. Exp. Eye Res. 2021, 213, 108846. [Google Scholar] [CrossRef]
- Niu, T.; Zhao, M.; Jiang, Y.; Xing, X.; Shi, X.; Cheng, L.; Jin, H.; Liu, K. Endomucin restores depleted endothelial glycocalyx in the retinas of streptozotocin-induced diabetic rats. FASEB J. 2019, 33, 13346–13357. [Google Scholar] [CrossRef]
- Kur, J.; Newman, E.A.; Chan-Ling, T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog. Retin. Eye Res. 2012, 31, 377–406. [Google Scholar] [CrossRef] [PubMed]
- Wright, W.S.; Eshaq, R.S.; Lee, M.; Kaur, G.; Harris, N.R. Retinal Physiology and Circulation: Effect of Diabetes. Compr. Physiol. 2020, 10, 933–974. [Google Scholar] [CrossRef] [PubMed]
- Leskova, W.; Warar, R.; Harris, N.R. Altered Retinal Hemodynamics and Mean Circulation Time in Spontaneously Hypertensive Rats. Investig. Opthalmology Vis. Sci. 2020, 61, 12. [Google Scholar] [CrossRef]
- Leskova, W.; Watts, M.N.; Carter, P.R.; Eshaq, R.S.; Harris, N.R. Measurement of Retinal Blood Flow Rate in Diabetic Rats: Disparity Between Techniques Due to Redistribution of Flow. Investig. Opthalmology Vis. Sci. 2013, 54, 2992–3018. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yadav, A.S.; Leskova, W.; Harris, N.R. Attenuation of streptozotocin-induced microvascular changes in the mouse retina with the endothelin receptor A antagonist atrasentan. Exp. Eye Res. 2010, 91, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Wright, W.S.; Messina, J.E.; Harris, N.R. Attenuation of diabetes-induced retinal vasoconstriction by a thromboxane receptor antagonist. Exp. Eye Res. 2009, 88, 106–112. [Google Scholar] [CrossRef]
- Wright, W.S.; Harris, N.R. Ozagrel attenuates early streptozotocin-induced constriction of arterioles in the mouse retina. Exp. Eye Res. 2008, 86, 528–536. [Google Scholar] [CrossRef]
- Labat-Gest, V.; Tomasi, S. Photothrombotic Ischemia: A Minimally Invasive and Reproducible Photochemical Cortical Lesion Model for Mouse Stroke Studies. J. Vis. Exp. 2013, e50370. [Google Scholar] [CrossRef]
- Talley Watts, L.; Zheng, W.; Garling, R.J.; Frohlich, V.C.; Lechleiter, J.D. Rose Bengal Photothrombosis by Confocal Optical Imaging In Vivo: A Model of Single Vessel Stroke. J. Vis. Exp. 2015, 100, e52794. [Google Scholar] [CrossRef]
- Baker, M.; Wayland, H. On-line volume flow rate and velocity profile measurement for blood in microvessels. Microvasc. Res. 1974, 7, 131–143. [Google Scholar] [CrossRef]
- Liu, Q.; Mirc, D.; Fu, B.M. Mechanical mechanisms of thrombosis in intact bent microvessels of rat mesentery. J. Biomech. 2008, 41, 2726–2734. [Google Scholar] [CrossRef] [PubMed]
- Tarbell, J.M.; Shi, Z.-D. Effect of the glycocalyx layer on transmission of interstitial flow shear stress to embedded cells. Biomech. Model. Mechanobiol. 2012, 12, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Pries, A.R.; Secomb, T.W. Microvascular blood viscosity in vivo and the endothelial surface layer. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2657–H2664. [Google Scholar] [CrossRef] [PubMed]
- Machin, D.R.; Phuong, T.T.; Donato, A.J. The role of the endothelial glycocalyx in advanced age and cardiovascular disease. Curr. Opin. Pharmacol. 2019, 45, 66–71. [Google Scholar] [CrossRef]
- Hu, Z.; Cano, I.; D’Amore, P.A. Update on the Role of the Endothelial Glycocalyx in Angiogenesis and Vascular Inflammation. Front. Cell Dev. Biol. 2021, 9, 734276. [Google Scholar] [CrossRef]
- Secomb, T.W.; Hsu, R.; Pries, A.R. Blood flow and red blood cell deformation in nonuniform capillaries: Effects of the endothelial surface layer. Microcirculation 2002, 9, 189–196. [Google Scholar] [CrossRef]
- Lanotte, L.; Tomaiuolo, G.; Misbah, C.; Bureau, L.; Guido, S. Red blood cell dynamics in polymer brush-coated microcapillaries: A model of endothelial glycocalyx in vitro. Biomicrofluidics 2014, 8, 014104. [Google Scholar] [CrossRef]
- Jiang, X.Z.; Goligorsky, M.S. Biomechanical properties of endothelial glycocalyx: An imperfect pendulum. Matrix Biol. Plus 2021, 12, 10087. [Google Scholar] [CrossRef]
- Paques, M.; Tadayoni, R.; Sercombe, R.; Laurent, P.; Genevois, O.; Gaudric, A.; Vicaut, E. Structural and Hemodynamic Analysis of the Mouse Retinal Microcirculation. Investig. Opthalmology Vis. Sci. 2003, 44, 4960–4967. [Google Scholar] [CrossRef]
- Bulpitt, C.J.; Dollery, C.T. Estimation of retinal blood flow by measurement of the mean circulation time. Cardiovasc. Res. 1971, 5, 406–412. [Google Scholar] [CrossRef]
- Russell, J.; Cooper, D.; Tailor, A.; Stokes, K.Y.; Granger, D.N. Low venular shear rates promote leukocyte-dependent recruitment of adherent platelets. Am. J. Physiol. Gastrointest Liver Physiol. 2003, 284, G123–G129. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bienvenu, K.; Granger, D.N. Molecular determinants of shear rate-dependent leukocyte adhesion in postcapillary venules. Am. J. Physiol. Circ. Physiol. 1993, 264, H1504–H1508. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, A.A.; Vink, H.; Spaan, J.A.E. Elevated capillary tube hematocrit reflects degradation of endothelial cell glycocalyx by oxidized LDL. Am. J. Physiol. Circ. Physiol. 2001, 280, H1051–H1057. [Google Scholar] [CrossRef] [PubMed]
- Mulivor, A.W.; Lipowsky, H.H. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1282–H1291. [Google Scholar] [CrossRef] [PubMed]
- Vink, H.; Constantinescu, A.A.; Spaan, J.A. Oxidized lipoproteins degrade the endothelial surface layer: Implications for platelet-endothelial cell adhesion. Circulation 2000, 101, 1500–1502. [Google Scholar] [CrossRef] [PubMed]
- Wright, W.S.; Yadav, A.S.; McElhatten, R.M.; Harris, N.R. Retinal blood flow abnormalities following six months of hyperglycemia in the Ins2(Akita) mouse. Exp. Eye Res. 2012, 98, 9–15. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, G.; Leskova, W.; Harris, N.R. The Endothelial Glycocalyx and Retinal Hemodynamics. Pathophysiology 2022, 29, 663-677. https://doi.org/10.3390/pathophysiology29040052
Kaur G, Leskova W, Harris NR. The Endothelial Glycocalyx and Retinal Hemodynamics. Pathophysiology. 2022; 29(4):663-677. https://doi.org/10.3390/pathophysiology29040052
Chicago/Turabian StyleKaur, Gaganpreet, Wendy Leskova, and Norman R. Harris. 2022. "The Endothelial Glycocalyx and Retinal Hemodynamics" Pathophysiology 29, no. 4: 663-677. https://doi.org/10.3390/pathophysiology29040052
APA StyleKaur, G., Leskova, W., & Harris, N. R. (2022). The Endothelial Glycocalyx and Retinal Hemodynamics. Pathophysiology, 29(4), 663-677. https://doi.org/10.3390/pathophysiology29040052






