Abstract
Oral carcinogenesis is also dependent on the balance of the oral microbiota. Candida albicans is a member oral microbiota that acts as an opportunistic pathogen along with changes in the epithelium that can predispose to premalignancy and/or malignancy. This systematic review uses the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines to analyze the role of Candida albicans in the process of oral carcinogenesis. Eleven articles qualified inclusion criteria, matched keywords, and provided adequate information about the carcinogenesis parameters of Candida albicans in oral cancer. Candida albicans in oral carcinogenesis can be seen as significant virulent factors for patients with oral squamous cell carcinoma (OSCC) or potentially malignant disorder (OPMD) with normal adjacent mucosa. Candida albicans have a role in the process of oral carcinogenesis concerning morphological phenotype changes in cell structure and genotype and contribute to the formation of carcinogenic substances that can affect cell development towards malignancy.
1. Introduction
Oral squamous cell carcinoma (OSCC) is the most prevalent cancer in the oral cavity. Oral cancers with OSCC are found in more than 90% of cases [1,2]. OSCC is the fifth most common form of malignancy worldwide, along with oropharyngeal cancer [3]. More than 70% of deaths from this cancer occur in Asia, and two-thirds of cases occur in Asian countries such as Sri Lanka, Indonesia, India, Pakistan, and Bangladesh. Oral cancer is the most common malignancy, accounting for more than 25% of all new cancer cases yearly [4]. There is also a similarity to numerous factors aggravating the development of OSCC. Alcohol consumption and tobacco smoking are predisposing factors included in lifestyle factors alongside obesity and nutrient deficiency [5].
Moreover, other predisposing factors such as microbiome environment, genetic infirmity, environmental factors, and exposure to chemicals, pesticides, and heavy metals can induce pro-oncogenic genetic and epigenetic alterations, which lead to the development of OSCC [6,7,8]. In contrast to exogenous factors such as tobacco and alcohol, oral microbes in OSCC may result from the microbe being a commensal or secondary infection in the cancerous tissue [5]. It has been previously reported how the oral microbiota is involved in carcinogenesis by primarily paying attention to chronic inflammation, microbial synthesis of carcinogens, and alteration of the integrity of the epithelial barrier [9,10,11,12]. The human papillomavirus (HPV) has been widely discussed for its involvement in the development of oral cancer [13]. The presence of HPV and Candida albicans (as the normal flora) is able to contribute to the development of oral cancer [14]. Among the other species of Candida, Candida albicans has the highest prevalence found in OSCC and always gained concerns in relation to the ability of pathogenic state shifting from the commensal condition [15]. Hence, Candida albicans is considered to be one of the most commonly researched of oral microbes in OSCC [15]. Its commensal condition can develop into an opportunistic pathogen linked explicitly with the initiation of oral neoplasia and the development of OSCC [16,17,18,19]. It has also been reported that Candida invasion is a significant risk factor for the malignant transformation of oral potentially malignant disorder (OPMD) to oral cancer [20,21].
However, it has always been challenging to distinguish Candida albicans in the commensal or pathogenic state. In contrast, non-Candida Albicans species such as Candida tropicalis and Candida glabrata has been classified as pathogenic microorganisms, and their dominance in the oral cavity is considered a sign of oral dysbacteriosis. The shift in oral Candida species towards non-Candida albicans in OSCC may provide preliminary evidence for a potential pathogenic associate and OSCC [21]. Therefore, it is important to review several studies on the ability of Candida albicans to progress to a pathogenic state. Only a few studies still report that Candida albicans have a parameter by which the pathogenic form can be seen and compared to those where it is still commensal. The purpose is to reduce distortion from Candida albicans in pathological conditions aggravated by other predisposing factors. Therefore, from OPMD and cancerous lesions obtained in this review, it is essential to examine the type and prevalence of the lesion, which has been proven that Candida albicans is one of the factors in the development of oral cancer. It is also essential to identify the factors increasing the carcinogenesis capability of Candida albicans. Thus, role of Candida spp. in OPMD and malignant can be accurately correlated and these factors can be intervened. In addition, the antifungal therapy that can be administered will also be more specific to the treatment, increasing the curative effect for preventing synergistic role in carcinogenesis and patient survival rate up to 80% along with treatment of other predisposing factors when it is appropriately diagnosed at an early stage [4].
2. Materials and Methods
2.1. Data Sources and Search Strategy
The preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) were used in this systematic review. A comprehensive search of the scientific literature was carried out in the following databases: PubMed, Scopus, and Web of Science for the studies published from 1987 to 2022.
The titles and keywords used were a combination of the following keywords adapted to each database: [(“Candida albicans” OR “Oral Candidiasis”) AND (“Oral Carcinogenesis” OR “Oral Cancer” OR “OSCC” or “Oral Squamous Cell Carcinoma”) AND (“Oral Potentially Malignant Disorder” OR “OPMD”)]. The keyword searches were run from 30 December 2021, to 1 December 2022. The manual searching and Embase cross-referencing method are also used to complete the investigation.
2.2. Study Selection
All studies including case-control, analytical cross-sectional studies, cohort, and randomized controlled trials that evaluated the role of Candida albicans in oral carcinogenesis were included. The inclusion criteria were articles describing Candida albicans as a predisposing factor for oral carcinogenesis.
2.3. Data Extraction
Three authors screened each study independently (MC, VKPA, and TASP). Both MC and VKPA are last year of undergraduate dentistry program. The authors first screened the title(s), abstracts, and full texts to determine whether the inclusion criteria had been accomplished. The following information was extracted from the studies to be included in the systematic review: study design, oral cancer diagnosis, oral candidiasis diagnosis, and Candida albicans virulence marker. In case of disagreement, third investigators (NFA, MDCS, and FYM) acted as a referral and reach a consensus through discussion.
2.4. Data Synthesis and Analysis
The data extracted from included articles were classified into four group types of data: study characteristics, case characteristics, diagnosis procedure, and result. The reports were identified based on virulence markers of Candida albicans related to carcinogenesis (i.e., phenotype, genotype and metabolic identification). The samples must mainly be taken from patients with oral cancer or OPMD diagnosis.
3. Results
3.1. Study Characteristic
Most samples of oral carcinoma were defined as oral cancer, oral dysplasia, OSCC without lymph node metastases (LNM), OSCC regional LNM, and adenocarcinoma. The OPMD was defined as leukoplakia (homogenous and non-homogenous), erythroplakia, oral lichen planus (OLP) (reticular, plaque, atrophic, or mixed), atypical lichen planus, oral lichenoid lesion (OLL) (reticular, plaque, atrophic, or mixed), and oral submucous fibrosis (OSF) (Table 1).

Table 1.
Sample characteristics in each study.
3.2. Candida Isolation and Culture Procedure
Most samples of OSCC and oral cancer were performed through tissue biopsy, oral rinse, mucosal swab, and saliva collection to detect Candida’s presence. Most studies cultured the specimen by CHROM-agar medium as well as the Hematoxylin and Eosin (HE) staining, and examination under fluorescent microscopy for analysis of Candida morphology. The assimilation test, agglutination test, Leicester and real-time polymerase chain reaction (RT-PCR) to identify Candida species was also performed (Table 2).

Table 2.
The Candida albicans isolation and the diagnosis procedure.
3.3. Candida Virulence Factor
3.3.1. Phenotype
The phenotype of Candida is defined as the presence of hyphae, spores, colonies, biofilm formation, and Cell Surface Hydrophobicity (CSH). Oral cancer and epithelial dysplasia showed a higher presence of Candida spp. than healthy gingiva tissue. Oral cancer itself showed higher colonies, biofilm formation, biofilm mass activity, and CSH (Table 3).

Table 3.
The Candida albicans phenotype identified in each lesion.
3.3.2. Genotype
The genotype of Candida has defined as Candida albicans alcohol dehydrogenase 1 (CaADH1) mRNA gene was strongly expressed in OSCC and oral dysplasia and especially with LNM. The identified Candida was dominated by Candida albicans genotype A. The Stratifin (SNF) was highly expressed in the Candida albicans infection both OPMD and OSCC (Table 4).

Table 4.
The Candida albicans genotype identified in each lesion.
3.3.3. Metabolic Factors
The metabolic factors of Candida were defined as the production of acetaldehyde, proteinase, proteolytic, lipolytic, phospolytic, esterase, and N-nitroso benzyl methylamine (NMBA) production (Table 5).

Table 5.
The Candida albicans metabolic product identified in each lesion.
4. Discussion
Candida albicans is the most virulent pathogen among other Candida species and is most commonly found in OSCC and OPMD patients [33,34]. However, Candida albicans is also an important component of the commensal microbiota in maintaining oral health and the general physiology of the human body and contributes to overall health [35]. Thus, even though this microbiota has been found in large numbers in healthy individuals with healthy oral cavities, the results found in comparisons between OSCC and healthy ones may be inconclusive and insignificant [5]. Therefore, to see the role of microorganisms in OSCC, research results with a highly significant difference between OSCC patients compared to healthy patients with normal oral mucosa are necessary [5]. Some of the articles reviewed in this systematic review are unable to show the course of the disease caused by patients with oral dysbiosis caused by a high prevalence of Candida albicans or by patients presenting the pathogenic phase of Candida albicans in the form of oral candidiasis and chronic hyperplastic candidiasis to OSCC [22,23,24,25,26,27,28,29,34]. Nevertheless, the role of Candida albicans in the process of oral carcinogenesis can still be seen from the importance of Candida albicans virulence factors generated between OSCC or OPMD patients compared to normal healthy mucosa [5,36,37,38].
In general, the role of Candida albicans in the process of carcinogenesis tends to be complex, such as the role of virulence factors, the host genome, influence on the immune response, and oral dysbiosis, as noted in a review conducted by Di Cosola et al. 2021 [35]. However, several studies have been conducted, and no direct study of Candida albicans with OSCC or OPMD [39,40,41,42,43,44,45]. In this systematic review, there are eight articles dominated by the Candida albicans virulence factor. In general, there are seven virulence factors of Candida albicans discussed in this article, mainly phenotype (Candida frequencies, hyphae, sphere, colonies, biofilm formation), genotype (Candida albicans alcohol dehydrogenase 1 (CaADH1) mRNA gene, genotypic diversity of Candida albicans strains, CSH), and metabolic production (acetaldehyde product, lipase, proteinase product, phospholipase, and esterase activity, NMBA production) [22,23,24,26,27,28,29,34].
Increased colonization of Candida albicans is one of the strong associations with oral epithelial dysplasia and neoplastic transformation leading to the OSCC process [46,47]. The number of colonies and excessive density of Candida albicans can damage host cells and promote the development of carcinogenesis [34]. The presence of Candida albicans in the form of colonies and biofilm formation found in the healthy mucosa group compared to moderate, severe dysplasia and OSCC showed high statistical significance. However, based on research conducted by Tamgadge et al., 2017 and Alnuaimi et al., 2016, the study did not concern Candida albicans in detail but rather Candida as a whole [22,29]. In addition, adhesiveness also influences the early stages of colony formation, one of which is CSH as a reference for measuring adhesiveness to hydrophobic substrates such as buccal keratinous tissue and oral prostheses [48,49]. Differences in CSH in all study groups (oral cancer, atypical lichen planus, chronic candidiasis) compared to healthy mucosa were statistically significant [26].
The genotypic diversity of Candida albicans in the oral cavity, with its relationship to high colonization in OSCC, means that variety may also influence the process of oral carcinogenesis [24,29,30,31,50]. At the subspecies level, only Candida albicans species have been extensively genotyped by the ABC genotyping method based on the size and presence of transposable introns in 25S ribosomal DNA in Candida albicans genotype A, genotype B, and genotype C [12,24,35]. Candida albicans genotype A strain observed in oral cancer patients had a significantly higher incidence compared to non-oral cancer patients. Furthermore, the genotype B Candida albicans strain found in non-cancer patients had a substantially higher incidence than in oral cancer patients [24]. However, regarding the ability to produce carcinogenic substances such as acetaldehyde, there is no significance between the individual genotypes of Candida albicans [29].
Candida albicans also have the potential to induce OSCC by producing carcinogenic compounds. Certain strains of Candida albicans and other yeasts play an essential role in developing oral cancer by creating endogenous nitrosamines. Candida albicans can convert both nitrite and/or nitrate into nitrosamines and other substances to produce acetaldehyde [25,50,51]. The Candida albicans strain showed the highest nitrosation potential compared to other strains. Strains with high nitrosation potential is generally isolated from lesions with more advanced oral precancerous changes [25]. However, it should also be noted that the study had a low-quality assessment of the risk of bias, including unclear details of the inclusion of research subjects and neglect of confounding factors that could affect study results [25]. Candida albicans has the ability to convert alcohol to acetaldehyde, which has carcinogenic role in oral cavity. This conversion is facilitated by Candida albicans Alcohol dehydrogenase 1 (CaADH1) mRNA gene [24]. The study results showed that the CaADH 1 gene could be significantly associated with OSCC with and without metastases compared to healthy patients but could not be predicted in patients with oral dysplasia compared to healthy patients. Studies examining the potential role of Candida and CaADH1 mRNA in the initiation and progression of oral dysplasia and OSCC and metastatic OSCC is relatively rare. In this study, not all isolates demonstrated the presence of the CaADH1 mRNA gene in Candida albicans [23].
One of the interesting points is the finding of SFN expression oral carcinogenesis [32]. SFN is a cell cycle checkpoint protein that has been reported to be involved in carcinogenesis [52]. In some cancers, such as ovarian cancer [53], breast cancer [54], ocular surface squamous neoplasia [55], and lung cancer [56], the SFN was significantly higher and determined as a prognosis [53]. The higher SFN expression is correlated with higher p53 expression [55]. In oral cancer, the SFN expression is influenced by Candida albicans [32]. Both OPMD and OSCC with Candida albicans expressed a higher SFN expression than those without Candida albicans and healthy mucosa [32]. The role of SFN in oral carcinogenesis is not yet fully understood. This discovery leads us to research more about the involvement of SNF in oral carcinogenesis.
Candida albicans acetaldehyde production occurs through the conversion of alcohol to carcinogenic metabolite acetaldehyde. Pure ethanol has no independent carcinogenic effects, but the first metabolite of acetaldehyde is an undeniable carcinogen. It can induce mutagenic impacts such as DNA adducts, cross-linking, aneuploidy, or chromosomal aberrations [24,57,58]. Acetaldehyde metabolism is significantly influenced by smoking behaviour and alcohol [59]. Therefore, these risk factors must also be included in the research variables of articles examining acetaldehyde production. All Candida albicans isolates were shown to produce high levels (>100 μM) of acetaldehyde in all incubations containing ethanol, ethanol–glucose or glucose incubation. The acetaldehyde-producing ability was significantly lower in OPMD lesions (OLP, OLL, and leukoplakia) than in non-cancer patients [27]. Among them, leukoplakia has acetaldehyde-producing ability higher than OLP and OLL [27]. This difference in acetaldehyde-producing ability is influenced by predisposing factors such as smoking and alcohol consumption. Where are the predisposing factors can induce adaptive changes that lead to the overregulation of acetaldehyde metabolism by Candida albicans. Hence, acetaldehyde production is influenced in candida mediated oral carcinogenesis [27].
Candida albicans can also affect the progression from OPMD to OSCC. Among other OPMD lesions, oral leukoplakia has the highest incidence and rate of malignant transformation. Oral leukoplakia and its association with Candida albicans may be associated with a higher risk of malignant transformation [22,27]. There is also a significant association between histologically oral mucosal fungal infections and epithelial dysplasia [25]. This is associated with increased exposure to other risk factors such as alcohol and smoking. Isolates from the oral leukoplakia group produced significantly higher levels of acetaldehyde than isolates from oral lichen planus (reticular, plaque, atrophic, or combination) when exposed to glucose–ethanol or glucose alone. In oral leukoplakia, higher Candida colonisation rates, higher smoking rates, and a cumulative increase in Candida acetaldehyde metabolism may contribute to malignant transformation [27]. Another study also confirmed that the leukoplakia with Candida albicans (or Candidal leukoplakia) has a high rate of cancer transformation, because it highly expressed a fibroblast activation protein (FAP) and α-smooth muscle actin (α-SMA). In addition, leukoplakia also secretes low a CX3CL1, a potential antifungal protein, which is more resistance to Candida albicans [60].
Factors affecting the ability of Candida albicans to accelerate the development of oral carcinogenesis also includes virulence factors, protein-degrading ability, and lipolytic activity. The persistence of Candida also depends on the capacity to secrete hydrolytic exoenzymes that facilitate further tissue invasion [26]. It has been shown statistically that Candida albicans proteinase, phospholipase and lipase activity was higher in oral cancer patients [26,28,29]. However, it was found that Candida albicans esterase activity was significantly higher in healthy patients than in oral cancer patients. In addition, in Castillo 2018, the research was not explicitly conducted on Candida albicans but on Candida in general [26].
Candida albicans can stimulate the origin and development of cancer. These studies examine several mechanisms by which this fungi type can trigger cancer. The more precise image of the mechanism is summarized as stated in Figure 1.
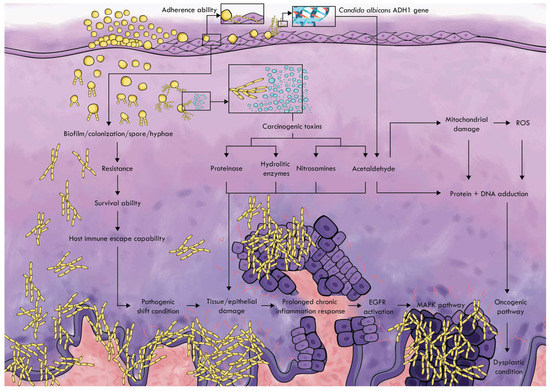
Figure 1.
Candida albicans carcinogenic pathway.
Adherence ability is the first step in colonization with Candida albicans. CSH is a method for determining the adherence ability of microorganisms to hydrophobic substrates, such as buccal keratin tissue and oral prostheses. This ability affects the formation of biofilms that contribute to maintaining the prolonged infection and avoiding the defense mechanism of the host [49,50]. Candida albicans in normal microbiota has a normal level of biofilm and colonization, while in the oral microbiota dominated by Candida albicans, the relationship between biofilm formation and colonization increases, which increases resistance and survival, which leads to invasive colonization and pathogenic shifting state [61,62]. The variety of Candida albicans strains also influences the ability of these microorganisms to form biofilms [29,63]. This leads to tissue damage and triggers a continued chronic inflammatory response [29,61,62].
Chronic contact with microorganisms and their products such as endotoxins, enzymes are toxic for host cells, which can either trigger mutations or alter the signaling pathways to influence on cell proliferation or the survival of the epithelial cells [64]. Candida albicans can produce carcinogens such as acetaldehyde, nitrosamines, and enzymes (proteases, lipases, esterases, and phospholipases) that can promote cancer formation [29,62,64,65].
One of the proteins identified in the mannoprotein infraction of Candida albicans, which increases tumor adhesion by triggering inflammation in endothelial cells, is alcohol hydrogenase (ADH1), which is associated with a cancer-stimulating mechanism by acetaldehyde production. Candida albicans use the enzyme alcohol hydrogenase (ADH1) to convert alcohol and other substances, such as carbohydrates into carcinogenic acetaldehyde [62]. This ability is encoded by the ADH gene, which is expressed in seven types of ADH genes in the genome database by Candida albicans [23]. Acetaldehyde can induce tumor development in various ways. This carcinogen binds to proteins and DNA, changes its structure and function, and the reduction in the antioxidant activity of glutathione increases the content of reactive oxygen species (ROS) in the cells. These changes can lead to genomic instability, inhibiting the apoptotic system and tumor development [62]. Nitrosamines produced by Candida albicans individually or in combination with other carcinogenic compounds can activate specific proto-oncogenes that can cause the development of cancer lesions that lead to changes in dysplastic conditions in oral epithelium and cancer [62,66]. Carcinogenic products and hydrolytic enzymes produced by Candida albicans also lead to further tissue destruction and trigger a continuous chronic inflammatory reaction [29].
Some of the results from these articles also show shortcomings that there are studies that are not being conducted specifically on the Candida albicans subspecies but focused on Candida species in general. However, Candida albicans, the most prevalent fungal microbiota in the oral cavity, are expected to be considered from the results obtained [33,34]. In addition, there are several articles that only list OPMD without OSCC; thus, a study group without OSCC can lead to different results [25,27]. However, from the significance obtained from the reviewed research, the comparison of OPMD with normal mucosa seems to be statistically significant, which will have a much higher importance in OSCC, whose histopathological and clinical condition is much more severe than in OPMD. Another thing to consider is the pathological state or condition of Candida albicans, which is difficult to determine when the microorganism is already pathogenic or still opportunistic commensal, characterized by the absence of articles containing oral candidiasis in the study group. However, some of the highly significant differences between OPMD or OSCC and normal mucosal patient groups must be considered when determining the role of Candida albicans in carcinogenesis.
5. Conclusions
Candida albicans were closely associated with oral potentially malignant and malignant oral lesions with various pathways. However, this systematic review study flags the important parameters involved in the oral carcinogenesis process, which include production of several phenotypes, genotype virulence factors, and carcinogenic metabolites.
In general, the relationship between fungal infections, especially Candida albicans, and oral cancer has been discussed in the literature for a long time. Many in vitro and in vivo studies show evidence of the parameters and markers involved in Candida albicans carcinogenesis. However, clinical evidence is still lacking, as it is difficult to find studies that deal specifically with this topic. Risk factors affecting the virulence factors of Candida albicans is also necessary to be researched in more articles involved. The exact mechanism by which Candida albicans is interested in developing OSCC also requires much research, particularly clinical research.
Author Contributions
Supervision, N.F.A., M.D.C.S. and F.Y.M.; validation, N.F.A., M.D.C.S., F.Y.M., A.B.R.S. and L.S.; project administration, N.F.A., M.D.C.S., F.Y.M. and T.A.S.P.; conceptualization, N.F.A., M.D.C.S., F.Y.M., M.C. and V.K.P.A.; resources, M.C., V.K.P.A. and T.A.S.P.; writing—original draft preparation, M.C., V.K.P.A. and T.A.S.P.; writing—review and editing; N.F.A., M.D.C.S., F.Y.M., A.B.R.S. and L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data available is a personal request to the corresponding author (nurina-ayu@fkg.unair.ac.id).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abati, S.; Bramati, C.; Bondi, S.; Lissoni, A.; Trimarchi, M. Oral Cancer and Precancer: A Narrative Review on the Relevance of Early Diagnosis. Int. J. Environ. Res. Public Health 2020, 17, 9160. [Google Scholar] [CrossRef]
- Markopoulos, A.K. Current Aspects on Oral Squamous Cell Carcinoma. Open Dent. J. 2012, 6, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Ferreira, F.; Nedel, F.; Etges, A.; Gomes, A.P.N.; Furuse, C.; Tarquinio, S.B.C. Etiologic factors associated with oral squamous cell carcinoma in non-smokers and non-alcoholic drinkers: A brief approach. Braz. Dent. J. 2012, 23, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.D.; Vedsted, P.; Kallestrup, P.; Neupane, D. Prevalence and incidence of oral cancer in low- and middle-income countries: A scoping review. Eur. J. Cancer Care 2019, 29, e13207. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Arakeri, G.; Alamir, A.W.H.; Patil, S.; Awan, K.H.; Baeshen, H.; Raj, T.; Fonseca, F.P.; Brennan, P.A. Is toombak a risk factor for oral leukoplakia and oral squamous cell carcinoma? A systematic review. J. Oral Pathol. Med. 2020, 49, 103–109. [Google Scholar] [CrossRef]
- Falzone, L.; Marconi, A.; Loreto, C.; Franco, S.; Spandidos, D.A.; Libra, M. Occupational exposure to carcinogens: Benzene, pesticides and fibers. Mol. Med. Rep. 2016, 14, 4467–4474. [Google Scholar] [CrossRef]
- Malfa, G.A.; Tomasello, B.; Sinatra, F.; Villaggio, G.; Amenta, F.; Avola, R.; Renis, M. “Reactive” response evaluation of primary human astrocytes after methylmercury exposure. J. Neurosci. Res. 2014, 92, 95–103. [Google Scholar] [CrossRef]
- Rapisarda, V.; Ledda, C.; Matera, S.; Fago, L.; Arrabito, G.; Falzone, L.; Marconi, A.; Libra, M.; Loreto, C. Absence of t(14;18) chromosome translocation in agricultural workers after short-term exposure to pesticides. Mol. Med. Rep. 2017, 15, 3379–3382. [Google Scholar] [CrossRef]
- Karpiński, T.M. Role of Oral Microbiota in Cancer Development. Microorganisms 2019, 7, 20. [Google Scholar] [CrossRef]
- Pang, X.; Tang, Y.J.; Ren, X.H.; Chen, Q.M.; Tang, Y.L.; Liang, X.H. Microbiota, Epithelium, Inflammation, and TGF-β Signaling: An Intricate Interaction in Oncogenesis. Front. Microbiol. 2018, 9, 1353. [Google Scholar] [CrossRef]
- Multhoff, G.; Molls, M.; Radons, J. Chronic Inflammation in Cancer Development. Front. Immunol. 2012, 2, 98. [Google Scholar] [CrossRef]
- Alnuaimi, A.; Wiesenfeld, D.; O’Brien-Simpson, N.; Reynolds, E.; Peng, B.; McCullough, M. The development and validation of a rapid genetic method for species identification and genotyping of medically important fungal pathogens using high-resolution melting curve analysis. Mol. Oral Microbiol. 2014, 29, 117–130. [Google Scholar] [CrossRef]
- Yete, S.; D’Souza, W.; Saranath, D. High-Risk Human Papillomavirus in Oral Cancer: Clinical Implications. Oncology 2018, 94, 133–141. [Google Scholar] [CrossRef]
- Muzio, L.L.; Ballini, A.; Cantore, S.; Bottalico, L.; Charitos, I.A.; Ambrosino, M.; Nocini, R.; Malcangi, A.; Dioguardi, M.; Cazzolla, A.P.; et al. Overview of Candida albicans and Human Papillomavirus (HPV) Infection Agents and their Biomolecular Mechanisms in Promoting Oral Cancer in Pediatric Patients. Biomed. Res. Int. 2021, 2021, 7312611. [Google Scholar] [CrossRef]
- Mallika, L.; Augustine, D.; Rao, R.S.; Patil, S.; Alamir, A.W.H.; Awan, K.H.; Sowmya, S.V.; Haragannavar, V.C.; Prasad, K. Does microbiome shift play a role in carcinogenesis? A systematic review. Transl. Cancer Res. 2020, 9, 3153–3166. [Google Scholar] [CrossRef]
- Williams, D.W.; Bartie, K.L.; Potts, A.; Wilson, M.J.; Fardy, M.J.; Lewis, M. Strain persistence of invasive Candida albican in chronic hyperplastic candidosis that underwent malignant change. Gerodontology 2001, 18, 73–78. [Google Scholar] [CrossRef]
- Cannon, R.D.; Chaffin, W.L. Oral Colonization By Candida albicans. Crit. Rev. Oral Biol. Med. 1999, 10, 359–383. [Google Scholar] [CrossRef]
- Rodríguez, M.J.; Schneider, J.; Moragues, M.-D.; Martínez-Conde, R.; Pontón, J.; Aguirre, J.M. Cross-reactivity between Candida albicans and oral squamous cell carcinoma revealed by monoclonal antibody C7. Anticancer Res. 2007, 27, 3639–3643. [Google Scholar]
- McManus, B.A.; Coleman, D.C. Molecular epidemiology, phylogeny and evolution of Candida albicans. Infect. Genet. Evol. 2014, 21, 166–178. [Google Scholar] [CrossRef]
- van der Waal, I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncol. 2009, 45, 317–323. [Google Scholar] [CrossRef]
- Arya, C.P.; Jaiswal, R.; Tandon, A.; Jain, A. Isolation and identification of oral Candida species in potentially malignant disorder and oral squamous cell carcinoma. Natl. J. Maxillofac. Surg. 2021, 12, 387. [Google Scholar] [CrossRef] [PubMed]
- Tamgadge, S.; Tamgadge, A.; Pillai, A.; Chande, M.; Acharya, S.; Kamat, N. Association of Candida sp. with the Degrees of Dysplasia and Oral Cancer: A Study by Calcofluor White under Fluorescent Microscopy. Iran J. Pathol. 2017, 12, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Hafed, L.; Farag, H.; El-Rouby, D.; Shaker, O.; Shabaan, H.-A. Candida Albicans Alcohol Dehydrogenase 1 gene in oral dysplasia and oral squamous cell carcinoma. Pol. J. Pathol. 2019, 70, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Alnuaimi, A.D.; Wiesenfeld, D.; O’Brien-Simpson, N.; Reynolds, E.; McCullough, M.J. Oral Candida colonization in oral cancer patients and its relationship with traditional risk factors of oral cancer: A matched case-control study. Oral Oncol. 2015, 51, 139–145. [Google Scholar] [CrossRef]
- Krogh, P.; Hald, B.; Holmstrup, P. Possible mycological etiology of oral mucosal cancer: Catalytic potential of infecting Candida aibicans and other yeasts in production of N-nitrosobenzylmethylamine. Carcinogenesis 1987, 8, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Del Valle Castillo, G.; de Blanc, S.L.; Sotomayor, C.E.; Azcurra, A.I. Study of virulence factor of Candida species in oral lesions and its association with potentially malignant and malignant lesions. Arch. Oral Biol. 2018, 91, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Gainza-Cirauqui, M.L.; Nieminen, M.T.; Novak Frazer, L.; Aguirre-Urizar, J.M.; Moragues, M.D.; Rautemaa, R. Production of carcinogenic acetaldehyde by Candida albicans from patients with potentially malignant oral mucosal disorders. J. Oral Pathol. Med. 2013, 42, 243–249. [Google Scholar] [CrossRef]
- Nawaz, A.; Mäkinen, A.; Pärnänen, P.; Meurman, J.H. Proteolytic activity of non-albicans Candida and Candida albicans in oral cancer patients. New Microbiol. 2018, 41, 296–301. [Google Scholar]
- Alnuaimi, A.D.; Ramdzan, A.N.; Wiesenfeld, D.; O’Brien-Simpson, N.M.; Kolev, S.D.; Reynolds, E.C.; McCullough, M.J. Candida virulence and ethanol-derived acetaldehyde production in oral cancer and non-cancer subjects. Oral Dis. 2016, 22, 805–814. [Google Scholar] [CrossRef]
- Sankari, S.L.; Mahalakshmi, K.; Kumar, V.N. A comparative study of Candida species diversity among patients with oral squamous cell carcinoma and oral potentially malignant disorders. BMC Res. Notes 2020, 13, 488. [Google Scholar] [CrossRef]
- Abidullah, M.; Bhosle, S.; Komire, B.; Sharma, P.; Swathi, K.; Karthik, L. Investigation of Candidal Species among People Who Suffer from Oral Potentially Malignant Disorders and Oral Squamous Cell Carcinoma. J. Pharm. Bioallied. Sci. 2021, 13 (Suppl. 2), S1050–S1054. [Google Scholar]
- Hsieh, Y.-P.; Wu, Y.-H.; Cheng, S.-M.; Lin, F.-K.; Hwang, D.-Y.; Jiang, S.-S.; Chen, K.-C.; Chen, M.-Y.; Chiang, W.-F.; Liu, K.-J.; et al. Single-Cell RNA Sequencing Analysis for Oncogenic Mechanisms Underlying Oral Squamous Cell Carcinoma Carcinogenesis with Candida albicans Infection. Int. J. Mol. Sci. 2022, 23, 4833. [Google Scholar] [CrossRef]
- Sankari, S.L.; Mahalakshmi, K. Oral Candidal Carriage Among Patients with Oral Potential Malignant Disorders: A Case-Control Study. Pesqui. Bras. Odontopediatr. Clin. Integr. 2019, 19, e4802. [Google Scholar] [CrossRef]
- Gallè, F.; Colella, G.; Di Onofrio, V.; Rossiello, R.; Angelillo, I.F.; Liguori, G. Candida spp. in oral cancer and oral precancerous lesions. New Microbiol. 2013, 36, 283–288. [Google Scholar]
- Di Cosola, M.; Cazzolla, A.P.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Santacroce, L. Candida albicans and oral carcinogenesis. A brief review. J. Fungi. 2021, 7, 476. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.-A. What Is a Host? Incorporating the Microbiota into the Damage-Response Framework. Infect. Immun. 2015, 83, 2–7. [Google Scholar] [CrossRef]
- Pirofski, L.-A.; Casadevall, A. The Damage–Response Framework as a Tool for the Physician-Scientist to Understand the Pathogenesis of Infectious Diseases. J. Infect. Dis. 2018, 218 (Suppl. 1), S7–S11. [Google Scholar] [CrossRef]
- Pirofski, L.-A.; Casadevall, A. The Damage-Response Framework of Microbial Pathogenesis and Infectious Diseases. Adv. Exp. Med. Biol. 2008, 635, 135–146. [Google Scholar]
- Ruiz-Herrera, J.; Elorza, M.V.; Valentín, E.; Sentandreu, R. Molecular organization of the cell wall of Candida albicans and its relation to pathogenicity. FEMS Yeast Res. 2006, 6, 14–29. [Google Scholar] [CrossRef]
- Mishra, P.; Bolard, J.; Prasad, R. Emerging role of lipids of Candida albicans, a pathogenic dimorphic yeast. Biochim. Biophys. Acta 1992, 1127, 1–14. [Google Scholar] [CrossRef]
- Forche, A.; Alby, K.; Schaefer, D.; Johnson, A.D.; Berman, J.; Bennett, R.J. The Parasexual Cycle in Candida albicans Provides an Alternative Pathway to Meiosis for the Formation of Recombinant Strains. PLoS Biol. 2008, 6, e110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Upritchard, J.E.; Holland, B.; Fenton, L.E.; Ferguson, M.M.; Cannon, R.; Schmid, J. Distribution of mutations distinguishing the most prevalent disease-causing Candida albicans genotype from other genotypes. Infect. Genet. Evol. 2009, 9, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.L.; Limon, J.J.; Underhill, D.M. Immunity to Commensal Fungi: Detente and Disease. Annu. Rev. Pathol. Mech. Dis. 2017, 12, 359–385. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jönsson, F. Expression, Role, and Regulation of Neutrophil Fcγ Receptors. Front. Immunol. 2019, 10, 1958. [Google Scholar] [CrossRef] [PubMed]
- Černáková, L.; Rodrigues, C.F. Microbial interactions and immunity response in oral Candida species. Future Microbiol. 2020, 15, 1653–1677. [Google Scholar] [CrossRef]
- McCullough, M.; Jaber, M.; Barrett, A.; Bain, L.; Speight, P.; Porter, S. Oral yeast carriage correlates with presence of oral epithelial dysplasia. Oral Oncol. 2002, 38, 391–393. [Google Scholar] [CrossRef]
- Nagy, K. The microflora associated with human oral carcinomas. Oral Oncol. 1998, 34, 304–308. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of Invasive Candidiasis: A Persistent Public Health Problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef]
- Tobudic, S.; Kratzer, C.; Lassnigg, A.; Graninger, W.; Presterl, E. In vitro activity of antifungal combinations against Candida albicans biofilms. J. Antimicrob. Chemother. 2010, 65, 271–274. [Google Scholar] [CrossRef]
- McCullough, M.J.; Clemons, K.V.; Stevens, D.A. Molecular and Phenotypic Characterization of Genotypic Candida albicans Subgroups and Comparison with Candida dubliniensis and Candida stellatoidea. J. Clin. Microbiol. 1999, 37, 417–421. [Google Scholar] [CrossRef]
- Gayathri, K.; Balachander, N.; Malathi, L.; Sankari, S. Candida in potentially malignant oral disorders. J. Pharm. Bioallied Sci. 2015, 7, 162–164. [Google Scholar] [CrossRef]
- Khongsti, S.; Shunyu, B.; Ghosh, S. Promoter-associated DNA methylation & expression profiling of genes (FLT 3, EPB41L3 & SFN) in patients with oral squamous cell carcinoma in the Khasi & Jaintia population of Meghalaya, India. Indian J. Med. Res. 2019, 150, 584. [Google Scholar]
- Hu, Y.; Zeng, Q.; Li, C.; Xie, Y. Expression profile and prognostic value of SFN in human ovarian cancer. Biosci. Rep. 2019, 39, BSR20190100. [Google Scholar] [CrossRef]
- Rishehri, M.; Etemadi, T.; Pisheh, L.; Koufigar, G.; Azadeh, M. Quantitative Expression of SFN, lncRNA CCDC18-AS1, and lncRNA LINC01343 in Human Breast Cancer as the Regulator Biomarkers in a Novel ceRNA Network: Based on Bioinformatics and Experimental Analyses. Genet. Res. 2022, 2022, 6787791. [Google Scholar] [CrossRef]
- Chauhan, S.; Sen, S.; Chauhan, S.S.; Pushker, N.; Tandon, R.; Kashyap, S.; Vanathi, M.; Bajaj, M.S. Stratifin in ocular surface squamous neoplasia and its association with p53. Acta Ophthalmol. 2021, 99, e1483–e1491. [Google Scholar] [CrossRef]
- Ma, Y.-S.; Hou, L.-K.; Yao, S.-H.; Liu, J.-B.; Yu, X.-C.; Shi, Y.; Yang, X.-L.; Wu, W.; Wu, C.-Y.; Jiang, G.-X.; et al. Elevated Stratifin promotes cisplatin-based chemotherapy failure and poor prognosis in non-small cell lung cancer. Mol. Ther. Oncolytics 2021, 22, 326–335. [Google Scholar] [CrossRef]
- Homann, N. High acetaldehyde levels in saliva after ethanol consumption: Methodological aspects and pathogenetic implications. Carcinogenesis 1997, 18, 1739–1743. [Google Scholar] [CrossRef]
- Homann, N.; Tillonen, J.; Meurman, J.; Rintamäki, H.; Lindqvist, C.; Rautio, M.; Jousimies-Somer, H.; Salaspuro, M. Increased salivary acetaldehyde levels in heavy drinkers and smokers: A microbiological approach to oral cavity cancer. Carcinogenesis 2000, 21, 663–668. [Google Scholar] [CrossRef]
- Uittamo, J.; Siikala, E.; Kaihovaara, P.; Salaspuro, M.; Rautemaa, R. Chronic candidosis and oral cancer in APECED-patients: Production of carcinogenic acetaldehyde from glucose and ethanol by Candida albicans. Int. J. Cancer 2009, 124, 754–756. [Google Scholar] [CrossRef]
- Cheng, R.; Li, D.; Shi, X.; Gao, Q.; Wei, C.; Li, X.; Li, Y.; Zhou, H. Reduced CX3CL1 Secretion Contributes to the Susceptibility of Oral Leukoplakia-Associated Fibroblasts to Candida albicans. Front. Cell. Infect. Microbiol. 2016, 6, 150. [Google Scholar] [CrossRef]
- Kabir, M.A.; Hussain, M.A.; Ahmad, Z. Candida albicans: A Model Organism for Studying Fungal Pathogens. ISRN Microbiol. 2012, 2012, 538694. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Garcia, A.; Rementeria, A.; Aguirre-Urizar, J.M.; Moragues, M.D.; Antoran, A.; Pellon, A.; Abad-Diaz-de-Cerio, A.; Hernando, F.L. Candida albicans and cancer: Can this yeast induce cancer development or progression? Crit. Rev. Microbiol. 2014, 42, 93–181. [Google Scholar] [PubMed]
- Sardi, J.C.O.; Duque, C.; Höfling, J.F.; Gonçalves, R.B. Genetic and phenotypic evaluation of Candida albicans strains isolated from subgingival biofilm of diabetic patients with chronic periodontitis. Med. Mycol. 2012, 50, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Kurago, Z.B.; Lam-Ubol, A.; Stetsenko, A.; De La Mater, C.; Chen, Y.; Dawson, D.V. Lipopolysaccharide-Squamous Cell Carcinoma-Monocyte Interactions Induce Cancer-Supporting Factors Leading to Rapid STAT3 Activation. Head Neck Pathol. 2008, 2, 1–12. [Google Scholar] [CrossRef]
- Lax, A.J. Bacterial toxins and cancer—A case to answer? Nat. Rev. Microbiol. 2005, 3, 343–349. [Google Scholar] [CrossRef]
- Sanjaya, P.; Gokul, S.; Patil, B.G.; Raju, R. Candida in oral pre-cancer and oral cancer. Med. Hypotheses 2011, 77, 1125–1128. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).