The Effect of TGFβ1 in Adipocyte on Inflammatory and Fibrotic Markers at Different Stages of Adipocyte Differentiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture and Differentiation of NIH3T3L1 Cells
2.2. TGFβ1 Treatment
2.3. RNA Extraction
2.4. Protein Extraction and Quantification
2.5. Oil Red O Staining
2.6. Statistical Analysis
3. Results
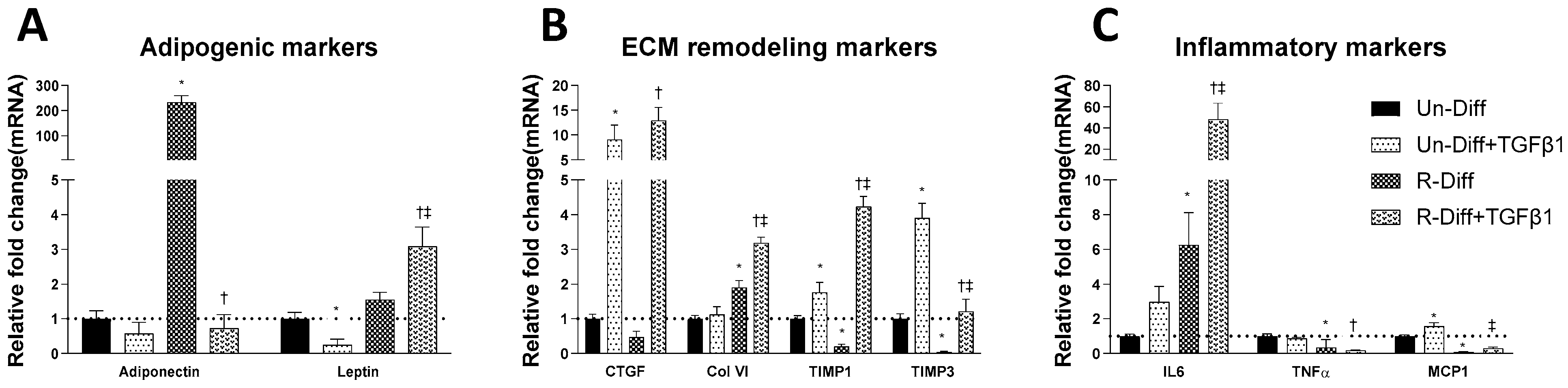
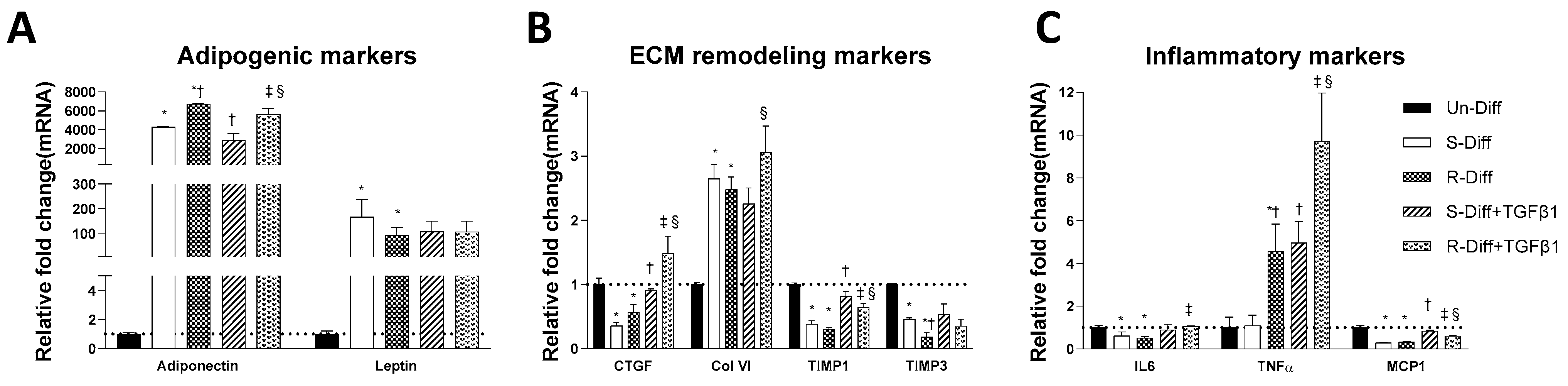
3.1. Changes in Markers at Different Stages of Adipocyte Differentiation
3.2. TGFβ1 Treatment Affects Both the Synthesis and Degradation of Fibrosis Differently at Certain Stages of Adipocyte Differentiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, C.K.; Leuenberger, N.; Tan, M.J.; Yan, Y.W.; Chen, Y.; Kambadur, R.; Wahli, W.; Tan, N.S. Smad3 deficiency in mice protects against insulin resistance and obesity induced by a high-fat diet. Diabetes 2011, 60, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Quijano, C.; Kamaraju, A.K.; Gavrilova, O.; Malek, R.; Chen, W.; Zerfas, P.; Zhigang, D.; Wright, E.C.; Stuelten, C.; et al. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell Metab. 2011, 14, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Budi, E.H.; Muthusamy, B.P.; Derynck, R. The insulin response integrates increased TGF-beta signaling through Akt-induced enhancement of cell surface delivery of TGF-beta receptors. Sci. Signal. 2015, 8, ra96. [Google Scholar] [CrossRef] [PubMed]
- Samad, F.; Yamamoto, K.; Pandey, M.; Loskutoff, D.J. Elevated expression of transforming growth factor-beta in adipose tissue from obese mice. Mol. Med. 1997, 3, 37–48. [Google Scholar] [CrossRef]
- Boney, C.M.; Sekimoto, H.; Gruppuso, P.A.; Frackelton, A.R.J. Src Family Tyrosine Kinases Participate in Insulin-like Growth Factor I Mitogenic Signaling in 3T3-L1 Cells1. Cell Growth Differ. 2001, 12, 379–386. [Google Scholar]
- Sekimoto, H.; Eipper-Mains, J.; Pond-Tor, S.; Boney, C.M. (alpha)v(beta)3 integrins and Pyk2 mediate insulin-like growth factor I activation of Src and mitogen-activated protein kinase in 3T3-L1 cells. Mol. Endocrinol. 2005, 19, 1859–1867. [Google Scholar] [CrossRef]
- Massague, J.; Wotton, D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000, 19, 1745–1754. [Google Scholar] [CrossRef]
- Siegel, P.M.; Massagué, J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat. Rev. Cancer 2003, 3, 807–821. [Google Scholar] [CrossRef]
- Heldin, C.H.; Landström, M.; Moustakas, A. Mechanism of TGF-beta signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Curr. Opin. Cell Biol. 2009, 21, 166–176. [Google Scholar] [CrossRef]
- Keophiphath, M.; Achard, V.; Henegar, C.; Rouault, C.; Clement, K.; Lacasa, D. Macrophage-secreted factors promote a profibrotic phenotype in human preadipocytes. Mol. Endocrinol. 2009, 23, 11–24. [Google Scholar] [CrossRef]
- Maharjan, B.R.; McLennan, S.V.; Twigg, S.M.; Williams, P.F. The effect of TGFbeta1 on thermogenic markers is dependent on the degree of adipocyte differentiation. Biosci. Rep. 2020, 40, BSR20194262. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.T.; McLennan, S.V.; Song, W.W.; Lo, L.W.; Bonner, J.G.; Williams, P.F.; Twigg, S.M. Connective tissue growth factor inhibits adipocyte differentiation. Am. J. Physiol. Cell Physiol. 2008, 295, C740–C751. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.R.; Murphy, G.; Reynolds, J.J.; Whitham, S.E.; Docherty, A.J.; Angel, P.; Heath, J.K. Transforming growth factor beta modulates the expression of collagenase and metalioproteinase inhibitor. EMBO J. 1987, 6, 1899–1904. [Google Scholar] [CrossRef]
- Sabatelli, P.; Sardone, F.; Traina, F.; Merlini, L.; Santi, S.; Wagener, R.; Faldini, C. TGF-β1 differentially modulates the collagen VI α5 and α6 chains in human tendon cultures. J. Biol. Regul. Homeost. Agents 2016, 30, 107–113. [Google Scholar]
- Park, S.A.; Kim, M.J.; Park, S.Y.; Kim, J.S.; Lim, W.; Nam, J.S.; Yhong Sheen, Y. TIMP-1 mediates TGF-beta-dependent crosstalk between hepatic stellate and cancer cells via FAK signaling. Sci. Rep. 2015, 5, 16492. [Google Scholar] [CrossRef]
- Eickelberg, O.; Pansky, A.; Mussmann, R.; Bihl, M.; Tamm, M.; Hildebrand, P.; Perruchoud, A.P.; Roth, M. Transforming growth factor-beta1 induces interleukin-6 expression via activating protein-1 consisting of JunD homodimers in primary human lung fibroblasts. J. Biol. Chem. 1999, 274, 12933–12938. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Chen, X.; Polhill, T.S.; Sumual, S.; Twigg, S.; Gilbert, R.E.; Pollock, C.A. TGF-beta1 induces IL-8 and MCP-1 through a connective tissue growth factor-independent pathway. Am. J. Physiol. Ren. Physiol. 2006, 290, F703–F709. [Google Scholar] [CrossRef]
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal. Biochem. 2012, 425, 88–90. [Google Scholar] [CrossRef]
- Song, W.W.; McLennan, S.V.; Tam, C.; Williams, P.F.; Baxter, R.C.; Twigg, S.M. CCN2 requires TGF-beta signalling to regulate CCAAT/enhancer binding proteins and inhibit fat cell differentiation. J. Cell Commun. Signal. 2014, 9, 27–36. [Google Scholar] [CrossRef]
- Colella, A.D.; Chegenii, N.; Tea, M.N.; Gibbins, I.L.; Williams, K.A.; Chataway, T.K. Comparison of Stain-Free gels with traditional immunoblot loading control methodology. Anal. Biochem. 2012, 430, 108–110. [Google Scholar] [CrossRef]
- Taylor, S.C.; Posch, A. The design of a quantitative western blot experiment. Biomed Res. Int. 2014, 2014, 361590. [Google Scholar] [CrossRef] [PubMed]
- Sparks, R.L.; Allen, B.J.; Strauss, E.E. TGF-beta Blocks Early but Not Late Differentiation-Specific Gene Expression and Morphologic Differentiation of 3T3 T Proadipocytes. J. Cell. Physiol. 1992, 150, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Choy, L.; Skillington, J.; Derynck, R. Roles of autocrine TGF-beta receptor and Smad signaling in adipocyte differentiation. J. Cell Biol. 2000, 149, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Hausman, D.B.; Dean, R.G.; Hausman, G.J. Hormonal Regulation of Leptin mRNA Expression and Preadipocyte Recruitment and Differentiation in Porcine Primary Cultures of S-V Cells. Obes. Res. 1998, 6, 164–172. [Google Scholar] [CrossRef]
- Nakajima, I.; Muroya, S.; Tanabe, R.; Chikuni, K. Extracellular matrix development during differentiation into adipocytes with a unique increase in type V and VI collagen. Biol. Cell 2002, 94, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Meissburger, B.; Stachorski, L.; Roder, E.; Rudofsky, G.; Wolfrum, C. Tissue inhibitor of matrix metalloproteinase 1 (TIMP1) controls adipogenesis in obesity in mice and in humans. Diabetologia 2011, 54, 1468–1479. [Google Scholar] [CrossRef]
- Bernot, D.; Barruet, E.; Poggi, M.; Bonardo, B.; Alessi, M.C.; Peiretti, F. Down-regulation of tissue inhibitor of metalloproteinase-3 (TIMP-3) expression is necessary for adipocyte differentiation. J. Biol. Chem. 2010, 285, 6508–6514. [Google Scholar] [CrossRef]
- Verrecchia, F.; Mauviel, A. Transforming growth factor-beta signaling through the Smad pathway: Role in extracellular matrix gene expression and regulation. J. Investig. Dermatol. 2002, 118, 211–215. [Google Scholar] [CrossRef]
- Leco, K.J.; Khokha, R.; Pavloff, N.; Hawkes, S.P.; Edwards, D.R. Tissue Inhibitor of Metalloproteinases-3 (Timp-3) Is an Extracellular Matrix-Associated Protein with a Distinctive Pattern of Expression in Mouse Cells and Tissues. J. Biol. Chem. 1994, 269, 9352–9360. [Google Scholar] [CrossRef]
- Qureshi, H.Y.; Sylvester, J.; El Mabrouk, M.; Zafarullah, M. TGF-beta-induced expression of tissue inhibitor of metalloproteinases-3 gene in chondrocytes is mediated by extracellular signal-regulated kinase pathway and Sp1 transcription factor. J. Cell. Physiol. 2005, 203, 345–352. [Google Scholar] [CrossRef]
- Menghini, R.; Menini, S.; Amoruso, R.; Fiorentino, L.; Casagrande, V.; Marzano, V.; Tornei, F.; Bertucci, P.; Iacobini, C.; Serino, M.; et al. Tissue inhibitor of metalloproteinase 3 deficiency causes hepatic steatosis and adipose tissue inflammation in mice. Gastroenterology 2009, 136, 663–672 e664. [Google Scholar] [CrossRef] [PubMed]
- Kassiri, Z.; Oudit, G.Y.; Kandalam, V.; Awad, A.; Wang, X.; Ziou, X.; Maeda, N.; Herzenberg, A.M.; Scholey, J.W. Loss of TIMP3 enhances interstitial nephritis and fibrosis. J. Am. Soc. Nephrol. 2009, 20, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Menghini, R.; Casagrande, V.; Menini, S.; Marino, A.; Marzano, V.; Hribal, M.L.; Gentileschi, P.; Lauro, D.; Schillaci, O.; Pugliese, G.; et al. TIMP3 overexpression in macrophages protects from insulin resistance, adipose inflammation, and nonalcoholic fatty liver disease in mice. Diabetes 2012, 61, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, B.; Smith, U. Cytokines promote Wnt signaling and inflammation and impair the normal differentiation and lipid accumulation in 3T3-L1 preadipocytes. J. Biol. Chem. 2006, 281, 9507–9516. [Google Scholar] [CrossRef] [PubMed]
- Pricola, K.L.; Kuhn, N.Z.; Haleem-Smith, H.; Song, Y.; Tuan, R.S. Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an ERK1/2-dependent mechanism. J. Cell Biochem. 2009, 108, 577–588. [Google Scholar] [CrossRef]
- Hube, F.; Hauner, H. The role of TNF-alpha in human adipose tissue: Prevention of weight gain at the expense of insulin resistance? Horm. Metab. Res. 1999, 31, 626–631. [Google Scholar] [CrossRef]
- Wernstedt Asterholm, I.; Tao, C.; Morley, T.S.; Wang, Q.A.; Delgado-Lopez, F.; Wang, Z.V.; Scherer, P.E. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014, 20, 103–118. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Zhang, T.; Wei, C.; Shi, W. TGF-β and NF-κB signaling pathway crosstalk potentiates cornealepithelial senescence through an RNA stress response. Aging 2016, 8, 2337–2350. [Google Scholar] [CrossRef]
- Torti, F.M.; Dieckmann, B.; Beutler, B.; Cerami, A.; Ringold, G.M. A macrophage factor inhibits adipocyte gene expression: An in vitro model of cachexia. Science 1985, 229, 867–869. [Google Scholar] [CrossRef]
- Sewter, C.; Berger, D.; Considine, R.V.; Medina, G.; Rochford, J.; Ciaraldi, T.; Henry, R.; Dohm, L.; Flier, J.S.; O’Rahilly, S.; et al. Human obesity and type 2 diabetes are associated with alterations in SREBP1 isoform expression that are reproduced ex vivo by tumor necrosis factor-alpha. Diabetes 2002, 51, 1035–1041. [Google Scholar] [CrossRef]
- Renes, J.; Bouwman, F.; Noben, J.P.; Evelo, C.; Robben, J.; Mariman, E. Protein profiling of 3T3-L1 adipocyte differentiation and (tumor necrosis factor alpha-mediated) starvation. Cell. Mol. Life Sci. 2005, 62, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Petkova, A.P.; Granneman, J.G. Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab. 2013, 18, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Sadler, D.; Mattacks, C.A.; Pond, C.M. Changes in adipocytes and dendritic cells in lymph node containing adipose depots during and after many weeks of mild inflammation. J. Anat. 2005, 207, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Gambero, A.; Marostica, M.; Abdalla Saad, M.J.; Pedrazzoli, J., Jr. Mesenteric adipose tissue alterations resulting from experimental reactivated colitis. Inflamm. Bowel. Dis. 2007, 13, 1357–1364. [Google Scholar] [CrossRef]
- Sheehan, A.L.; Warren, B.F.; Gear, M.W.; Shepherd, N.A. Fat-wrapping in Crohn’s disease: Pathological basis and relevance to surgical practice. Br. J. Surg. 1992, 79, 955–958. [Google Scholar] [CrossRef]
- Rodriguez, T.M.; Saldias, A.; Irigo, M.; Zamora, J.V.; Perone, M.J.; Dewey, R.A. Effect of TGF-beta1 Stimulation on the Secretome of Human Adipose-Derived Mesenchymal Stromal Cells. Stem Cells Transl. Med. 2015, 4, 894–898. [Google Scholar] [CrossRef]


| Primers | Forward | Reverse |
|---|---|---|
| Adiponectin | 5′-CGACACCAAAAGGGCTCAGG-3′ | 5′-ACGTCATCTTCGGCATGACT-3′ |
| Leptin | 5′-GCTGCAAGGTGCAAGAAGAAG-3′ | 5′-TAGGACCAAAGCCACAGGAAC-3′ |
| MCP1 | 5′-CACTCACCTGCTGCTACTCA-3′ | 5′-GCTTGGTGACAAAAACTACAGC-3′ |
| IL6 | 5′-TCCTCTCTGCAAGAGACTTCC-3′ | 5′-TTGTGAAGTAGGGAAGGCCG-3′ |
| TNFα | 5′-GACCCTCACACTCACAAACCA-3′ | 5′-ACAAGGTACAACCCATCGGC-3′ |
| Collagen VI | 5′-GAACTTCCCTGCCAAACAGA-3′ | 5′-CACCTTGTGGAAGTTCTGCTC-3′ |
| CCN2/CTGF | 5′-GAGTGTGCACTGCCAAAGATG-3′ | 5′-TCCAGGCAAGTGCATTGG T-3′ |
| TIMP1 | 5′-CACAAGTCCCAGAACCGC-3′ | 5′-GGATTCCGTGGCAGGC-3′ |
| TIMP3 | 5′-CTTCTGCAACTCCGACATCGTGAT-3′ | 5′-CAGCAGGTACTGGTACTTGTTGAC-3′ |
| NoNo | 5′-TGCTCCTGTGCCACCTGGTACTC-3′ | 5′-CCGGAGCTGGACGGTTGAATGC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maharjan, B.R.; McLennan, S.V.; Twigg, S.M.; Williams, P.F. The Effect of TGFβ1 in Adipocyte on Inflammatory and Fibrotic Markers at Different Stages of Adipocyte Differentiation. Pathophysiology 2022, 29, 640-649. https://doi.org/10.3390/pathophysiology29040050
Maharjan BR, McLennan SV, Twigg SM, Williams PF. The Effect of TGFβ1 in Adipocyte on Inflammatory and Fibrotic Markers at Different Stages of Adipocyte Differentiation. Pathophysiology. 2022; 29(4):640-649. https://doi.org/10.3390/pathophysiology29040050
Chicago/Turabian StyleMaharjan, Babu Raja, Susan V. McLennan, Stephen M. Twigg, and Paul F. Williams. 2022. "The Effect of TGFβ1 in Adipocyte on Inflammatory and Fibrotic Markers at Different Stages of Adipocyte Differentiation" Pathophysiology 29, no. 4: 640-649. https://doi.org/10.3390/pathophysiology29040050
APA StyleMaharjan, B. R., McLennan, S. V., Twigg, S. M., & Williams, P. F. (2022). The Effect of TGFβ1 in Adipocyte on Inflammatory and Fibrotic Markers at Different Stages of Adipocyte Differentiation. Pathophysiology, 29(4), 640-649. https://doi.org/10.3390/pathophysiology29040050






