Dietary Supplementation with D-Ribose-L-Cysteine Prevents Hepatic Stress and Pro-Inflammatory Responses in Male Wistar Rats Fed a High-Fructose High-Fat Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
2.2. Animal Care
2.3. Feed Formulation
2.4. Experimental Design
2.5. Termination of the Experiment
2.6. Statistical Analysis
3. Results
3.1. Effects of D-Ribose-L-Cysteine (DRLC) on Antioxidant/Pro-Oxidant Indices in the Serum of Male Wistar Rats Fed with High Fat-High Fructose (HFHF) Diet
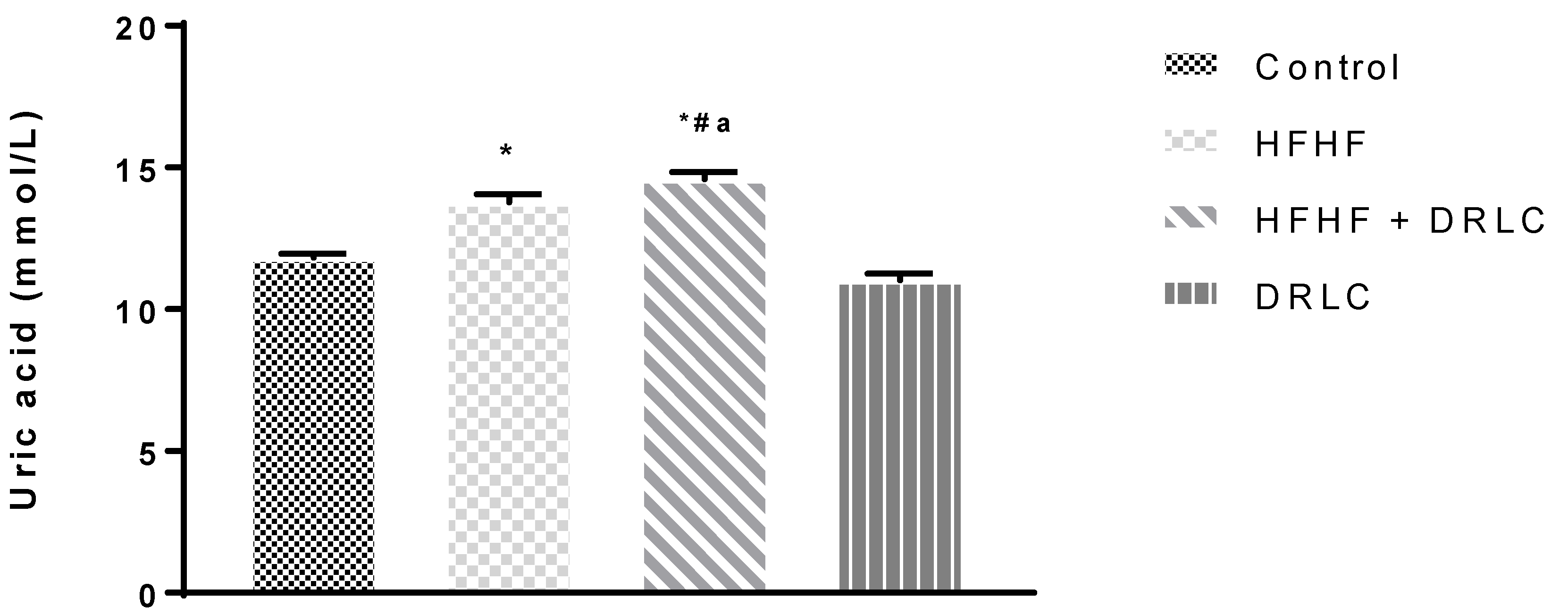
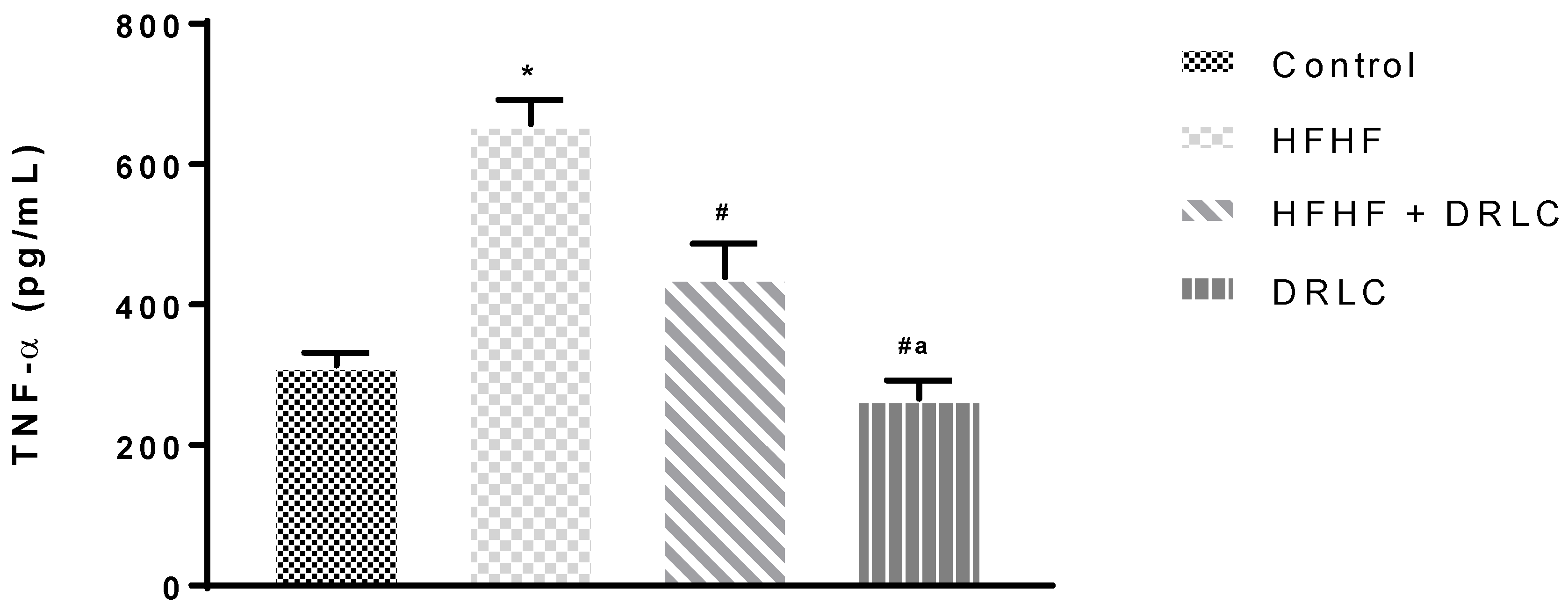
3.2. Effects of D-Ribose-L-Cysteine (DRLC) on Inflammatory Indices and Selected Enzymes in the Serum of Male Wistar Rats Fed with High Fat-High Fructose (HFHF) Diet
3.3. Effects of D-Ribose-L-Cysteine (DRLC) on Hepatic Oxidative Indices, Absolute and Relative Liver Weight in Male Wistar Rats Fed with High Fat-High Fructose (HFHF) Diet
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mamikutty, N.; Thent, Z.C.; Sapri, S.R.; Sahruddin, N.N.; Yusof, M.R.M.; Suhaimi, F.H. The Establishment of Metabolic Syndrome Model by Induction of Fructose Drinking Water in Male Wistar Rats. BioMed. Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.; Elshazly, S.M. Ursodeoxycholic Acid Ameliorates Fructose-Induced Metabolic Syndrome in Rats. PLoS ONE 2014, 9, e106993. [Google Scholar] [CrossRef]
- Di Luccia, B.; Crescenzo, R.; Mazzoli, A.; Cigliano, L.; Venditti, P.; Walser, J.C. Rescue of fructose-induced metabolic syndrome by antibiotics or faecal transplantation in a rat model of obesity. PLoS ONE 2015, 10, e0134893. [Google Scholar] [CrossRef] [PubMed]
- Lian, C.; Zhai, Z.; Li, Z.; Wang, L. High fat diet-triggered non-alcoholic fatty liver disease: A review of proposed mechanisms. Chem. Biol. Interact. 2020, 330, 109199. [Google Scholar] [CrossRef] [PubMed]
- Ojetola, A.A.; Adeyemi, W.J.; David, U.E.; Ajibade, T.O.; Adejumobi, O.A.; Omobowale, T.O.; Oyagbemi, A.A.; Fasanmade, A.A. D-ribose-L-cysteine prevents oxidative stress and cardiometabolic syndrome in high fructose high fat diet fed rats. Biomed. Pharmacother. 2021, 142, 112017. [Google Scholar] [CrossRef]
- Stocker, R.; Keaney, J.F., Jr. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, F.; Rodella, L.F.; Rezzani, R. Metabolic Syndrome, Aging and Involvement of Oxidative Stress. Aging Dis. 2015, 6, 109. [Google Scholar] [CrossRef]
- Palmieri, V.O.; Grattagliano, I.; Portincasa, P.; Palasciano, G. Systemic oxidative alterations are associated with visceral adiposity and liver steatosis in patients with metabolic syndrome. J. Nutr. 2006, 136, 3022–3026. [Google Scholar] [CrossRef]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef]
- Ghezzi, P.; Lemley, K.V.; Andrus, J.P.; de Rosa, S.C.; Holmgren, A.; Jones, D.; Jahoor, F.; Kopke, R.; Cotgreave, I.; Bottiglieri, T.; et al. Cysteine/glutathione deficiency: A significant and treatable corollary of disease. In The Therapeutic Use of N-Acetylcysteine (NAC) in Medicine; Springer Nature: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-kostova, A.T.; Tew, K.D. Review Oxidative Stress in Cancer. Cancer Cell 2020, 2, 1–31. [Google Scholar] [CrossRef]
- Vasdev, S.; Longerich, L.; Gill, V. Prevention of fructose- induced hypertension by dietary vitamins. Clin. Biochem. 2004, 37, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vasdev, S.; Singal, P.; Gill, V. The antihypertensive effect of cysteine. Int. J. Angiol. 2009, 18, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Elshorbagy, A.K.; Valdivia-Garcia, M.; Mattocks, D.A.; Plummer, J.D.; Smith, A.D.; Drevon, C.A.; Refsum, H.; Perrone, C.E. Cysteine supplementation reverses methionine restriction effects on rat adiposity: Significance of stearoyl-coenzyme A desaturase. J. Lipid. Res. 2011, 52, 104–112. [Google Scholar] [CrossRef]
- Vale, J.A.; Proudfoot, A.T. Paracetamol (acetaminophen) poisoning. Lancet 1995, 346, 547–552. [Google Scholar] [CrossRef]
- Roberts, J.C.; Nagasawa, H.T.; Zera, R.T.; Fricke, R.F.; Goon, D.J.W. Prodrugs of L- cysteine as protective agents against acetaminophen-induced hepatotoxicity. J. Med. Chem. 1987, 30, 1891–1896. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.C.; Francetic, D.J. Time course for the elevation of glutathione in numerous organs of L1210-bearing CDF1 mice given the L-cysteine prodrug, RibCys. Toxicol. Lett 1991, 59, 245–251. [Google Scholar] [CrossRef]
- Treweeke, A.T.; Winterburn, T.J.; Mackenzie, I.; Barrett, F.; Barr, C.; Rushworth, G.F.; Dransfield, I.; MacRury, S.M.; Megson, I.L. N-Acetylcysteine inhibits platelet-monocyte conjugation in patients with type 2 diabetes with depleted intraplatelet glutathione: A randomised controlled trial. Diabetologia 2012, 55, 2920–2928. [Google Scholar] [CrossRef]
- Ojetola, A.A.; Adedeji, T.G.; Fasanmade, A.A. 2021b. Changes in antioxidants status, atherogenic index and cardiovascular variables after prolonged doses of D-ribose-L-cysteine in male Wistar rats. Heliyon 2021, 7, e0628. [Google Scholar] [CrossRef]
- Dröge, W. Oxidative stress and ageing: Is ageing a cysteine deficiency syndrome? Phil. Trans. R. Soc. 2005, 360, 2355–2372. [Google Scholar] [CrossRef]
- Oz, H.S.; Chen, T.S.; Nagasawa, H. Comparative efficacies of 2 cysteine prodrugs and a glutathione delivery agent in a colitis model. Transl. Res. 2007, 150, 122–129. [Google Scholar] [CrossRef]
- Bajic, V.P.; Neste, C.N.; Obradovic, M.; Zafirovic, S.; Radak, D.; Bajic, V.B.; Essack, M.; Isenovic, E.R. Glutathione Redox Homeostasis and Its Relation to Cardiovascular Disease. Hindawi 2019, 5028181. [Google Scholar] [CrossRef] [PubMed]
- Suarez, C.; del Arco, C.; Lahera, V.; Ruilope, L.M. N-acetylcysteine potentiates the antihypertensive effect of angiotensin converting enzyme inhibitors. Am. J. Hypertens. 1995, 8, 859. [Google Scholar] [CrossRef]
- Cabassi, A.; Dumont, E.C.; Girouard, H. Effects of chronic N-acetylcysteine treatment on the actions of peroxynitrite on aortic vascular reactivity in hypertensive rats. J. Hypertens. 2001, 19, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Barrios, V.; Calderon, A.; Navarro-Cid, J.; Lahera, V.; Ruilope, L.M. N-acetylcysteine potentiates the antihypertensive effect of ACE inhibitors in hypertensive patients. Blood Press. 2002, 11, 235–239. [Google Scholar] [CrossRef]
- Robin, S.; Maupoil, V.; Groubatch, F.; Laurant, P.; Jacqueson, A.; Berthelot, A. Effect of a methionine-supplemented diet on the blood pressure of wistar-kyoto and spontaneously hypertensive rats. Br. J. Nutr. 2003, 89, 148–539. [Google Scholar] [CrossRef]
- Adeyemi, W.J.; Olayaki, L.A.; Abdussalam, T.A.; Ige, S.F.; Okesina, B.K.; Abolarin, P.O.; Usman, H.; Tiamiyu, A.O.; Seidu, M.O.; Opabode, A.O. Comparative evaluation of the pharmacological value of virgin coconut oil, omega 3 fatty acids, and orlistat in experimental study on obesity with normo/hyper-lipidaemic diet. PharmaNutrition 2020, 13, 100192. [Google Scholar] [CrossRef]
- Hamza, R.Z.; El-Shenawy, N.S. The beneficial effects of L–cysteine on brain antioxidants of rats affected by sodium valproate. Hum. Exp. Toxicol. 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Aderemi, A.S.; Dare, O.O.; Akomaye, A.J. Modulating Role of D-Ribose-L-Cysteine on Oxidative Stress in Streptozotocin Induced Diabetes on Plasma Lipoprotein, Oxidative Status, Spermatogenesis and Steroidogenesis in Male Wistar Rats. Curr. Res. Diabetes Obes. J. 2018, 9, 55–61. [Google Scholar] [CrossRef]
- Osinubi, A.A.; Medubia, L.J.; Akanga, E.N.; Sodiq, L.K.; Samuel, T.A.; Kusemiju, T.; Osolu, J.; Madu, D.; Fasanmade, O.A. Comparison of the anti-diabetic potential of D-ribose-L-cysteine with insulin, and oral hypoglycaemic agents on pregnant rats. Toxicol. Rep. 2018, 5, 823–838. [Google Scholar] [CrossRef] [PubMed]
- National Academy of Sciences. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Olayaki, L.A.; Adeyemi, W.J.; Adeyemi, E.; Osawaru, O.; Busura, I.; Jimoh, S. Melatonin enhanced the restoration of biochemical profile in chlorambucil treated-rats: Examination of after withdrawal effects of the drug. J. Afr. Ass. Physiol. Sci. 2019, 7, 80–87. [Google Scholar]
- Adeyemi, W.J.; Abdussalam, T.A.; Abdulrahim, A.; Olayaki, L.A. Elevated, sustained, and yet reversible biotoxicity effects of lead on cessation of exposure: Melatonin is a potent therapeutic option. Toxicol. Ind. Health 2020, 36, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Zagrodzki, P.; Joniec, A.; Gawlik, M.; Gawlik, M.A.Ł.; Krosniak, M.; Folta, M.; Bartoń, H.; Paśko, P.; Chłopicka, J.; Zachwieja, Z.O. ‘High fructose model of oxidative stress and metabolic disturbances in rats. Part I. Antioxidant status of rats’ tissues’. Bull. Vet. Inst. Pulawy 2009, 51, 407–412. [Google Scholar]
- Li, J.; Tsuprykov, O.; Yang, X.; Hocher, B. Paternal programming of offspring cardiometabolic diseases in later life. J. Hypertens. 2006, 34, 2111–2126. [Google Scholar] [CrossRef] [PubMed]
- Emokpae, O.; Ben-Azu, B.; Abayomi, M.; Ajayi, A.M.; Umukoro, S. D -Ribose- L -cysteine attenuates lipopolysaccharide-induced memory deficits through inhibition of oxidative stress, release of proinflammatory cytokines, and nuclear factor-kappa B expression in mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 909–925. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione Metabolism and Its Implications for Health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Inal, M.E.; Kanbak, G.; Sunal, E. Antioxidant enzyme activities and malondialdehyde levels related to aging. Clin. Chim. Acta 2001, 305, 75–80. [Google Scholar] [CrossRef]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. (Qassim). 2018, 12, 88–93. [Google Scholar]
- Slitt, A.M.L.; Dominick, P.K.; Roberts, J.C.; Cohen, S.D. Effect of ribose cysteine pretreatment on hepatic and renal acetaminophen metabolite formation and glutathione depletion. Basic Clin. Pharmacol. Toxicol. 2005, 96, 487–494. [Google Scholar] [CrossRef]
- Avelar, T.M.T.; Storch, A.S.; Castro, L.A.; Azevedo, G.V.M.M.; Ferraz, L.; Lopes, P.F. Oxidative stress in the pathophysiology of metabolic syndrome: Which mechanisms are involved? J. Bras. Patol. Med. Lab. 2015, 51, 231–239. [Google Scholar] [CrossRef]
- Nakagawa, T.; Hu, H.; Zharikov, S.; Tuttle, K.R.; Short, R.A.; Glushakova, O.; Ouyang, X.; Feig, D.I.; Block, E.R.; Herrera-Acosta, J.; et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am. J. Physiol. Ren. Physiol. 2006, 290, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lozada, L.G.; Tapia, E.; Jimenez, A.; Jiménez, A.; Bautista, P.; Cristóbal, M.; Nepomuceno, T.; Soto, V.; Ávila-Casado, C.; Nakagawa, T.; et al. Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. AJP Ren. Physiol. 2006, 292, F423–F429. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, M.; Imhof, A.; Berg, G.; Hutchinson, W.L.; Pepys, M.B.; Boeing, H.; Muche, R.; Brenner, H.; Koenig, W. Association between C-reactive protein and features of the metabolic syndrome: A population-based study. Diabetes Care 2000, 23, 1835–1839. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Rodriguez-Iturbe, B.; Kang, D.H.; Feig, D.I.; Herrera-Acosta, J. A unifying pathway for essential hypertension. Am. J. Hypertens. 2005, 18, 431–440. [Google Scholar] [CrossRef] [PubMed]

| Groups/Parameters | SOD (u/mL) | CAT (μmol/min/mL) | GSH (mM) | GPX (u/L) | TAC (mmol/mL) | MDA (μM) |
|---|---|---|---|---|---|---|
| 1. Control | 0.16 ± 0.01 | 17.97 ± 0.72 | 1.24 ± 0.14 | 1.90 ± 0.16 | 1.75 ± 0.17 | 3.39 ± 0.32 |
| 2. HFHF | 0.04 ± 0.02 * | 16.42 ± 0.84 | 0.77 ± 0.26 | 0.35 ± 0.04 * | 0.79 ± 0.14 * | 3.35 ± 0.43 |
| 3. HFHF + DRLC | 0.19 ± 0.03 # | 18.29 ± 0.37 | 0.97 ± 0.19 | 1.42 ± 0.15 # | 1.33 ± 0.32 | 3.33 ± 0.38 |
| 4. DRLC | 0.39 ± 0.03 *#a | 18.81 ± 0.72 | 1.49 ± 0.21 | 1.61 ± 0.14 # | 1.44 ± 0.14 | 0.83 ± 0.12 *#a |
| Groups/Parameters | ALB (g/dL) | GLOB (g/dL) | ALT (mg/dL) | ALP (mg/dL) | AST (mg/dL) |
|---|---|---|---|---|---|
| 1. Control | 2.92 ± 0.09 | 4.18 ± 0.06 | 14.44 ± 0.41 | 48.46 ± 0.54 | 4.72 ± 0.21 |
| 2. HFHF | 3.48 ± 0.15 | 4.24 ± 0.12 | 15.69 ± 0.59 | 47.13 ± 0.06 | 5.62 ± 0.23 |
| 3. HFHF + DRLC | 3.32 ± 0.19 | 4.08 ± 0.04 | 15.07 ± 0.66 | 48.73 ± 0.68 | 6.08 ± 0.28 * |
| 4. DRLC | 3.22 ± 0.22 | 4.08 ± 0.12 | 15.26 ± 0.19 | 46.66 ± 0.60 | 5.22 ± 0.37 |
| Groups/ Parameters | Liver SOD (U/mL) | Liver CAT (μmol/min/mL) | Liver GSH (mM) | Liver GPX (U/L) | Liver THIOL (nmol/mg) | Liver GGT (U/L) | Liver XO (U/L) | Liver Weight (g) | Relative Liver Weight (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1. Control | 0.78 ± 0.07 | 6.72 ± 0.52 | 3.92 ± 0.47 | 4.30 ± 0.57 | 3.58 ± 0.59 | 4.45 ± 0.81 | 18.74 ± 1.79 | 6.47 ± 0.30 | 2.41 ± 0.15 |
| 2. HFHF | 0.42 ± 0.05 * | 6.73 ± 0.65 | 2.33 ± 0.52 | 1.63 ± 0.30 * | 3.09 ± 0.64 | 4.95 ± 0.98 | 28.55 ± 2.49 * | 8.29 ± 0.12* | 2.69 ± 0.07 |
| 3. HFHF + DRLC | 0.53 ± 0.14 | 4.43 ± 0.92 | 2.56 ± 0.33 | 0.98 ± 0.22 * | 3.68 ± 0.29 | 2.60 ± 0.45 | 22.29 ± 3.68 | 7.52 ± 0.43 | 2.96 ± 0.15 * |
| 4. DRLC | 0.55 ± 0.07 | 5.95 ± 0.64 | 4.11 ± 0.54 # | 3.52 ± 0.95 a | 4.15 ± 0.85 | 2.35 ± 0.57# | 15.14 ± 2.47 # | 6.95 ± 0.14 # | 2.67 ± 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojetola, A.A.; Asiwe, J.N.; Adeyemi, W.J.; Ogundipe, D.J.; Fasanmade, A.A. Dietary Supplementation with D-Ribose-L-Cysteine Prevents Hepatic Stress and Pro-Inflammatory Responses in Male Wistar Rats Fed a High-Fructose High-Fat Diet. Pathophysiology 2022, 29, 631-639. https://doi.org/10.3390/pathophysiology29040049
Ojetola AA, Asiwe JN, Adeyemi WJ, Ogundipe DJ, Fasanmade AA. Dietary Supplementation with D-Ribose-L-Cysteine Prevents Hepatic Stress and Pro-Inflammatory Responses in Male Wistar Rats Fed a High-Fructose High-Fat Diet. Pathophysiology. 2022; 29(4):631-639. https://doi.org/10.3390/pathophysiology29040049
Chicago/Turabian StyleOjetola, Abodunrin Adebayo, Jerome Ndudi Asiwe, Wale Johnson Adeyemi, Dare Joshua Ogundipe, and Adesoji Adedipe Fasanmade. 2022. "Dietary Supplementation with D-Ribose-L-Cysteine Prevents Hepatic Stress and Pro-Inflammatory Responses in Male Wistar Rats Fed a High-Fructose High-Fat Diet" Pathophysiology 29, no. 4: 631-639. https://doi.org/10.3390/pathophysiology29040049
APA StyleOjetola, A. A., Asiwe, J. N., Adeyemi, W. J., Ogundipe, D. J., & Fasanmade, A. A. (2022). Dietary Supplementation with D-Ribose-L-Cysteine Prevents Hepatic Stress and Pro-Inflammatory Responses in Male Wistar Rats Fed a High-Fructose High-Fat Diet. Pathophysiology, 29(4), 631-639. https://doi.org/10.3390/pathophysiology29040049






