Abstract
Limb girdle muscular dystrophy type R1 disease is a progressive disease that is caused by mutations in the CAPN3 gene and involves the extremity muscles of the hip and shoulder girdle. The CAPN3 protein has proteolytic and non-proteolytic properties. The functions of the CAPN3 protein that have been determined so far can be listed as remodeling and combining contractile proteins in the sarcomere with the substrates with which it interacts, controlling the Ca2+ flow in and out through the sarcoplasmic reticulum, and regulation of membrane repair and muscle regeneration. Even though there are several gene therapies, cellular therapies, and drug therapies, such as glucocorticoid treatment, AAV- mediated therapy, CRISPR-Cas9, induced pluripotent stem cells, MYO-029, and AMBMP, which are either in preclinical or clinical phases, or have been completed, there is no final cure. Inhibitors and small molecules (tauroursodeoxycholic acid, salubrinal, rapamycin, CDN1163, dwarf open reading frame) targeting ER stress factors that are thought to be effective in muscle loss can be considered potential therapy strategies. At present, little can be done to treat the progressive muscle wasting, loss of function, and premature mortality of patients with LGMDR1, and there is a pressing need for more research to develop potential therapies.
1. Introduction
Calpainopathy is a progressive disease that causes weakness over time in the muscles that affect the upper extremities, including the hip and shoulder girdle muscles [1]. This disease, also known as limb girdle muscular dystrophy type R1 (LGMDR1; 253600 [2] or LGMD2A, as it used to be known), which is caused by defects in the calpain-3 (CAPN3; 114240) [2] gene, which is localized on 15q15.1, is an inherited autosomal recessive condition [3]. However, it was recently shown that there are autosomal dominant inherited variants [4]. Although the onset of the disease varies, it usually occurs in adolescence (8–15 years of age) [5]. There is a loss of ambulation in patients 10–20 years after the onset of the disease. Loss of ambulation is seen after the age of 60 in mild forms [4]. Patients exhibit muscle pain, cramps, fatigue, and exercise intolerance. Although muscle loss is symmetrical, it causes a waddling gait, wing scapula, and hyperlordosis in patients [6]. In addition, various muscle contractures are seen, especially involving the Achilles tendon [6]. According to reports in the Leiden Open Variation database as of October 2020, there are more than 490 pathogenic variants of CAPN3 [7].
Although the function of the CAPN3 protein in cells has not been fully elucidated, studies so far have revealed the proteolytic and non-proteolytic activities of the CAPN3 protein. The full-length form of CAPN3 is expressed mainly in skeletal muscle [8]. CAPN3 demonstrates its non-proteolytic activity by stabilizing Ca2+-handling proteins (CSQ [9], SERCA [10], RyR1 [11], CaMKII [11], and NCX3 [12]) and maintaining Ca2+ homeostasis, which is extremely important and essential for muscle structure and function [5]. CAPN3 shows its proteolytic activity by targeting proteins (myostatin, titin, α-actinin-3, tropomyosin, and LIM-domain binding protein 3 [13]) that play major roles in the regulation of sarcomere stability/integrity and muscle contraction as substrates [14]. CAPN3 is responsible for muscle restructuring and function, not muscle formation [15]. Interestingly, CAPN3 also has an autodegradation property that prevents it from being detected by biochemical tests [16]. The CAPN3 protein is important for the functionalization of many proteins that it targets through its proteolytic feature. In addition, its relationship with calcium and sodium makes it indispensable for muscle cells [14]. For these reasons, CAPN3 is of critical importance in the muscle cell, not only due to its primary function but also due to its secondary functions regarding the proteins and molecules it interacts with.
2. Current Clinical and Experimental Studies
Among the hereditary diseases that are already difficult to treat, gene therapy for hereditary muscle diseases is an especially challenging area because of the fact that muscles make up about 40 percent of the human body. To date, mostly palliative or symptomatic treatment strategies have been presented to LGMDR1 patients. Furthermore, few experimental studies and fewer clinical study strategies have been carried out by researchers for the development of therapies in LGMDR1. In particular, the prognosis of LMGDR1 varies widely according to the location of the mutation in the CAPN3 gene, the type of mutation, and homozygous/heterozygous status [17], such that even siblings with identical mutations might have different phenotypes and prognosis [18,19].
An ongoing phase II trial study involving drug therapy is focused on the effect of weekly oral glucocorticoid steroid (prednisone) administration in LGMDR1 patients [20]. Various steroid applications have been successful in DMD in the past [21,22,23]. Due to the immunosuppressive properties of glucocorticoids, it is estimated that steroids may reduce muscle damage that may occur due to the inflammatory response in muscle diseases [24,25]. In another drug treatment phase I/II study, MYO-029, an antibody that neutralizes the myostatin protein, which has an inhibitory role in muscle growth, was tested in patients with various types of muscular dystrophy [26]. Although the MYO-029 drug was found to be safe, it has been determined that it is incapable of increasing muscle strength [26]. A gene therapy experimental study has also been performed concerning this therapeutic agent [27]. In that study, by performing the inhibition of myostatin via the AAV-mediated expression of a mutated propeptide (pAAV-CMV-mSeAPpropmyoD76A), researchers identified an increase in absolute power, in addition to an increase in muscle mass in CAPN3-deficient mice [27] (Figure 1). However, in a recent study, researchers who performed the genetic inhibition of myostatin by increasing the expression of follistatin, an endogenous inhibitor of myostatin in the C3KO model, reported that this intervention was not effective in developing muscle strength in proximal limb muscles, finding that it even worsened exercise intolerance and decreased the oxidative capacity of the muscle, while only increasing muscle mass 1.5–2-fold [28]. Although myostatin inhibitors are good therapeutic agents for muscle diseases, the lack of consensus in studies using myostatin inhibitors as therapeutic agents for LGMDR1 shows that this strategy has poor validity since the pathophysiology of LGMDR1 has not been completely elucidated and the exact way in which LGMDR1 makes changes is not known [29,30]. One of the gene therapy strategies designed to reverse the CAPN3 defect is to systemically or locally (intramuscularly) administer AAV-associated CAPN3 gene transfer in the murine model [31,32,33]. However, it has been reported that the increase in CAPN3 expression in extra-muscular cells due to intravenous administration causes a cardiotoxic effect, which leads to cell death and especially to heart hypertrophy [31]. In the same study, a strategy was successfully developed to overcome this toxicity by adding cardiac-specific microRNA-208a to the CAPN3 regulatory cassette in the heart to prevent CAPN3 expression [31]. In another study using a strategy with the aim of overcoming this cardiac toxicity, heart damage previously seen in the murine model was not seen in a primate model, and this strategy led to a therapeutic effect in CAPN3 deficiency [34]. In addition, mice were thought to be more susceptible to cardiac toxicity due to the difference in titin (containing binding sites of CAPN3) transcripts in the murine model compared to primates and humans [34]. Researchers have used a serotype (AAVrh74) that can target skeletal muscle and cardiac muscle without off-target delivery in the in vivo transfer of AAV in neuromuscular diseases [35,36]. A recent study tested the biodelivery and stability of this vector system in LGMDR1 by loading the CAPN3 gene into the AAVrh74 serotype with the tMCK promoter, which was applied intravenously. According to their results, the authors been stated that this vector (AAVrh74.tMCK.hCAPN3) has no off-target effects and no toxic effects and has a successful therapeutic effect even at low doses [37].
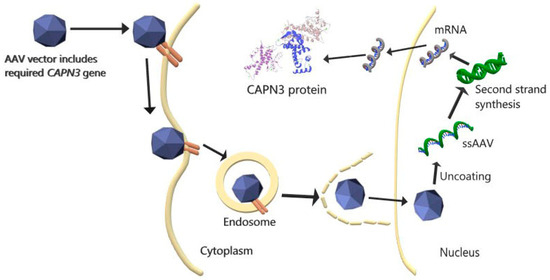
Figure 1.
AAV-mediated CAPN3 gene therapy. rAAV, containing the desired CAPN3 gene, binds to the appropriate receptor and enters the cell via the endosome. AAV enters the nucleus after escaping from the endosome in the cytoplasm. After the AAV enters the nucleus and separates it from its capsid, the single-stranded (ss) DNA is transformed into double-stranded DNA, and the desired CAPN3 mRNA is transcribed by the cell. The mRNA, when leaving the nucleus and entering the cytoplasm, is translated into the CAPN3 protein [38].
In LGMDR1 patients, the expression of FRZB, which prevents the translocation of β-catenin into the nucleus, is upregulated via inhibition of the Wnt pathway [39]. Normal CAPN3 regulates the localization of β-catenin [40]. The WNT signal is activated by its ability to activate the multimerized TCF/LEF luciferase reporter structure of 2-Amino-4-(3,4-(methylenedioxy)benzylamino)-6-(3-methoxyphenyl) pyrimidine (AMBMP), a WNT agonist that activates the canonical signal [41,42]. AMBMP activates CaMKII in metabolically altered C3K0 muscles and reprograms the muscle towards the slow oxidative muscle phenotype [43]. AMBMP reversed the LGMDR1 phenotype in vivo by improving oxidative properties, increasing slow fiber size, and improving exercise performance [43].
The CRISPR-Cas9 system, which won the 2020 Nobel Prize in Chemistry, is a groundbreaking area of research for in vivo gene therapy. Using the stem cell method, a wide variety of diseases can be treated [44]. In a study combining these two strategies, muscle engenderment and increases in CAPN3 mRNA were observed in mice as a result of the transplantation of corrected LGMDR1 myogenic progenitors through the use of IPSCs, which were gene-corrected with the CRISPR-Cas9 method (Table 1) [45] (Figure 2).

Table 1.
Current therapy strategies.
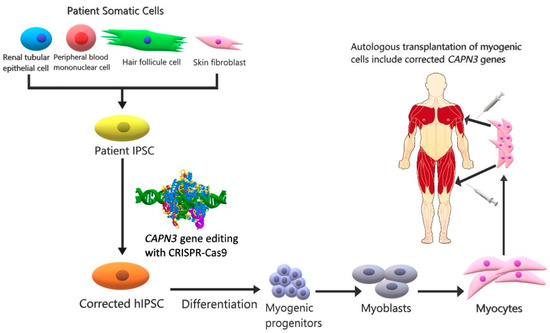
Figure 2.
CAPN3 cell therapy with a combination of IPSCs and CRISPR-Cas9. In IPSCs derived from the somatic cells of the LGMDR1 patient (renal tubular epithelial cell, peripheral blood mononuclear cell, hair follicle cell, skin fibroblast) using reprogramming factors, the CAPN3 gene is corrected by means of the CRISPR-Cas9 method. Genetically modified IPS cells are then stimulated with various factors (Pax3/Pax7 or MyoD) to differentiate into myogenic progenitors that can be multiplied in number. Cellular therapy is applied to the patient by injecting the proliferated myocytes intramuscularly into the muscles [46].
3. Future Therapy Strategies
It is known that mitochondrial damage is involved in the pathophysiology of LGMDR1 [47,48]. A new muscle-specific protein (Mss51) has recently been identified [49]. Considering that when the gene encoding Mss51 is deleted in mice, energy production increases and mitochondrial activity improves, researchers are investigating whether the elimination of this protein would be a suitable target in a calpainopathy model [50]. Although the pathophysiology of calpainopathy has not been fully elucidated yet, some experimental and bioinformatic results show that CAPN3 targets the E3 ubiquitin proteins MuRF1 [51] and TRIM32 [52] as substrates. In addition, a recent study reported that LGMDR1 is associated with endoplasmic reticulum (ER) stress [5]. In the ER (called the sarcoplasmic reticulum in muscle cells), protein synthesis, folding, maturation and transport, calcium storage, and lipid biosynthesis take place. ER stress is defined as the disruption of the balance between the protein folding capacity of the ER and the processed protein load, resulting in the accumulation of incorrectly folded or unfolded proteins [53]. Excessive synthesis of secretory proteins, mutations in proteins that play a role in protein folding, abnormal changes in the amount of Ca+2 in the ER, and viral infections are some of the factors that cause protein accumulation in the ER [54,55]. It would not be wrong to say that this plays a role in the pathophysiology of LGMR1, as the deterioration of homeostasis in the ER contributes to ER stress. Considering that the perturbation of calcium flux causes ER stress, a study reported that the SERCA2 protein, responsible for the reuptake of calcium into the ER, is decreased in LGMDR1 patients [56]. In this context, it should not be ignored that targeting ER stress may be therapeutic. In order to eliminate the ER stress caused by an imbalance between the load of unfolded proteins in the ER and the capacity of the cellular mechanism that manages this load, cells activate three mechanisms. The first of these is to reduce the protein load entering the ER via a temporary adaptation by reducing the synthesis of the protein in the cell and its translocation to the ER. Secondly; the unfolded protein response (UPR) is switched on. To this end, an increase in the capacity of the ER emerges to overcome unfolded proteins for a longer-term adaptation, which requires transcriptional activation of UPR target genes. Lastly, if homeostasis cannot be restored, a cell death response occurs to protect the organism from cells displaying unfolded proteins [57]. In eukaryotes, the ubiquitin-proteosome system is responsible for most of the protein degradation in cells, aimed at maintaining protein homeostasis. UPR, pancreatic ER kinase-like (PERK), inositol requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6) activate three important signaling pathways, initiated by stress sensors localized in the ER [58,59]. PERK phosphorylates and inactivates the eukaryotic initiation factor 2α (elF2α) with the formation of the PERK oligomer in the ER membrane [60]. Thus, mRNA transcription in the ER stops and the protein load is reduced [60]. Furthermore, when the cell encounters ER stress, ATF6 undergoes posttranscriptional modification. ATF6 sent to the Golgi apparatus undergoes clipping by interacting with site1 protease. Thus, an attempt to protect it against stress is made by increasing the ER folding capacity [61]. IRE1, which is bound to GRP78 under normal physiological conditions, becomes active through either transphosphorylation and RNAase activation or by directly binding to unfolded proteins. It stimulates the insertion of a 26-nucleotide segment from the mRNA of X-box binding protein 1 (XBP1) by cutting the activated IRE1 intron. XBP1, transformed from the spliced mRNA, eventually passes into the nucleus. XBP1 increases protein folding, ER biogenesis, and transcription of genes involved in ER-associated protein degradation (ERAD) to correct ER homeostasis [62]. As a result, through these pathways, unfolded proteins or misfolded proteins are degraded by the proteasome system or the process of cell death begins [63].
Using ER stress inhibitory agents or eliminating the causes of ER stress may be therapeutic in LGMDR1. The chemical chaperone mimetic drug tauroursodeoxycholic acid (TUDCA) has been reported in previous studies to have a reducing effect on ER stress-related molecules, such as ATF6α, IRE1α, PERK, CHOP, and GRP78 [64,65]. Salubrinal, which can be used as another therapeutic agent, can cooperate with transformational attenuation to reduce ER protein overload by inducing degradation of non-translated ER-targeted protein mRNAs [66]. Rapamycin, another agent that targets ER stress, provides the inhibition of mTORC1, triggering the autophagic process that targets toxic products, and this process provides a reduction in ER stress and a decrease in fibrosis and inflammation, in addition to an increase in contraction and strength in dystrophic muscles [67,68,69]. These results show that rapamycin may be therapeutic in muscular dystrophies.
Some small molecules targeted in various diseases are preferred due to their therapeutic potential [70]. In LGMDR1, the small molecule SERCA2 activator CDN1163, which acts on the SERCA enzyme directly through the allosteric mechanism, can be used to increase the activity of SERCA2 to both reduce ER stress and maintain Ca+2 homeostasis [71,72]. Another molecule in which a SERCA regulatory property was discovered is a putative muscle-specific long non-coding RNA which is called dwarf open reading frame (DWORF) that encodes a 34-amino-acid peptide [73]. DWORF is localized in the SR membrane and increases SERCA activity by modifying SERCA inhibitors phospholamban, sarcolipin, and myoregine [73]. DWORF is an endogenous peptide that is known to be effective in increasing muscle contraction, and it activates the SERCA pump through a physical interaction routine (Table 2) [73] (Figure 3).

Table 2.
Future therapy strategies.
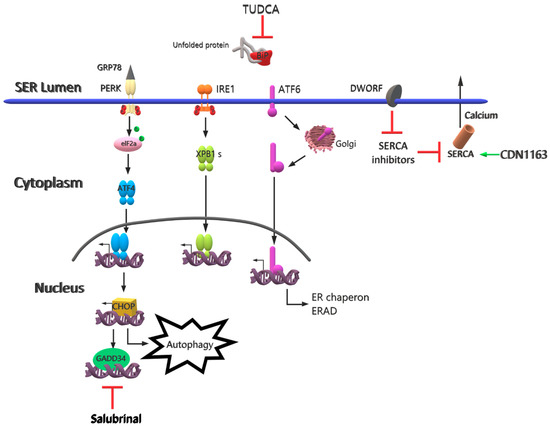
Figure 3.
Future therapy strategies for LGMDR1. Calcium imbalance, thought to play a role in the pathophysiology of LGDMR1, can be repaired by inhibiting SERCA inhibitors through DWORF and by activating SERCA2 through CDN1163. ER stress can be reduced by targeting the degradation of proteins at the mRNA level through salubrinal and by preventing unfolded proteins associated with ER stress by means of TUDCA.
4. Conclusions
In conclusion, therapy strategies for LGMDR1 disease, which is caused by CAPN3 defects, continue to be developed at both clinical and pre-clinical stages. In this article, we have summarized potential therapy strategies, in addition to actual therapies, such as cell therapy, gene therapy, and drug strategies. Although gene correction is the first strategy that comes to mind in single-gene diseases, the literature offers researchers several therapeutic agents that target many factors that are effective in the pathophysiology of LGMDR1. These agents consist of inhibitors and small molecules that target ER stress, which is thought to play a role in muscle loss in LGMDR1. Although several strategies have been attempted, no definitive conclusion has yet been reached. In viral systems used in gene therapies, failure or unexpected systemic effects may occur due to delivery and serotype compatibility problems. In addition, the problem of off-target effects must be overcome. Similarly, in cell therapies, the material produced for therapy should be able to target the desired organ and perform permanent treatment there. Furthermore, the success of a drug in a similar disease does not guarantee that it will always be effective for the target disease. Disease pathophysiology is important in this context. Such problems have also been observed in the drug strategies used against LGMDR1, a disease of which the pathophysiology has not been fully clarified yet. As mentioned above, although some strategies may seem effective in achieving goals in single-gene diseases, things do not always go as expected in science. However, this should not be daunting for scientists. It should not be forgotten that the tools used in science are increasing day by day and strategies for treatment can be obtained through various combinations of tools. More researchers need to work on LGMDR1 in order to achieve the desired end and restore the health of people suffering from this disease.
Author Contributions
İ.O.Ş. wrote the article, prepared the Tables and drew the Figures. M.D. and Y.Ö. revised the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest with regard to the research described in this manuscript.
Abbreviations
| AAV | Adeno-associated virus |
| AAVrh74.tMCK.hCAPN3 | AAV rhesus 74 truncated muscle creatine kinase human CAPN3 |
| AMBMP | 2-amino-4-(3,4-(methylenedioxy)benzylamino)-6-(3-methoxyphenyl) pyrimidine |
| ATF6 | Activating transcription factor 6 |
| C3KO | Calpain 3 knock out |
| CaMKII | Ca2+/calmodulin-dependent protein kinase II |
| CaMKII | Ca2+/calmodulin (CaM)-dependent protein kinase II |
| CAPN3 | Calpain 3 |
| Cas9 | Native Cas9 nuclease |
| CHOP | C/-EBP homologous protein |
| CRISPR-Cas9 | Clustered regularly interspaced short palindromic repeats CRISPR-associated proteins 9 |
| CSQ | Calsequestrin |
| DMD | Duchenne muscular dystrophy |
| elF2α | Eukaryotic initiation factor 2α |
| ER | Endoplasmic reticulum |
| ERAD | ER-associated protein degradation |
| FRZB | Frizzled-related protein |
| GRP78 | Glucose-regulated protein |
| IPSC | Induced pluripotent stem cell |
| IRE1α | Inositol-requiring enzyme 1α |
| LGMDR1 | Limb girdle muscular dystrophy R1 |
| LIM | Lin-11 Isl-1 Mec-3 |
| Mss51 | Mitochondrial translational activator |
| MuRF1 | Muscle RING-finger protein-1 |
| MYO-029 | Stamulumab |
| MyoD | Myogenic differentiation antigen |
| NCX3 | Na+-Ca2+ exchanger 3 |
| pAAV-CMV-mSeAPpropmyoD76A | Plasmid AAV-cytomegalovirus- murine-secreted alkaline phosphatase myogenic differentiation antigen murine-secreted alkaline phosphatase |
| Pax3/Pax7 | Paired box gene 3/Paired box gene 7 |
| PERK | PKR-like ER kinase |
| RyR1 | Ryanodine receptor 1 |
| SERCA | Sarcoplasmic/endoplasmic reticulum Ca2+ ATPase |
| TCF/LEF | T-cell factor/lymphoid enhancer factor |
| TRIM32 | Tripartite motif-containing protein 32 |
| TUDCA | Tauroursodeoxycholic acid |
| UPR | Unfolded protein response |
| WNT | Wingless-related integration site |
| XBP1 | X-box binding protein 1 |
References
- Richard, I.; Hogrel, J.Y.; Stockholm, D.; Payan, C.A.M.; Fougerousse, F.; Eymard, B.; Mignard, C.; Lopez de Munain, A.; Fardeau, M.; Urtizberea, J.A. Natural history of LGMD2A for delineating outcome measures in clinical trials. Ann. Clin. Transl. Neurol. 2016, 3, 248–265. [Google Scholar] [CrossRef] [PubMed]
- OMIM—Online Mendelian Inheritance in Man. Available online: https://www.omim.org/ (accessed on 20 October 2020).
- Richard, I.; Broux, O.; Allamand, V.; Fougerousse, F.; Chiannilkulchai, N.; Bourg, N.; Brenguier, L.; Devaud, C.; Pasturaud, P.; Roudaut, C.; et al. Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell 1995, 81, 27–40. [Google Scholar] [CrossRef]
- Vissing, J.; Barresi, R.; Witting, N.; Van Ghelue, M.; Gammelgaard, L.; Bindoff, L.A.; Straub, V.; Lochmüller, H.; Hudson, J.; Wahl, C.M.; et al. A heterozygous 21-bp deletion in CAPN3 causes dominantly inherited limb girdle muscular dystrophy. Brain 2016, 139, 2154–2163. [Google Scholar] [CrossRef]
- Lasa-Elgarresta, J.; Mosqueira-Martín, L.; Naldaiz-Gastesi, N.; Sáenz, A.; de Munain, A.L.; Vallejo-Illarramendi, A. Calcium Mechanisms in Limb-Girdle Muscular Dystrophy with CAPN3 Mutations. Int. J. Mol. Sci. 2019, 20, 4548. [Google Scholar] [CrossRef]
- Calpainopathy-GeneReviews®-NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1313/#lgmd2a.Molecular_Genetics (accessed on 4 May 2021).
- The CAPN3 gene homepage—Global Variome shared LOVD. Available online: https://databases.lovd.nl/shared/genes/CAPN3 (accessed on 10 October 2020).
- Sorimachi, H.; Imajoh-Ohmi, S.; Emori, Y.; Kawasaki, H.; Ohno, S.; Minami, Y.; Suzuki, K. Molecular cloning of a novel mammalian calcium-dependent protease distinct from both m- and μ-types. Specific expression of the mRNA in skeletal muscle. J. Biol. Chem. 1989, 264, 20106–20111. [Google Scholar] [CrossRef]
- Ojima, K.; Ono, Y.; Ottenheijm, C.; Hata, S.; Suzuki, H.; Granzier, H.; Sorimachi, H. Non-proteolytic functions of calpain-3 in sarcoplasmic reticulum in skeletal muscles. J. Mol. Biol. 2011, 407, 439–449. [Google Scholar] [CrossRef]
- Toral-Ojeda, I.; Aldanondo, G.; Lasa-Elgarresta, J.; Lasa-Fernandez, H.; Vesga-Castro, C.; Mouly, V.; de Munain, A.L.; Vallejo-Illarramendi, A. A Novel Functional In Vitro Model that Recapitulates Human Muscle Disorders. In Muscle Cell and Tissue-Current Status of Research Field; InTech: London, UK, 2018. [Google Scholar]
- Kramerova, I.; Kudryashova, E.; Ermolova, N.; Saenz, A.; Jaka, O.; López de munain, A.; Spencer, M.J. Impaired calcium calmodulin kinase signaling and muscle adaptation response in the absence of calpain 3. Hum. Mol. Genet. 2012, 21, 3193–3204. [Google Scholar] [CrossRef]
- Michel, L.Y.M.; Hoenderop, J.G.J.; Bindels, R.J.M. Calpain-3-mediated regulation of the Na+-Ca2+ exchanger isoform 3. Pflugers Arch. Eur. J. Physiol. 2016, 468, 243–255. [Google Scholar] [CrossRef]
- Ono, Y.; Ojima, K.; Torii, F.; Takaya, E.; Doi, N.; Nakagawa, K.; Hata, S.; Abe, K.; Sorimachi, H. Skeletal muscle-specific calpain is an intracellular Na+- dependent protease. J. Biol. Chem. 2010, 285, 22986–22998. [Google Scholar] [CrossRef]
- Ono, Y.; Ojima, K.; Shinkai-Ouchi, F.; Hata, S.; Sorimachi, H. An eccentric calpain, CAPN3/p94/calpain-3. Biochimie 2016, 122, 169–187. [Google Scholar] [CrossRef]
- Beckmann, J.S.; Spencer, M. Calpain 3, the “gatekeeper” of proper sarcomere assembly, turnover and maintenance. Neuromuscul. Disord. 2008, 18, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Sorimachi, H.; Toyama-Sorimachi, N.; Saido, T.C.; Kawasaki, H.; Sugita, H.; Miyasaka, M.; Arahata, K.I.; Ishiura, S.; Suzuki, K. Muscle-specific calpain, p94, is degraded by autolysis immediately after translation, resulting in disappearance from muscle. J. Biol. Chem. 1993, 268, 10593–10605. [Google Scholar] [CrossRef]
- de Paula, F.; Vainzof, M.; Passos-Bueno, M.R.; Pavanello, R.d.C.; Matioli, S.R.; Anderson, L.V.B.; Nigro, V.; Zatz, M. Clinical variability in calpainopathy: What makes the difference? Eur. J. Hum. Genet. 2002, 10, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Schessl, J.; Walter, M.C.; Schreiber, G.; Schara, U.; Müller, C.R.; Lochmüller, H.; Bönnemann, C.G.; Korinthenberg, R.; Kirschner, J. Phenotypic variability in siblings with calpainopathy (LGMD2A). Acta Myol. 2008, 27, 54–58. [Google Scholar] [PubMed]
- de Albuquerque, M.A.V.; Abath Neto, O.; da Silva, F.M.A.; Zanoteli, E.; Reed, U.C. Limb-girdle muscular dystrophy type 2A in Brazilian children. Arq. Neuropsiquiatr. 2015, 73, 993–997. [Google Scholar] [CrossRef][Green Version]
- Weekly Steroids in Muscular Dystrophy-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04054375 (accessed on 12 October 2020).
- Merlini, L.; Cicognani, A.; Malaspina, E.; Gennari, M.; Gnudi, S.; Talim, B.; Franzoni, E. Early prednisone treatment in Duchenne muscular dystrophy. Muscle Nerve 2003, 27, 222–227. [Google Scholar] [CrossRef]
- Mesa, L.E.; Dubrovsky, A.L.; Corderi, J.; Marco, P.; Flores, D. Steroids in duchenne muscular dystrophy—Deflazacort trial. Neuromuscul. Disord. 1991, 1, 261–266. [Google Scholar] [CrossRef]
- Hussein, M.R.; Hamed, S.A.; Mostafa, M.G.; Abu-Dief, E.E.; Kamel, N.F.; Kandil, M.R. The effects of glucocorticoid therapy on the inflammatory and Dendritic cells in muscular dystrophies. Int. J. Exp. Pathol. 2006, 87, 451–461. [Google Scholar] [CrossRef]
- Spencer, M.J.; Tidball, J.G. Do immune cells promote the pathology of dystrophin-deficient myopathies? Neuromuscul. Disord. 2001, 11, 556–564. [Google Scholar] [CrossRef]
- Gaud, A.; Simon, J.M.; Witzel, T.; Carre-Pierrat, M.; Wermuth, C.G.; Ségalat, L. Prednisone reduces muscle degeneration in dystrophin-deficient Caenorhabditis elegans. Neuromuscul. Disord. 2004, 14, 365–370. [Google Scholar] [CrossRef]
- Wagner, K.R.; Fleckenstein, J.L.; Amato, A.A.; Barohn, R.J.; Bushby, K.; Escolar, D.M.; Flanigan, K.M.; Pestronk, A.; Tawil, R.; Wolfe, G.I.; et al. A phase I/II trial of MYO-029 in adult subjects with muscular dystrophy. Ann. Neurol. 2008, 63, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, M.; Poupiot, J.; Vulin, A.; Fougerousse, F.; Arandel, L.; Daniele, N.; Roudaut, C.; Noulet, F.; Garcia, L.; Danos, O.; et al. AAV-mediated delivery of a mutated myostatin propeptide ameliorates calpain 3 but not α-sarcoglycan deficiency. Gene Ther. 2007, 14, 733–740. [Google Scholar] [CrossRef][Green Version]
- Kramerova, I.; Marinov, M.; Owens, J.; Lee, S.J.; Becerra, D.; Spencer, M.J. Myostatin inhibition promotes fast fibre hypertrophy but causes loss of AMP-activated protein kinase signalling and poor exercise tolerance in a model of limb-girdle muscular dystrophy R1/2A. J. Physiol. 2020, 598, 3927–3939. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, M.; Ishida, J.; Ebner, N.; Anker, S.D.; Von Haehling, S. Myostatin inhibitors as pharmacological treatment for muscle wasting and muscular dystrophy. JCSM Clin. Rep. 2017, 2, 1–10. [Google Scholar] [CrossRef]
- Mariot, V.; Joubert, R.; Hourdé, C.; Féasson, L.; Hanna, M.; Muntoni, F.; Maisonobe, T.; Servais, L.; Bogni, C.; Le Panse, R.; et al. Downregulation of myostatin pathway in neuromuscular diseases may explain challenges of anti-myostatin therapeutic approaches. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Roudaut, C.; Le Roy, F.; Suel, L.; Poupiot, J.; Charton, K.; Bartoli, M.; Richard, I. Restriction of calpain3 expression to the skeletal muscle prevents cardiac toxicity and corrects pathology in a murine model of limb-girdle muscular dystrophy. Circulation 2013, 128, 1094–1104. [Google Scholar] [CrossRef]
- Yalvac, M.E.; Amornvit, J.; Braganza, C.; Chen, L.; Hussain, S.R.A.; Shontz, K.M.; Montgomery, C.L.; Flanigan, K.M.; Lewis, S.; Sahenk, Z. Impaired regeneration in calpain-3 null muscle is associated with perturbations in mTORC1 signaling and defective mitochondrial biogenesis. Skelet. Muscle 2017, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, M.; Roudaut, C.; Martin, S.; Fougerousse, F.; Suel, L.; Poupiot, J.; Gicquel, E.; Noulet, F.; Danos, O.; Richard, I. Safety and efficacy of AAV-mediated calpain 3 gene transfer in a mouse model of limb-girdle muscular dystrophy Type 2A. Mol. Ther. 2006, 13, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Lostal, W.; Roudaut, C.; Faivre, M.; Charton, K.; Suel, L.; Bourg, N.; Best, H.; Smith, J.E.; Gohlke, J.; Corre, G.; et al. Titin splicing regulates cardiotoxicity associated with calpain 3 gene therapy for limb-girdle muscular dystrophy type 2A. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Chicoine, L.G.; Al-Zaidy, S.A.; Sahenk, Z.; Lehman, K.; Lowes, L.; Miller, N.; Alfano, L.; Galliers, B.; Lewis, S.; et al. Gene Delivery for Limb-Girdle Muscular Dystrophy Type 2D by Isolated Limb Infusion. Hum. Gene Ther. 2019, 30, 794–801. [Google Scholar] [CrossRef]
- Chicoine, L.G.; Rodino-Klapac, L.R.; Shao, G.; Xu, R.; Bremer, W.G.; Camboni, M.; Golden, B.; Montgomery, C.L.; Shontz, K.; Heller, K.N.; et al. Vascular delivery of rAAVrh74.MCK.GALGT2 to the gastrocnemius muscle of the rhesus macaque stimulates the expression of dystrophin and laminin α2 surrogates. Mol. Ther. 2014, 22, 713–724. [Google Scholar] [CrossRef]
- 1135—Biopotency and Biodistribution/ Toxicology Studies Following Systemic Gene Therapy with AAVrh74.tMCK.hCAPN3 in the Mouse Model for LGMD2A-ASGCT 23rd Annual Meeting. Available online: https://cslide-us.ctimeetingtech.com/asgct23/attendee/eposter/poster/466 (accessed on 16 October 2020).
- Li, C.; Samulski, R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Jaka, O.; Casas-Fraile, L.; Azpitarte, M.; Aiastui, A.; López de Munain, A.; Sáenz, A. FRZB and melusin, overexpressed in LGMD2A, regulate integrin β1D isoform replacement altering myoblast fusion and the integrin-signalling pathway. Expert Rev. Mol. Med. 2017, 19. [Google Scholar] [CrossRef]
- Kramerova, I.; Kudryashova, E.; Wu, B.; Spencer, M.J. Regulation of the M-Cadherin-β-Catenin Complex by Calpain 3 during Terminal Stages of Myogenic Differentiation. Mol. Cell. Biol. 2006, 26, 8437–8447. [Google Scholar] [CrossRef]
- Liu, J.; Wu, X.; Mitchell, B.; Kintner, C.; Ding, S.; Schultz, P.G. A small-molecule agonist of the Wnt signaling pathway. Angew. Chem. Int. Ed. 2005, 44, 1987–1990. [Google Scholar] [CrossRef]
- Lim, J.C.; Kania, K.D.; Wijesuriya, H.; Chawla, S.; Sethi, J.K.; Pulaski, L.; Romero, I.A.; Couraud, P.O.; Weksler, B.B.; Hladky, S.B.; et al. Activation of β-catenin signalling by GSK-3 inhibition increases p-glycoprotein expression in brain endothelial cells. J. Neurochem. 2008, 106, 1855–1865. [Google Scholar] [CrossRef]
- Liu, J.; Campagna, J.; John, V.; Damoiseaux, R.; Mokhonova, E.; Becerra, D.; Meng, H.; McNally, E.M.; Pyle, A.D.; Kramerova, I.; et al. A Small-Molecule Approach to Restore a Slow-Oxidative Phenotype and Defective CaMKIIβ Signaling in Limb Girdle Muscular Dystrophy. Cell Rep. Med. 2020, 1. [Google Scholar] [CrossRef]
- Alenzi, F.Q.; Lotfy, M.; Tamimi, W.G.; Wyse, R.K.H. Review: Stem cells and gene therapy. Lab. Hematol. 2010, 16, 53–73. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Dhoke, N.R.; Kiley, J.; Mateos-Aierdi, A.J.; Tungtur, S.; Mondragon-Gonzalez, R.; Killeen, G.; Oliveira, V.K.P.; López de Munain, A.; Perlingeiro, R.C.R. Gene Correction of LGMD2A Patient-Specific iPSCs for the Development of Targeted Autologous Cell Therapy. Mol. Ther. 2019, 27, 2147–2157. [Google Scholar] [CrossRef]
- Peng, G.-Y.; Lin, Y.; Li, J.-J.; Wang, Y.; Huang, H.-Y.; Shen, Z.-Y. The Application of Induced Pluripotent Stem Cells in Pathogenesis Study and Gene Therapy for Vascular Disorders: Current Progress and Future Challenges. Stem Cells Int. 2019, 2019, 9613258. [Google Scholar] [CrossRef]
- Nilsson, M.I.; Macneil, L.G.; Kitaoka, Y.; Alqarni, F.; Suri, R.; Akhtar, M.; Haikalis, M.E.; Dhaliwal, P.; Saeed, M.; Tarnopolsky, M.A. Redox state and mitochondrial respiratory chain function in skeletal muscle of LGMD2A patients. PLoS ONE 2014, 9, e102549. [Google Scholar] [CrossRef] [PubMed]
- Kramerova, I.; Kudryashova, E.; Wu, B.; Germain, S.; Vandenborne, K.; Romain, N.; Haller, R.G.; Verity, M.A.; Spencer, M.J. Mitochondrial abnormalities, energy deficit and oxidative stress are features of calpain 3 deficiency in skeletal muscle. Hum. Mol. Genet. 2009, 18, 3194–3205. [Google Scholar] [CrossRef] [PubMed]
- Moyer, A.L.; Wagner, K.R. Mammalian Mss51 is a Skeletal Muscle-Specific Gene Modulating Cellular Metabolism. J. Neuromuscul. Dis. 2015, 2, 371–385. [Google Scholar] [CrossRef]
- Research–Coalition to Cure Calpain 3. Available online: https://www.curecalpain3.org/research/ (accessed on 16 October 2020).
- Fanin, M.; Nascimbeni, A.C.; Angelini, C. Muscle atrophy in Limb Girdle Muscular Dystrophy 2A: A morphometric and molecular study. Neuropathol. Appl. Neurobiol. 2013, 39, 762–771. [Google Scholar] [CrossRef]
- CAPN3 Protein (Human)-STRING İnteraction Network. Available online: https://string-db.org/network/9606.ENSP00000380349 (accessed on 6 February 2021).
- Kaneko, M.; Imaißumi, K.; Saito, A.; Kanemoto, S.; Asada, R.; Matsuhisa, K.; Ohtake, Y. ER stress and disease: Toward prevention and treatment. Biol. Pharm. Bull. 2017, 40, 1337–1343. [Google Scholar] [CrossRef]
- Zhang, K.; Kaufman, R.J. Signaling the unfolded protein response from the endoplasmic reticulum. J. Biol. Chem. 2004, 279, 25935–25938. [Google Scholar] [CrossRef]
- Kincaid, M.M.; Cooper, A.A. ERADicate ER stress or die trying. Antioxid. Redox Signal. 2007, 9, 2373–2387. [Google Scholar] [CrossRef]
- Toral-Ojeda, I.; Aldanondo, G.; Lasa-Elgarresta, J.; Lasa-Fernández, H.; Fernández-Torrón, R.; De Munain, A.L.; Vallejo-Illarramendi, A. Calpain 3 deficiency affects SERCA expression and function in the skeletal muscle. Expert Rev. Mol. Med. 2016, 18. [Google Scholar] [CrossRef]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef]
- Malhotra, J.D.; Kaufman, R.J. The endoplasmic reticulum and the unfolded protein response. Semin. Cell Dev. Biol. 2007, 18, 716–731. [Google Scholar] [CrossRef]
- Nakka, V.P.; Prakash-babu, P.; Vemuganti, R. Crosstalk Between Endoplasmic Reticulum Stress, Oxidative Stress, and Autophagy: Potential Therapeutic Targets for Acute CNS Injuries. Mol. Neurobiol. 2016, 53, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, S.J.; Ron, D. Endoplasmic reticulum stress signaling in disease. Physiol. Rev. 2006, 86, 1133–1149. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, N. ER and aging-Protein folding and the ER stress response. Ageing Res. Rev. 2009, 8, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Sarvani, C.; Sireesh, D.; Ramkumar, K.M. Unraveling the role of ER stress inhibitors in the context of metabolic diseases. Pharmacol. Res. 2017, 119, 412–421. [Google Scholar] [CrossRef]
- Liu, M.Q.; Chen, Z.; Chen, L.X. Endoplasmic reticulum stress: A novel mechanism and therapeutic target for cardiovascular diseases. Acta Pharmacol. Sin. 2016, 37, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhou, C.; Chi, J.; Pan, S.; Lin, H.; Gao, F.; Ni, T.; Meng, L.; Zhang, J.; Jiang, C.; et al. The Role of Tauroursodeoxycholic Acid on Dedifferentiation of Vascular Smooth Muscle Cells by Modulation of Endoplasmic Reticulum Stress and as an Oral Drug Inhibiting In-Stent Restenosis. Cardiovasc. Drugs Ther. 2019, 33, 25–33. [Google Scholar] [CrossRef]
- Mesbah Moosavi, Z.S.; Hood, D.A. The unfolded protein response in relation to mitochondrial biogenesis in skeletal muscle cells. Am. J. Physiol. Cell Physiol. 2017, 312, C583–C594. [Google Scholar] [CrossRef]
- Romero-Ramírez, L.; Nieto-Sampedro, M.; Barreda-Manso, M.A. All roads go to salubrinal: Endoplasmic reticulum stress, neuroprotection and glial scar formation. Neural Regen. Res. 2015, 10, 1926–1927. [Google Scholar] [CrossRef]
- Foltz, S.J.; Luan, J.; Call, J.A.; Patel, A.; Peissig, K.B.; Fortunato, M.J.; Beedle, A.M. Four-week rapamycin treatment improves muscular dystrophy in a fukutin-deficient mouse model of dystroglycanopathy. Skelet. Muscle 2016, 6. [Google Scholar] [CrossRef]
- Bibee, K.P.; Cheng, Y.J.; Ching, J.K.; Marsh, J.N.; Li, A.J.; Keeling, R.M.; Connolly, A.M.; Golumbek, P.T.; Myerson, J.W.; Hu, G.; et al. Rapamycin nanoparticles target defective autophagy in muscular dystrophy to enhance both strength and cardiac function. FASEB J. 2014, 28, 2047–2061. [Google Scholar] [CrossRef]
- Kawakami, Y.; Hambright, W.S.; Takayama, K.; Mu, X.; Lu, A.; Cummins, J.H.; Matsumoto, T.; Yurube, T.; Kuroda, R.; Kurosaka, M.; et al. Rapamycin Rescues Age-Related Changes in Muscle-Derived Stem/Progenitor Cells from Progeroid Mice. Mol. Ther. Methods Clin. Dev. 2019, 14, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, E.V.; Gurevich, V.V. Therapeutic potential of small molecules and engineered proteins. Handb. Exp. Pharmacol. 2014, 219, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Dahl, R.; Hsieh, W.; Shin, A.; Zsebo, K.M.; Buettner, C.; Hajjar, R.J.; Lebeche, D. Small molecular allosteric activator of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) attenuates diabetes and metabolic disorders. J. Biol. Chem. 2016, 291, 5185–5198. [Google Scholar] [CrossRef]
- Dahl, R. A new target for Parkinson’s disease: Small molecule SERCA activator CDN1163 ameliorates dyskinesia in 6-OHDA-lesioned rats. Bioorg. Med. Chem. 2017, 25, 53–57. [Google Scholar] [CrossRef]
- Nelson, B.R.; Makarewich, C.A.; Anderson, D.M.; Winders, B.R.; Troupes, C.D.; Wu, F.; Reese, A.L.; McAnally, J.R.; Chen, X.; Kavalali, E.T.; et al. Muscle physiology: A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science 2016, 351, 271–275. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).