An Adaptive Motivation Approach to Understanding the ‘How’ and ‘Why’ of Wellbeing
Abstract
:1. Introduction
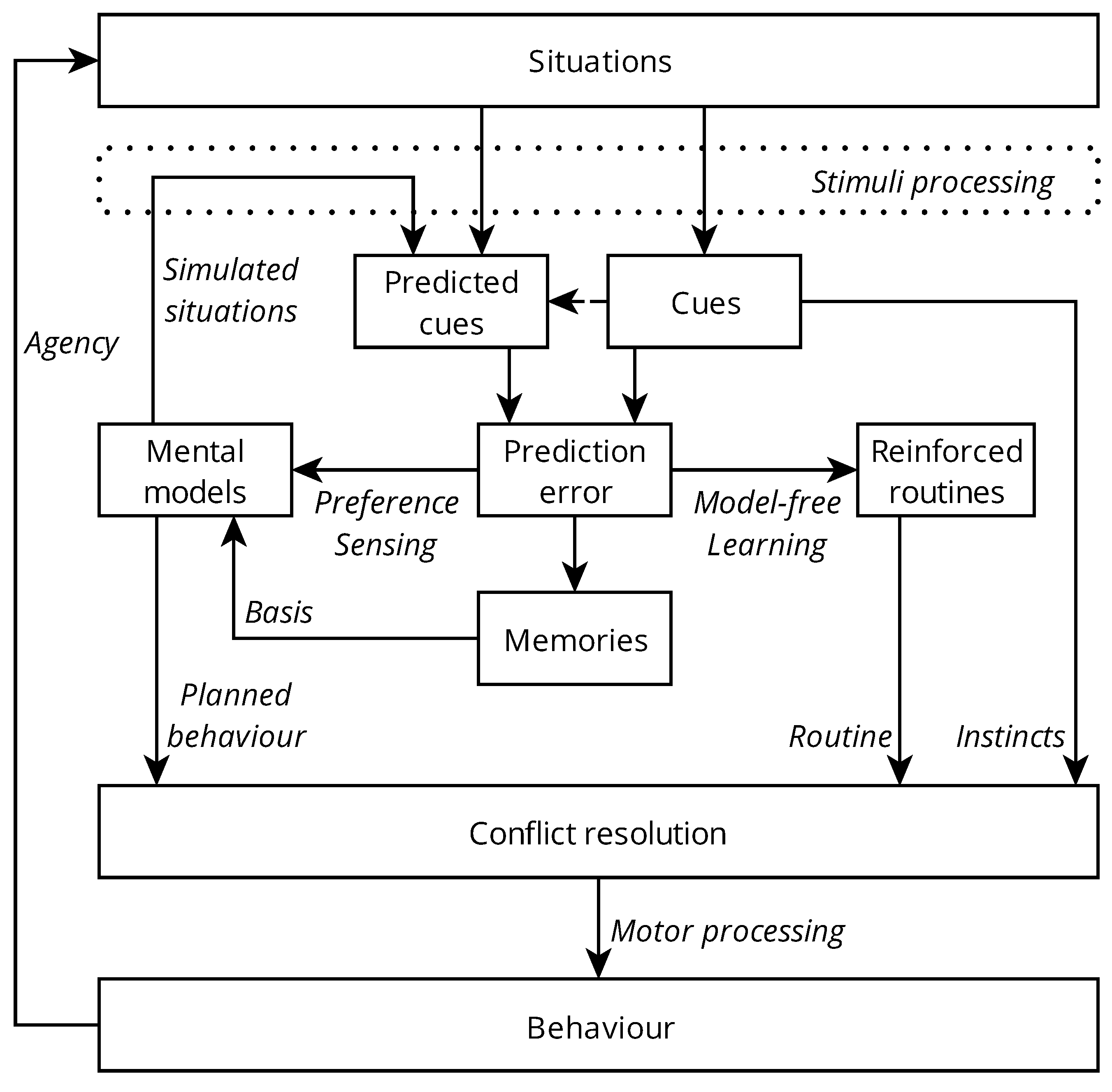
1.1. Model Overview
1.2. Situations, Behaviour, and Agency
1.3. Cues
1.4. Cue Value
1.5. Prediction Error
1.6. Motivation
1.6.1. Instincts
1.6.2. Routines and Reinforcement Learning
1.6.3. Planned Control
1.7. Conflict Resolution
1.8. Memory and Forgetting
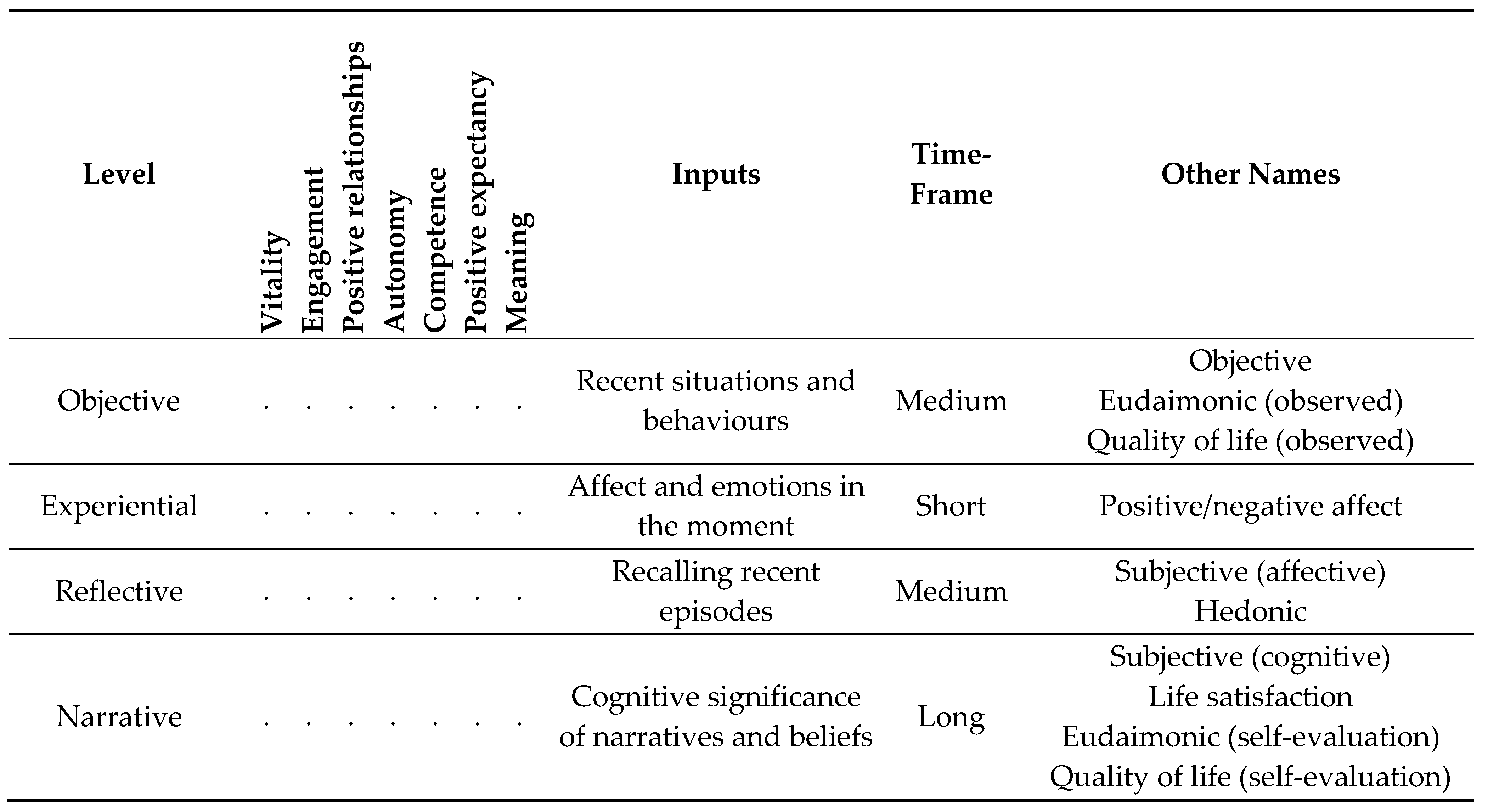
1.9. Wellbeing
1.10. Proposed Integration with Existing Literature
1.10.1. Wellbeing Terms
1.10.2. Motives
| Wellbeing Aspect | Proposed Association with Fitness | Wellbeing Terms |
|---|---|---|
| Vitality | Physically capable of agency to increase fitness | Vitality [5,72,73] |
| Energy [74] | ||
| Engagement | Behaviour is likely to be increasing fitness (through learning, skill development, use of skills, etc.) | Interest [75] |
| Engagement [72,73,76,77] | ||
| Involvement [5,78] | ||
| Effort in pursuing excellence [78] | ||
| Enjoyment [78] | ||
| Positive relationships | Increase in fitness due to social factors | Positive relationships [72,75,76,77,79] |
| Relatedness [80] | ||
| Connection [5] | ||
| Social belonging/trust [5,73] | ||
| Supportive relationships [73] | ||
| Autonomy | Fitness is less limited by dominance of others | Autonomy [73,74,75,79,80] |
| Self-congruence [5] | ||
| Competence | A particular type of agency will be more effective in increasing fitness | Competence [73,74,76,80] |
| Accomplishment [77] | ||
| Environmental mastery [79] | ||
| Self-esteem [72,73,76] | ||
| Manageability [81] | ||
| Comprehensibility [81] | ||
| Clear thinking [74] | ||
| Positive expectancy | Fitness is more likely to increase in the future, or not decrease | Optimism [5,72,73,76] |
| Meaning | Planned behaviour is/was worthwhile to increase fitness, (particularly through social support) | Purpose [5,73,75,76,77,78,79] |
| Meaning [5,72,73,77,78] | ||
| Meaningfulness [81] | ||
| Significance [5] | ||
| Contribution [75,76] | ||
| Positive emotion (Experiential and reflective layers, covering all cues) | General increase in fitness, or absence of decrease | Positive emotion/feelings [73,76,77] |
| Happiness [5,75] | ||
| Emotional stability [72] | ||
| Calmness [5] | ||
| Absence of negative feelings [73] | ||
| Positive narrative (Narrative layer, covering all cues) | Indirect | Self-acceptance [5,74,75,76,79] |
| Self-worth [5] | ||
| (Personal) Growth [75,79] | ||
| (Personal) Development [5,74,78] | ||
| Self-discovery [78] | ||
| Satisfying life [73] | ||
| Resilience [5,73] |
| Aspect | Motives |
|---|---|
| Vitality | Health and fitness, physical strength and endurance [82]; acquiring metabolic resources (1), maintaining body, avoiding infection (1) [40]; physiological needs [83]; health [84]; healthy, clean [85] |
| Engagement | Curiosity and exploration (1), mental knowledge and skills (1) [82]; acquiring knowledge about the world (1), honing skills (1) [40]; exploration, appreciating beauty, effort [84]; creativity, curiosity [85] |
| Positive relationships | Affection/commitment, altruism, social exchange, curiosity and exploration (2) [82]; affiliation, status improvement, maintaining functioning of large non-kin groups [40]; affiliation, status/esteem, parenting [83]; social values, social giving, interpersonal care, respect, avoiding rejection, interpersonal effectiveness, socialising, social life and friendship, being liked, being close to parents’ family [84]; social recognition (1), sense of belonging, politeness, honouring of parents and elders, devoutness, humility, helpfulness, forgiving, honest, loyal, mature love, true friendship, equality, social justice [85] |
| Autonomy | Dominance and aggression (giving or receiving) [82]; control of others, leadership, confidence and autonomy, avoiding conflict [84]; freedom, independence, choosing own goals, authority, social power, obedience, respect for tradition, accepting one’s portion in life [85] |
| Competence | Mental knowledge & skills (2) [82]; honing skills (2), acquiring knowledge about the world (2) [40]; self-knowledge, fastidiousness, mastery and perseverance, avoiding failure, self-regulation, smartness and rationality, organisation and efficiency, analysis & technical know-how, intellectual growth, occupational success [84]; self-respect, ambition, influence, capability, success, intelligence, self-discipline, wisdom, broad-mindedness [85] |
| Positive expectancy | Safety [82]; avoiding predation, avoiding infection (2) [40]; self-protection [83]; avoiding harm, stability and safety [84]; national security, family security, social order, moderation, protecting the environment, a world at peace [85] |
| Meaning | Legacy, meaning [82]; inspiring others, wisdom and serenity, pursuing ideas and passions [84]; social recognition (2), responsibility, meaning in life, inner harmony, detachment, unity with nature, a world of beauty [85] |
| Mating | Sex, appearance [82]; mating, acquiring high quality sexual relationships, maintaining high quality sexual relationships [40]; mate acquisition, mate retention [83]; Sexual intimacy [84] |
| Resources | Wealth [82]; acquiring metabolic resources (2), accumulating surplus resources [40]; money and wealth, financial freedom [84]; wealth [85] |
| Positive emotion | Happiness, avoiding stress and anxiety [84]; pleasure, daring [85] |
| Positive narrative | Enjoying life, religion and spirituality, being better than others, personal morals [84]; enjoying life, an exciting life, a varied life, a spiritual life, public image [85] |
1.10.3. Cues and Emotions
2. Methods
2.1. Plain-Language Overview
2.2. Situation Frequency
2.3. Motivational Processes
2.4. Fitness and Reproduction
2.5. Wellbeing
2.6. Determination of Parameters
3. Results
3.1. Wellbeing and Fitness
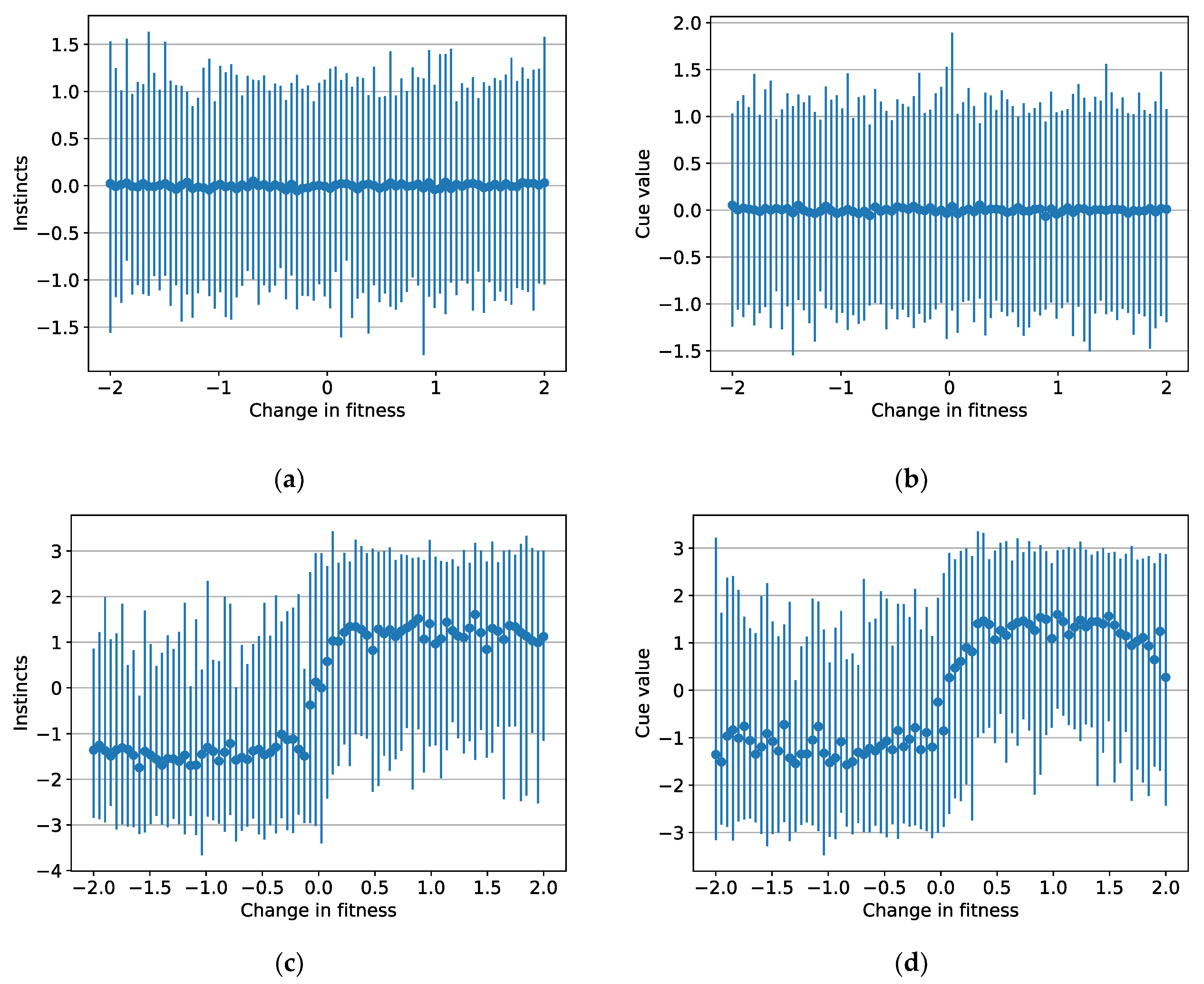
3.2. Instincts and Cue Values
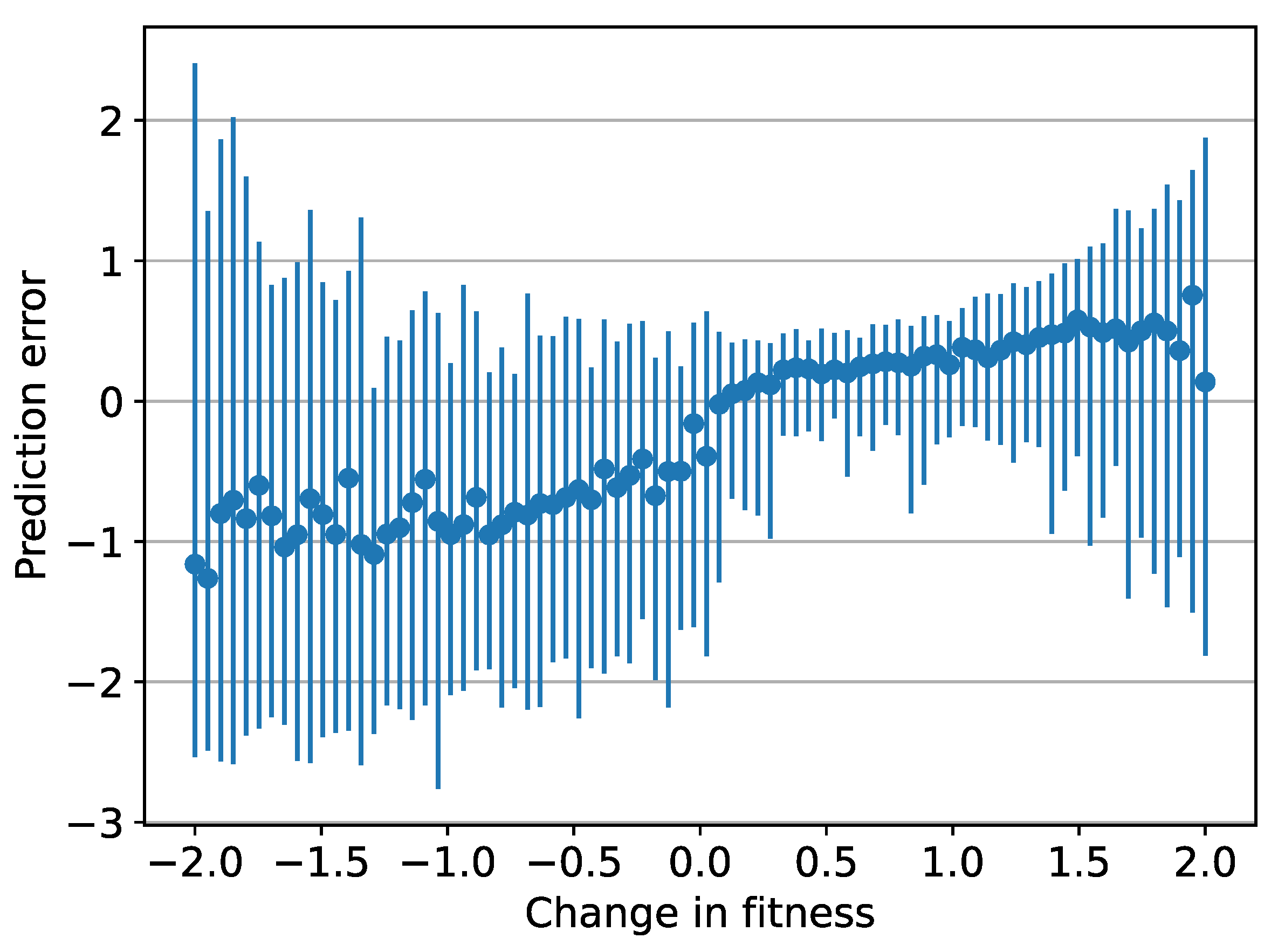
3.3. Prediction Errors
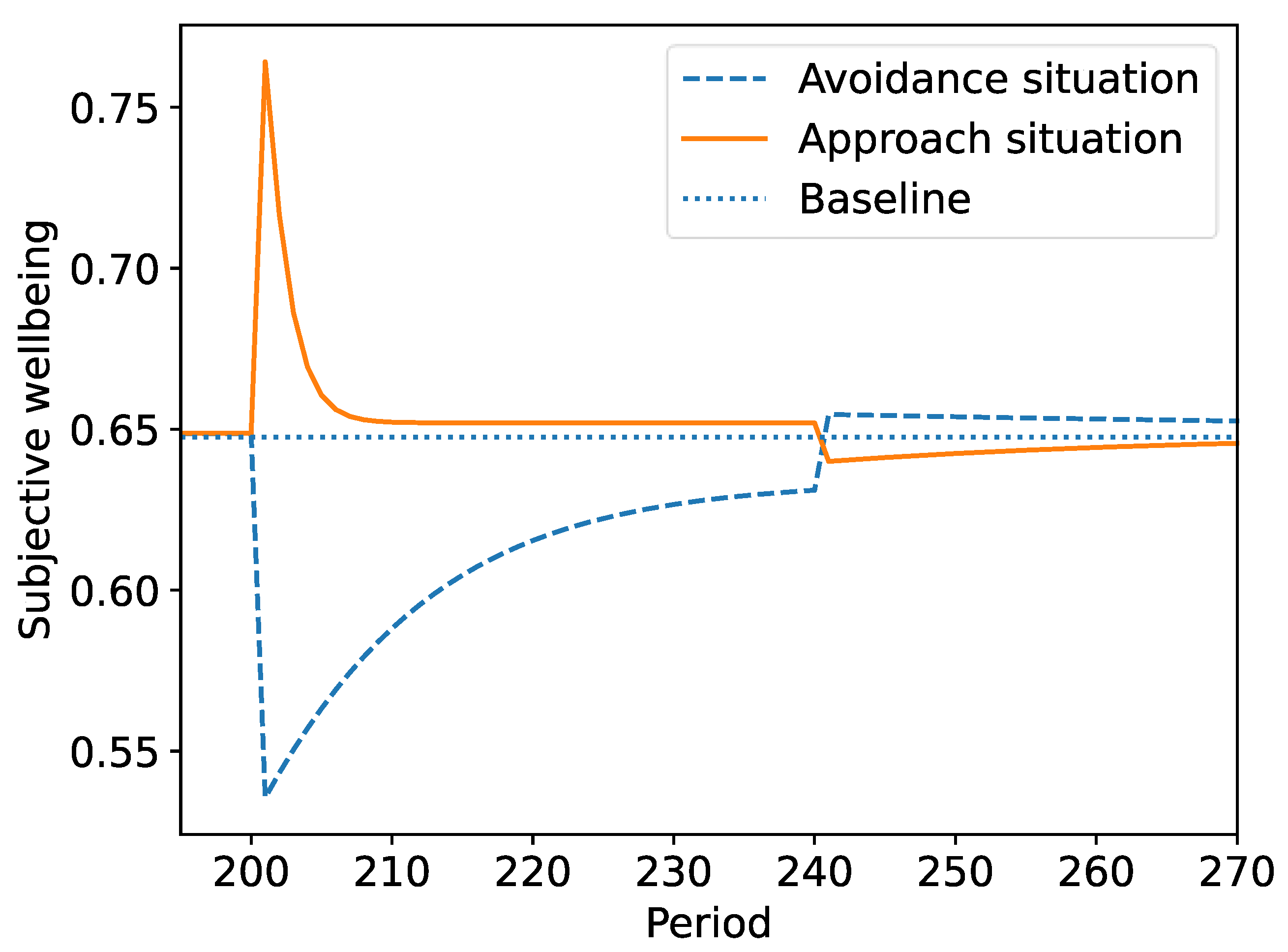
3.4. Hedonic Adaptation
4. Discussion
4.1. Wellbeing Dynamics
4.2. Individual Differences
4.3. Understanding Wellbeing
4.4. Limitations and Future Research
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diener, E.; Lucas, R.E.; Oishi, S. Advances and Open Questions in the Science of Subjective Well-Being. Collabra Psychol. 2018, 4, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medvedev, O.N.; Landhuis, C.E. Exploring Constructs of Well-Being, Happiness and Quality of Life. PeerJ 2018, 6, e4903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tov, W. Well-Being Concepts and Components. In Handbook of Well-Being; Diener, E., Oishi, S., Tay, L., Eds.; DEF Publishers: Salt Lake City, UT, USA, 2018; p. 15. [Google Scholar]
- Hone, L.C.; Jarden, A.; Schofield, G.M.; Duncan, S. Measuring Flourishing: The Impact of Operational Definitions on the Prevalence of High Levels of Wellbeing. Int. J. Wellbeing 2014, 4, 62–90. [Google Scholar] [CrossRef] [Green Version]
- Longo, Y.; Coyne, I.; Joseph, S. The Scales of General Well-Being (SGWB). Personal. Individ. Differ. 2017, 109, 148–159. [Google Scholar] [CrossRef]
- Martela, F.; Sheldon, K.M. Clarifying the Concept of Well-Being: Psychological Need Satisfaction as the Common Core Connecting Eudaimonic and Subjective Well-Being. Rev. Gen. Psychol. 2019, 23, 458–474. [Google Scholar] [CrossRef]
- Kim-Prieto, C.; Diener, E.; Tamir, M.; Scollon, C.; Diener, M. Integrating The Diverse Definitions of Happiness: A Time-Sequential Framework of Subjective Well-Being. J. Happiness Stud. 2005, 6, 261–300. [Google Scholar] [CrossRef]
- Dodge, R.; Daly, A.; Huyton, J.; Sanders, L. The Challenge of Defining Wellbeing. Int. J. Wellbeing 2012, 2, 222–235. [Google Scholar] [CrossRef] [Green Version]
- Alexander, R.; Aragón, O.R.; Bookwala, J.; Cherbuin, N.; Gatt, J.M.; Kahrilas, I.J.; Kästner, N.; Lawrence, A.; Lowe, L.; Morrison, R.G.; et al. The Neuroscience of Positive Emotions and Affect: Implications for Cultivating Happiness and Wellbeing. Neurosci. Biobehav. Rev. 2021, 121, 220–249. [Google Scholar] [CrossRef]
- Berridge, K.; Kringelbach, M. Towards a Neuroscience of Well-Being: Implications of Insights from Pleasure Research. In Human Happiness and the Pursuit of Maximization: Is More Always Better? Brockmann, H., Delhey, J., Eds.; Springer Science + Business Media: Berlin, Germany, 2013; pp. 81–100. ISBN 978-94-007-6608-2. [Google Scholar]
- Dolcos, S.; Moore, M.; Katsumi, Y. Neuroscience and Well-Being. In Handbook of Well-Being; Diener, E., Oishi, S., Tay, L., Eds.; DEF Publishers: Salt Lake Cit, UT, USA, 2018; p. 27. [Google Scholar]
- Carrero, G.; Makin, J.; Malinowski, P. A Mathematical Model for the Dynamics of Happiness. Math. Biosci. Eng. 2021, 19, 2002–2029. [Google Scholar] [CrossRef]
- Kopsov, I. A New Model of Subjective Well-Being. Open Psychol. J. 2019, 12, 102–115. [Google Scholar] [CrossRef]
- Barkow, J.H. Happiness in Evolutionary Perspective. In Uniting Psychology and Biology: Integrative Perspectives on Human Development.; Segal, N.L., Weisfeld, G.E., Weisfeld, C.C., Eds.; American Psychological Association: Washington, DC, USA, 1997; pp. 397–418. ISBN 978-1-55798-428-9. [Google Scholar]
- Hill, S.E.; Buss, D.M. Evolution and Subjective Well-Being. In The Science of Subjective Well-Being; Eid, M., Larsen, R.J., Eds.; Guilford Press: New York, NY, USA, 2008. [Google Scholar]
- Kenrick, D.T.; Krems, J.A. Well-Being, Self-Actualization, and Fundamental Motives: An Evolutionary Perspective. In Handbook of Well-Being; Diener, E., Oishi, S., Tay, L., Eds.; DEF Publishers: Salt Lake Cit, UT, USA, 2018; p. 13. [Google Scholar]
- Nesse, R.M. Natural Selection and the Elusiveness of Happiness. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2004, 359, 1333–1347. [Google Scholar] [CrossRef] [PubMed]
- King, P.E.; Barrett, J.L.; Greenway, T.S.; Schnitker, S.A.; Furrow, J.L. Mind the Gap: Evolutionary Psychological Perspectives on Human Thriving. J. Posit. Psychol. 2018, 13, 336–345. [Google Scholar] [CrossRef]
- Laland, K.N. Niche Construction, Human Behavioural Ecology and Evolutionary Psychology; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Bargh, J.A.; Gollwitzer, P.M.; Oettingen, G. Motivation. In Handbook of Social Psychology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; ISBN 978-0-470-56111-9. [Google Scholar]
- Heckhausen, J. Evolutionary Perspectives on Human Motivation. Am. Behav. Sci. 2000, 43, 1015–1029. [Google Scholar] [CrossRef]
- Todd, P.M.; Gigerenzer, G. Mechanisms of Ecological Rationality: Heuristics and Environments That Make Us Smart; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Cosmides, L.; Tooby, J. Evolutionary Psychology: New Perspectives on Cognition and Motivation. Annu. Rev. Psychol. 2013, 64, 201–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jellema, T.; Perrett, D.I. Neural Pathways of Social Cognition. In Neural Pathways of Social Cognition; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Panksepp, J. The Neuroevolutionary and Neuroaffective Psychobiology of the Prosocial Brain. In Oxford Handbook of Evolutionary Psychology; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Neel, R.; Brown, N.A.; Sng, O. Evolutionary Perspectives on Situations. In The Oxford Handbook of Psychological Situations; Rauthmann, J.F., Sherman, R.A., Funder, D.C., Eds.; Oxford University Press: Oxford, UK, 2020; pp. 111–123. ISBN 978-0-19-026334-8. [Google Scholar]
- Velasco, P.F.; Loev, S. Affective Experience in the Predictive Mind: A Review and New Integrative Account. Synthese 2021, 198, 10847–10882. [Google Scholar] [CrossRef]
- Bar, M. The Proactive Brain: Memory for Predictions. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1235–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diederen, K.M.J.; Fletcher, P.C. Dopamine, Prediction Error and Beyond. Neurosci. 2021, 27, 30–46. [Google Scholar] [CrossRef]
- Glimcher, P.W. Understanding Dopamine and Reinforcement Learning: The Dopamine Reward Prediction Error Hypothesis. Proc. Natl. Acad. Sci. USA 2011, 108, 15647–15654. [Google Scholar] [CrossRef] [Green Version]
- Nasser, H.M.; Calu, D.J.; Schoenbaum, G.; Sharpe, M.J. The Dopamine Prediction Error: Contributions to Associative Models of Reward Learning. Front. Psychol. 2017, 8, 244. [Google Scholar] [CrossRef] [Green Version]
- Schultz, W. Dopamine Reward Prediction Error Coding. Dialogues Clin. Neurosci. 2016, 18, 23–32. [Google Scholar] [CrossRef]
- Schultz, W. Neuronal Reward and Decision Signals: From Theories to Data. Physiol. Rev. 2015, 95, 853–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daw, N.D.; Shohamy, D. The Cognitive Neuroscience of Motivation and Learning. Soc. Cogn. 2008, 26, 593–620. [Google Scholar] [CrossRef] [Green Version]
- Darvas, M.; Fadok, J.P.; Palmiter, R.D. Requirement of Dopamine Signaling in the Amygdala and Striatum for Learning and Maintenance of a Conditioned Avoidance Response. Learn. Mem. 2011, 18, 136–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dombrowski, P.A.; Maia, T.V.; Boschen, S.L.; Bortolanza, M.; Wendler, E.; Schwarting, R.K.W.; Brandão, M.L.; Winn, P.; Blaha, C.D.; Da Cunha, C. Evidence That Conditioned Avoidance Responses Are Reinforced by Positive Prediction Errors Signaled by Tonic Striatal Dopamine. Behav. Brain Res. 2013, 241, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Ferreira-Santos, F.; Satpute, A.B. Predictive Processing Models and Affective Neuroscience. Neurosci. Biobehav. Rev. 2021, 131, 211–228. [Google Scholar] [CrossRef]
- Hutchinson, J.B.; Barrett, L.F. The Power of Predictions: An Emerging Paradigm for Psychological Research. Curr. Dir. Psychol. Sci. 2019, 28, 280–291. [Google Scholar] [CrossRef]
- Villano, W.J.; Otto, A.R.; Ezie, C.E.C.; Gillis, R.; Heller, A.S. Temporal Dynamics of Real-World Emotion Are More Strongly Linked to Prediction Error than Outcome. J. Exp. Psychol. Gen. 2020, 149, 1755–1766. [Google Scholar] [CrossRef]
- Aunger, R.; Curtis, V. The Anatomy of Motivation: An Evolutionary-Ecological Approach. Biol. Theory 2013, 8, 49–63. [Google Scholar] [CrossRef]
- Sutton, R.S.; Barto, A.G. Toward a Modern Theory of Adaptive Networks: Expectation and Prediction. Psychol. Rev. 1981, 88, 135–170. [Google Scholar] [CrossRef]
- Sutton, R.S.; Barto, A.G. Reinforcement Learning: An Introduction; Adaptive computation and machine learning; 2nd ed.; MIT Press: Cambridge, MA, USA, 1998; ISBN 978-0-262-19398-6. [Google Scholar]
- Benoit, R.G.; Gilbert, S.J.; Burgess, P.W. A Neural Mechanism Mediating the Impact of Episodic Prospection on Farsighted Decisions. J. Neurosci. 2011, 31, 6771–6779. [Google Scholar] [CrossRef]
- Schacter, D.L.; Benoit, R.G.; Szpunar, K.K. Episodic Future Thinking: Mechanisms and Functions. Curr. Opin. Behav. Sci. 2017, 17, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Fellows, L.K. The Cognitive Neuroscience of Human Decision Making: A Review and Conceptual Framework. Behav. Cogn. Neurosci. Rev. 2004, 3, 159–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinmetz, J.; Tausen, B.M.; Risen, J.L. Mental Simulation of Visceral States Affects Preferences and Behavior. Pers. Soc. Psychol. Bull. 2018, 44, 406–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barkley, R.A. The Executive Functions and Self-Regulation: An Evolutionary Neuropsychological Perspective. Neuropsychol. Rev. 2001, 11, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, R.F.; Vohs, K.D. Self-Regulation, Ego Depletion, and Motivation. Soc. Personal. Psychol. Compass 2007, 1, 115–128. [Google Scholar] [CrossRef] [Green Version]
- Calderon, C.B.; Loof, E.D.; Ergo, K.; Snoeck, A.; Boehler, C.N.; Verguts, T. Signed Reward Prediction Errors in the Ventral Striatum Drive Episodic Memory. J. Neurosci. 2021, 41, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Ergo, K.; De Vilder, L.; De Loof, E.; Verguts, T. Reward Prediction Errors Drive Declarative Learning Irrespective of Agency. Psychon. Bull. Rev. 2021, 28, 2045–2056. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, N.; Niv, Y. Signed and Unsigned Reward Prediction Errors Dynamically Enhance Learning and Memory. eLife 2021, 10, e61077. [Google Scholar] [CrossRef]
- Ergo, K.; De Loof, E.; Verguts, T. Reward Prediction Error and Declarative Memory. Trends Cogn. Sci. 2020, 24, 388–397. [Google Scholar] [CrossRef] [Green Version]
- Bein, O.; Plotkin, N.A.; Davachi, L. Mnemonic Prediction Errors Promote Detailed Memories. Learn. Mem. 2021, 28, 422–434. [Google Scholar] [CrossRef]
- Mason, A.; Farrell, S.; Howard-Jones, P.; Ludwig, C.J.H. The Role of Reward and Reward Uncertainty in Episodic Memory. J. Mem. Lang. 2017, 96, 62–77. [Google Scholar] [CrossRef] [Green Version]
- Benoit, R.G.; Schacter, D.L. Specifying the Core Network Supporting Episodic Simulation and Episodic Memory by Activation Likelihood Estimation. Neuropsychologia 2015, 75, 450–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kent, C.; Lamberts, K. The Encoding–Retrieval Relationship: Retrieval as Mental Simulation. Trends Cogn. Sci. 2008, 12, 92–98. [Google Scholar] [CrossRef]
- Hardt, O.; Nader, K.; Nadel, L. Decay Happens: The Role of Active Forgetting in Memory. Trends Cogn. Sci. 2013, 17, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.H. Neural, Cellular and Molecular Mechanisms of Active Forgetting. Front. Syst. Neurosci. 2018, 12, 490–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubin, D.C.; Schulkind, M.D. The Distribution of Autobiographical Memories across the Lifespan. Mem. Cognit. 1997, 25, 859–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meeter, M.; Murre, J.M.J.; Janssen, S.M.J. Remembering the News: Modeling Retention Data from a Study with 14,000 Participants. Mem. Cognit. 2005, 33, 793–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Averell, L.; Heathcote, A. The Form of the Forgetting Curve and the Fate of Memories. J. Math. Psychol. 2011, 55, 25–35. [Google Scholar] [CrossRef]
- Lemaire, B.; Portrat, S. A Computational Model of Working Memory Integrating Time-Based Decay and Interference. Front. Psychol. 2018, 9, 416. [Google Scholar] [CrossRef]
- Takiyama, K. Context-Dependent Memory Decay Is Evidence of Effort Minimization in Motor Learning: A Computational Study. Front. Comput. Neurosci. 2015, 9, 4. [Google Scholar] [CrossRef]
- Tov, W. Daily Experiences and Well-Being: Do Memories of Events Matter? Cogn. Emot. 2012, 26, 1371–1389. [Google Scholar] [CrossRef] [PubMed]
- Yonelinas, A.P.; Ritchey, M. The Slow Forgetting of Emotional Episodic Memories: An Emotional Binding Account. Trends Cogn. Sci. 2015, 19, 259–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diener, E.; Lucas, R.E.; Oishi, S. Subjective Well-Being: The Science of Happiness and Life Satisfaction. In Handbook of Positive Psychology; Snyder, C.R., Lopez, S.J., Eds.; Oxford University Press: New York, NY, USA, 2002; Volume 2, pp. 63–73. [Google Scholar]
- Cohn-Sheehy, B.I.; Delarazan, A.I.; Crivelli-Decker, J.E.; Reagh, Z.M.; Mundada, N.S.; Yonelinas, A.P.; Zacks, J.M.; Ranganath, C. Narratives Bridge the Divide between Distant Events in Episodic Memory. Mem. Cognit. 2022, 50, 478–494. [Google Scholar] [CrossRef] [PubMed]
- Diener, E.; Lucas, R.E.; Scollon, C.N. Beyond the Hedonic Treadmill: Revising the Adaptation Theory of Well-Being. In The Science of Well-Being; Diener, E., Ed.; Springer: Dordrecht, Netherlands, 2009; pp. 103–118. ISBN 978-90-481-2350-6. [Google Scholar]
- Lucas, R.E. Adaptation and the Set-Point Model of Subjective Well-Being Does Happiness Change After Major Life Events? Curr. Dir. Psychol. Sci. 2007, 16, 75–79. [Google Scholar] [CrossRef]
- Diener, E.; Ng, W.; Harter, J.; Arora, R. Wealth and Happiness across the World: Material Prosperity Predicts Life Evaluation, Whereas Psychosocial Prosperity Predicts Positive Feeling. J. Pers. Soc. Psychol. 2010, 99, 52. [Google Scholar] [CrossRef] [Green Version]
- Biswas-Diener, R. Material Wealth and Subjective Well-Being. In The Science of Subjective Well-Being; Eid, M., Larsen, R.J., Eds.; Guilford Press: New York, NY, USA, 2008; pp. 307–322. ISBN 978-1-60623-073-2. [Google Scholar]
- Huppert, F.A.; So, T.T.C. Flourishing Across Europe: Application of a New Conceptual Framework for Defining Well-Being. Soc. Indic. Res. 2013, 110, 837–861. [Google Scholar] [CrossRef] [Green Version]
- Michaelson, J.; Abdallah, S.; Steuer, N.; Thompson, S.; Marks, N.; Aked, J.; Cordon, C.; Potts, R. National Accounts of Well-Being: Bringing Real Wealth onto the Balance Sheet; New Economics Foundation: London, UK, 2009. [Google Scholar]
- Tennant, R.; Hiller, L.; Fishwick, R.; Platt, S.; Joseph, S.; Weich, S.; Parkinson, J.; Secker, J.; Stewart-Brown, S. The Warwick-Edinburgh Mental Well-Being Scale (WEMWBS): Development and UK Validation. Health Qual. Life Outcomes 2007, 5, 63. [Google Scholar] [CrossRef] [Green Version]
- Keyes, C.L.M. Mental Illness and/or Mental Health? Investigating Axioms of the Complete State Model of Health. J. Consult. Clin. Psychol. 2005, 73, 539–548. [Google Scholar] [CrossRef] [Green Version]
- Diener, E.; Wirtz, D.; Tov, W.; Kim-Prieto, C.; Choi, D.; Oishi, S.; Biswas-Diener, R. New Well-Being Measures: Short Scales to Assess Flourishing and Positive and Negative Feelings. Soc. Indic. Res. 2010, 97, 143–156. [Google Scholar] [CrossRef]
- Seligman, M.E.P. Flourish: A Visionary New Understanding of Happiness and Well-Being; Simon & Schuster: New York, NY, USA, 2011; ISBN 978-1-86471-297-1. [Google Scholar]
- Waterman, A.S.; Schwartz, S.J.; Zamboanga, B.L.; Ravert, R.D.; Williams, M.K.; Bede Agocha, V.; Yeong Kim, S.; Brent Donnellan, M. The Questionnaire for Eudaimonic Well-Being: Psychometric Properties, Demographic Comparisons, and Evidence of Validity. J. Posit. Psychol. 2010, 5, 41–61. [Google Scholar] [CrossRef]
- Ryff, C.D. Happiness Is Everything, or Is It? Explorations on the Meaning of Psychological Well-Being. J. Pers. Soc. Psychol. 1989, 57, 1069. [Google Scholar] [CrossRef]
- Ryan, R.M.; Deci, E.L. On Happiness and Human Potentials: A Review of Research on Hedonic and Eudaimonic Well-Being. Annu. Rev. Psychol. 2001, 52, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Antonovsky, A. Unraveling the Mystery of Health: How People Manage Stress and Stay Well, 1st ed.; Jossey-Bass: San Francisco, CA, USA, 1987; ISBN 978-1-55542-028-4. [Google Scholar]
- Bernard, L.C.; Mills, M.; Swenson, L.; Walsh, R.P. An Evolutionary Theory of Human Motivation. Genet. Soc. Gen. Psychol. Monogr. 2005, 131, 129–184. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.; Kenrick, D.T.; Neel, R.; Neuberg, S.L. Evolution and Human Motivation: A Fundamental Motives Framework. Soc. Personal. Psychol. Compass 2017, 11, e12319. [Google Scholar] [CrossRef]
- Talevich, J.R.; Read, S.J.; Walsh, D.A.; Iyer, R.; Chopra, G. Toward a Comprehensive Taxonomy of Human Motives. PLoS ONE 2017, 12, e0172279. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, S.H. Universals in the Content and Structure of Values: Theoretical Advances and Empirical Tests in 20 Countries. In Advances in Experimental Social Psychology; Academic Press: Cambridge, MA, USA, 1992; Volume 25, pp. 1–65. [Google Scholar]
- Solomon, R.L.; Corbit, J.D. An Opponent-Process Theory of Motivation: I. Temporal Dynamics of Affect. Psychological 1974, 81, 119–145. [Google Scholar] [CrossRef]
- Biswas-Diener, R.; Vittersø, J.; Diener, E. Most People Are Pretty Happy, but There Is Cultural Variation: The Inughuit, The Amish, and The Maasai. J. Happiness Stud. 2005, 6, 205–226. [Google Scholar] [CrossRef]
- Trampe, D.; Quoidbach, J.; Taquet, M. Emotions in Everyday Life. PLoS ONE 2015, 10, e0145450. [Google Scholar] [CrossRef] [Green Version]
- Baumeister, R.F.; Bratslavsky, E.; Finkenauer, C.; Vohs, K.D. Bad Is Stronger than Good. Rev. Gen. Psychol. 2001, 5, 323–370. [Google Scholar] [CrossRef]
- Fujita, F.; Diener, E. Life Satisfaction Set Point: Stability and Change. J. Pers. Soc. Psychol. 2005, 88, 158. [Google Scholar] [CrossRef]
- Lyubomirsky, S. Hedonic Adaptation to Positive and Negative Experiences. In The Oxford Handbook of Stress, Health, and Coping; Folkman, S., Ed.; Oxford University Press: Oxford, UK, 2011; pp. 200–224. [Google Scholar]
- Luhmann, M.; Intelisano, S. Hedonic Adaptation and the Set Point for Subjective Well-Being. In Handbook of Well-Being; Diener, E., Oishi, S., Tay, L., Eds.; DEF Publishers: Salt Lake City, UT, USA, 2018; p. 26. [Google Scholar]
- Buchanan, K.E.; Bardi, A. Acts of Kindness and Acts of Novelty Affect Life Satisfaction. J. Soc. Psychol. 2010, 150, 235–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldon, K.; Boehm, J.; Lyubomirsky, S. Variety Is the Spice of Happiness: The Hedonic Adaptation Prevention (HAP) Model. In Oxford Handbook of Happiness; David, S.A., Boniwell, I., Ayers, A.C., Eds.; Oxford University Press: Oxford, UK, 2013; pp. 901–914. ISBN 978-0-19-955725-7. [Google Scholar]
- Sheldon, K.M.; Lyubomirsky, S. The Challenge of Staying Happier: Testing the Hedonic Adaptation Prevention Model. Pers. Soc. Psychol. Bull. 2012, 38, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Keyes, C.L.M.; Myers, J.M.; Kendler, K.S. The Structure of the Genetic and Environmental Influences on Mental Well-Being. Am. J. Public Health 2010, 100, 2379–2384. [Google Scholar] [CrossRef] [PubMed]
- Nes, R.B.; Røysamb, E. The Heritability of Subjective Well-Being: Review and Meta-Analysis. In Genetics of Psychological Well-Being; Pluess, M., Ed.; Oxford University Press: Oxford, UK, 2015; pp. 75–96. ISBN 978-0-19-968667-4. [Google Scholar]
- Archontaki, D.; Lewis, G.J.; Bates, T.C. Genetic Influences on Psychological Well-Being: A Nationally Representative Twin Study. J. Pers. 2013, 81, 221–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scollon, C.N.; Diener, E.; Oishi, S.; Biswas-Diener, R. Emotions Across Cultures and Methods. J. Cross-Cult. Psychol. 2004, 35, 304–326. [Google Scholar] [CrossRef]
- Gilbert, D.T.; Driver-Linn, E.; Wilson, T.D. The Trouble with Vronsky: Impact Bias in the Forecasting of Future Affective States. In The Wisdom in Feeling: Psychological Processes in Emotional Intelligence; Barrett, L.F., Salovey, P., Eds.; The Guilford Press: New York, NY, USA, 2002; pp. 114–143. [Google Scholar]
- Gilbert, D.T.; Wilson, T.D. Prospection: Experiencing the Future. Science 2007, 317, 1351–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, R.E. Exploring the Associations Between Personality and Subjective Well-Being. In Handbook of Well-Being; Diener, E., Oishi, S., Tay, L., Eds.; DEF Publishers: Salt Lake City, UT, USA, 2018; p. 15. [Google Scholar]
- Buss, D.M. The Evolution of Happiness. Am. Psychol. 2000, 55, 15. [Google Scholar] [CrossRef]

| Aspect | Examples of Possible Approach Emotions or Cues | Examples of Possible Avoidance Emotions or Cues |
|---|---|---|
| Vitality | Vigour, strength, speed | Tiredness, physical difficulty, pain, discomfort |
| Engagement | Interest, curiosity, flow, novelty | Boredom, frustration, overwhelm |
| Positive relationships | Love, playfulness, touch, gratitude, trust, kindness | Loneliness, grief, deceit, anger, exploitation, exclusion, rejection |
| Autonomy | Respect, freedom, independence | Coercion, dominance, aggression, obligation |
| Competence | Goal completion, problem solving, influence | Goal failure, confusion, uncertainty, loss, making mistakes |
| Positive expectancy | Hopefulness, excitement | Anxiety, fear |
| Meaning | Praise, appreciation, receiving attention, gratification | Ridicule, embarrassment, criticism |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusk, R.D. An Adaptive Motivation Approach to Understanding the ‘How’ and ‘Why’ of Wellbeing. Int. J. Environ. Res. Public Health 2022, 19, 12784. https://doi.org/10.3390/ijerph191912784
Rusk RD. An Adaptive Motivation Approach to Understanding the ‘How’ and ‘Why’ of Wellbeing. International Journal of Environmental Research and Public Health. 2022; 19(19):12784. https://doi.org/10.3390/ijerph191912784
Chicago/Turabian StyleRusk, Reuben D. 2022. "An Adaptive Motivation Approach to Understanding the ‘How’ and ‘Why’ of Wellbeing" International Journal of Environmental Research and Public Health 19, no. 19: 12784. https://doi.org/10.3390/ijerph191912784
APA StyleRusk, R. D. (2022). An Adaptive Motivation Approach to Understanding the ‘How’ and ‘Why’ of Wellbeing. International Journal of Environmental Research and Public Health, 19(19), 12784. https://doi.org/10.3390/ijerph191912784





