Evaluation of Three Marine Algae on Degradability, In Vitro Gas Production, and CH4 and CO2 Emissions by Ruminants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algae Harvesting
2.2. Evaluations of Volatile Organic Compounds (VOCs) in Kelp, Silk, and Ulva by Flash Gas Chromatography Electronic Nose
2.3. Chemical Composition Analysis and In Vitro Fermentation Kinetics
2.4. Methane (CH4) and Carbon Dioxide (CO2) Production
2.5. Statistical Analysis
3. Results
3.1. The VOCs Present in Kelp, Silk, and Ulva Algae Determined by FGC- E-Nose
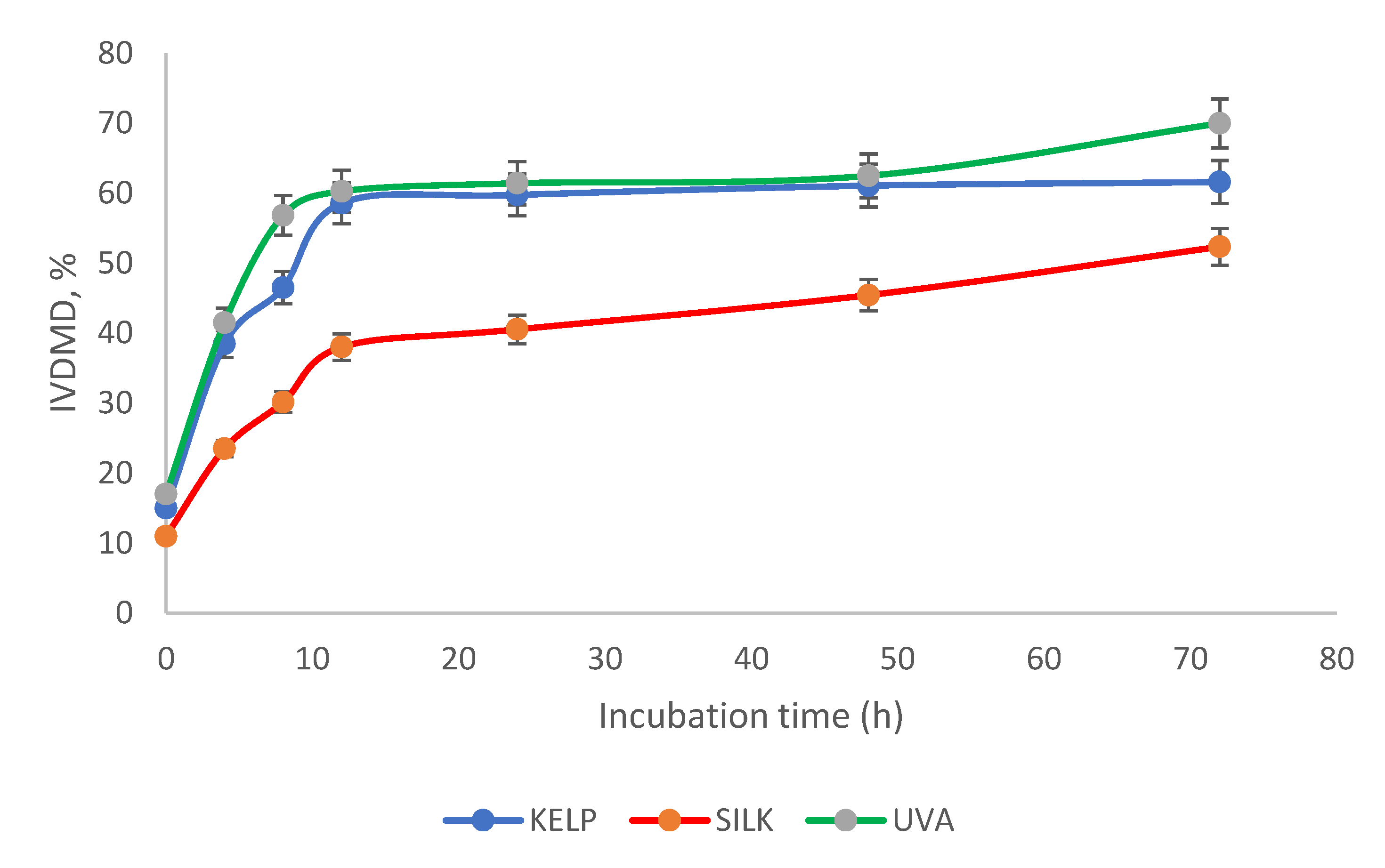
3.2. Chemical Composition, In vitro Dry Matter Degradability (IVDMD), and In Vitro Fermentation Kinetics
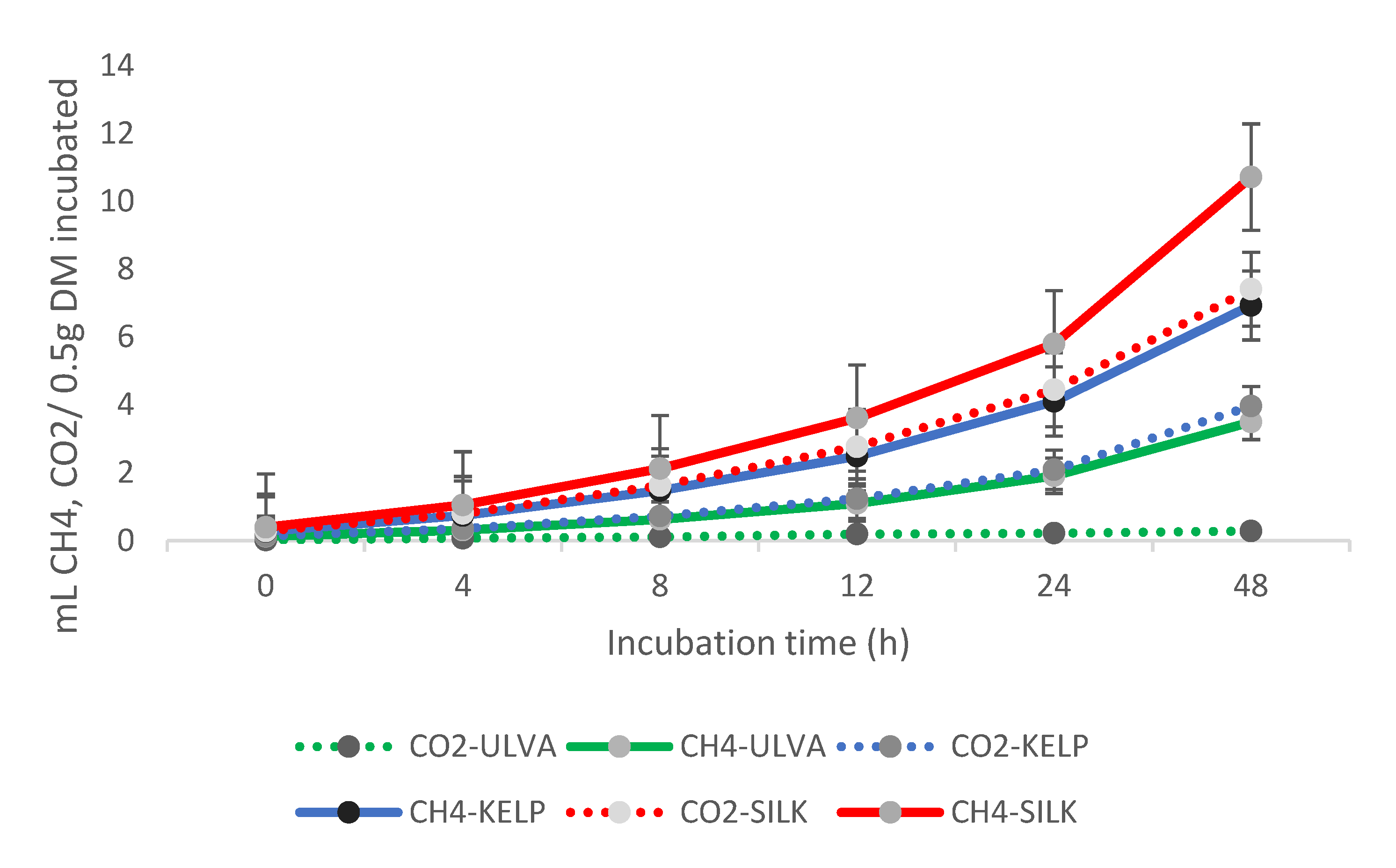
3.3. Ruminal CO2 and CH4 Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delgado, C.L.; Rosegrant, M.W.; Steinfeld, H.; Ehui, S.K.; Courbois, C. Livestock to 2020: The Next Food Revolution; International Food Policy Research Institute: Washington, DC, USA; FAO: Québec City, Canada; International Livestock Research Institute: Nairobi, Kenya, 1999. [Google Scholar]
- Beauchemin, K.A.; Ungerfeld, E.M.; Eckard, R.J.; Wang, M. Review: Fifty years of research on rumen methanogenesis: Lessons learned and future challenges for mitigation. Animals 2020, 14, s2–s16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinfeld, H.; Gerber, P.; Wassenaar, T.; Castel, V.; Rosales, M.; de-Haan, C. Livestock’s Long Shadow. FAO: Rome, Italy; p. 416.
- Eckard, R.; Grainger, C.; de Klein, C.A.M. Options for the abatement of methane and nitrous oxide from ruminant production: A review. Livest. Sci. 2010, 130, 47–56. [Google Scholar] [CrossRef]
- Benaouda, M.; González, M.; Molina, L.T.; Castelán, O.A. Estado de la investigación sobre emisiones de metano entérico y estrategias de mitigación en América Latina. Rev. Mex. Cienc. Agrícolas 2017, 8, 965–974. [Google Scholar] [CrossRef] [Green Version]
- Ku-Vera, J.C.; Castelán-Ortega, O.A.; Galindo-Maldonado, F.A. Review: Strategies for enteric methane mitigation in cattle fed tropical forages. Animal 2020, 14, s453–s463. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Giger-Reverdin, S.; Lessire, M.; Lebas, F. Seaweeds for livestock diets: A review. Anim. Feed Sci. Tech. 2016, 212, 1–17. [Google Scholar] [CrossRef]
- Rey-Crespo, F.; López-Alonso, M.; Miranda, M. The use of seaweed from the Galician coast as a mineral supplement in organic dairy cattle. Animal 2014, 8, 580–586. [Google Scholar] [CrossRef]
- Li, X.; Norman, H.C.; Kinley, R.D.; Laurence, M.; Wilmot, M.; Bender, H.; de Nys, R.; Tomkins, N. Asparagopsis taxiformis decreases enteric methane production from sheep. Anim. Prod. Sci. 2018, 58, 681–688. [Google Scholar] [CrossRef]
- Melucci, D.A.; Bendini, F.; Tesini, S.; Barbieri, A.; Zappi, S.; Vichi, T.; Toschi, G. Rapid direct analysis to discriminate geographic origin of extra virgin olive oils by flash gas chromatography electronic nose and chemometrics. Food Chem. 2016, 204, 263–273. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Rockville, Maryland; The William Byrd Press Inc.: Richmond, VA, USA, 1990. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and non–starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Cobos, M.; Yokoyama, M. Clostridium paraputrificum var ruminantium: Colonization and degradation of shrimp carapaces in vitro observed by scanning electron microscopy. In Proceedings of the a Workshop, Addis Ababa, Ethiopia; International Livestock Research Institute: Nairobi, Kenya, 1995; pp. 151–161. [Google Scholar]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 9–52. [Google Scholar]
- Miranda, L.A.; Lee-Rangel, H.A.; Mendoza-Martínez, G.D.; Crosby-Galván, M.M.; Relling, A.E.; Pinos-Rodríguez, J.M.; Rojo Rubio, R.; González Hernandez, M. Influence of calcium propionate on in vitro fermentation of sorghum-based diets Revista de la Facultad de Ciencias Agrarias. DOAJ 2017, 49, 185–192. [Google Scholar]
- Sall, J.; Lehman, A.; Stephens, M.; Creighton, L. JMP® Start Statistics: A Guide to Statistics and Data Analysis, 5th ed.; SAS Institute Inc.: Cary, NC, USA, 2012. [Google Scholar]
- Jerković, I.; Marijanović, Z.; Roje, M.; Kuś, P.M.; Jokić, S.; Čož-Rakovac, R. Phytochemical study of the headspace volatile organic compounds of fresh algae and seagrass from the Adriatic Sea (single point collection). PLoS ONE 2018, 13, e0196462. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Kumar, M.; Gupta, V. Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem. 2010, 120, 749–757. [Google Scholar] [CrossRef]
- Berneira, L.M.; da Silva, C.C.; Passos, L.F.; Mansilla, A.; dos Santos, M.A.Z.; de Pereira, C.M.P. Evaluation of volatile organic compounds in brown and red sub-Antarctic macroalgae. Rev. Bras. Bot. 2021, 44, 79–84. [Google Scholar] [CrossRef]
- Yamamoto, M.; Baldermann, S.; Yoshikawa, K. Determination of volatile compounds in four commercial samples of japanese green algae using solid phase microextraction gas chromatography mass spectrometry. Sci. World J. 2014, 2014, 289780. [Google Scholar] [CrossRef]
- Kamenarska, Z.; Ivanova, A.; Stancheva, R. Volatile compounds from some red algae and their chemotaxonomic application. Bot. Mar. 2006, 49, 47–56. [Google Scholar] [CrossRef]
- Balbas, J.; Hamid, N.; Liu, T. Comparison of physicochemical characteristics, sensory properties and volatile composition between commercial and New Zealand made wakame from Undaria pinnatifida. Food Chem. 2015, 186, 168–175. [Google Scholar] [CrossRef]
- Berneira, L.; da Silva, C.; Poletti, T. Evaluation of the volatile composition and fatty acid profle of seven Antarctic macroalgae. J. Appl. Phycol. 2020, 32, 3319–3329. [Google Scholar] [CrossRef]
- Haznedaroglu, M.Z.; Zeybec, U. HPLC determination of chicoric acid in leaves of Posidonia oceanica. Pharm Biol. 2007, 45, 745–748. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Lee, S.J.; Lee, Y.J.; Kim, H.S.; Eom, J.S.; Kim, S.C.; Kim, E.T.; Lee, S.S. New challenges for efficient usage of Sargassum fusiforme for ruminant production. Sci. Rep. 2020, 10, 19655. [Google Scholar] [CrossRef]
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a Functional Ingredient for a Healthy Diet. Mar. Drugs 2020, 18, 301. [Google Scholar]
- Zhou, A.Y.; Robertson, J.; Hamid, N.; Ma, Q.; Lu, J. Changes in Total Nitrogen and Amino Acid Composition of New Zealand Undaria pinnatifida with Growth, Location and Plant Parts. Food Chem. 2015, 186, 319–325. [Google Scholar] [CrossRef]
- Biancarosa, I.; Espe, M.; Bruckner, C.G.; Heesch, S.; Liland, N.; Waagbø, R.; Torstensen, B.; Lock, E.J. Amino Acid Composition, Protein Content, and Nitrogen-to-Protein Conversion Factors of 21 Seaweed Species from Norwegian Waters. J. Appl. Phycol. 2017, 29, 1001–1009. [Google Scholar] [CrossRef]
- Kendel, M.; Wielgosz-Collin, G.; Bertrand, S.; Roussakis, C.; Bourgougnon, N.; Bedoux, G. Lipid composition, fatty acids and sterols in the seaweeds Ulva armoricana, and Solieria chordalis from Brittany (France): An analysis from nutritional, chemotaxonomic, and antiproliferative activity perspectives. Mar. Drugs 2015, 13, 5606–5628. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Machado, D.I.; Lopez-Cervantes, J.; Lopez-Hernandez, J.; Paseiro-Losada, P. Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem. 2004, 85, 439–444. [Google Scholar] [CrossRef]
- Pardilhó, S.; Cotas, J.; Pereira, L.; Oliveira, M.B.; Dias, J.M. Marine macroalgae in a circular economy context: A comprehensive analysis focused on residual biomass. Biotechnol. Adv. 2022, 107987. [Google Scholar] [CrossRef]
- Sudhakar, K.; Mamat, R.; Samykano, M.; Azmi, W.H.; Ishak, W.F.W.; Yusaf, T. An overview of marine macroalgae as bioresource. Renew. Sust. Energ. Rev. 2018, 91, 165–179. [Google Scholar] [CrossRef]
- Filote, C.; Santos, S.C.R.; Popa, V.I.; Botelho, C.M.S.; Volf, I. Biorefinery of marine macroalgae into high-tech bioproducts: A review. Environ. Chem. Lett. 2020, 19, 969–1000. [Google Scholar] [CrossRef]
- Dubois, B.; Tomkins, N.W.; Kinley, R.D.; Bai, M.; Seymour, S.; Paul, N.A.; de Nys, R. Effect of tropical algae as additives on rumen in vitro gas production and fermentation characteristics. Am. J. Plant Sci. 2013, 4, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Ventura, M.R.; Castañón, J.I.R. The nutritive value of seaweed (Ulva lactuca) for goats. Small Rumin. Res. 1998, 29, 325–327. [Google Scholar] [CrossRef]
- Zitouni, H.; Arhab, R.; Boudry, C.; Bousseboua, H.; Beckers, Y. Chemical and biological evaluation of the nutritive value of Algerian green seaweed Ulva lactuca using in vitro gas production technique for ruminant animals. Int. J. Advan. Res. 2014, 2, 916–925. [Google Scholar]
- Hansen, H.R.; Hector, B.L.; Feldmann, J. A qualitative and quantitative evaluation of the seaweed diet of North Ronaldsay sheep. Anim. Feed Sci. Tech. 2003, 105, 21–28. [Google Scholar] [CrossRef]
- Mora Castro, N.; Casas Valdez, M.; Águila Ramírez, R.N.; Sánchez Rodríguez, I.; Hernández Contreras, H.; Sanginés García, L. The kelp Macrocystis pyrifera as nutritional supplement for goats. Rev. Científica 2009, 19, 63–70. [Google Scholar]
- Casas-Valdez, M.; Hernández-Contreras, H.; Marín-Alvarez, A. El alga marina Sargassum (Sargassaceae): Una alternativa tropical para la alimentación de ganado caprino [The seaweed Sargassum (Sargassaceae) as tropical alternative for goats’ feeding]. Rev. Biol. Trop. 2006, 54, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marín, A.A. Utilización del alga Sargassum spp. Como Complemento Alimenticio de Ganado Ovino. Master’s Thesis, Centro Interdisciplinario de Ciencias Marinas, I.P.N. La Paz, México, 1999. [Google Scholar]
- Rodriguez, M.P.; Mariezcurrena, M.D.; Mariezcurrena, M.A.; Lagunas, B.C.; Elghandour, M.M.M.Y.; Kholif, A.M.; Kholif, A.E.; Almaraz, E.M.; Salem, A.Z.M. Influence of live cells or cells extract of Saccharomyces cerevisiae on in vitro gas production of a total mixed ration. Ital. J. Anim. Sci. 2015, 14, 590–595. [Google Scholar] [CrossRef]
- Elghandour, M.M.Y.; Kholif, A.E.; Hernández, J.; Mariezcurrena, M.D.; López, S.; Camacho, L.M.; Márquez, O.; Salem, A.Z.M. Influence of the addition of exogenous xylanase with or without pre-incubation on the in vitro ruminal fermentation of three fibrous feeds. Czech J. Anim. Sci. 2016, 61, 262–272. [Google Scholar] [CrossRef] [Green Version]
- Hamid, P.; Akbar, T.; Hossein, J.; Ali, M.G. Nutrient Digestibility and Gas Production of Some Tropical Feeds Used in Ruminant Diets Estimated by the in vivo and in vitro Gas Production Techniques. Am. J. Anim. Vet. Sci 2007, 2, 108–113. [Google Scholar] [CrossRef] [Green Version]
- Burtin, P. Nutritional value of seaweeds. Electron. J. Environ. Agric. Food Chem. 2003, 2, 498–503. [Google Scholar]
- Deal, M.S.; Hay, M.E.; Wilson, D.; Fenical, W. Galactolipids rather than phlorotannins as herbivore deterrents in the brown seaweed Fucus vesiculosus. Oecologia 2003, 136, 107–114. [Google Scholar] [CrossRef]
- Wang, Y.; Alexander, T.W.; Mcallister, T.A. In vitro effects of phlorotannins from Ascophyllum nodosum (brown seaweed) on rumen bacterial populations and fermentation. J. Sci. Food Agric. 2009, 89, 2252–2260. [Google Scholar] [CrossRef]
- Belanche, A.; Ramos-Morales, E.; Newbold, C.J. In vitro screening of natural feed additives from crustaceans, diatoms, seaweeds and plant extracts to manipulate rumen fermentation. J. Sci. Food Agric. 2016, 96, 3069–3078. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.; Morgavi, D.P.; Doreau, M. Methane mitigation in ruminants: From microbe to the farm scale. Animal. 2010, 4, 351–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee-Rangel, H.A.; Vázquez Valladolid, A.; Mendez-Cortes, H.; Garcia-Lopez, J.C.; Álvarez-Fuentes, G.; Roque-Jimenez, J.A.; Mejia-Delgadillo, M.A.; Negrete-Sánchez, L.O.; Cifuentes-López, O.; Ramírez-Tobías, H.M. Influence of Copra Meal in the Lambs Diet on In Vitro Ruminal Kinetics and Greenhouse Gases Production. Agriculture 2021, 11, 925. [Google Scholar] [CrossRef]
- Min, B.R.; Pinchak, W.E.; Anderson, R.C.; Callaway, T.R. Effect of tannins on the in vitro growth of Escherichia coli O157: H7 and in vivo growth of generic Escherichia coli excreted from steers. J. Food Prot. 2007, 70, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Machado, L.; Tomkins, N.; Magnusson, M.; Midgley, D.J.; de Nys, R.; Rosewarne, C.P. In vitro response of rumen microbiota to the antimethanogenic red macroalga Asparagopsis taxiformis. Microb. Ecol. 2018, 75, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Roque, B.M.; Salwen, J.K.; Kinley, R.; Kebreab, E. Inclusion of Asparagopsis armata in lactating dairy cows’ diet reduces enteric methane emission by over 50 percent. J. Clean. Prod. 2019, 234, 132–138. [Google Scholar] [CrossRef]
- Tayyab, U.; Novoa-Garrido, M.; Roleda, M.Y.; Lind, V.; Weisbjerg, M.R. Ruminal and intestinal protein degradability of various seaweed species measured in situ in dairy cows. Anim. Feed Sci. Technol. 2016, 213, 44–54. [Google Scholar] [CrossRef]
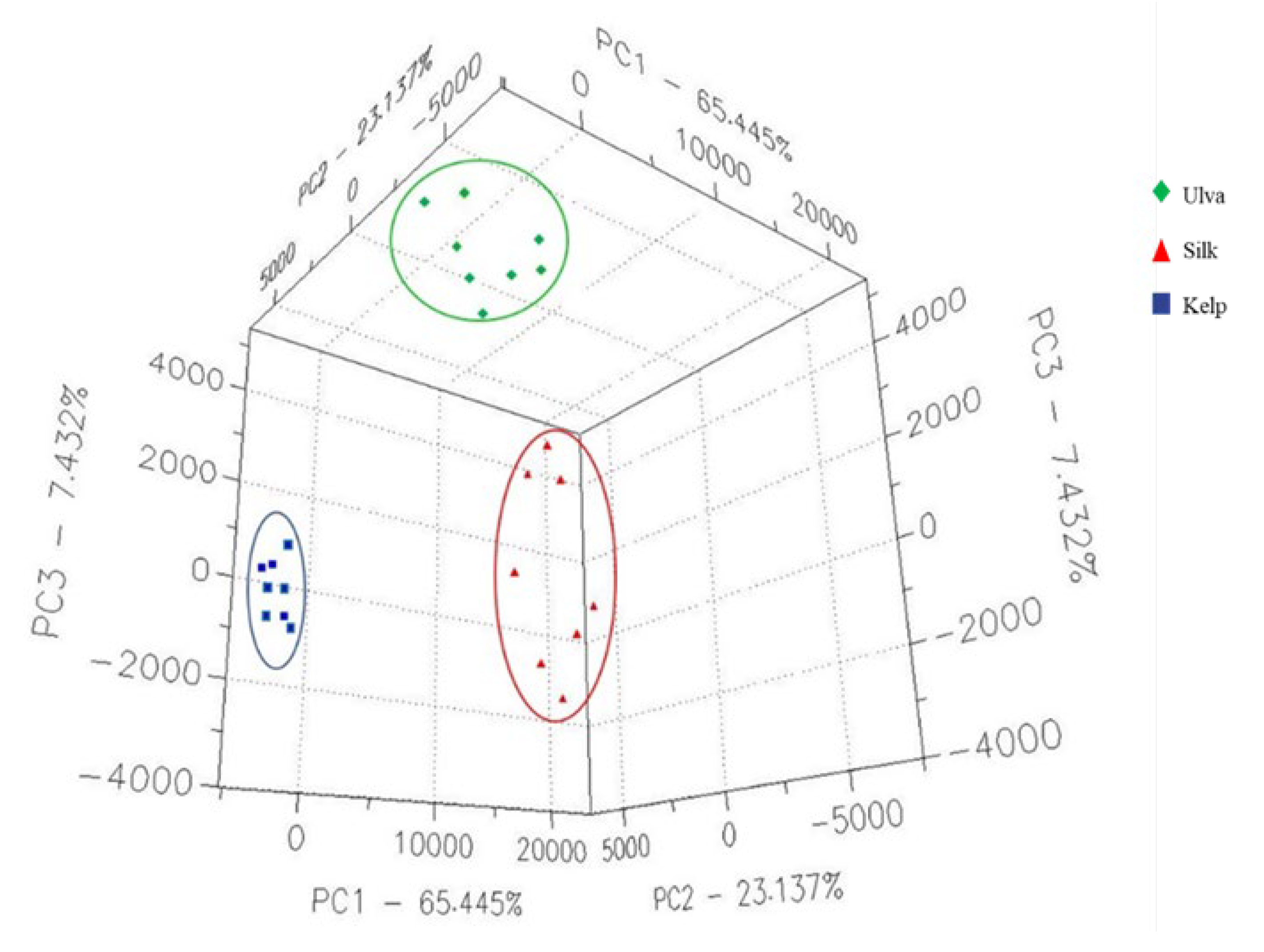
| Kelp | Ulva | Silk | ||||||
|---|---|---|---|---|---|---|---|---|
| RT min | Compound | Relevance Index | RT min | Compound | Relevance Index | RT min | Compound | Relevance Index |
| 12.69 | Butane | 44.45 | 12.7 | Butane | 51.14 | 15.11 | Butane | 56.94 |
| 13.02 | Butane | 50.93 | 13.02 | Butane | 56.94 | 17.67 | propanon-2-one | 79.55 |
| 14.57 | Trimethylamine | 44.82 | 14.57 | Trimethylamine | 49.06 | 18.68 | Diethyl ether | 57.98 |
| 15.53 | 2-Methylbutane | 39.77 | 15.53 | 2-Methylbutane | 43.72 | 19.78 | 1-Propanol | 83.79 |
| 17.01 | Diethyl ether | 57.97 | 17.01 | Diethyl ether | 57.98 | 20.85 | Carbon disulfide | 73.05 |
| 18.15 | 1,1-Dichloroethene | 55.69 | 19 | Propanon-2-one | 79.55 | 22.38 | butan-2-one | 80.93 |
| 19.04 | propanon-2-one | 57.39 | 20.26 | Diisopropyl ether | 70.53 | 24.21 | Trichloroethane | 39.48 |
| 20.27 | Ethene, 1,2-dichloro-, (E) - | 71.32 | 21.67 | 2-methylfuran | 79.10 | 25.13 | 2-Octenal, (E) | 68.34 |
| 21.69 | 1-Propanol | 62.40 | 22.5 | 1-Propanol | 83.79 | 27.17 | Acetoin | 77.74 |
| 22.55 | Carbon disulfide | 72.87 | 23.33 | butan-2-one | 80.93 | 28.3 | Methyl butanoate | 86.03 |
| 23.35 | 2-butanol | 85.82 | 24.16 | 2-butanol | 60.47 | 30.54 | pentanol | 81.53 |
| 24.17 | 2-butanol | 58.07 | 26.91 | Methyl butanoate | 86.03 | 32.39 | Octane | 70.26 |
| 25.11 | 1,2-Dichloroethane | 86.97 | 27.62 | 2,3-Pentanedione | 71.64 | 35.01 | E-2-Hexen-1-ol | 93.20 |
| 26.93 | 1,2-Dichloropropane | 80.11 | 30.04 | Acetoin | 77.74 | 37.09 | pentanoic acid | 82.72 |
| 27.66 | pental-2-ol | 76.88 | 30.82 | pentanol | 81.53 | 38.21 | Methyl hexanoate | 87.25 |
| 30.11 | Acetoin | 57.89 | 31.52 | Ethyl isovalerate | 83.32 | 39.86 | 1-Heptanol | 86.44 |
| 30.83 | pentanol | 72.40 | 33.89 | ethyl pentanoate | 73.53 | 40.63 | 2,3-Octanedione | 91.82 |
| 31.57 | Ethyl isovalerate | 86.62 | 34.44 | E-2-Hexen-1-ol | 93.20 | 41.46 | 2-Ethyl-3-methylpyrazine | 86.08 |
| 35.43 | (-) – beta,-Pinene | 84.61 | 35.38 | sabinene | 73.92 | 42.56 | acetilpyrazine | 77.41 |
| 36.03 | alfa-Phellandrene | 89.23 | 35.98 | alpha-Pheladrene | 83.34 | 43.43 | (Z)-2-octenal | 83.08 |
| 37.86 | Putrescine | 91.25 | 37.79 | 2,3-Octanedione | 91.82 | 44.11 | 1,2-Cyclopentanedione | 85.73 |
| 39.08 | Benzene, 1,2-dichloro | 80.26 | 38.59 | Undecane | 92.20 | 45.23 | Undecane | 92.20 |
| 39.76 | 2-Isopropyl-3-methoxypyrazine | 85.22 | 39.01 | terpinolene | 76.18 | 45.72 | p-menthatriene | 85.28 |
| 40.45 | p-menthatriene | 82.41 | 39.69 | acetilpyrazine | 77.41 | 46.19 | Limonene oxide | 79.92 |
| 41.36 | ethenyl-dimethylpyrazine | 85.79 | 40.41 | p-menthatriene | 85.28 | 47.73 | 2,3-Diethyl-5-methylpyrazine | 42.15 |
| 42.65 | ethyl 3-(methylthio)propanoate | 90.90 | 41.27 | ethenyl-dimethylpyrazine | 82.34 | 49.74 | Decanal | 88.12 |
| 43.19 | 1,2-Cyclopentanedione, 3,4… | 74.89 | 42.58 | Limonene oxide | 79.92 | 50.5 | 2,6-Dichlorophenol | 87.04 |
| 45.03 | Triethyl phosphate | 77.13 | 43.16 | 1-2-Cyclopentanedione, 3,4 | 85.73 | 52.32 | Anethole | 81.06 |
| 45.85 | p-Cresol | 79.15 | 44.96 | Decanal | 88.12 | 53.17 | ndecane-2-one | 72.60 |
| 47.45 | N,N-dimethylacetamide | 65.62 | 45.78 | Methyl salicylate | 79.22 | 56.09 | trans-2-Undecenal | 79.06 |
| 48.78 | Tetradecane | 87.87 | 47.38 | Nerol | 78.86 | 57.22 | Tetradecane | 80.74 |
| 49.85 | 5-ethyl-3-hydroxy-4-methyl-2… | 95.20 | 48.72 | 2,6-Dichlorophenol | 87.04 | 59.49 | Carbamothioic acid, butyleth | 33.39 |
| 51.62 | trans-2-Undecenal | 81.81 | 49.77 | 2,4-Decadienal, (E;Z) | 72.25 | 60.94 | beta-Himachalene | 41.58 |
| 54.41 | beta-Himachalene | 38.97 | 51.57 | trans-2-Undecenal | 79.06 | 63.91 | Rheosmin | 30.65 |
| 55.57 | beta-ionone | 35.18 | 54.25 | gamma-nonalactona | 48.98 | 74.38 | 1,4-Naphthalenedione | 28.00 |
| 56.88 | Mevinphos | 75.53 | 55.51 | beta-ionone | 34.85 | |||
| 57.56 | wine lactone | 87.26 | 67.13 | 1,4-Naphtalenedione, 2,3… | 28.00 | |||
| 58.44 | delta-decalactone | 84.64 | 70.3 | Chlorothalonil | 24.51 | |||
| 60.53 | Tebuthiuron | 76.31 | ||||||
| 61.95 | Acetamide, 2-chloro-N | 50.55 | ||||||
| 63.44 | 4(4-hydroxy-3-methoxypheny | 54.14 | ||||||
| 64.44 | 3-oxo-alpha-ionone | 29.49 | ||||||
| 67.04 | 1,4-Naphtalendione, 2,3 | 25.03 | ||||||
| Ulva | Silk | Kelp | |
|---|---|---|---|
| DM, % | 94.5 | 96.1 | 99.3 |
| OM, % | 78.6 | 79.1 | 77.1 |
| CP, % | 6.6 | 10.5 | 10.4 |
| EE, % | 0.33 | 0.19 | 0.41 |
| NDF, % | 32.3 | 39.2 | 38.6 |
| ADF, % | 43.4 | 46.2 | 40.1 |
| Ash, % | 21.4 | 20.9 | 22.9 |
| Kelp | Ulva | Silk | SEM | |
|---|---|---|---|---|
| The volume of gas produced (v), mL | 63.4a | 44.37ab | 30.33c | 12.9 |
| Production rate (s), mL/g−1 | 0.017b | 0.015b | 0.025a | 0.001 |
| Lag time (L), h | 17.29a | 9.49b | 9.47b | 2.3 |
| % CO2 at 48 h | 52.27a | 38.97ab | 28.38c | 4.81 |
| % CH4 at 48 h | 47.73c | 61.03b | 71.62a | 9.78 |
| ME, MJ/kg of DM | 4.06 | 3.54 | 3.48 | 0.85 |
| mmol SCFA | 0.205 | 0.295 | 0.196 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee-Rangel, H.A.; Roque-Jiménez, J.A.; Cifuentes-López, R.O.; Álvarez-Fuentes, G.; Cruz-Gómez, A.D.l.; Martínez-García, J.A.; Arévalo-Villalobos, J.I.; Chay-Canul, A.J. Evaluation of Three Marine Algae on Degradability, In Vitro Gas Production, and CH4 and CO2 Emissions by Ruminants. Fermentation 2022, 8, 511. https://doi.org/10.3390/fermentation8100511
Lee-Rangel HA, Roque-Jiménez JA, Cifuentes-López RO, Álvarez-Fuentes G, Cruz-Gómez ADl, Martínez-García JA, Arévalo-Villalobos JI, Chay-Canul AJ. Evaluation of Three Marine Algae on Degradability, In Vitro Gas Production, and CH4 and CO2 Emissions by Ruminants. Fermentation. 2022; 8(10):511. https://doi.org/10.3390/fermentation8100511
Chicago/Turabian StyleLee-Rangel, Héctor Aarón, José Alejandro Roque-Jiménez, Rubén Oswaldo Cifuentes-López, Gregorio Álvarez-Fuentes, Adriana De la Cruz-Gómez, José Antonio Martínez-García, Jaime Iván Arévalo-Villalobos, and Alfonso Juventino Chay-Canul. 2022. "Evaluation of Three Marine Algae on Degradability, In Vitro Gas Production, and CH4 and CO2 Emissions by Ruminants" Fermentation 8, no. 10: 511. https://doi.org/10.3390/fermentation8100511





