Effects of Different Extraction Methods on the Gelatinization and Retrogradation Properties of Highland Barley Starch
Abstract
1. Introduction
2. Results and Discussion
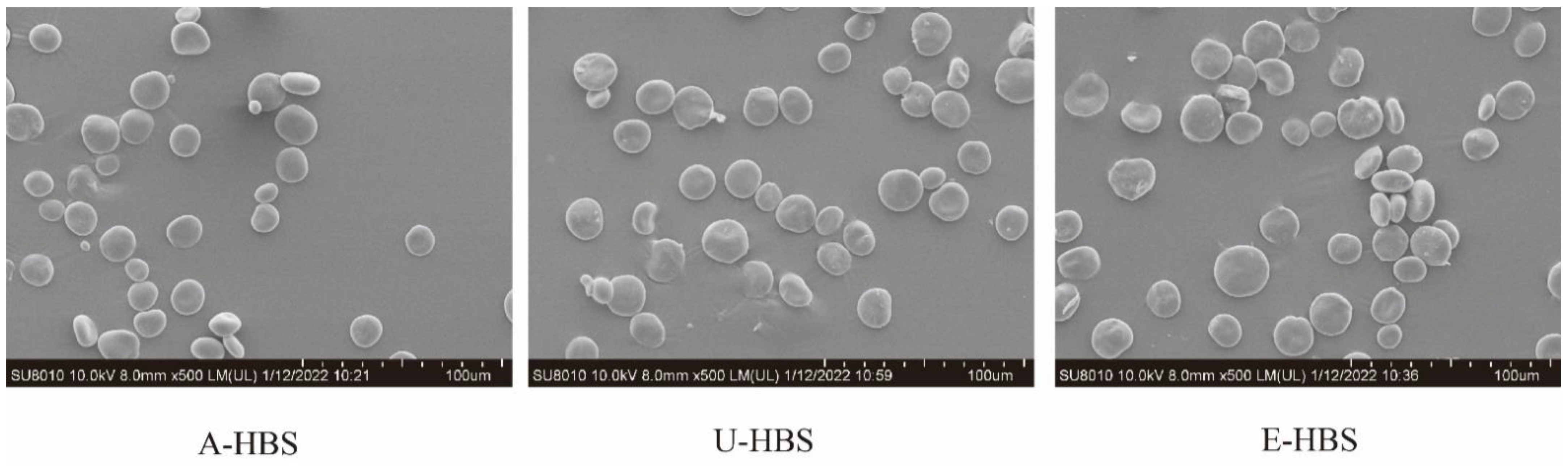
2.1. Morphology and Chemical Compositions of HBS
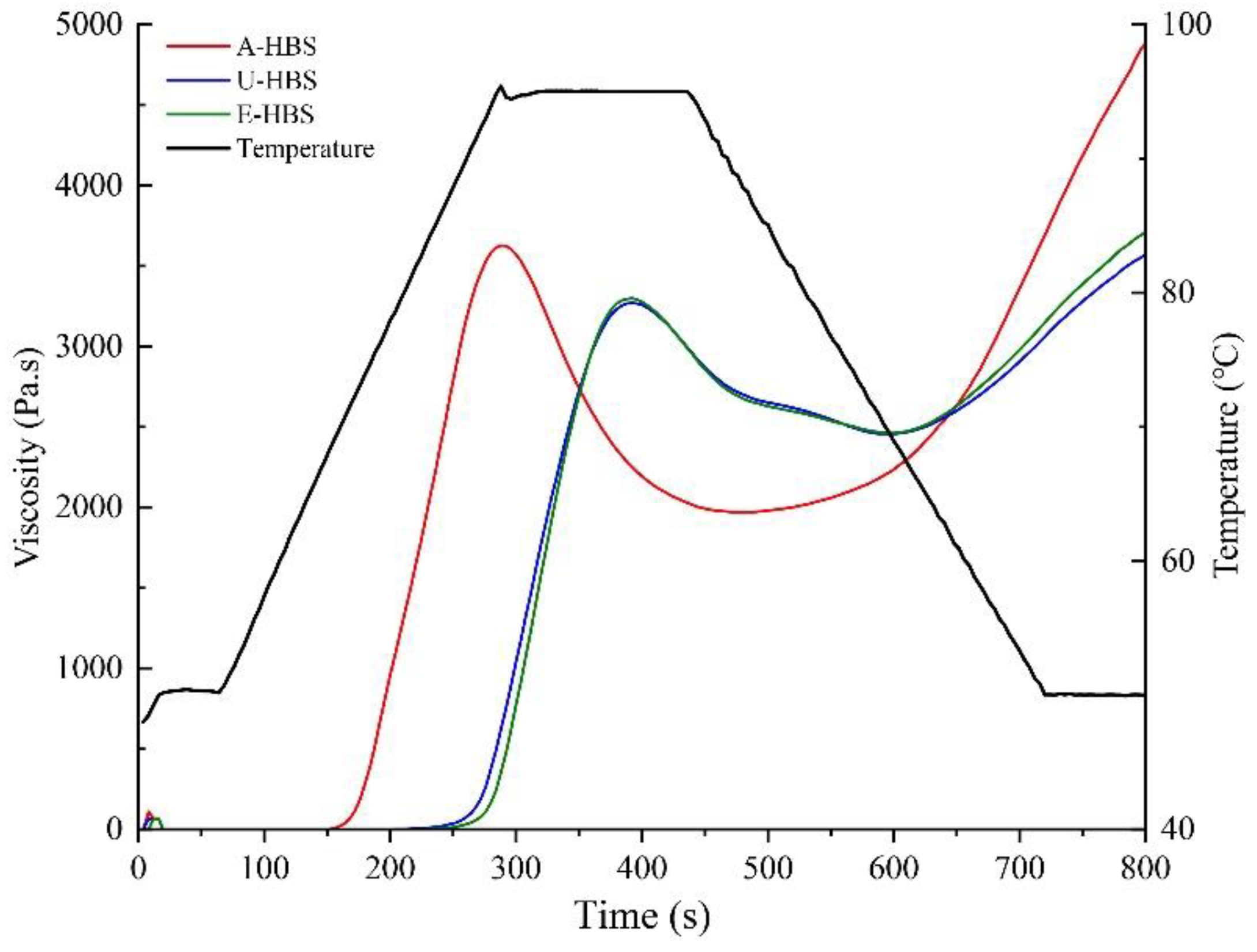
2.2. Pasting Properties
2.3. Gel Properties
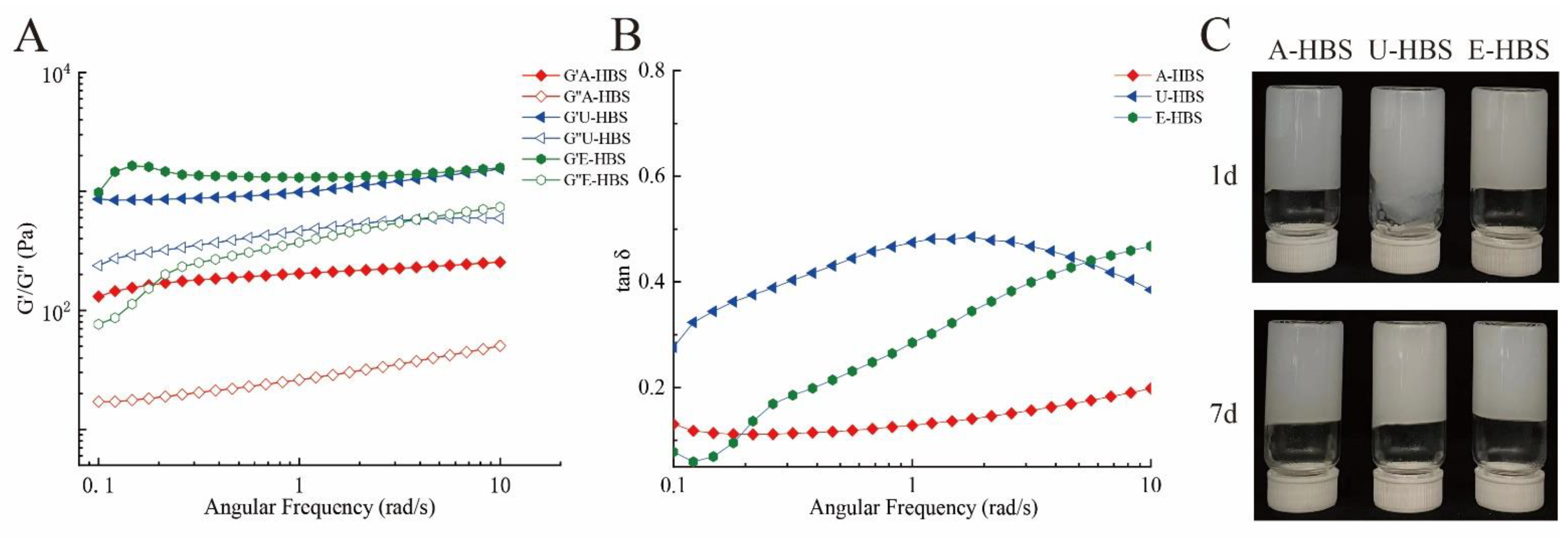
2.4. Rheological Properties of HBS
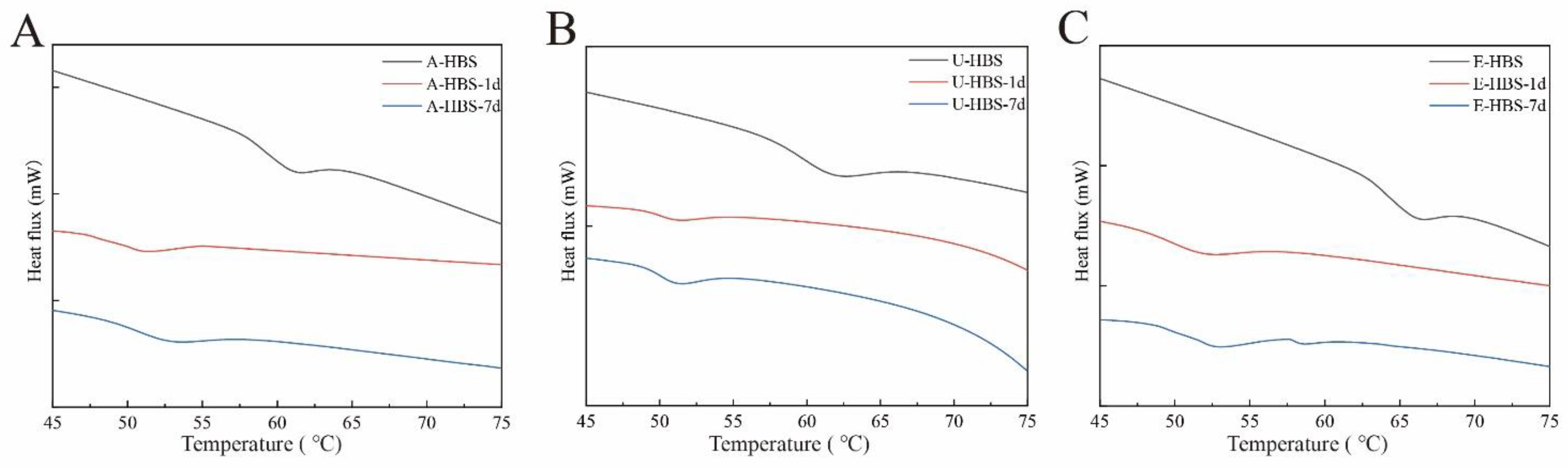
2.5. Thermal Properties
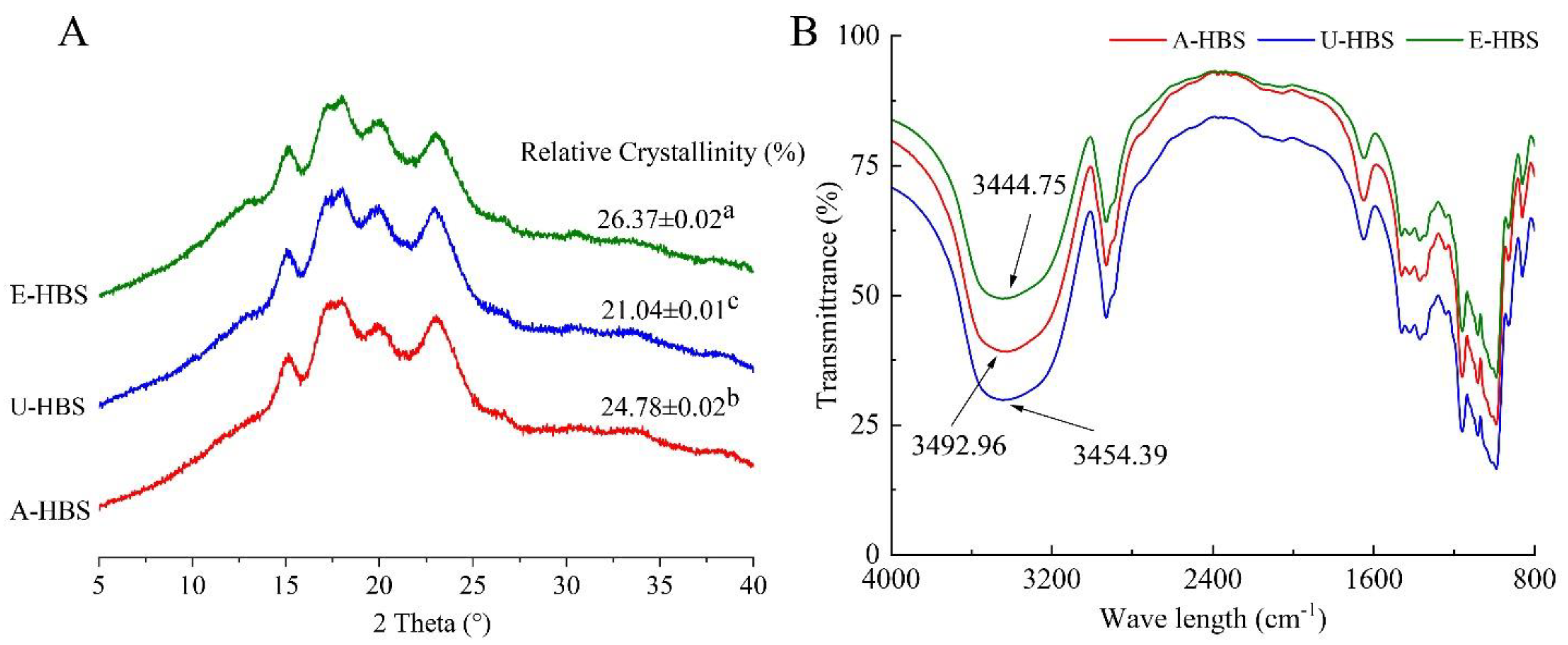
2.6. X-ray Diffraction Pattern
2.7. Short-Range Ordered Structure
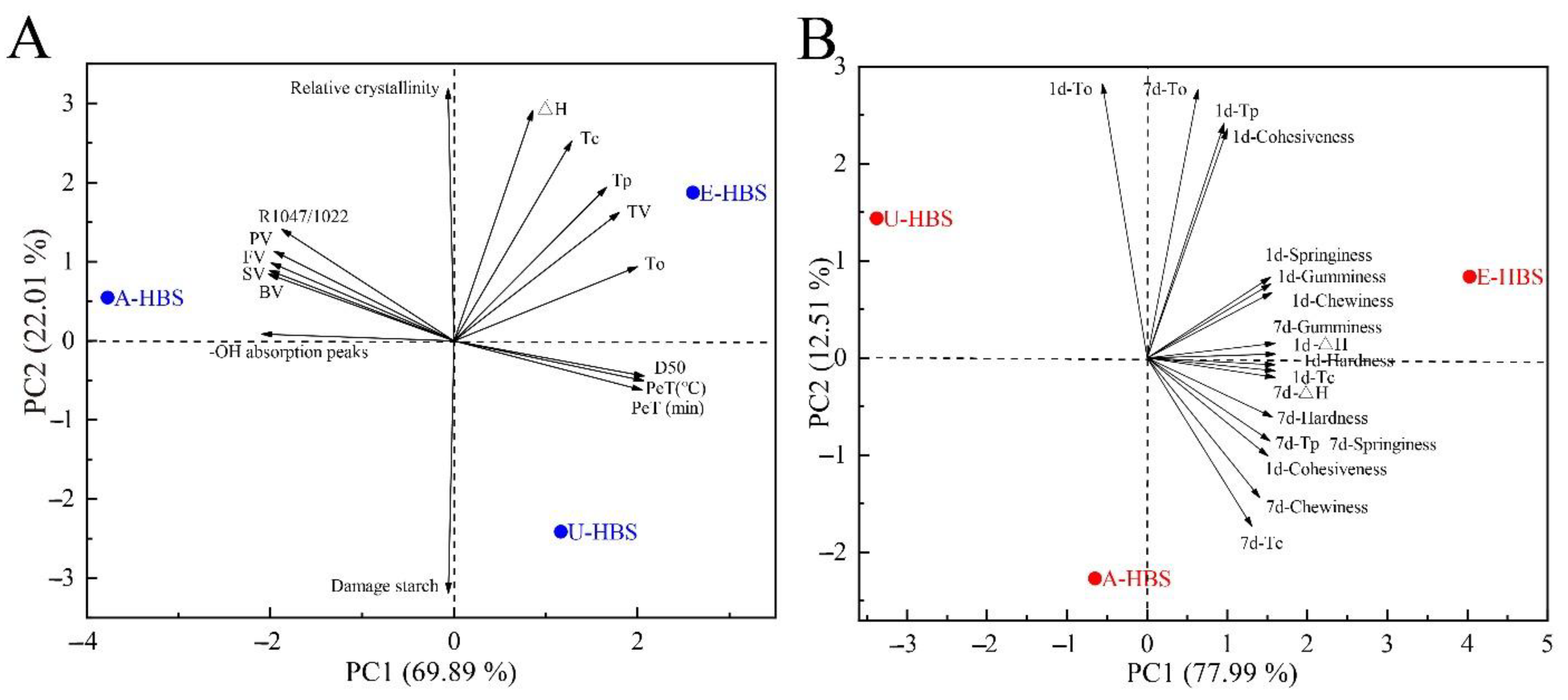
2.8. Principal Component Analysis (PCA)
3. Materials and Methods
3.1. Materials
3.2. Samples Preparation
3.2.1. Alkali Extraction
3.2.2. Ultrasound Extraction
3.2.3. Enzyme Extraction
3.3. General Compositions and Particle Size
3.4. Scanning Electron Microscopy (SEM)
3.5. Pasting Property
3.6. Texture Profile Analysis (TPA)
3.7. Rheological Characterization
3.8. Thermal Analysis
3.9. X-ray Diffraction (XRD) Analysis
3.10. Fourier-Transform Infrared Spectrometry (FTIR) Analysis
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Obadi, M.; Sun, J.; Xu, B. Highland barley: Chemical composition, bioactive compounds, health effects, and applications. Food Res. Int. 2021, 140, 110065. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.A.; Sarbini, S.R. The potential of resistant starch as a prebiotic. Crit. Rev. Biotechnol. 2016, 36, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, B.; Chen, L.; Li, X.; Zheng, B. Hierarchical structure and physicochemical properties of highland barley starch following heat moisture treatment. Food Chem. 2019, 271, 102–108. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Wang, L.; Wang, L.; Li, Z.; Qiu, J. Multi-scale structure, rheological and digestive properties of starch isolated from highland barley kernels subjected to different thermal treatments. Food Hydrocoll. 2022, 129, 107630. [Google Scholar] [CrossRef]
- Yang, Y.; Jiao, A.; Zhao, S.; Liu, Q.; Fu, X.; Jin, Z. Effect of removal of endogenous non-starch components on the structural, physicochemical properties, and in vitro digestibility of highland barley starch. Food Hydrocoll. 2021, 117, 106698. [Google Scholar] [CrossRef]
- Pires, M.B.; Amante, E.R.; Oliveira Petkowicz, C.L.; Esmerino, E.A.; Cruz Rodrigues, A.M.; Silva, L.H. Impact of extraction methods and genotypes on the properties of starch from peach palm (Bactris gasipaes Kunth) fruits. LWT-Food Sci. Technol. 2021, 150, 111983. [Google Scholar] [CrossRef]
- Umego, E.C.; He, R.; Ren, W.; Xu, H.; Ma, H. Ultrasonic-assisted enzymolysis: Principle and applications. Process Biochem. 2021, 100, 59–68. [Google Scholar] [CrossRef]
- Wang, L.Y.; Wang, M.; Zhou, Y.; Wu, Y.; Ouyang, J. Influence of ultrasound and microwave treatments on the structural and thermal properties of normal maize starch and potato starch: A comparative study. Food Chem. 2022, 377, 131990. [Google Scholar] [CrossRef]
- Lin, X.; Sun, S.; Wang, B.; Zheng, B.; Guo, Z. Structural and physicochemical properties of lotus seed starch nanoparticles prepared using ultrasonic-assisted enzymatic hydrolysis. Ultrason. Sonochemistry 2020, 68, 105199. [Google Scholar]
- Buksa, K. Extraction and characterization of rye grain starch and its susceptibility to resistant starch formation. Carbohydr. Polym. 2018, 194, 184–192. [Google Scholar] [CrossRef]
- Ozturk, O.K.; Kaasgaard, S.G.; Palmén, L.G.; Vidal, B.C.; Hamaker, B.R. Enzyme treatments on corn fiber from wet-milling process for increased starch and protein extraction. Ind. Crops Prod. 2021, 168, 113622. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Chen, Z.; Yu, Q.; Feng, W. Impact of binding interaction characteristics on physicochemical, structural, and rheological properties of waxy rice flour. Food Chem. 2018, 266, 551–556. [Google Scholar] [CrossRef]
- Chang, Q.; Zheng, B.; Zhang, Y.; Zeng, H. A comprehensive review of the factors influencing the formation of retrograded starch. Int. J. Biol. Macromol. 2021, 186, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, L.; Qian, Y.; Wang, X.; Zhang, S.; Chang, J.; Ruan, Y.; Ma, B. Influences of Extraction Methods on Physicochemical and Functional Characteristics of Three New Bulbil Starches from Dioscorea opposita Thunb. cv. Tiegun. Molecules 2019, 24, 2232. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Obadi, M.; Qi, Y.; Xu, B. Highland barley starch (Qingke): Structures, properties, modifications, and applications. Int. J. Biol. Macromol. 2021, 185, 725–738. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Yi, C.; Quan, K.; Lin, B. Chemical composition, structure, physicochemical and functional properties of rice bran dietary fiber modified by cellulase treatment. Food Chem. 2021, 342, 128352. [Google Scholar] [CrossRef]
- Bernardes, A.; Pellegrini, V.O.A.; Curtolo, F.; Camilo, C.M.; Mello, B.L.; Johns, M.A.; Scott, J.L.; Guimaraes, F.E.C.; Polikarpov, I. Carbohydrate binding modules enhance cellulose enzymatic hydrolysis by increasing access of cellulases to the substrate. Carbohydr. Polym. 2019, 211, 57–68. [Google Scholar] [CrossRef]
- Li, M.; Daygon, V.D.; Solah, V.; Dhital, S. Starch granule size: Does it matter? Crit. Rev. Food Sci. Nutr. 2021, 1–21. [Google Scholar] [CrossRef]
- Punia, S. Barley starch: Structure, properties and in vitro digestibility—A review. Int. J. Biol. Macromol. 2020, 155, 868–875. [Google Scholar] [CrossRef]
- Liu, S.; Xiao, Y.; Shen, M.; Zhang, X.; Wang, W.; Xie, J. Effect of sodium carbonate on the gelation, rheology, texture and structural properties of maize starch-Mesona chinensis polysaccharide gel. Food Hydrocoll. 2019, 87, 943–951. [Google Scholar] [CrossRef]
- Ma, Y.-S.; Pan, Y.; Xie, Q.-T.; Li, X.-M.; Zhang, B.; Chen, H.-Q. Evaluation studies on effects of pectin with different concentrations on the pasting, rheological and digestibility properties of corn starch. Food Chem. 2019, 274, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Sun, R.; Liu, Y.; Wang, X.; Guan, X.; Huang, K.; Zhang, Y. Appropriate microwave improved the texture properties of quinoa due to starch gelatinization from the destructed cyptomere structure. Food Chem.-X 2022, 14, 100347. [Google Scholar] [CrossRef] [PubMed]
- Devraj, L.; Natarajan, V.; Ramachandran, S.V.; Manicakam, L.; Sarvanan, S. Influence of microwave heating as accelerated aging on physicochemical, texture, pasting properties, and microstructure in brown rice of selected Indian rice varieties. J. Texture Stud. 2020, 51, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Hug-Iten, S.; Escher, F.; Conde-Petit, B. Structural properties of starch in bread and bread model systems: Influence of an antistaling alpha-amylase. Cereal Chem. 2001, 78, 421–428. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Y.-S.; Chen, H.-H.; Li, Q.-Q.; Wang, Q. The gelatinization and retrogradation properties of wheat starch with the addition of stearic acid and sodium alginate. Food Hydrocoll. 2018, 81, 77–86. [Google Scholar] [CrossRef]
- Xie, H.; Ying, R.; Huang, M. Effect of arabinoxylans with different molecular weights on the gelling properties of wheat starch. Int. J. Biol. Macromol. 2022, 209, 1676–1684. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y.; Manthey, F.A.; Xu, X.; Jin, Z.; Deng, L. Influence of beta-cyclodextrin on the short-term retrogradation of rice starch. Food Chem. 2009, 116, 54–58. [Google Scholar] [CrossRef]
- Dudu, O.E.; Oyedeji, A.B.; Oyeyinka, S.A.; Ma, Y. Impact of steam-heat-moisture treatment on structural and functional properties of cassava flour and starch. Int. J. Biol. Macromol. 2019, 126, 1056–1064. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, F.; Liu, J.; Zhou, Y.; Lei, L.; Zhao, G. Rheological nature and dropping performance of sweet potato starch dough as influenced by the binder pastes. Food Hydrocoll. 2018, 85, 39–50. [Google Scholar] [CrossRef]
- Wang, N.; Wu, L.; Zhang, F.; Kan, J.; Zheng, J. Modifying the rheological properties, in vitro digestion, and structure of rice starch by extrusion assisted addition with bamboo shoot dietary fiber. Food Chem. 2022, 375, 131900. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Yeh, A.I.; Li, C.Y. Correlation between starch retrogradation and water mobility as determined by differential scanning calorimetry (DSC) and nuclear magnetic resonance (NMR). Cereal Chem. 2001, 78, 647–653. [Google Scholar] [CrossRef]
- Gong, B.; Cheng, L.; Gilbert, R.G.; Li, C. Distribution of short to medium amylose chains are major controllers of in vitro digestion of retrograded rice starch. Food Hydrocoll. 2019, 96, 634–643. [Google Scholar] [CrossRef]
- Qin, W.; Lin, Z.; Wang, A.; Xiao, T.; He, Y.; Chen, Z.; Wang, L.; Liu, L.; Wang, F.; Tong, L.-T. Influence of damaged starch on the properties of rice flour and quality attributes of gluten-free rice bread. J. Cereal Sci. 2021, 101, 103296. [Google Scholar] [CrossRef]
- Qin, W.; Xi, H.; Wang, A.; Gong, X.; Chen, Z.; He, Y.; Wang, L.; Liu, L.; Wang, F.; Tong, L. Ultrasound Treatment Enhanced Semidry-Milled Rice Flour Properties and Gluten-Free Rice Bread Quality. Molecules 2022, 27, 5403. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, D.; Xiang, J.; Xu, W.; Xu, B.; Li, P.; Huang, J. Structural Variations of Wheat Proteins under ultrasound treatment. J. Cereal Sci. 2021, 99, 103219. [Google Scholar] [CrossRef]
- Zhou, D.; Ma, Z.; Yin, X.; Hu, X.; Boye, J.I. Structural characteristics and physicochemical properties of field pea starch modified by physical, enzymatic, and acid treatments. Food Hydrocoll. 2019, 93, 386–394. [Google Scholar] [CrossRef]
- Qin, W.; Lin, Z.; Wang, A.; Chen, Z.; He, Y.; Wang, L.; Liu, L.; Wang, F.; Tong, L.-T. Influence of particle size on the properties of rice flour and quality of gluten-free rice bread. LWT-Food Sci. Technol. 2021, 151, 112236. [Google Scholar] [CrossRef]
- Cheng, W.; Sun, Y.; Xia, X.; Yang, L.; Fan, M.; Li, Y.; Wang, L.; Qian, H. Effects of β-amylase treatment conditions on the gelatinization and retrogradation characteristics of wheat starch. Food Hydrocoll. 2022, 124, 107286. [Google Scholar] [CrossRef]
- Iqbal, S.; Wu, P.; Kirk, T.V.; Chen, X.D. Amylose content modulates maize starch hydrolysis, rheology, and microstructure during simulated gastrointestinal digestion. Food Hydrocoll. 2021, 110, 106171. [Google Scholar] [CrossRef]

| A-HBS | U-HBS | E-HBS | |
|---|---|---|---|
| Total starch (%) | 92.33 ± 0.23 a | 92.15 ± 0.26 a | 90.68 ± 0.12 b |
| Amylose starch (%) | 23.37 ± 0.01 a | 23.71 ± 0.29 a | 23.76 ± 0.03 a |
| Damaged starch (%) | 1.66 ± 0.02 b | 2.57 ± 0.06 a | 1.20 ± 0.03 c |
| Moisture (%) | 8.46 ± 0.06 b | 8.65 ± 0.05 b | 9.14 ± 0.10 a |
| Crude protein (%) | 0.18 ± 0.01 a | 0.24 ± 0.02 a | 0.23 ± 0.02 a |
| Crude lipid (%) | 0.18 ± 0.04 a | 0.25 ± 0.00 a | 0.26 ± 0.02 a |
| Ash (%) | 0.35 ± 0.06 a | 0.25 ± 0.02 a | 0.20 ± 0.01 a |
| Total dietary fiber (%) | 3.04 ± 0.11 a | 3.14 ± 0.02 a | 2.96 ± 0.01 a |
| Yield (%) | 42.15 ± 0.33 a | 30.12 ± 0.27 c | 36.91 ± 0.18 b |
| D10 (μm) | 11.35 ± 0.13 b | 12.02 ± 0.03 a | 12.20 ± 0.13 a |
| D50 (μm) | 19.10 ± 0.12 b | 20.70 ± 0.00 a | 20.81 ± 0.10 a |
| D90 (μm) | 26.90 ± 0.13 b | 30.40 ± 0.03 a | 30.7 ± 0.00 a |
| R1047/1022 | 1.15 ± 0.00 a | 1.11 ± 0.07 a | 1.12 ± 0.02 a |
| A-HBS | U-HBS | E-HBS | |
|---|---|---|---|
| Pasting properties | |||
| PV (cP) | 3597 ± 35 a | 3258 ± 16 b | 3311 ± 11 b |
| TV (cP) | 1935 ± 34 b | 2437 ± 43 a | 2489 ± 36 a |
| FV (cP) | 5180 ± 77 a | 3604 ± 59 c | 3773 ± 21 b |
| BV (cP) | 1662 ± 16 a | 820 ± 29 b | 822 ± 7 b |
| SV (cP) | 3244 ± 16 a | 1167 ± 24 c | 1284 ± 13 b |
| PeT (min) | 4.76 ± 0.14 b | 6.56 ± 0.11 a | 6.60 ± 0.10 a |
| PeT (°C) | 70.76 ± 0.01 c | 90.50 ± 0.04 b | 92.34 ± 0.10 a |
| Textural properties | |||
| 1 d | |||
| Hardness (g) | 210.28 ± 0.90 b | 183.47 ± 0.77 c | 258.58 ± 0.39 a |
| Cohesiveness | 0.43 ± 0.05a | 0.44 ± 0.00 a | 0.45 ± 0.01 a |
| Springiness (%) | 91.03 ± 1.79 a | 90.61 ± 0.43 a | 94.74 ± 0.37 a |
| Gumminess | 92.58 ± 1.77 b | 89.43 ± 0.34 b | 114.28 ± 1.29 a |
| Chewiness | 84.32 ± 1.28 b | 80.76 ± 0.07 b | 103.34 ± 0.52 a |
| 7 d | |||
| Hardness (g) | 283.77 ± 0.86 b | 219.75 ± 0.56 c | 348.30 ± 0.71 a |
| Cohesiveness | 0.58 ± 0.02 a | 0.54 ± 0.04 a | 0.60 ± 0.01 a |
| Springiness (%) | 94.33 ± 1.60 a | 92.53 ± 0.33 a | 95.83 ± 0.21 a |
| Gumminess | 127.45 ± 1.19 b | 112.85 ± 1.02 c | 153.91 ± 1.75 a |
| Chewiness | 134.54 ± 0.08 b | 112.05 ± 1.05 c | 139.74 ± 0.45 a |
| To (°C) | Tp (°C) | Tc (°C) | ΔH (J/g) | |
|---|---|---|---|---|
| A-HBS | 57.03 ± 0.53 b | 61.15 ± 0.61 b | 65.46 ± 0.77 b | 5.69 ± 0.11 b |
| U-HBS | 59.76 ± 0.16 b | 61.99 ± 0.42 b | 65.08 ± 0.45 b | 5.32 ± 0.10 b |
| E-HBS | 62.41 ± 0.08 a | 65.63 ± 0.18 a | 69.30 ± 0.96 a | 6.48 ± 0.37 a |
| A-HBS-1d | 47.32 ± 0.74 b | 50.35 ± 0.53 a | 52.56 ± 1.93 a | 1.46 ± 0.18 b |
| U-HBS-1d | 48.10 ± 0.34 a | 50.92 ± 0.39 a | 51.88 ± 0.29 a | 1.13 ± 0.04 b |
| E-HBS-1d | 47.72 ± 0.64 b | 51.43 ± 0.78 a | 53.55 ± 1.63 a | 2.15 ± 0.12 a |
| A-HBS-7d | 47.62 ± 0.17 b | 52.35 ± 0.89 a | 54.97 ± 0.08 a | 2.27 ± 0.16 b |
| U-HBS-7d | 48.01 ± 0.28 a | 51.10 ± 0.56 b | 52.93 ± 0.32 b | 1.54 ± 0.18 c |
| E-HBS-7d | 48.15 ± 0.16 a | 53.15 ± 1.13 a | 55.12 ± 0.35 a | 3.25 ± 0.09 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, M.; Piao, C.; Li, J.; He, Y.; Xi, H.; Chen, Z.; Wang, L.; Liu, L.; Huang, Y.; Wang, F.; et al. Effects of Different Extraction Methods on the Gelatinization and Retrogradation Properties of Highland Barley Starch. Molecules 2022, 27, 6524. https://doi.org/10.3390/molecules27196524
Nie M, Piao C, Li J, He Y, Xi H, Chen Z, Wang L, Liu L, Huang Y, Wang F, et al. Effects of Different Extraction Methods on the Gelatinization and Retrogradation Properties of Highland Barley Starch. Molecules. 2022; 27(19):6524. https://doi.org/10.3390/molecules27196524
Chicago/Turabian StyleNie, Mengzi, Chunhong Piao, Jiaxin Li, Yue He, Huihan Xi, Zhiying Chen, Lili Wang, Liya Liu, Yatao Huang, Fengzhong Wang, and et al. 2022. "Effects of Different Extraction Methods on the Gelatinization and Retrogradation Properties of Highland Barley Starch" Molecules 27, no. 19: 6524. https://doi.org/10.3390/molecules27196524
APA StyleNie, M., Piao, C., Li, J., He, Y., Xi, H., Chen, Z., Wang, L., Liu, L., Huang, Y., Wang, F., & Tong, L. (2022). Effects of Different Extraction Methods on the Gelatinization and Retrogradation Properties of Highland Barley Starch. Molecules, 27(19), 6524. https://doi.org/10.3390/molecules27196524








