1. Introduction
Twisted bilayer graphene (TwBLG) is a robust correlation system obtained by stacking coupling and interlayer rotation between one layer of graphene nanosheets and another layer of graphene nanosheets. This interlayer interaction will cause the symmetry of the electron to break [
1]. For example, most Mott insulators exhibit an antiferromagnetic arrangement of electron spin [
2,
3]. In 2018, Pablo Jarrilo–Herrero et al. experimentally observed for the first time that magnetic angle graphene could achieve insulation and low-temperature superconductivity under strong electric field conditions slightly above absolute zero, which aroused considerable interest among researchers [
4]. Defective bilayer graphene (BLG) with a small twisted angle will generate the early experimental preparation process [
5]. The distortion angle causes the slight dislocation of the cellular lattice to produce a long period Moiré superlattice. This induces a periodic potential field and a microgap at the edge of the Briviine region of the Moiré superlattice (MS) [
6]. As the interaction increases, the Quantum Anomalous Hall Effect (QAHE) and chiral edge states will occur near the insulating state [
7]. The MS can be produced in a variety of ways, the latest being by cold-pressing graphite sheets [
8]. The Moiré superlattice can effectively regulate the distribution of the magnetic transition dipole moment to control the chirality [
9]. The MS structure has a circular dichroism due to its differential response to polarized light [
10]. This optical activity originates from the relative rotation of the top and underlying layers, leading to the rotation of the helicity of Dirac Fermions [
11,
12]. The transition from a perfect tunnel reflection to a partial reflection by the height and energy of the barrier demonstrates the adjustable chirality of twisted bilayer graphene [
13,
14]. However, the physical mechanism related to the modulation of the twisted angle on the chirality of BLG has not been studied, in particular, circular dichroism with antisymmetric properties. The main purpose of this paper is to study the antisymmetric chirality of zigzag-edge TwBLG-QDs (Z-TwBLG-QDs) based on periodic complementary twist angles by first principles and wave function analysis, and to study the physical mechanism of the chirality of the mole in superlattices by the topological analysis of electron density.
We simulated the Z-TwBLG-QDs based on the periodic complementary twisted angle by precisely controlling the twisted angle between layers and the stacking mode of polar 2D graphene nanosheets [
15,
16,
17,
18]. One-photon absorption revealed the optical absorption properties of quantum dots. The visualizing electromagnetic interaction of the light-quantum dot system [
19] is used to study the axisymmetric circular dichroism in ECD. Atomic orbital transitions of DOS reveal the anti-symmetric distribution of the transition electric dipole moment (TEDM) and the transition magnetic dipole moment (TMDM). A topological analysis of electron density reveals the physical mechanism by which MS determines chirality.
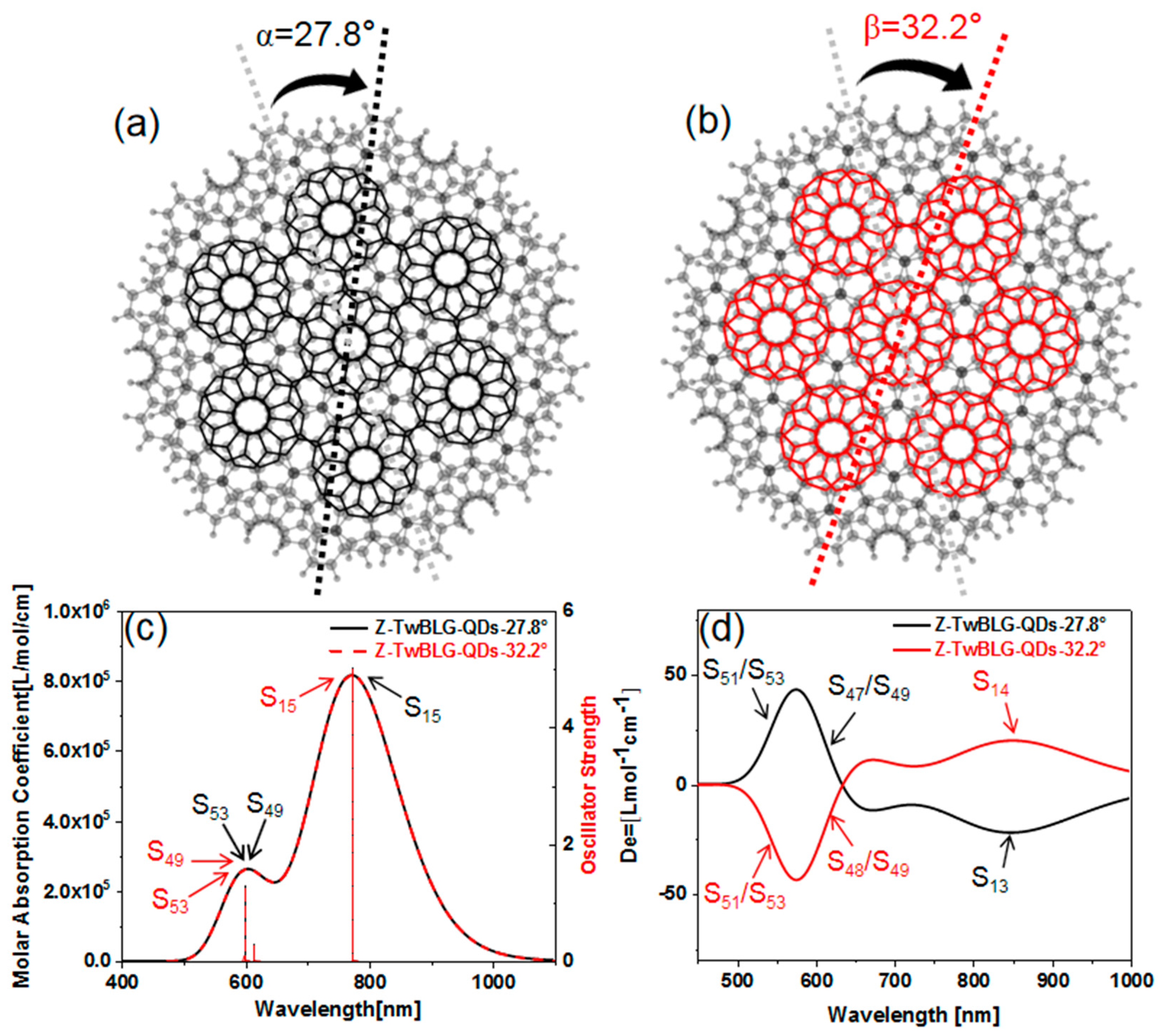
The graphene monolayer is a regular hexagon structure, and the edge is composed of seven benzene rings. We use the modeling software MS to adjust the two graphene nanosheets to the appropriate layer spacing, and through the Van der Waals force between layers, the micro nano “Van der Waals superposition structure” quantum dots are formed. This structure makes BLG exhibit a strong interaction between coupling layers [
20]. Keep the geometric center of the graphene nanosheet as the origin, and rotate the top graphene nanosheet through a specific “magic angle”. When the twisted angles are 27.8° and 32.2°, the periodic complimentary relationship (the sum of angles are 60°, and the Moiré superlattice is the same during the rotation of every 60° of the top layer graphene) is satisfied. Two structures of MS with the same shape but different rotation directions (Z-TwBLG-QDs-27.8° and Z-TwBLG-QDs-32.2°) are obtained, see
Figure 1a,b.
2. Results
The UV–vis and ECD spectra are calculated to study the optical absorption characteristics of quantum dot system. As shown in
Figure 1c, the spectra of Z-TwBLG-QDs-27.8° and Z-TwBLG-QDs-32.2° overlap. The main absorption peak is near 772.3 nm, which is contributed to by S
15, and the second absorption peak is contributed to by two excited states (S
49 and S
53). The main absorption peak of Z-TwBLG-QDs-32.2° is near 772.4 nm, which is contributed to by two excited states (S
15 and S
16), and the secondary peak is contributed to by two excited states (S
49 and S
53). As shown in
Figure 1d, ECD shows two regions with high rotatory strength in the visible light region. When the twisted angle is 27.8°, the corresponding ECD excited states are S
51/S
53 and S
47/S
49. When the twisted angle is 32.2°, the corresponding excited states are S
51/S
53 and S
48/S
49. The excited states corresponding to the absorption peaks in the near-infrared region are S
13 and S
14. In addition, the rotatory intensity of the excited states of the two systems at the same wavelength is axisymmetric, and the absolute value is almost the same (see
Table S2 in supplementary). Therefore, the electromagnetic interaction intensity of their incident light-quantum dots is very close. The symmetrical rotatory strength makes the molar absorption coefficients of Z-TwBLG-QDs-27.8° and Z-TwBLG-QDs-32.2° satisfy an axisymmetric relationship, indicating that the molecular system exhibits symmetric circular dichroism in response to polarized light.
Circular dichroism is the asymmetric response of the electric and magnetic transition dipole moments when the system interacts with electromagnetic waves, which can reflect the chiral properties of the system. The TEDM and TMDM are plotted to reveal the physical mechanism of the axial symmetry of the ECD spectrum. The transition dipole moment density is the product of the transition density and the coordinate variables in the three directions x, y, and z of the cartesian coordinates [
21]:
The transition dipole moment density here is connected to the transition electric dipole moment, specifically. The contribution of the x, y, and z components to TEDM is obtained. The component of the transition magnetic dipole moment can be defined as
The component of the transition magnetic dipole moment
is defined to visualize the transition magnetic dipole moment. The explicit expression is
Its full-space integral is the transition magnetic dipole moment as follows:
To make the distribution characteristics of TEDM and TMDM more precise and more intuitive, the smoothing electric/magnetic moments (TEDM
S and TMDM
S) of the system on the right side of TEDM and TMDM in
Figure 2,
Figure 3 and
Figure 4 are performed. TEDMS and TMDM
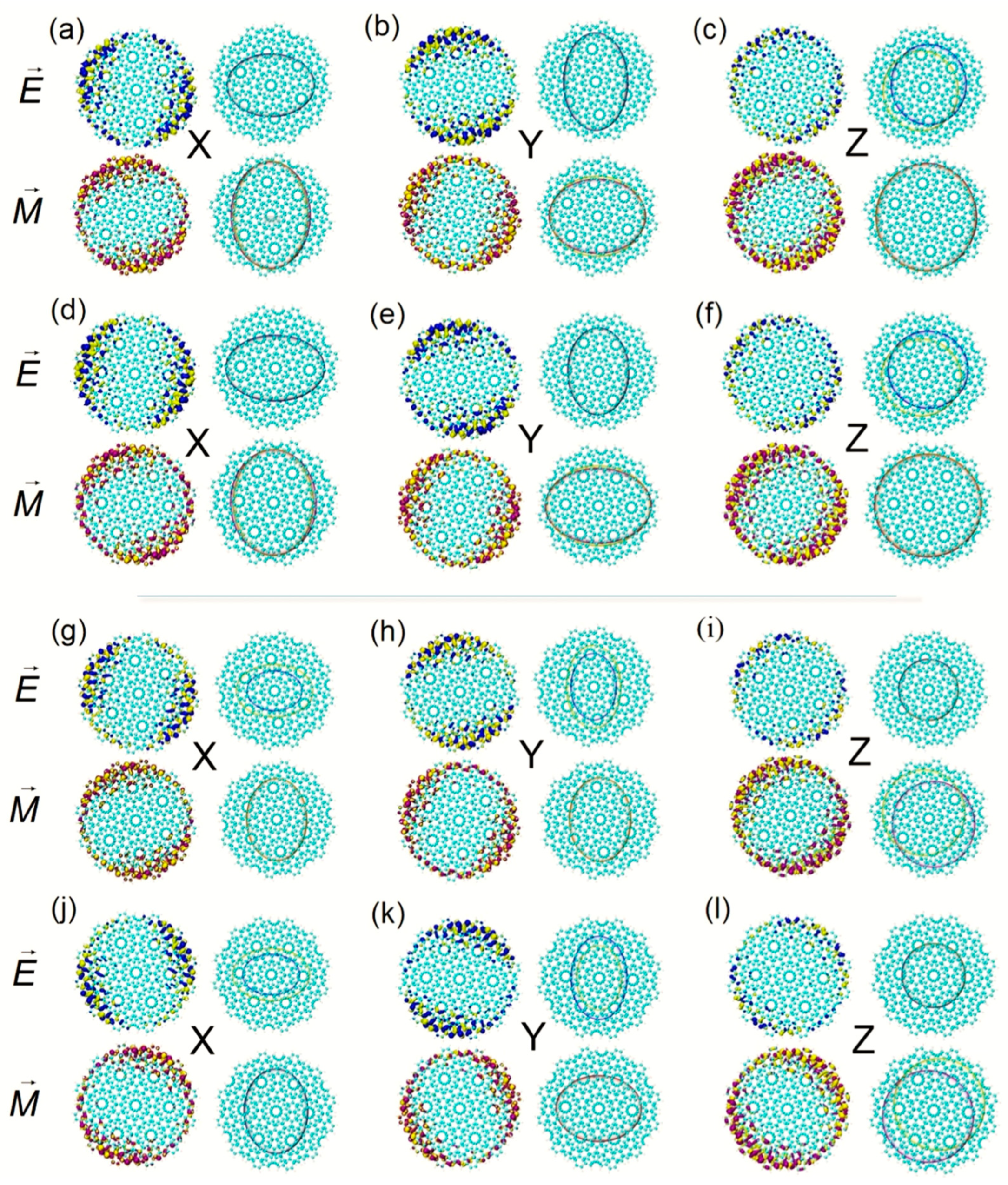
S are equivalent to describing the distribution of TEDM and TMDM with Gaussian functions equivalently, and the distribution details are erased. At this time, the isosurface map of TEDM and TMDM becomes an ellipse. When the twisted angle is 27.8°, the TEDM of S
13 at 844.5 nm is symmetrically distributed in the x, y, and z components. The isosurface is gathered at the edge of the BLG, which corresponds to the long axis of the smoothing isosurface of the ellipse. The positive and negative isosurfaces of TEDM on the z component are wholly separated. The TMDM is distributed on the axis of symmetry on the x and y components. The distribution is uniform in the z component and tends to the Moiré superlattice, see
Figure 2a–c. S
14 is the degenerate state of S
13, and their TEDM and TMDM on the x and y components are perpendicular to each other. ECD shows that the rotatory intensities of the two excited states contribute negatively to ECD. When the twisted angle changes to 32.2°, the absorption peak at the same wavelength is contributed to by S
13. Compared with S
14, the TEDM and TMDM on the x, y, and z components are anti-symmetric, so S
13 is responsible for ECD absorption. S
14 is responsible for chirality.
Comparing the ECD excited state of the two systems at 844.5 nm, the distribution range of TEDM
S in S
13 is more extensive in the x component, and the TEDM of S
14 is more extensive in the y component, see
Figure 2d,e,g,h. On the z component, the TEDM and TMDM distributions are also anti-symmetric. The TEDM
S and TMDM
S show that the positive and negative isosurface face have opposite directions, see
Figure 2f,i, which indicates that the axisymmetric ECD spectrum at 844.5 nm is mainly contributed to by the anti-symmetric distribution of the TEDM in the z component. The excited states of Z-TwBLG-QDs-27.8° and Z-TwBLG-QDs-32.2° of chiral reversal at 614.5 nm are S
47 and S
48, respectively. Their sizes of the TEDM and TMDM isosurface in Cartesian coordinates and distributions are very close, so these two excited states are responsible for optical absorption in the ECD, see
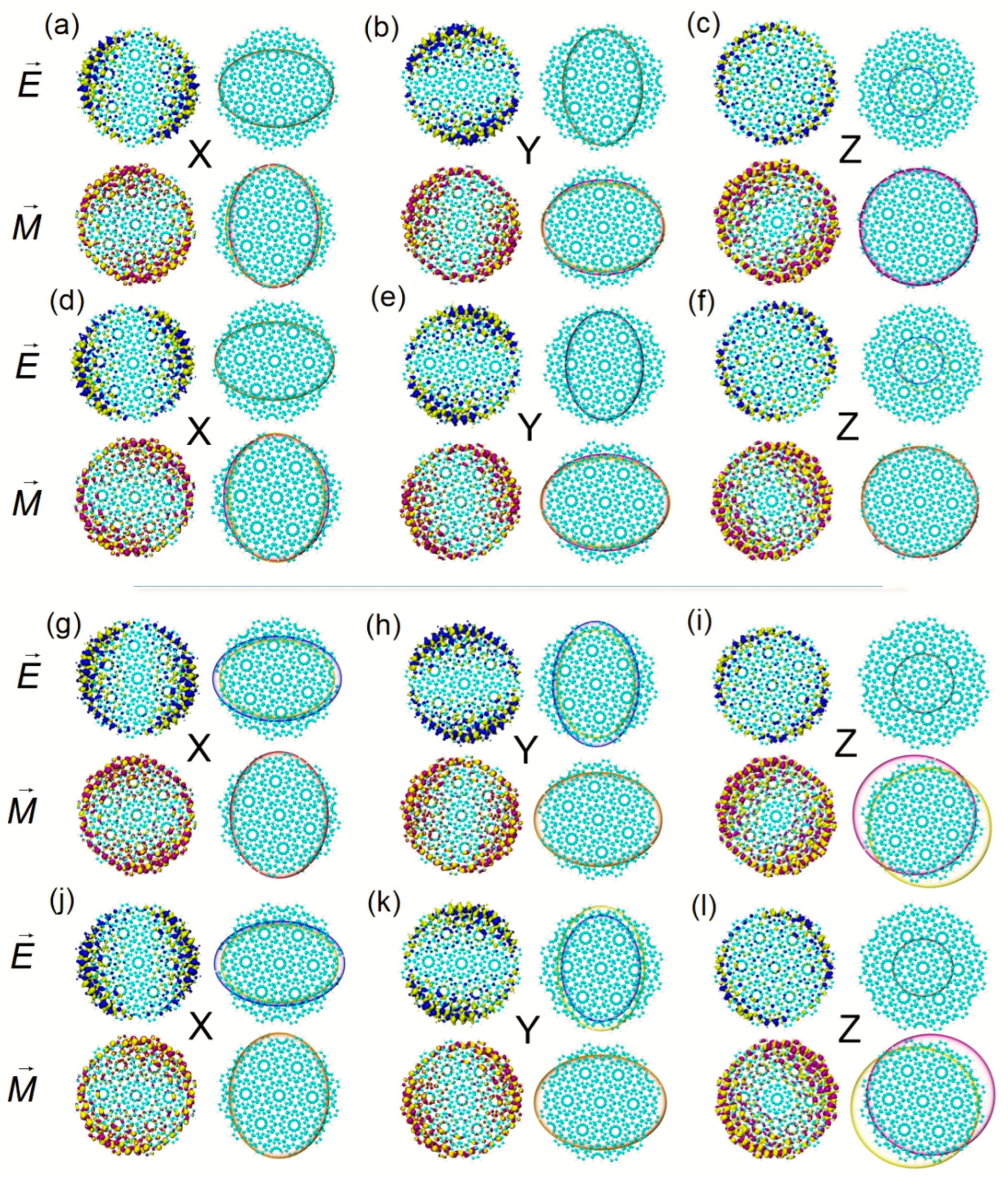
Figure 3a–f.
The ECD excited state of the two systems at 612.8 nm is contributed to by S
49. When the twisted angle is 27.8°, the TEDMs on the x and y components are distributed axisymmetrically on the edge of the BLG, TEDM
S, and TMDM
S shows that the negative value is greater than the positive value, the TEDM isosurface on the z component is significantly reduced, and the TEDM
S shows the more significant degree of separation of positive and negative values. The isosurface distribution of TMDM also has axial symmetry. The proportions of positive and negative values on the x and the y component are similar, and the positive and negative values on the z component are separated to a greater extent, see
Figure 3g–i. When the twisted angle is 32.2°, the negative value of TEDM on the x component is greater than the positive value, and the situation on the y component is the opposite. Additionally, the positive–negative value ratio of TMDM changes. On the z component, the positive and negative isosurfaces also separate to a greater extent and the directions are opposite, see
Figure 4j–l. Therefore, the axisymmetric ECD of the two systems at 612.7 nm is mainly caused by the anti-symmetric distribution of the positive and negative isosurfaces on the z component.
The ECD excited state of Z-TwBLG-QDs-27.8° at 602.2 nm is S
51. TEDM and TMDM are axisymmetrically distributed in the x and y components. The TEDM in the z component is significantly reduced, but the TMDM is significantly increased. The edge distribution spreads to the Moiré superlattice, and the positive and negative values of the TEDM
S and TMDM
S are more evenly distributed, see
Figure 4a–c. When the twisted angle is 32.2°, TEDM and TMDM have the same changing trend. The difference is that the ratio of the positive and negative values of the TMDM
S on the x component changes, the TEDM on the y component changes, and the orientation of TEDM
S on the z component is opposite, see
Figure 4d–f. Another group of ECD excited states near 598.2 nm is S
53. The distribution characteristics of TEDM and TMDM are similar to S
51. The TMDM
S of Z-TwBLG-QDs-27.8° on the x component are larger, and the negative value of TEDM
S on the y component is larger, see
Figure 4g–h, while for Z-TwBLG-QDs-32.2°, the negative value of the TEDM
S on the x component is larger, the positive value of TMDM on the y component is more significant, and the direction of TMDM
S on the z component is opposite, see
Figure 4j–l. This situation is widespread in other ECD excited states with axisymmetric circular dichroism, and it intuitively reveals the physical mechanism of the anti-symmetric distribution of the transition electric dipole moment and the transition magnetic dipole moment.
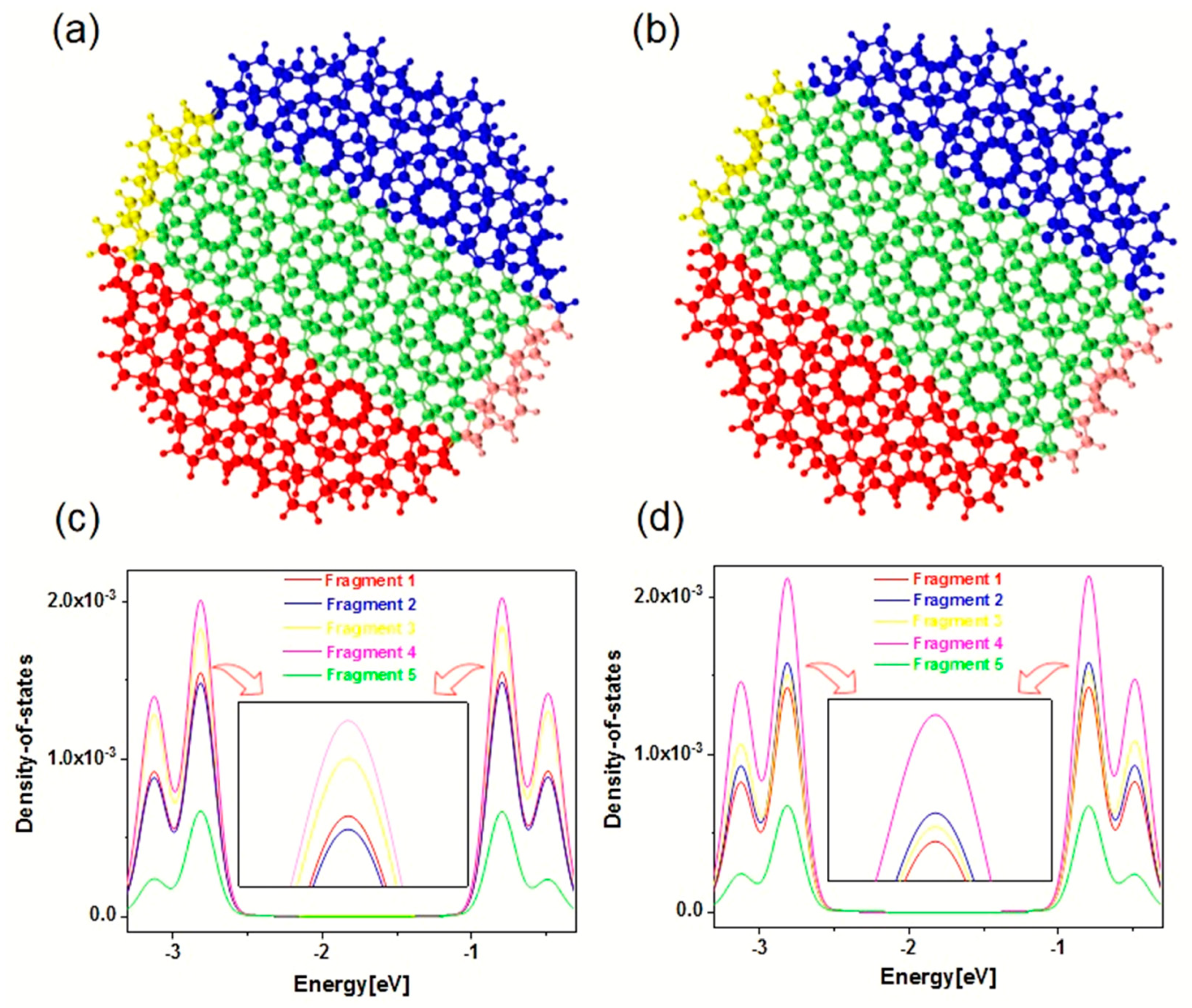
The above analysis discusses the electromagnetic interaction mechanism of Z-TwBLG-QDs based on the periodic complementary twisted angles. It is necessary to investigate the DOS analysis of the area with anti-symmetric distribution of TEDM and TMDM to reveal the physical mechanism of the anti-symmetric distribution of TEDM and TMDM, corresponding to fragments 1–5, see
Figure 5a,b. Fragment definition adds the basis functions on the atom to the fragment, which is marked with different colors. As shown in
Figure 5c,d, the normalized integral curve of PDOS represents the contribution of each fragment’s atomic orbital, the relative positions of the blue curve, and the yellow curve alternate near the energy of −2.77 eV. According to Mulliken division, the contribution of the r-th basis function to the transition dipole moment is as follows:
where
is the transition density matrix between certain two states and
is the electric dipole moment integral between the
and
basis functions.
The contribution of the atoms to the transition dipole moment is to sum the basis function contributions of all atoms since the transition electric dipole moment is the transition dipole moment from the ground state to the excited state. Therefore, the alternating phenomenon of the contribution of the fragment atomic orbits will cause significant changes in the response mode of the transition electric dipole during the light-quantum dot interaction of the system. Combining Formulas (1) and (2), alternating atomic orbital contributions will change the orbital wavefunctions and , thereby causing the changes in the transition electric dipole moment and the transition magnetic dipole moment. These changes will indicate the anti-symmetric distribution of TEDM and TMDM for the two systems. The MS structure that is based on the periodic complementary twisted angles on the atomic orbitals of the edge region are the cause of this situation.
The above discussion qualitatively reveals the physical mechanism of the axisymmetric ECD of the quantum dot system. To investigate the origin of the symmetric circular dichroism quantitatively, it is necessary to calculate the helical properties of chiral interactions in molecular systems. These can be investigated by the polarization (the sensitivity of electron density to deformation in an electric field) of atomic fragments in chiral molecules according to the theory of molecular polarizabilities [
21]. The electron density is defined as
where
is the occupied number of orbital
,
is the orbital wavefunction,
is the basis function, and
is the coefficient matrix. The element in the
row and
column correspond to the expansion coefficient of orbit
relative to the basis function
. When the twisted angle is 27.8°, the MS structure contains an axisymmetrically distributed Moiré superlattice along the central benzene ring of the graphene layer. The RCP of the Moiré superlattice is found in the chemical bond formed by the benzene ring on the top and underlying graphene.
As shown in
Tables S4–S6 in supplementary, the real space function of the RCP of the Coronene and the two Z-TwBLG-QDs are calculated through the topological analysis of the electron density. The
of the two systems are very close. All RCP are numbered to analyze the correlation between the
and the chirality of the system. The red serial numbers correspond to the RCP of the Moiré superlattice in the top graphene, and the blue serial numbers correspond to the RCP of the Moiré superlattice in the underlying graphene.
Figure 6a indicates the changing trend of the normalized
. The polarization of the Moiré superlattice increases as the electron density increases after removing an RCP with the smallest polarization. The red circular arrow on the top layer of the graphene is the reduced order of the electron density, see
Figure 6c. The rotation direction is clockwise, so the top layer graphene is a right-handed spiral, while the underlying graphene is a left-handed spiral, see
Figure 6b,d. The Laplace function of the electron density (
) is to calculate the gradient of
:
The normalized
is the same as the curve of normalized
because
is the second derivative of
, see
Figure S3a,b. The numerical value indicates that
is positive, indicating that the interlayer interaction originates from the closed-shell interaction of the π-π bond in the Moiré superlattice. The interaction that is different from the covalent bond comes from the shared electron pair, so there is no electron accumulation in the bonding area. The increase in the degree of aggregation will reduce the surging ability of electrons and weaken the role of electrons in the electrostatic interaction of the region of MS, making the nuclear electrostatic potential of Z-TwBLG-QDs relative to Coronene increase, see
Figure 6e,f.
The values of
between the Coronene and the two Z-TwBLG-QDs reflect that the MS will enhance the localization. It is necessary to analyze the localized orbital locator (LOL) of the Coronene and the two systems of Z-TwBLG-QDs to further reveal the influence of the MS on the localization of the system.
Tables S4–S6 in supplementary show that the value of LOL at the central RCP of the two systems is significantly greater than that of Coronene. The color-filled map of LOL-pi at 1.2 Bohr above the plane of the molecular system was drawn to visually analyze this difference. As shown in
Figure 7c, the LOL-pi coloring diagram of Coronene shows that the difference from RCP is that the outer-shell electrons of the carbon atom of the central six-membered ring have no significant changes due to the stable conjugated structure.
On the contrary, the π electrons delocalize between adjacent carbon atoms at the six protruding points of the zigzag edge. After adding the Moiré, the delocalization path of the π electron does not include the six-cornered outer benzene ring. Additionally, the delocalization gradually weakens from the outside to the inside because the gradient of orbital wavefunction of the system at the boundary is larger than the inside, see
Figure 7d,e.
Another tool for analyzing the delocalization of electrons is the electron localization function (ELF), which has a similar physical meaning to LOL. The changing trend of the ELF value of the three systems’ RCP is the same as that of LOL, see
Figure 7b. The previous discussion indicates that the electrons at the edge of Z-TwBLG-QDs are more active than the internal ones under the edge effect. The ELF_pi isosurface can intuitively reflect the delocalization characteristics of the molecular system contributed to by π electrons, see
Figure 7f. When the pi isovalue of the six-membered ring in the Coronene center is broken, the isovalue is 0.495. When the pi isovalue of the six-member ring at the edge is broken, the isovalue is 0.518. Therefore, the conjugation degree of the central six-membered ring is lower than the edge, see
Figure S4a,b. When the isovalue is set to 0.7, it can be clearly seen that the π isosurface at the edge is more significant than the internal one, and this law also applies to the more extensive system such as Z-TwBLG-QDs. As shown in
Figure 7g,h, the π isosurface of Z-TwBLG-QDs-27.8 gradually decreases from the edge to the internal, and the degree of π conjugation decreases. This situation is the same when the twisted angle is 32.2°.